ABSTRACT
Background
The Parkinson's disease (PD) patient population, with an already reduced life expectancy, is rendered particularly vulnerable by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2).
Objectives
We determined the risk factors that increase the risk of death in patients with Parkinson's disease who are infected by SARS‐CoV‐2.
Methods
Patients with a diagnosis of PD admitted to Montefiore Hospital (Bronx, New York) and tested for SARS‐CoV‐2 were identified. Retrospective review of electronic medical records confirmed the diagnosis; patients were classified by severity of PD. PD severity, demographic, socioeconomic factors, and co‐morbidities were correlated with mortality rates in patients with SARS‐CoV‐2.
Results
We identified 162 patients meeting criteria; chart review confirmed a diagnosis of PD in 70 patients. Of the 70 patients, 53 were positive for SARS‐CoV‐2 and 17 were negative. PD patients with SARS‐CoV‐2 infection had a higher mortality rate (35.8%) compared to PD patients without the infection (5.9%, P = 0.028). PD patients older than 70 years of age, those with advanced Parkinson's disease, those with reductions in their medications, and non‐Hispanics (largely comprised of Black/African‐ Americans) had a statistically significant higher mortality rate, if infected.
Conclusions
PD did not increase mortality rates from SARS‐CoV‐2 infection when age was controlled. However, certain unalterable factors (advanced disease and age greater than 70 years) and alterable ones (reductions in PD medications) placed PD patients at increased risk for mortality. Also several socioeconomic factors contributed to mortality, for example, non‐Hispanic patients with SARS‐CoV‐2 infection fared worse, likely driven by poorer outcomes in the Black/African‐American cohort.
Keywords: Parkinson's disease, SARS‐CoV‐2, mortality, socioeconomic, ethnicity
Parkinson's disease (PD) is the second most common neurodegenerative disorder that reduces life expectancy.1 The features most commonly found to reduce survival are the akinetic‐rigid phenotype, PD dementia or mild cognitive impairment (MCI), and early autonomic dysfunction.1, 2, 3, 4
Based on Medicare data, in New York approximately 1.78% of individuals (1781/100,000) aged 65 or older have PD, and the annual inpatient hospital admission rate for PD patients is greater than 558/1000, for both neurologic and non‐neurologic causes.5 One study found that the prevalence of PD in the US is 1.6% in patients over the age of 65 years. Of those patients, 7% to 28% were hospitalized ‐ approximately 1.5 times more often than non‐PD patients.6
The PD patient population, with an already reduced life expectancy, is rendered particularly vulnerable by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2). The Bronx has had the highest rates of infection, hospitalizations, and deaths from coronavirus disease‐2019 (COVID‐19) in New York City,7 which was an epicenter of the pandemic. Up to the point of preparation of this manuscript, 5.9% of residents in the Bronx had contracted COVID‐19, and 9% of them had died.8 Only the most severe cases were advised to come to the hospital.
Physicians and scientists at our institution, Montefiore Medical Center/Albert Einstein College of Medicine, the Bronx's largest medical complex and the center of a large tertiary network serving Westchester and several other nearby populous counties, published the impact of COVID‐19 on patients who previously were healthy and those who had pre‐existing illnesses.9 A more recent study demonstrated a higher in‐hospital mortality rate in patients with COVID‐19 with neurologic presentations.10
At the outbreak of the epidemic, much of the information about patients with PD who contracted COVID‐19 was reported from other countries. A survey, including over 700 patients, conducted in the Tuscany region among multiple centers, found an increased prevalence of COVID‐19 in patients with PD.11 However, it could not be concluded that PD is a risk factor for COVID‐19. A case series of 10 patients in Padua, Italy and London, UK showed patients with older age and longer duration of PD (12.7 years) with COVID‐19 had a high (40%) mortality rate.12 A larger case series from PD centers across Italy, Iran, UK, and Spain, also demonstrated a high mortality rate in PD patients with COVID‐19, and advanced age and hypertension increased the likelihood of death.13 A case–control study done in a tertiary center in Madrid found a 21% mortality rate in PD patients with COVID‐19; being institutionalized and having a comorbid malignancy increased the risk of acquiring SARS‐CoV‐2 infection and impacted its severity.14 Another study found a higher mortality rate (36%) in patients with all types of movement disorders compared to the general population.15
The aim of our study was to determine the risk factors that make a PD patient more likely to succumb if infected by SARS‐CoV‐2. We analyzed the records of patients with PD and SARS‐CoV‐2 infection who survived or had died at the time of discharge from our medical center. In addition to assessing factors that have previously been assessed (age, cognitive impairment, non‐neurological co‐morbidities), we assessed factors specific to the population in the Bronx that also play a role in increased morbidity and mortality, particularly ethnicity and socioeconomic status.16, 17 Our secondary objective was to determine the degree of risk PD adds to all patients contracting COVID‐19. A tertiary objective was to compare mortality in PD inpatients with and without a COVID‐19 diagnosis.
