INTRODUCTION
In experimental conditions, all Food and Drug Administration‐approved vaccines have demonstrated vaccine‐related reductions in COVID‐19 hospitalizations. 1 , 2 While the literature is rapidly evolving, several observational studies have evaluated the efficacy of COVID‐19 vaccination on particular populations. Studies of healthcare workers in Jerusalem, 3 Italy, 4 and the United States found reduced COVID‐19 infection rates soon (generally within 1–2 weeks) 5 following vaccination. National observational studies of the general population in Scotland 6 and Israel 7 found lower rates of symptomatic infection, hospitalization, and death soon (withing 2–3 weeks) following vaccination.
Starting January 18, 2021, Washington prioritized Washingtonians aged 65 and older (65+) for COVID‐19 vaccination. 8 We sought to explore whether reduced COVID‐19 hospitalizations could be discerned in real‐world conditions following vaccination prioritization for that population. To do this, we used historical trends of Washington State COVID‐19 hospitalizations for the 65+ and other age groups to generate estimates of COVID‐19 hospitalization in the 65+ years age group had prioritization not occurred, and compared these estimates with actual hospitalization in the first 6 weeks following prioritization.
METHODS
We analyzed 16,511 Washington Department of Health COVID‐19 hospitalizations that occurred between March 1, 2020 and March 1, 2021.
Using COVID‐19 hospitalizations for the 65+ and four other age groups (0–19, 20–34, 35–49, and 50–64) before vaccination prioritization (March 1, 2020–January 17, 2021), we generated a synthetic control 9 using a Bayesian structural time‐series synthetic control model. 10 Extending that model to the post‐prioritization period (January 18, 2021–March 1, 2021), we used actual hospitalization in the non‐65+ age groups to generate an estimate of the number of hospitalizations among the 65+ population had vaccination prioritization not occurred. From that, we subtracted actual hospitalizations to estimate the number of hospitalizations avoided (with 95% confidence intervals [CIs]) following prioritization. We used the age‐specific Washington State COVID‐19 hospitalization fatality rate for October 1, 2020–December 31, 2020 (28% = 983 deaths/3509 hospitalizations among those 65+) to estimate the number of avoided deaths associated with vaccine prioritization for older Washingtonians.
Because we used publicly available data, IRB approval was not required.
RESULTS
Before vaccination prioritization, hospitalization rates were highly correlated across age groups, with Pearson's correlation coefficients ranging between 0.79 and 0.96, depending on the age group examined (data not shown).
For the 65+ age group, the synthetic model tightly tracked actual hospitalizations in the pre‐prioritization period (Figure 1). By January 29, 2021, the actual numbers of hospitalizations and the synthetic model's estimates of hospitalizations for that group statistically significantly diverged. Between January 18, 2021 and March 1, 2021, the synthetic model predicted 1604 (95% CI: 1521–1691) hospitalizations for the 65+ age group; actual hospitalizations numbered 1170, or 434 (95% CI: 351–521) fewer and 27.1% (95% CI: 22.9%–32.0%) lower than expected. Applying the age‐specific case‐fatality rate from the pre‐prioritization period, we estimate that vaccination prioritization was associated with 122 (95% CI: 98–146) avoided deaths in the 65+ age group in its first 6 weeks. The association between vaccination prioritization and hospitalization reduction among Washingtonians aged 65+ was statistically significant (Bayesian one‐sided tail‐area probability < 0.001).
FIGURE 1.
