Abstract
Adequate maternal selenium level is essential for immune response and healthy pregnancy. This study aimed to shed light on the selenium status of pregnant women with COVID‐19 and the effects of potential deficiency in serum selenium levels. Totally 141 pregnant women, 71 of them were COVID‐19 patients, in different trimesters were included in the study. Maternal serum selenium levels, demographic and clinical parameters were determined. Serum selenium levels of pregnant women in the second (p: .0003) and third (p: .001) trimesters with COVID‐19 were significantly lower than in the healthy group. Maternal selenium level was found to be negatively correlated with gestational week (p < .0001, r: −.541), D‐dimer (p: .0002, r: −.363) and interleukin‐6 (IL‐6) level (p: .02, r: −.243). In the second trimester, serum selenium level positively correlated with white blood cell (p: .002, r: .424), neutrophil (p: .006, r: .39), lymphocyte (p: .004, r: .410) count and hemoglobin (p: .02, r: .323), hematocrit (p: .008, r: .38) status. In the third trimester, it was found that maternal selenium level positively correlated with monocyte (p: .04, r: .353) and negatively correlated with C‐reactive protein level (p: .03, r: −.384). Serum selenium level was gradually decreased during the pregnancy period, however, this natural decrease was enhanced together with COVID‐19 infection. The reason might be increased selenium needs depended on the immune response against infection. The decrease in maternal selenium level was found to be related to IL‐6 and D‐dimer levels, which indicate selenium's role in disease progression.
Keywords: COVID‐19, immunity, infection, pregnancy, selenium
1. INTRODUCTION
Selenium has an important place among the trace elements that are essential to maintain the homeostasis of the human body. Its most vital function is its role in the creation of an immune response against oxidative stress due to its antioxidant properties.1, 2 Selenium presents as seleno‐proteins for its biological function. It is known that selenium supplements provide protection against many harmful chemical and biological factors for the body. They can provide a wide range of protection from drug side effects, heavy metals, pesticides, toxins, and other oxidative stressors.3, 4, 5 The most important enzyme that selenium cofactors is glutathione peroxidase. The main function of glutathione peroxidase is to ensure that hydrogen peroxidase and lipid peroxidase, which are strong oxidative stress factors, are removed from the body in a harmless way.6, 7
Oxidative stress markers and reactive oxygen radicals, which are also revealed in viral infections, aim to break this antioxidant defense mechanism in human cells, which are the host for viruses, and reduce the immunity of the host. 8 Selenium, is the constituent of different seleno‐proteins and especially the intracellular antioxidative enzyme glutathione peroxidase, which prevents oxidant stress in the cell. Increased oxidative stress during the infection and inflammation may require more selenium use leading to a decrease in selenium level. 9 Also, the levels of trace elements such as selenium depend on several dietary factors. 10 Another reason for selenium decrease might be the dysfunction of the liver, which is responsible for the biosynthesis of seleno‐proteins. 11 All the above reasons could be possible as a consequence of COVID‐19 infection. Furthermore, selenium was observed to suppress the release of the inflammatory precursor cytokine that is activated by pathogens.12, 13 In cell studies, it was observed that cells infected with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) decreased selenoprotein synthesis and this decrease was found to be associated with the increase of IL‐6, an inflammatory marker. 14
On the other hand, it was mentioned that selenium supplementation was beneficial for the regulation of immune response against thyroid autoimmunity during pregnancy and the post‐partum period. 15 In an animal study, maternal selenium deficiency during pregnancy caused reduced fetal growth by reduced fetal glucose concentrations. Also, placental dysfunction was observed due to reduced placental seleno‐dependent deiodinase expression and increased placental glycogen content by changing the mRNA expression of solute carrier family 2 which is facilitated by glucose transporter member 3 (SLC2A3). These findings indicate that selenium deficiency dysregulates placental nutrient transport. 16 Serum selenium level was also considered as a risk marker for pregnancy‐induced hypertension (OR, 15.34; p: .002). 17 It has been shown that maternal serum selenium deficiency significantly affects not only pregnancy complications but also the health parameters and cognitive functions of infants in the first few years of life.18, 19
Maternal selenium level is observed as an important parameter for pregnancy, mother and infant health. As the vital risk factor for the pregnancy period, selenium effects on infectious diseases especially in HIV infection was also investigated in several studies.20, 21 Selenium deficiency and its effects in HIV‐infected pregnant women were investigated and it was observed that women with selenium deficiency had approximately 8‐fold higher preterm delivery and low birth weight risk compared to women with normal selenium level. 22
However, according to current literature knowledge, the maternal selenium status role in the recent COVID‐19 pandemic hasn't been investigated yet. This study aimed to shed light on the selenium status of pregnant women with COVID‐19 and the effects of potential deficiency in serum selenium levels on disease outcomes in each trimester of the pregnancy period for the first time to date.