Methods
Study Population
This study analyzed the risk factors for mortality among patients with PD who were tested for SARS‐CoV‐2, admitted between March 1st and May 31st 2020 at Montefiore Medical Center. In order to capture and analyze the data, the charts of all patients admitted during this period and tested for SARS‐CoV‐2 virus through real‐time PCR detection, in nasopharyngeal swabs, using FDA‐approved assays (Abbott, Luminex Aries, Cepheid Xpert Xpress SARS‐CoV‐2, or Hologic Panther Fusion real‐time RT‐PCR SARS‐CoV‐2 assay) were searched for PD ICD 10 diagnosis codes. We identified 162 patients and reviewed the electronic charts to determine if these patients had PD or other conditions where PD had to be excluded. Two movement disorders neurologists independently reviewed all the charts and where disagreement existed, a third movement disorders specialist adjudicated the case. The third movement disorders neurologist was needed for 6 cases out of the 162 charts. Mortality was determined by the status at hospital discharge of the index admission, when COVID‐19 testing was done.
Based on chart review, patients with PD were classified into three stages of severity, as determined by the complexity of PD treatment and the patient's functionality. The usual staging system, specifically the Hoehn and Yahr (H&Y) classification could not be applied because these patients were generally not evaluated by movement disorders specialists during their hospitalization because their infectious illness was their primary problem. The three stages were defined as follows: Stage 1 (mild), patient taking <1000 mg of levodopa, and possibly one other medication, for example, a dopamine agonist, amantadine, or a monoamine oxidase ‐ B (MAO‐B) inhibitor, and living at home; Stage 2 (moderate), patient taking 1000‐1500 mg of levodopa, and 2 or more other PD medications, living at home; Stage 3 (severe), institutionalized or dependent on basic activities of daily living, and minimally ambulatory, wheelchair bound, or bedbound.
Statistical Analysis
We analyzed data using SPSS v27 (IBM, Armonk, NY) to determine statistically significant association between the variables and mortality rate in patients with PD. The associations between mortality risk factors in patients with PD and COVID‐19 and categorical variables were tested for significance using a Fisher exact test. Continuous variables were tested for significance using a Mann–Whitney U test. Statistically significant p‐value was preset at 0.05.
Results
During the three‐month period of our study, March 1, 2020 to May 31, 2020, 7619 subjects were identified as admitted and tested for COVID‐19, out of which 3877 tested positive for SARS‐CoV‐2 qPCR during the admission. In the same period 70 patients with previously diagnosed PD were admitted to the hospital and tested for SARS‐CoV‐2 qPCR. Out of 70, 53 tested positive and 17 tested negative (Fig. 1). No patients were diagnosed with new‐onset PD during their admission.
FIG. 1.
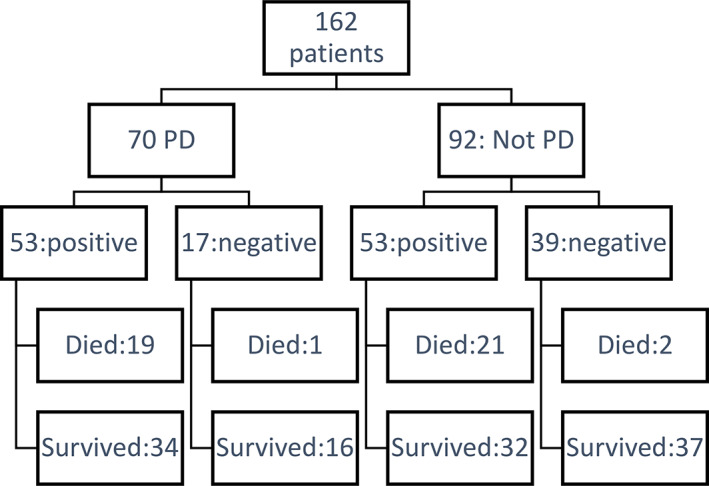
Overall patients assessed and categorized into PD, COVID‐19 status, and survivor status.
Patient Demographics
The age distribution was similar among COVID‐19 positive and negative PD patients (Table 1). The distribution of race/ethnicities was similar in both the COVID‐19 positive and negative PD cohorts (Table 1). More PD patients were institutionalized among the patients with COVID‐19, 25 (47%) compared to the patients without COVID‐19, 6 (35.3%). Of the PD patients who came from home and had COVID‐19, the median annual household income, based on the respective zip codes of residence, was lower ($48,685) than in patients without COVID‐19 ($63,952), which was not significantly different (P = 1.0).
