Funding sources
None.
Conflict of interest
Nothing to disclose.
Dear Editor,
Lately, we have witnessed sporadic reports of Pityriasis rosea (PR) and PR‐like eruptions associated with SARS‐CoV‐2. 1 Such relationship has raised debate on the possible aetiopathogenic mechanism underlying this exanthematous disease. Our aim is to reinforce the link between PR and coronaviruses based on our finding of two cases following COVID‐19 vaccination – the first to our knowledge – and two cases related to SARS‐CoV‐2 infection.
Clinical pictures of the patients hereby reported are described in Figs 1 and 2. In all of them, morphology, distribution and evolution of skin lesions were compatible with the diagnosis of PR. The two patients with the vaccine‐associated PR developed the herald patch 24 h and 7 days after the second dose of the Pfizer BioNTech vaccine (BioNTech Manufacturing GmbH, Mainz, Germany), respectively. ELISA serology revealed positive SARS‐CoV‐2 IgG antibodies in both cases. The remaining two patients recalled symptoms or signs suggesting COVID‐19 in the preceding weeks, although serologic confirmation was possible in just one of them. All four patients had negative nasopharyngeal PCR test and absence of systemic symptoms at the moment of the dermatologic evaluation. We also obtained positive HHV‐6 serology in three cases, with titres ranging from 1 : 160 to 1 : 640.
Figure 1.
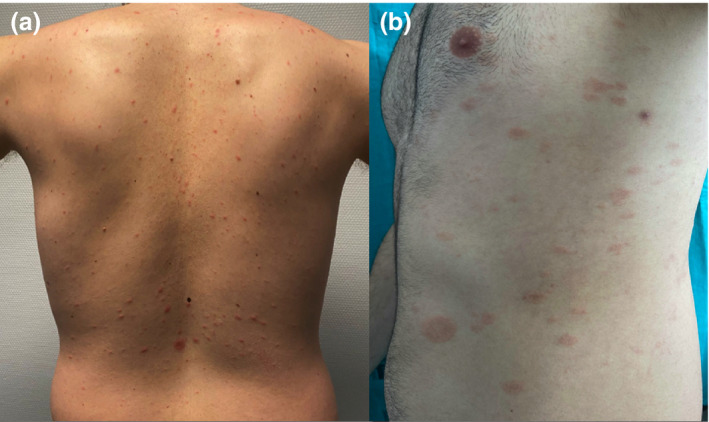
(a) Herald patch followed by typical oval‐shaped macules appeared along skin tension lines in this 26‐year‐old patient seven days after the second dose of COVID‐19 vaccine. Biopsy revealed mild spongiosis with foci of parakeratosis and a lymphohistiocytic infiltrate around superficial vessels. (b) This 29‐year‐old man presented similar findings, but the rash started 24 h after complete vaccination. RPR was non‐reactive in both cases.
Figure 2.
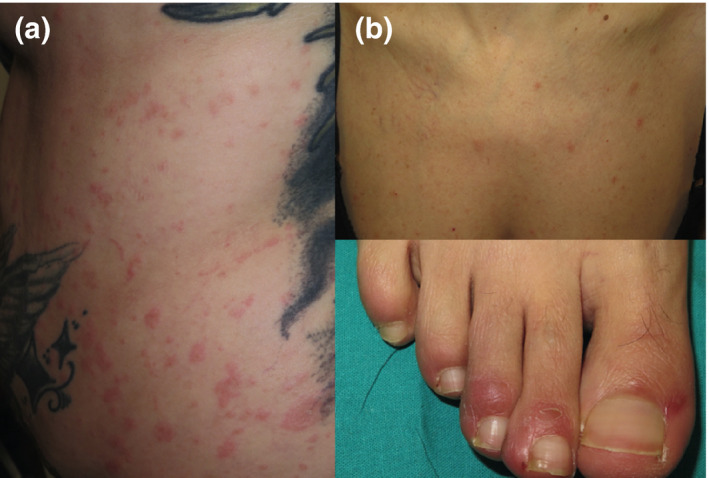
(a) This 26‐year‐old woman presented pruritic erythematous patches with mild, peripheral scaling in trunk and proximal aspects of the limbs. She had symptoms suggesting COVID‐19 a month before and positive IgG antibodies. (b) This 48‐year‐old woman presented chilblain‐like macules on the toes after a high‐risk contact with an infected patient. She subsequently developed a rash suggesting PR but had no COVID‐19 symptoms nor positive serology. However, SARS‐CoV‐2 infection was presumed based on clinical and epidemiological factors.