2. MATERIALS AND METHODS
2.1. Study groups and ethics
Pregnant women who were admitted to the Ministry of Health Ankara City Hospital, Department of Obstetrics and Gynecology between July 15, 2020 and December 15, 2020 were included in the study. The groups consist of SARS‐CoV‐2 positive and control groups with similar clinical and demographic characteristics. For the diagnosis of SARS‐CoV‐2 infection, real‐time polymerase chain reaction (RT‐PCR) testing of nasopharyngeal and oropharyngeal samples was evaluated. Both the Turkish Ministry of Health as well as the institutional review board of the study protocol (E2‐20‐22) was approved, and informed consent was obtained from all patients. All COVID‐19 cases were managed under the guidance of national guidelines.
2.2. Clinical characteristics
Blood samples were taken from the patients along with the first laboratory tests at the first admission to the hospital. Demographic characteristics, clinical characteristics, and laboratory parameters were analyzed. Maternal age, body mass index (BMI) (kg/m2), gravity, parity, comorbid conditions, gestational age, pregnancy status, obstetric complications, vital outcomes of patients, hemoglobin (Hb), hematocrit (Hct), WBC, thrombocyte, lymphocyte, neutrophil counts, erythrocyte sedimentation rate (ESR), D‐dimer, C‐reactive protein (CRP), procalcitonin, interleukin‐6 (IL‐6), ferritin, blood urea nitrogen (BUN), creatine, liver function tests (aspartate aminotransferase, alanine aminotransferase) were obtained to determine the biochemical and clinical profile of the control group and study group.
2.3. Maternal selenium level measurements
Selenium levels were analyzed with atomic absorption spectroscopy. A Perkin Elmer Analyst 800 device and the “WinLab32” program were used for the atomic absorption spectroscopy method. The calibration curve was generated following guidelines with standard solutions and selenium levels were determined with two repetitive measurements of each sample.
2.4. Statistical analysis
IBM SPSS Statistics 25 (Armonk) software was used for statistical analysis and the data were expressed as mean ± SD. The student's T‐test was used to analyze differences between study groups, and Pearson correlation was used for correlation analysis between data. p < .05 was considered statistically significant. Graphpad PRISM 6.0 was used for visualization of the data.
3. RESULTS
Clinical characteristics of patients are given in Table 1. Our results indicate that there are several outcomes which change with COVID‐19 infection in different stages of pregnancy. There were significant decreases in white blood cell (WBC), lymphocytes, and neutrophil count with COVID‐19 infection in each trimester. CRP levels were increased in the COVID‐19 group compared to the control group in the second and third trimesters. Similarly, IL‐6 and ferritin levels were increased with COVID‐19 in the third trimester (p values were given in Table 1). D‐dimer level was found significantly higher in COVID‐19 patients in the second trimester however D‐dimer increase with COVID‐19 infection wasn′t significant in the third trimester due to high standard deviation.
Table 1.