TABLE 1.
Demographics of PD patients with and without COVID‐19 and PD stage
| Parkinson's disease and COVID‐19 positive | Parkinson's disease and COVID‐19 negative | Distribution COVID‐19 positive vs. COVID‐19 negative | Effect on mortality in COVID‐19 positive | Effect on mortality in COVID‐19 negative | Effect on mortality COVID‐19 positive vs. COVID‐19 negative | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall number (N, %) | Lived (N, %) | Died (N, %) | Overall number (N, %) | Lived (N, %) | Died (N, %) | P value | ||||
| Patients (N, %) | 53 | 34 (64.2) | 19 (35.8) | 17 | 16 (94.1) | 1 (5.9) | 0.028 | |||
| Age | ||||||||||
| Average age (range, IQR) | Average 78.7 (61–92, 12) | 78.5 (65–92, 12) | 82 (61–88, 12) | Average 77.8 (66–93, 11) | 76.5 (66–93, 12) | 80 | 0.593 | 0.446 | 0.706 | 0.9 |
| 60–70 yr | 9 (17) | 7 (77.8) | 2 (22.2) | 3 (17.6) | 3 (100) | 0 | 1 | 0.463 | 1 | 1 |
| >70 yr old | 44 (83) | 27 (61.4) | 17 (38.6) | 14 (82.3) | 13 (92.8) | 1 (7.2) | 1 | 0.463 | 1 | 0.044 |
| Gender | ||||||||||
| Male | 31 (58.5) | 19 (61.3) | 12 (38.7) | 10 (58.8) | 9 (90) | 1 (10) | 1 | 0.773 | 1 | 0.129 |
| Female | 22 (41.5) | 15 (68.2) | 7 (31.8) | 7 (41.2) | 7 (100) | 0 | 1 | 0.773 | 1 | 0.147 |
| Race | ||||||||||
| Asian | 1 (1.8) | 1 (100) | 0 | 0 | 0 | 0 | ||||
| Black or African American | 15 (28.3) | 8 (53.3) | 7 (46.7) | 5 (29.4) | 5 (100) | 0 | 0.696 | 0.4 | 0.114 | |
| White | 8 (15.1) | 6 (75) | 2 (25) | 4 (23.5) | 4 (100) | 0 | 0.696 | 0.4 | 0.515 | |
| Other | 26 (49.1) | 17 (65.4) | 9 (34.6) | 6 (35.3) | 5 (83.3) | 1 (16.7) | ||||
| Unavailable | 3 (5.7) | 2 (66.6) | 1 (33.4) | 2 (11.8) | 0 | 0 | ||||
| Ethnicity | ||||||||||
| Hispanic | 20 | 12 (60) | 8 (40) | 7 (41.2) | 6 (85.7) | 1 (14.3) | 1 | 0.768 | 0.412 | 0.363 |
| Not Hispanic | 29 | 19 (65.5) | 10 (34.5) | 10 (58.8) | 10 (100) | 0 | 1 | 0.768 | 0.412 | 0.04 |
| Declined/Unavailable | 4 (7.6) | 3 (75) | 1 (25) | 0 | 0 | 0 | ||||
| Stage of Parkinson Disease | ||||||||||
| I | 16 (30.2) | 12 (75) | 4 (25) | 4 (23.6) | 4 (100) | 0 | 0.761 | 0.358 | 1 | 0.538 |
| II | 10 (18.9) | 5 (50) | 5 (50) | 3 (17.6) | 3 (100) | 0 | 1 | 0.465 | 1 | 0.231 |
| III | 27 (50.9) | 17 (63) | 10 (37) | 10 (58.8) | 9 (90) | 1 (10) | 0.591 | 1 | 1 | 0.224 |
| Stage of Parkinson Disease (combined) | ||||||||||
| I | 16 (30.2) | 12 (75) | 4 (25) | 4 (23.6) | 4 (100) | 0 | 0.761 | 0.358 | 1 | 0.538 |
| II & III | 37 (69.8) | 22 (59.5) | 15 (40.5) | 13(76.4) | 12 (92.3) | 1 (7.7) | 0.761 | 0.358 | 1 | 0.039 |
The median age of PD patients with or without SARS‐CoV‐2 infection who died was not statistically different from the median age of those who survived (Table 1). There was a statistically significant higher mortality rate in patients older than 70 years with COVID‐19 than in 60–70 years old PD patients (P = 0.044).
Gender distribution was equivalent in COVID‐19 positive and negative PD cohorts and it did not impact mortality in either COVID‐19 positive or COVID‐19 negative patients (Table 1).