Aetiopathogenesis of PR has never been clarified. A viral origin was proposed based on clinical aspects (seasonal pattern and flu‐like prodromal symptoms) and laboratory findings (intracellular virus‐like particles, cytopathic degeneration of keratinocytes and elevation of IFN‐γ). 2 Although multiple candidates were initially considered, the reiterated finding of DNA sequences of HHV‐6/7 in plasma, skin lesions and peripheral blood mononuclear cells (PBMCs) pointed to these agents as the main aetiological factors. Positive tissue immunohistochemistry, viral mRNA in situ hybridization and neutralizing or high‐avidity IgG antibodies against HHV‐6/7 have also been detected in patients with PR, 3 supporting the idea of an endogenous reactivation. Regression of lesions in response to acyclovir therapy strengthens that hypothesis. 4
Nonetheless, other studies have failed to detect such findings, so PR appearing in association with COVID‐19 may question the link between the former and herpesviruses. Given that HHV‐6/7 reactivation might represent an epiphenomenon in the setting of immune dysregulation, a doubt arises on whether PR could be a direct manifestation of viral primoinfections, like SARS‐CoV‐2. The increasing number of reports relating PR to COVID‐19 should also raise the question whether seasonal coronaviruses might have been the actual cause of PR cases observed in the past. However, this should not be incompatible with HHV‐6 reactivation as the main aetiopathogenic pathway for PR. Drago et al. 5 reported that viral DNA and high‐avidity HHV‐6 and EBV antibodies were detected in a case of COVID‐19‐related PR, suggesting that SARS‐CoV‐2 might have triggered a chained viral reactivation. In three of our patients, we also demonstrated high HHV‐6 antibody titres, supporting this hypothesis. This phenomenon is yet to be understood but probably plays a role in other skin eruptions like drug reaction with eosinophilia and systemic symptoms. 6 Furthermore, herpes simplex virus and cytomegalovirus reactivations have also been found in severe COVID‐19 patients, and they could be related to an acquired state of immunodeficiency determined by SARS‐CoV‐2‐associated lymphopaenia. 7
Pityriasis rosea‐like rashes have been described after H1N1 influenza, HPV, diphtheria, poliomyelitis, smallpox, pneumococcal, hepatitis B and BCG vaccinations. 8 In some cases, antibodies against HHV‐6/7 as well as viral DNA in plasma and PBMCs were detected. 9 , 10 These findings suggest that immune‐induced viral reactivation – rather than viral primoinfection – may be the primary cause of this disease and not a mere epiphenomenon. Like some investigators have proposed, the elicitation of a specific immune response against an infectious agent triggered by vaccines might distract the cell‐mediated control on latent infections such as HHV‐6/7. 8
The fundaments of this immunomodulatory effect of vaccinations remain obscure and such connection can only be speculated. In spite of that, our report reinforces the idea that immune‐related herpesvirus reactivation – driven by infections, vaccines, drugs or other factors – may be involved in the genesis of PR. More studies are needed to detail the pathogenic mechanisms through which this event might occur. Also, we consider that the ongoing connection between COVID‐19 and PR should encourage clinicians to explore the relationship between this exanthematous disease and seasonal coronaviruses.
Acknowledgement
The patients in this manuscript have given written informed consent to the publication of their case details.
References
- 1. Ehsani AH, Nasimi M, Bigdelo Z. Pityriasis rosea as a cutaneous manifestation of COVID‐19 infection. J Eur Acad Dermatol Venereol 2020; 34: 436–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Drago F, Ciccarese G, Broccolo F et al. The role of cytokines, chemokines, and growth factors in the pathogenesis of Pityriasis Rosea. Mediators Inflamm 2015; 2015: 438963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Watanabe T, Kawamura T, Jacob SE et al. Pityriasis rosea is associated with systemic active infection with both human herpesvirus‐7 and human herpesvirus‐6. J Invest Dermatol 2002; 119: 793–797. [DOI] [PubMed] [Google Scholar]
- 4. Rodriguez‐Zuniga M, Torres N, Garcia‐Perdomo H. Effectiveness of acyclovir in the treatment of pityriasis rosea. A systematic review and meta‐analysis. An Bras Dermatol 2018; 93: 686–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Drago F, Ciccarese G, Rebora A, Parodi A. Human herpesvirus‐6, ‐7, and Epstein‐Barr virus reactivation in pityriasis rosea during COVID‐19. J Med Virol 2021; 93: 1850–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cho YT, Yang CW, Chu CY. Drug reaction with eosinophilia and systemic symptoms (DRESS): an interplay among drugs, viruses, and immune system. Int J Mol Sci 2017; 18: 1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Le Balc'h P, Pinceaux K, Pronier C, Seguin P, Tadié JM, Reizine F. Herpes simplex virus and cytomegalovirus reactivations among severe COVID‐19 patients. Crit Care 2020; 24: 530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Drago F, Ciccarese G, Javor S, Parodi A. Vaccine‐induced pityriasis rosea and pityriasis rosea‐like eruptions: a review of the literature. J Eur Acad Dermatol Venereol 2016; 30: 544–545. [DOI] [PubMed] [Google Scholar]
- 9. Papakostas D, Stavropoulos PG, Papafragkaki D, Grigoraki E, Avgerinou G, Antoniou C. An atypical case of pityriasis rosea gigantea after influenza vaccination. Case Rep Dermatol 2014; 6: 119–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Drago F, Ciccarese G, Rebora A, Parodi A. Pityriasis rosea following human papillomavirus vaccination. Braz J Infect Dis 2015; 19: 224–225. [DOI] [PMC free article] [PubMed] [Google Scholar]