Characteristics of study groups
| Variables | 1st Trimester | 2nd Trimester | 3rd Trimester | |||
|---|---|---|---|---|---|---|
| Control n: 26 mean (SD) | COVID‐19 n: 24 mean (SD) | Control n: 22 mean (SD) | COVID‐19 n: 26 mean (SD) | Control n: 22 mean (SD) | COVID‐19 n: 21 mean (SD) | |
| Age (year) | 26.34 (4.02) | 28.37 (4.70) | 28.04 (6.27) | 29.76 (6.66) | 26.3 (4.11) | 28.95 (4.77) |
| Gestational age (week) | 9.11 (3.14) | 9.83 (3.11) | 22 (4.45) | 24.69 (3.06) | 34.69 (3.27) | 35.28 (3.39) |
| Gravidity | 1.9 (0.8) | 2.3 (1.5) | 2.54 (1.29) | 2.65 (1.38) | 1.9 (0.95) | 2.61 (1.68) |
| Parity | 0.73 (0.72) | 1.04 (1.32) | 1.22 (1.15) | 1.34 (1.29) | 0.769 (0.725) | 1.47 (1.40) |
| WBC per ml | 8192 (2343)a | 5905 (3018)a | 10,422 (2777)b | 6407 (2030)b | 10,240 (1745)c | 7301 (2619)c |
| Neutrophil per ml | 5587 (1982)d | 4162 (2768)d | 7930 (2329)b | 4705 (1853)b | 7371 (1382)e | 5446 (2391)e |
| Lymphocyte per ml | 1970 (552)f | 1257 (442)f | 1807 (542)b | 1181 (509)b | 1965 (435)g | 1292 (613)g |
| Monocyte per ml | 381 (128) | 345 (170) | 444 (126)h | 327 (203)h | 591 (224)g | 360 (209)g |
| CRP, mg/dl | 9.81 (8.85) | 14.02 (14.26) | 8.48 (5)h | 24.49 (33.64)h | 4.69 (3.45)g | 17.45 (15.28)g |
| ESR | NA | NA | 29.38 (16.5) | 13.27 (27.86) | 33.72 (10.12) | 29.81 (34.65) |
| IL‐6, pg/ml | NA | NA | 7.17 (11.98) | 8.84 (10.99) | 3.87 (1.08)e | 14.90 (16.56)e |
| Ferritin, ng/ml | 26.17 (22.75) | 47.04 (59.29) | 13.38 (10.58) | 35.53 (55.10) | 9.38 (5.62)e | 31.63 (45.39)e |
| Hemoglobulin, g/dl | 12.9 (1.06) | 12.35 (0.96) | 12.10 (1.08)i | 11.22 (1.03)i | 11.60 (0.93) | 10.86 (1.29) |
| Hematokrit, % | 38.83 (3.26) | 37.46 (2.79) | 37.04 (2.98)i | 34.29 (3.60)i | 35.53 (2.84) | 33.42 (3.57) |
| Platelet count | 267,692 (64,728)d | 225,250 (57,912)d | 259,181 (56,120)i | 208,240 (68,492)i | 244,076 (58,377) | 222,904 (54,701) |
| BUN, mg/dl | 19.03 (5.86) | 18.78 (5.0) | 17.65 (3.74)i | 14.34 (3.11)i | 17.58 (5.16) | 15.76 (5.34) |
| Creatinine, mg/dl | 0.552 (0.06) | 0.533 (0.09) | 0.50 (0.08) | 0.45 (0.07) | 0.45 (0.07) | 0.47 (0.08) |
| ALT, IU | 17.84 (8.40) | 28.86 (27.83) | 18.1 (5.09) | 30.69 (33.49) | 13.33 (3.91)e | 21.33 (15.94)e |
| AST, IU | 13.52 (4.11)d | 22.34 (15.96)d | 14.45 (3.76) | 38.8 (67.06) | 14.66 (2.64)g | 24.95 (12.38)g |
| D‐dimer, mcg/ml | 0.468 (0.391) | 0.761 (0.739) | 0.818 (0.354)h | 1.5 (0.849)h | 1.95 (0.98) | 3.13 (2.05) |
| Fibrinogen, g/L | 3.11 (1.70) | 3.72 (0.81) | 4.52 (0.25)h | 3.74 (0.86)h | 4.49 (0.62) | 3.81 (0.91) |
| Maternal selenium level, mcg/L | 44.59 (8.40) | 46.52 (8.17) | 46.15 (8.15)b | 36.03 (9.68)b | 36.15 (6.25)g | 27.01 (7.82)g |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BUN, blood urinary nitrogen; CRP, C‐reactive protein; ESR, erythrocyte sedimentation rate; IL‐6, interleukin‐6; WBC, white blood cell.