Racial/ethnic distribution among both COVID‐19 positive and negative cohorts was similar in regard to Black/African‐American, Hispanic, and non‐Hispanic groups (Table 1). The mortality rate of Black/African‐American patients was higher than that of white patients in the COVID‐19 positive cohort, but this difference was not statistically significant. Non‐Hispanic patients had a higher mortality rate (P = 0.04) in the COVID‐19 positive group than the COVID‐19 negative group (Table 1).
Of the 28 patients admitted from home, the median household income of the areas where they resided was $48,685, which is less than the median household income of people living in the US ($68,703). PD patients without COVID 19 had a median annual household income similar ($63,952) to that of the US median. Ten of the 28 patients admitted from home, died. These 10 patients came from areas where the median household income was $41,454, which is lower than that of the median household income of people in the US, but was not statistically significant. Only one patient died in the COVID‐19 negative group; however, because this patient came from an institution, his household income could not be determined.
Overall Mortality
Of the total 3878 patients who tested positive for SARS‐CoV‐2, 971 (25.4%) died during their initial admission to the hospital. Nineteen of the 53 patients with PD and COVID‐19 died, while only 1 of the 17 PD patients without COVID‐19 died (Table 1). There was a statistically significant higher mortality rate in patients with COVID‐19 compared to those without COVID‐19 (P = 0.028) (Table 1). The mortality rate in patients with SARS‐CoV‐2 infection and PD (35.8%) was similar to age‐matched patients with COVID‐19, but no PD (34.7%).
Among the patient charts that were reviewed, who had other diagnoses, not PD, 53 were COVID‐19 positive, with a mortality rate of 39.6%. Within this group of COVID‐19 positive patients, 45 were aged 60 or older, with a mortality rate of 35.6%, which is similar to the PD patients with COVID‐19 (Fig. 2).
FIG. 2.
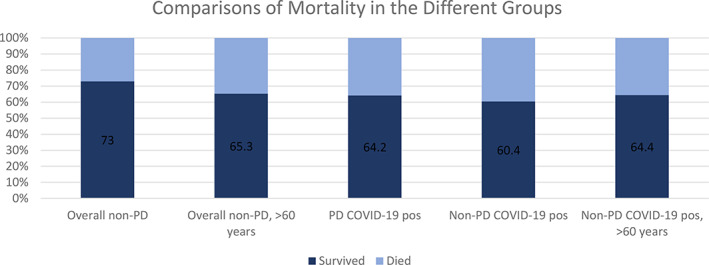
Comparison of mortality rates in patients with COVID‐19, with or without PD and older age.
PD specific Risk Factors for Mortality
The distribution of patients by stage of PD severity was similar in the COVID‐19 positive and negative patients (Table 1). When combined, patients in more advanced stages (Stage 2 and 3) had a significantly higher mortality rate in the COVID‐19 positive compared to the COVID‐19 negative cohort.
Effect of Comorbidities on PD Mortality
There were more PD patients with dementia in the COVID‐19 negative cohort (100%), compared to the COVID‐19 positive cohort (P = 0.0201). Although PD patients with COVID‐19 and dementia had a higher mortality rate, there was no statistical difference compared to the COVID‐19 negative cohort. Patients without any other neurologic comorbidity had a higher mortality rate in the COVID‐19 positive PD cohort (P = 0.038) (Table 2).
TABLE 2.
Effect of Comorbidities on mortality rate in PD patients with and without COVID‐19
| Parkinson's disease and COVID‐19 positive | Parkinson's disease and COVID‐19 negative | Distribution COVID‐19 positive vs. COVID‐19 negative | Effect on mortality in COVID‐19 positive | Effect on mortality in COVID‐19 negative | Effect on mortality COVID‐19 positive vs. COVID‐19 negative | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Overall number (N, %) | Lived (N, %) | Died (N, %) | Overall number (N, %) | Lived (N, %) | Died (N, %) | P value | ||||
| Neurologic comorbidities (before COVID‐19) | ||||||||||
| Overall neurologic comorbidities (N, %) | 33 (62.3) | 25 (75.8) | 8 (24.2) | 13 (76.5) | 12 (92.3) | 1 (7.7) | 0.383 | 0.038 | 1 | 0.41 |
| Dementia | 14 | 9 (64.3) | 5 (35.7) | 10 | 9 (90) | 1 (10) | 0.02 | 1 | 1 | 0.341 |
| Stroke or ICH history | 1 | 1 (100) | 0 | 1 | 1 (100) | 0 | 0.429 | 1 | 1 | |
| Schizophrenia | 5 | 4 (80) | 1 (20) | 1 | 1 (100) | 0 | 1 | 0.643 | 1 | 1 |
| Neuropathy | 11 | 10 (91) | 1 (9) | 4 | 4 (100) | 0 | 1 | 0.074 | 1 | 1 |
| Migraine & other headaches | 9 | 6 (66.7) | 3 (33.3) | 4 | 4 (100) | 0 | 0.72 | 1 | 1 | 0.497 |
| Sleep disturbance (REM sleep behavior, OSA, insomnia) | 11 | 8 (72.7) | 3 (27.3) | 4 | 4 (100) | 0 | 1 | 0.726 | 1 | 0.516 |
| Seizure history | 5 | 4 (80) | 1 (20) | 2 | 2 (100) | 0 | 1 | 0.643 | 1 | 1 |
| Non‐neurologic comorbidities (before COVID‐19) | ||||||||||
| Overall non‐neurologic comorbidities (N, %) | 40 (75.5) | 27 (67.5) | 13 (32.5) | 12 (70.6) | 11 (91.7) | 1 (8.3) | 0.753 | 0.144 | ||
| Hypertension | 33 | 24 (72.7) | 9 (27.2) | 12 | 11 (91.7) | 1 (8.3) | 0.577 | 0.14 | 1 | 0.246 |
| Diabetes | 17 | 14 (82.4) | 3 (17.6) | 4 | 4 (100) | 0 | 0.561 | 0.072 | 1 | 1 |
| COPD | 2 | 2 (100) | 0 | 17 | 16 (94.1) | 1 (5.9) | 0.001 | 0.531 | ||
| Asthma | 8 | 5 (62.5) | 3 (37.5) | 4 | 4 (100) | 0 | 0.467 | 1 | 1 | 0.491 |
| CHF | 10 | 8 (80) | 2 (20) | 6 | 6 (100) | 0 | 0.191 | 0.299 | 1 | 0.5 |
| CKD | 12 | 9 (75) | 3 (25) | 4 | 3 (75) | 1 (25) | 1 | 0.502 | 0.235 | 1 |
| History of malignancy | 11 | 7 (63.6) | 4 (36.4) | 3 | 3 (100) | 0 | 1 | 1 | 1 | 1 |
| Smoking history | ||||||||||
| Current or former smoker | 5 | 4 (80) | 1 (20) | 4 | 3 (75) | 1 (25) | 0.408 | 0.615 | 0.364 | 1 |
| Never smoker | 20 | 11 (55) | 9 (45) | 7 | 7 (100) | 0 | 0.408 | 0.615 | 0.364 | 0.059 |
| Unknown | 2 | 2 (100) | 0 | 1 | 1 (100) | 0 | ||||
| BMI | ||||||||||
| Average BMI (range, IQR) | Average 26.2 (16–40.2, 8.8) | 26.89 (17.4–40.2, 8.3) | 21.99 (16–34.3, 8.7) | Average 23 (16–33.6, 7.7) | 23 (16–33.6, 7.7) | 19 | 0.034 | 0.718 | 0.375 | 0.211 |
Abbreviations: COPD, chronic obstructive pulmonary disease; CHF, congestive heart failure; BMI, body mass index; CKD, chronic kidney disease.
The proportion of patients with non‐neurologic comorbidities in the COVID‐19 positive and negative group was similar, except in regards to COPD, where there were more patients in the COVID‐19 negative group with COPD (P = 0.001) (Table 2). PD patients with COVID‐19 and other non‐neurologic comorbidities had a similar mortality rate (34.8%) compared to COVID‐19 positive non‐PD, age matched patients (34.7%). The mortality rates for PD patients with hypertension, diabetes, or congestive heart failure (CHF), were lower, compared to the overall cohort, but not statistically different (Table 2). Due to the smaller cohort, no significant conclusions could be drawn about the effect of non‐neurologic comorbidities in patients without COVID‐19.
Presentation and Evolution of COVID‐19 in PD patients
In the COVID‐19 positive cohort, patients presented with respiratory symptoms (62%) more often than with neurologic (17%), cardiac (6%), or gastroenterologic (15%) ones, but in the COVID‐19 negative cohort, neurologic presentations (47%) were more common. Patients with neurologic symptoms—a change in mental state, physical neurologic deficit, or generalized weakness—had a higher mortality rate (44.4%) than patients with respiratory (36.4%) or gastroenterologic symptoms (25%) (P = 0.87). Of eight patients in the COVID‐19 positive cohort who were intubated (15%), seven died (P = 0.01, vs. those not requiring intubation). There was no significant difference in the median length of stay of patients who survived (6 days, range 1–18 days) compared to those who died (6 days, range 1–14 days).