p < .01 between control and COVID‐19 group in 1st trimester.
p < .001 between control and COVID‐19 group in 2nd trimester.
p < .001 between control and COVID‐19 group in 3rd trimester.
p < .05 between control and COVID‐19 group in 1st trimester.
p < .05 between control and COVID‐19 group in 3rd trimester.
p < .001 between control and COVID‐19 group in 1st trimester.
p < .01 between control and COVID‐19 group in 3rd trimester.
p < .05 between control and COVID‐19 group in 2nd trimester.
p < .01 between control and COVID‐19 group in 2nd trimester.
Our results showed that the serum selenium level of both healthy and SARS‐CoV‐2 (+) pregnant women was decreased, and it was shown by the correlation analysis between serum selenium level and gestational week (p < .0001, r: −.541) (Figure 2A). However, especially in the second (p: .0003) and third (p: .001) trimesters, serum selenium levels of pregnant women with COVID‐19 were significantly lower than that in the healthy group (Figure 1). There wasn′t any significant difference in serum selenium level between groups in the first trimester (p: .413).
Figure 1.
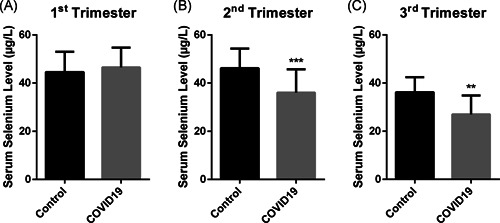
Maternal selenium levels of pregnant women with COVID‐19 and control groups in (A) first, (B) second, and (C) third trimesters
Figure 2.
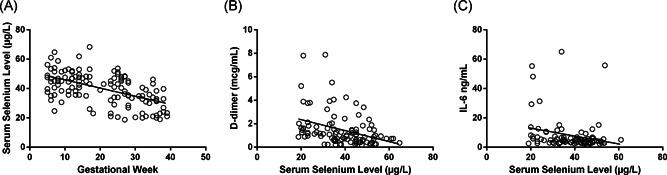
Maternal selenium levels relation with (A) gestational week, (B) D‐dimer, and (C) IL‐6 levels
According to cumulative correlation analysis of all pregnant women at different trimesters, maternal selenium level was found to be correlated with D‐dimer (p: .0002, r:−.363) and IL‐6 level (p: .02, r:−.243) (Figure 2).
Subgroup correlation analysis for each trimesters showed that, in the second trimester, serum selenium level correlated with WBC (p: .002, r: .424), neutrophil (p: .006, r: .39), lymphocyte (p: .004, r: .410) count and hemoglobin (p: .02, r: .323), hematocrit (p: .008, r: .38) status (Figure 3). In the third trimester, it was found that maternal selenium level correlated with monocyte (p: .04, r: .353) and CRP level (p: .03, r: −.384) (Figure 4).
Figure 3.
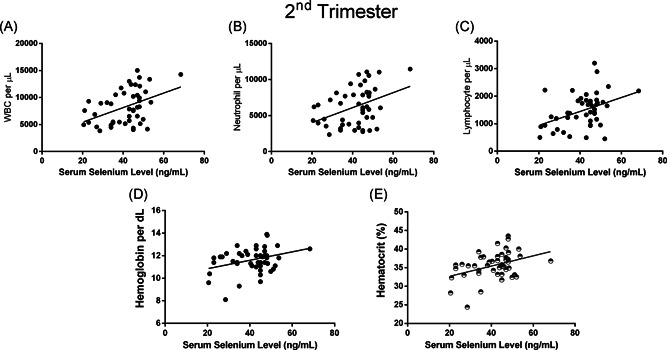
Maternal selenium levels of pregnant women in second‐trimester relationship with (A) white blood cell, (B) neutrophil, (C) lymphocyte count, (D) hemoglobin (per dl), and (E) hematocrit (%) levels
Figure 4.