Mortality rates in the COVID‐19 negative patients, based on presentation, could not be compared. In this cohort, only one patient was intubated, and survived. Though the median length of stay was lower (4.5 days, range 1–14), this was not statistically significant (P = 0.27).
There were significant differences in laboratory results among the COVID 19 positive patients between those who died and those who survived. The mean and maximum concentrations of creatinine kinase (CK), D‐dimer, creatinine, neutrophils, and aspartate aminotransferase (AST) were significantly higher in patients who died than those who survived. In contrast, the lymphocyte mean and maximum values were lower in patients who died, compared to those who survived (Supplement S1). In the COVID‐19 negative cohort, comparisons for the same laboratory test results were either not checked or not significant between patients who survived and those who died.
Medication Changes and Mortality
Within the cohort of PD patients with COVID‐19, 8 underwent reductions in their PD medications. Of these patients, 75% (6) died, which was statistically significant when compared to those who died without PD medication changes 31% (14) (p = 0.042). These patients did not receive their PD medications for different reasons: due to mental status being impaired to the point that they could not safely take any oral medications; patients being made comfort care, and therefore no oral medications were administered; and all medications being held not related to the current COVID‐19 illness. Since the reasons for medication changes differed, or was prompted by already aggravated prognosis, and was not limited to the PD medications, it is difficult to infer that the change in the PD medications was the cause of the increased mortality rate.
Discussion
We found statistical significance in our data, some at odds with what has been reported in the literature, and some not previously reported.
Our sample size of PD patients with or without COVID‐19 was modest and derived exclusively from hospitalized patients, but the data shows that contracting SARS‐CoV‐2 is a significant risk factor for death and disability for patients with PD. Among patients with PD infected with COVID‐19 35.8% died and, of those who survived, only 33% returned home. In contrast to a prior study that demonstrated a lower mortality rate (5.7%) in PD patients compared to non‐PD patients,18 in our age matched patients with COVID‐19 and with or without PD, the mortality rate was similar (35.5%). This similarity contrasts with a prior study that demonstrated a lower mortality rate (5.7%) in PD patients compared to non‐PD patients.18 Earlier studies, like ours, demonstrated higher mortality rates in PD patients with COVID‐19 compared to the overall mortality rate of non‐PD patients with COVID 19: 40%,12 20%,13 21%.14
Among patients with PD and COVID‐19, those 70 years and older had a 38.6% mortality rate compared to those younger than 70 years, which is statistically significant. Similarly, other studies demonstrated advanced age as a risk factor associated with poor outcome in PD patients with COVID‐19.12, 13 One study, through multivariate regression, showed that the risk of in‐hospital death from COVID‐19 in all patients increased with age.19 Another study attributed poor outcome in PD patients aged 55 years and older with COVID‐19 to concurrent medical co‐morbidities.20
Although several groups have reported that being male was a risk factor for contracting COVID‐19, poor outcome, or both,20, 21, 22, 23 our data showed no significant difference in outcome by gender.
Our cohort was comprised of predominantly Black/African‐American and Hispanic patients living in the Bronx where the annual median household income ($38,085) is considerably lower than across the US ($68,703). Compared to the general population of the US, Black/African‐Americans have had a higher death rate from COVID‐19. For example, three‐quarters of COVID‐19 deaths in Milwaukee, WI were Black/African‐American.16 Drawing on CDC data, Latino and Black/African‐American residents of the US have been three times as likely to contract COVID‐19 and almost two times as likely to die from it compared to white residents.17 At another institution in New York City, a greater incidence of critical illness was found in racial and ethnic minorities, with similar mortality rates in Black/African‐American patients (40%) and Hispanic patients (38%) compared to our study.23 Williamson and colleagues found a higher mortality rate among Black and South Asian patients with COVID‐19.24 In contrast, in our PD patients with COVID‐19, the mortality rate of Black/African‐American patients was 46.7% and 40% for Hispanic patients, which were not statistically significant. There was, however, a statistically significant higher mortality rate among patients of non‐Hispanic origin in the COVID‐19 positive cohort, likely due to the trend of higher mortality in Black/African‐American patients.
When considering the effect of different stages of PD, our data showed that patients with advanced PD (Stages 2 and 3) and COVID‐19 compared to mild PD (Stage 1) and COVID‐19 had a statistically significantly higher mortality. Similarly, defining advanced PD by longer disease duration, the presence of dementia, or greater incapacity, several studies found it a risk factor for death among patients infected with COVID‐19.12, 13, 25 Furthermore, another recent study demonstrated that PD conferred a higher risk of dying from COVID‐19 compared with patients without PD.26
Several studies found dementia to be a significant risk factor for death in PD patients with COVID 19,12, 13, 24 but our data showed PD patients and COVID‐19 with dementia had a lower mortality rate than the overall group. Studies prior to the COVID‐19 pandemic also found dementia1, 2 and mild cognitive impairment (MCI)4 to be risk factors for mortality in PD patients.