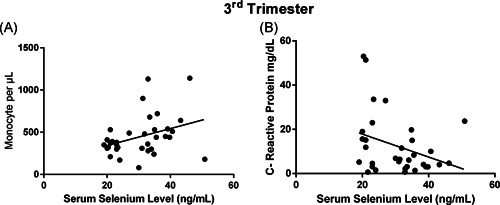
Maternal selenium levels of pregnant women in third‐trimester relationship with (A) monocyte and (B) CRP levels
COVID‐19 vital outcomes are given in Table 2. Serum selenium level correlated only with diastolic blood pressure (p: .003, r: .354).
Table 2.
Vital outcomes of pregnant women with COVID‐19
| 1st Trimester n: 24 | 2nd Trimester n: 26 | 3rd Trimester n: 21 | |
|---|---|---|---|
| Temperature, °C | 36.5 (0.49) | 36.58 (0.52) | 36.59 (0.47) |
| Heart rate per minute | 95.95 (9.19) | 98.88 (15.09) | 92.57 (8.88) |
| Systolic blood pressure, mm Hg | 110.35 (13.5) | 108.76 (11.81) | 116.85 (9.10) |
| Diastolic blood pressure, mm Hg | 69 (10.51) | 63.68 (7.08) | 63.85 (7.08) |
| O2saturation, % | 97.05 (0.91) | 96.79 (1.58) | 97.15 (0.87) |
4. DISCUSSION
The recent COVID‐19 pandemic affects the pregnant women population as well as the entire community. According to patients’ data in the literature, COVID‐19 infection during pregnancy should not be underestimated due to its potential to cause complications threatening mother and neonatal health. In this study, serum selenium level was investigated because of its role in immunity and maternal health.
Management of COVID‐19 in pregnant women is challenging. Physiological and immunological changes during pregnancy make pregnant women more susceptible to various viral infections. The increase in the transverse diameter of the thorax and the elevation of the diaphragm decrease the tolerance of the pregnant women against hypoxia. Changes in lung volumes and vasodilation cause mucosal edema and increased secretions in the respiratory tract. In addition, changes in cellular immunity increase susceptibility to infection with intracellular organisms such as viruses. Although the data of the limited number of pregnant cases in the literature indicate that the clinical status caused by COVID‐19 during pregnancy is similar to the general population, pregnant women should take similar precautions. An association between COVID‐19 infection and adverse pregnancy outcomes such as preterm birth, spontaneous abortion, and intrauterine growth restriction have been reported in studies.23, 24, 25
There was a significant decrease in maternal selenium level during the pregnancy period which indicates selenium needs are increased depending on fetal growth. Also, a gradual decrease in serum selenium concentration in subsequent trimesters of pregnancy was shown in the prior literature. 26 However, due to significant differences in serum selenium level between COVID‐19 and the control group (given in Figure 1), it should be mentioned that this selenium needs might be enhanced in COVID‐19 infection in the second and third trimester. Our results showed that the serum selenium level is decreased in pregnant women with COVID‐19. It might be supposed that COVID‐19 might increase selenium needs dependent on the immune response of the body or pathogenesis of infection or it might decrease serum selenium level by increasing selenium transport into the cell or selenium excretion.
In a study examining the potential roles of selenium and seleno‐proteins on COVID‐19 infection, it was found that the selenium level of survival patients was significantly higher than non‐survival patients with COVID‐19. 27 Another study about selenium effects on COVID‐19 mortality was found similar results. 28 Also, there is a study that shows the linear correlation between selenium level and the cure rate of patients with COVID‐19. 29
Our study showed that maternal selenium level correlated with D‐dimer and IL‐6 level in COVID‐19. IL‐6 is well known as one of the most important acute phase reactants and disease severity predictors of COVID‐19.30, 31 It was also shown before that COVID‐19 infection induces coagulopathy and secondary hyper‐fibrinolysis with disease severity.31, 32 A high level of D‐dimer on admission was observed to be related to worse outcomes of COVID‐19.33, 34 In H1N1 infection, the reason for the increase in D‐dimer level was observed mostly due to pulmonary thrombosis. 35 Furthermore, selenium‐dependent glutathione peroxidase deficiency was observed to lead to arterial thrombosis by enhanced platelet aggregation in an animal study. 36 Together with our results, it might be thought that the serum selenium status might be a preventative factor for COVID‐19 severity by regulatory roles of selenium on inflammation and thrombosis.
Maternal selenium level was found to be correlated with lymphocyte, neutrophil, monocyte count, and CRP levels in different stages of pregnancy (given in Figures 3 and 4), which indicates serum selenium level deficiency affects immunity through immune cells and CRP level. It was mentioned that selenium status together with zinc level was related to the severity of critically ill patients. 37 Our previous research has also shown that other trace elements such as zinc copper and magnesium were affected with COVID‐19 infection in pregnancy. We observed that zinc and copper levels were significantly decreased and magnesium levels were increased in pregnant women with COVID‐19 infection compared to healthy ones. 38 Also in a mice model, low selenium status has been shown to contribute the influenza virus mutations. 39 This potential of selenium on virus mutations might have importance also for SARS‐CoV‐2 mutations which are recently seen around the world.40, 41
5. CONCLUSION
Serum selenium level was gradually decreased during the pregnancy period however this natural decrease was enhanced together with COVID‐19 infection. The reason might be increased selenium needs depended on the immune response against infection. Maternal selenium deficiency was found to be related to IL‐6 and D‐dimer levels, which indicate selenium's role in disease progression. Also, pregnant women in the second or third trimesters were affected more from COVID‐19 infection compared to women in the first trimester. The adequate level of maternal selenium levels should be taken into consideration along with its potential protective roles in COVID‐19 disease.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Seyit Ahmet Erol: data collection, writing—review and editing; Naci Polat: investigation and data analysis; Sevginur Akdas: writing the first draft, editing, data analysis, visualization; Pelin Aribal Ayral: supervision, writing—review and editing; Ali Taner Anuk: data collection, writing—review and editing; Eda Ozden Tokalioglu: writing—review and editing; Sule Goncu Ayhan: writing—review and editing; Burcu Kesikli: investigation and data analysis; Merve Nur Ceylan: writing—review and editing; Atakan Tanacan: writing—review and editing; Ozlem Moraloglu Tekin: supervision, writing— review and editing; Nuray Yazihan: supervision, investigation, methodology, writing—review and editing; Dilek Sahin: conceptualization, writing—original draft, supervision.
ETHICS APPROVAL STATEMENT
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving research study participants were approved by the Turkish Ministry of Health Ankara City Hospital Ethics Committee (E2‐20‐22). Written informed consent was obtained from all subjects/patients.
Erol SA, Polat N, Akdas S, et al. Maternal selenium status plays a crucial role on clinical outcomes of pregnant women with COVID‐19 infection. J Med Virol. 2021;93:5438‐5445. 10.1002/jmv.27064
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Broome CS, McArdle F, Kyle JA, et al. An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am J Clin Nutr. 2004;80(1):154‐162. [DOI] [PubMed] [Google Scholar]
- 2. Kiełczykowska M, Kocot J, Paździor M, Musik I. Selenium—a fascinating antioxidant of protective properties. Adv Clin Exp Med. 2018;27(2):245‐255. [DOI] [PubMed] [Google Scholar]
- 3. Harsini SG, Habibiyan M, Moeini MM, Abdolmohammadi AR. Effects of dietary selenium, vitamin E, and their combination on growth, serum metabolites, and antioxidant defense system in skeletal muscle of broilers under heat stress. Biol Trace Elem Res. 2012;148(3):322‐330. [DOI] [PubMed] [Google Scholar]
- 4. Han Y‐S, Chang G‐G, Juo C‐G, et al. Papain‐like protease 2 (PLP2) from severe acute respiratory syndrome coronavirus (SARS‐CoV): expression, purification, characterization, and inhibition. Biochemistry. 2005;44(30):10349‐10359. [DOI] [PubMed] [Google Scholar]