Counterintuitively, in our group, PD patients with COVID‐19 accompanied by other neurologic comorbidities, compared to those without these comorbidities, had a lower mortality rate. Moreover, the proportion of patients with several non‐neurologic comorbidities had lower mortality rates than the overall COVID‐19 positive cohort (35.8%): hypertension (27%), CHF (20%), and diabetes (18%). These same comorbidities in other studies were found to be more common in patients who died from COVID‐19.22, 27 This finding led to the conclusion that underlying diseases increase the risk for more severe illness, which in turn can lead to death. In our study, patients with underlying diseases had lower mortality rates, therefore we concluded that having PD put our cohort at a higher risk of mortality.
Interestingly, mortality was higher among PD patients with COVID‐19 who had undergone reduction or withholding of their oral PD medications (75%) versus those who continued their medications (31%). PD medication reductions were prompted for safety reasons when oral intake was unsafe, was not always limited to PD meds, or was prompted by a change to comfort care status. Since the reasons for medication changes differed, or was prompted by an already aggravated prognosis, and was not limited to the PD medications, it is difficult to infer that the change in the PD medications increased the patients' mortality rate.
Comparing laboratory results among PD patients with COVID 19, we discovered significant differences between those who died and those who survived. The mean and maximum concentrations for CK, D‐dimer, creatinine, neutrophils, and AST were significantly higher among patients who died than in those who survived. The lymphocyte mean and maximum values were lower in patients with COVID‐19 who died, compared to those who survived. Our findings are similar to those seen in the original studies in China.27, 28 Since these abnormalities were present in all patients, they likely reflect the systemic nature of COVID‐19 rather than being specific to patients with PD.
Our study has some limitations. Our sample size of PD patients with and without COVID‐19, compared to non‐PD patients, was relatively small. Also, because our sample was drawn from hospitalized patients, PD patients who remained home with COVID‐19, with presumably less severe illness, could not be included. Therefore, some of our findings did not reach statistical significance and may not apply to the entire Parkinson disease population.
In conclusion, certain unalterable factors—advanced PD and age greater than 70 years—put patients with PD and COVID‐19 at higher risk of mortality. Even patients with mild PD who survived were unable to function independently after the hospitalization. Although we did not find statistically significant differences in economic factors, non‐Hispanic PD patients with COVID‐19 fared worse, likely due to the outcomes in the Black/African‐American cohort.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript: A. Writing of the first draft, B. Review and Critique.
R.P.: MD: 1B, 1C, 2A, 2B, 3A, 3B
V.F.: MD: 2A, 2B, 3B
A.G.: MD, PhD: 2A, 2C, 3B
H.L.G.: MD, PhD: 2A, 2B
D.M.K.: MD: 1A, 1C, 3B
Disclosures
Ethical Compliance Statement: This study was approved by the Albert Einstein College of Medicine IRB. The reference number for this study is IRB #2020–11,621. Since this study was a retrospective review of patient charts, informed consent was not obtained. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflict of Interest: The authors did not have any conflicts of interests or funding sources to complete the research presented in this manuscript.
Financial Disclosures for the Previous 12 Months: AS Galanopoulou acknowledges grant support by NINDS RO1 NS091170, U54 NS100064, the US Department of Defense. (W81XWH‐18‐1‐0612), NICHD U54HD090260, and research funding from the Heffer Family and the Segal Family Foundations and the Abbe Goldstein/Joshua Lurie and Laurie Marsh/Dan Levitz families. AS Galanopoulou is Editor in Chief of Epilepsia Open and has received royalties for publications from Elsevier and Morgan & Claypool publishers.
Supporting information
Table S1. Comparison of laboratory results in PD patients with and without COVID‐19.
Relevant disclosures and conflicts of interest are listed at the end of this article.
References
- 1.Macleod AD, Taylor KS, Counsell CE. Mortality in Parkinson's disease: a systematic review and meta‐analysis. Mov Disord 2014;29:1615–1622. [DOI] [PubMed] [Google Scholar]
- 2.Levy G, Tang MX, Louis ED, et al. The association of incident dementia with mortality in PD. Neurology 2002;59(11):1708–1713. 10.1212/01.wnl.0000036610.36834.e0. [DOI] [PubMed] [Google Scholar]
- 3.De Pablo‐Fernandez E, Tur C, Revesz T, et al. Association of autonomic dysfunction with disease progression and survival in Parkinson disease. JAMA Neurol 2017;74:970–976. 10.1001/jamaneurol.2017.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Backstrom D, Granasen G, Domellof ME, et al. Early predictors of mortality in parkinsonism and Parkinson disease: a population base stud. Neurology 2018;91:e2045–e2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantri S, Fullard ME, Beck J, Willis AW. State‐level prevalence, health service use, and spending vary widely among Medicare beneficiaries with Parkinson disease. NPJ Parkinsons Dis 2019;5:1. 10.1038/s41531-019-0074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oguh O, Videnovic A. Inpatient management of Parkinson disease: current challenges and future directions. Neurohospitalist 2012;2(1):28–35. 10.1177/1941874411427734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Freytas‐Tamura K, Hu W, Cook LR. Newspaper Article. It's the death towers: How the Bronx became New York's virus hot spot. New York Times 2020 May 26https://www.nytimes.com/2020/05/26/nyregion/bronx‐coronavirus‐outbreak.html
- 8.NYC Health . https://www1.nyc.gov/site/doh/covid/covid-19-data-totals.page#boro. Accessed December 2020.
- 9.Correa DJ, Labovitz DL, Milstein MJ, et al. Folding a neuroscience center into streamlined COVID‐19 response teams. Neurology 2020;95:583–592. 10.1212/wnl.0000000000010542. [DOI] [PubMed] [Google Scholar]
- 10.Eskandar EN, Altschul DJ, De la Garza Ramos R, et al. Neurologic syndromes predict higher in‐hospital mortality in COVID‐19. Neurology 2021;96(11):e1527–e1538. 10.1212/WNL.0000000000011356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Del Prete E, Francesconi A, Palermo G, et al. Prevalence and impact of COVID 19 in Parkinson's disease: evidence from a multi‐center survey in Tuscany region. J Neurol 2020;3:1–9. 10.1007/s00415-020-10002-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antonini A, Leta V, Teo J, et al. Outcome of Parkinson's disease patients affected by COVID‐19. Mov Disord 2020. Jun;35(6):905–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fasano A, Elia AE, Dallocchio C, et al. Predictors of COVID 19 outcome in Parkinson's disease. Parkinsonism Relat Disord 2020;78:134–147. 10.1016/j.parkreldis.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sainz‐Amo R, Baena‐Alvarez B, Parees I, et al. COVID‐19 in Parkinson's disease: what holds the key? J Neurol 2020;1–5. 10.1007/s00415-020-10272-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Marcaida JA, Lahrmann J, Machado D, et al. Clinical characteristics of coronovirus disease 19 (COVID‐19) among patients at a movement disorders center. Geriatrics (Basel) 2020;5(3):54. 10.3390/geriatrics5030054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Dorn A, Cooney RE, Sabin ML. COVID‐19 exacerbating inequalities in the US. Lancet 2020;395:1243–1244. 10.1016/s0140-6736(20)30893-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oppel RA, Gebeloff R, Lai KKR, et al. Newspaper article. The fullest look yet at the racial inequity of coronavirus. New York Times 2020 Jul 5. https://www.nytimes.com/interactive/2020/07/05/us/coronavirus‐latinos‐african‐americans‐cdc‐data.html?searchResultPosition=1. Accessed 5 July 2020.
- 18.Fasano A, Cereda E, Cassani E, et al. COVID‐19 in Parkinson disease patients living in Lombardy, Italy. Mov Disord 2020;35:1089–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–1062. 10.1016/s0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papa SM, Brundin P, Fung VSC, et al. Impact of the COVID‐19 pandemic on Parkinson's disease and movement disorders. Mov Disord 2020;25:711–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA 2020;323:1061–1069. 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID‐19 in New York City: a prospective cohort study. Lancet 2020;395:1763–1770. 10.1016/s0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williamson EJ, Walker AJ, Bhaskaran K, et al. OpenSAFELY: factors associated with COVID‐19 death in 17 million patients. Nature 2020;584:430–436. 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown EG, Chahine LM, Goldman SM, et al. The effect of the COVID‐19 pandemic on people with Parkinson's disease. J Parkinsons Dis 2020;10(4):1365–1377. 10.3233/jpd-202249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Q, Schultz JL, Aldridge GM, et al. Coronavirus disease 2019 case fatality and Parkinson's disease. Mov Disord 2020;35(11):1914–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang B, Zhou X, Qui Y, et al. Clinical characteristics of 82 cases of death from COVID 19. PLoS One 2020;15(7):e0235458. 10.1371/journal.pone.0235458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 2020;368:m1091. 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparison of laboratory results in PD patients with and without COVID‐19.