- 5. McKelvey SM, Horgan KA, Murphy RA. Chemical form of selenium differentially influences DNA repair pathways following exposure to lead nitrate. J Trace Elem Med Biol. 2015;29:151‐169. [DOI] [PubMed] [Google Scholar]
- 6. Labunskyy VM, Hatfield DL, Gladyshev VN. Selenoproteins: molecular pathways and physiological roles. Physiol Rev. 2014;94(3):739‐777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brigelius‐Flohé R, Maiorino M. Glutathione peroxidases. Biochim Biophys Acta. 2013;1830(5):3289‐3303. [DOI] [PubMed] [Google Scholar]
- 8. Molteni C, Principi N, Esposito S. Reactive oxygen and nitrogen species during viral infections. Free Radic Res. 2014;48(10):1163‐1169. [DOI] [PubMed] [Google Scholar]
- 9. Hiffler L, Rakotoambinina B. Selenium and RNA virus interactions: potential implications for SARS‐CoV‐2 infection (COVID‐19). Front Nutr. 2020;7:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tóth RJ, Csapó J. The role of selenium in nutrition—a review. Acta Univ Sapientiae Alimentaria. 2018;11(1):128‐144. [Google Scholar]
- 11. Himoto T, Masaki T. Current trends of essential trace elements in patients with chronic liver diseases. Nutrients. 2020;12(7):2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dhanjal NIK, Sharma S, Prabhu KS, Tejo, Prakash N. Selenium supplementation through Se‐rich dietary matrices can upregulate the anti‐inflammatory responses in lipopolysaccharide‐stimulated murine macrophages. Food Agric Immunol. 2017;28(6):1374‐1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mahmoodpoor A, Hamishehkar H, Shadvar K, et al. The effect of intravenous selenium on oxidative stress in critically ill patients with acute respiratory distress syndrome. Immunol Invest. 2019;48(2):147‐159. [DOI] [PubMed] [Google Scholar]
- 14. Wang Y, Huang J, Sun Y, et al. SARS‐CoV‐2 suppresses mRNA expression of selenoproteins associated with ferroptosis. ER stress and DNA synthesis. BioRxiv. 2020. 10.1101/2020.07.31.230243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mantovani G, Isidori AM, Moretti C, et al. Selenium supplementation in the management of thyroid autoimmunity during pregnancy: results of the “SERENA study”, a randomized, double‐blind, placebo‐controlled trial. Endocrine. 2019;66(3):542‐550. [DOI] [PubMed] [Google Scholar]
- 16. Hofstee P, Bartho LA, McKeating DR, et al. Maternal selenium deficiency during pregnancy in mice increases thyroid hormone concentrations, alters placental function and reduces fetal growth. J Physiol. 2019;597(23):5597‐5617. [DOI] [PubMed] [Google Scholar]
- 17. Lewandowska M, Sajdak S, Lubiński J. Serum selenium level in early healthy pregnancy as a risk marker of pregnancy induced hypertension. Nutrients. 2019;11(5):1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Skröder HM, Hamadani JD, Tofail F, Persson LÅ, Vahter ME, Kippler MJ. Selenium status in pregnancy influences children's cognitive function at 1.5 years of age. Clin Nutr. 2015;34(5):923‐930. [DOI] [PubMed] [Google Scholar]
- 19. Varsi K, Bolann B, Torsvik I, Rosvold Eik TC, Høl PJ, Bjørke‐Monsen A‐L. Impact of maternal selenium status on infant outcome during the first 6 months of life. Nutrients. 2017;9(5):486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Okunade KS, John‐Olabode S, Akinsola OJ, Akinajo O, Akanmu SA, Kanki PJ. Effects of selenium supplementation on pregnancy outcome and disease progression in HIV‐infected pregnant women in Lagos, Nigeria: Study protocol for a randomised, double‐blind, placebo‐controlled trial. Medicine. 2019;98(3):12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang C, Wang H, Luo J, et al. Selenium deficiency impairs host innate immune response and induces susceptibility to Listeria monocytogenes infection. BMC Immunol. 2009;10(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Okunade KS, Olowoselu OF, Osanyin GE, John‐Olabode S, Akanmu SA, Anorlu RI. Selenium deficiency and pregnancy outcome in pregnant women with HIV in Lagos, Nigeria. Int J Gynecol Obstet. 2018;142(2):207‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dey M, Singh S, Tiwari R, Nair VG, Arora D, Tiwari S. Pregnancy outcome in first 50 Sars‐Cov‐2 positive patients at our center. Gynecol Obstet Reprod Med. 2021:1‐6. [Google Scholar]
- 24. Abdelazim IA, AbuFaza M, Al‐Munaifi S. COVID‐19 positive woman presented with preterm labor: case report. Gynecol Obstet Reprod Med. 2021:1‐3. [Google Scholar]
- 25. Sahin D, Tanacan A, Erol SA, et al. Updated experience of a tertiary pandemic center on 533 pregnant women with COVID‐19 infection: a prospective cohort study from Turkey. Int J Gynecol Obstet. 2021;152(3):328‐334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pieczyńska J, Płaczkowska S, Sozański R, Orywal K, Mroczko B, Grajeta H. Is maternal dietary selenium intake related to antioxidant status and the occurrence of pregnancy complications? J Trace Elem Med Biol. 2019;54:110‐117. [DOI] [PubMed] [Google Scholar]
- 27. Hasani M, Djalalinia S, Khazdooz M, et al. Effect of selenium supplementation on antioxidant markers: a systematic review and meta‐analysis of randomized controlled trials. Hormones. 2019;18(4):451‐462. [DOI] [PubMed] [Google Scholar]
- 28. Moghaddam A, Heller RA, Sun Q, et al. Selenium deficiency is associated with mortality risk from COVID‐19. Nutrients. 2020;12(7):2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang J, Taylor EW, Bennett K, Saad R, Rayman MP. Association between regional selenium status and reported outcome of COVID‐19 cases in China. Am J Clin Nutr. 2020;111(6):1297‐1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Han H, Ma Q, Li C, et al. Profiling serum cytokines in COVID‐19 patients reveals IL‐6 and IL‐10 are disease severity predictors. Emerg Microbes Infect. 2020;9(1):1123‐1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Herold T, Jurinovic V, Arnreich C, et al. Elevated levels of interleukin‐6 and CRP predict the need for mechanical ventilation in COVID‐19. J Allergy Clin Immunol. 2020;146:128‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ji H‐L, Zhao R, Matalon S, Matthay MA. Elevated plasmin (ogen) as a common risk factor for COVID‐19 susceptibility. Physiol Rev. 2020;100:1065‐1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li Y, Zhao K, Wei H, et al. Dynamic relationship between D‐dimer and COVID‐19 severity. Br J Haematol. 2020;190:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang ZF, Su F, Lin XJ, et al. Serum D‐dimer changes and prognostic implication in 2009 novel influenza A (H1N1). Thromb Res. 2011;127(3):198‐201. [DOI] [PubMed] [Google Scholar]
- 36. Pang P, Abbott M, Abdi M, et al. Pre‐clinical model of severe glutathione peroxidase‐3 deficiency and chronic kidney disease results in coronary artery thrombosis and depressed left ventricular function. Nephrol Dial Transplant. 2018;33(6):923‐934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ruocco MAC, Cechinatti EDP, Barbosa F Jr, Navarro AM. Zinc and selenium status in critically ill patients according to severity stratification. Nutrition. 2018;45:85‐89. [DOI] [PubMed] [Google Scholar]
- 38. Anuk AT, Polat N, Akdas S, et al. The relation between trace element status (zinc, copper, magnesium) and clinical outcomes in COVID‐19 Infection During Pregnancy. Biol Trace Elem Res. 2020:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nelson HK, Shi Q, Van Dael P, et al. Host nutritional selenium status as a driving force for influenza virus mutations. FASEB J. 2001;15(10):1727‐1738. [PubMed] [Google Scholar]
- 40. Jung S. COVID‐19 mutation, a critical variable between Infectivity and Toxicity. Eng Sci. 2020;5(4):279‐282. [Google Scholar]
- 41. Wang R, Chen J, Gao K, Hozumi Y, Yin C, Wei G‐W. Characterizing SARS‐CoV‐2 mutations in the United States. arXiv. 2020:200712692. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