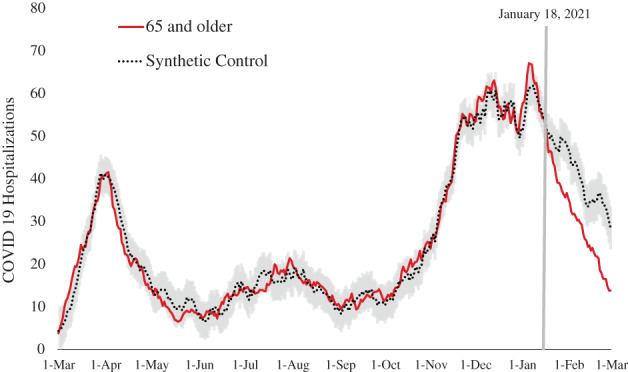
Synthetic control (dotted line with 95% confidence intervals in gray) compared with the actual number of hospitalizations for the 65 and older age group (solid red line) during the entire period examined (March 1, 2020–March 1, 2021). The vertical line indicates January 18, 2021, the date when the 65 and older population was prioritized for COVID‐19
DISCUSSION
In Washington State, vaccination prioritization of those aged 65+ was associated with a substantial and statistically significant decrease in COVID‐19 hospitalizations, potentially saving between 98 and 146 older Washingtonians' lives. Our findings suggest that prioritizing vaccination of the most vulnerable populations based on age alone was an effective strategy at mitigating overall COVID‐19 hospitalization and mortality in the older Washington population.
Our study has several limitations. First, we conducted our analysis in a dynamic time period. Because Washington's initial vaccination efforts began on December 14, 2020, 3 vaccination of frontline healthcare workers (few of whom are likely to be 65+) and long‐term care facility residents (many of whom are likely to be 65+) might have influenced our synthetic model before January 17, 2021. However, as those same populations continued to get vaccinated after January 18, 2021, our findings may be conservative: the synthetic model's post‐vaccination prioritization hospitalization estimates for the 65+ age group might have been “pulled down” as other groups' hospitalization rates decreased. Second, our model assumes that the association between hospitalizations in the age 65+ years population and the control time series were consistent over time and were influenced only by vaccination prioritization; it is possible that unmeasured confounders influenced our model. Finally, our data are administratively collected; to the extent there were errors in data collection, our model may be flawed.
Despite these limitations, although early, our findings mimic those of the large Phase III trials that found rapid and dramatic reductions in infection, hospitalization, and mortality rates among those vaccinated. 1 , 2 Our study suggests that, in the United States, local policymakers might see brisk declines in hospitalization rates among those prioritized for vaccination.
CONFLICT OF INTEREST
The authors have no conflicts of interest to report.
AUTHOR CONTRIBUTIONS
Mr. Lavista conceptualized the paper, completed data analytics, and contributed to the initial draft and rewritings of the paper. Dr. Richardson helped with statistical review and rewritings of the paper. Dr. Weeks helped conceptualize the paper, contributed to the initial draft and rewritings of the paper, and contributed administrative efforts.
SPONSOR'S ROLE
There were no sponsors for this work.
ACKNOWLEDGMENTS
REFERENCES
- 1. Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on immunization Practices' interim recommendation for use of Pfizer‐BioNTech COVID‐19 vaccine – United States, December 2020. MMWR Morb Mortal Wkly Rep. 2020;69(50):1922‐1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Oliver SE, Gargano JW, Marin M, et al. The Advisory Committee on Immunization Practices' interim recommendation for use of Moderna COVID‐19 vaccine – United States, December 2020. MMWR Morb Mortal Wkly Rep. 2021;69(5152):1653‐1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Benenson S, Oster Y, Cohen MJ, Nir‐Paz R. BNT162b2 mRNA Covid‐19 vaccine effectiveness among health care workers. N Engl J Med. 2021;384(18):1775‐1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fabiani M, Ramigni M, Gobbetto V, Mateo‐Urdiales A, Pezzotti P, Piovesan C. Effectiveness of the Comirnaty (BNT162b2, BioNTech/Pfizer) vaccine in preventing SARS‐CoV‐2 infection among healthcare workers, Treviso province, Veneto region, Italy, 27 December 2020 to 24 March 2021. Euro Surveill. 2021;26(17). 10.2807/1560-7917.ES.2021.26.17.2100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pilishvili T, Fleming‐Dutra KE, Farrar JL, et al. Interim estimates of vaccine effectiveness of Pfizer‐BioNTech and Moderna COVID‐19 vaccines among health care personnel – 33 U.S. sites, January‐March 2021. MMWR Morb Mortal Wkly Rep. 2021;70(20):753‐758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vasileiou E, Simpson CR, Shi T, et al. Interim findings from first‐dose mass COVID‐19 vaccination roll‐out and COVID‐19 hospital admissions in Scotland: a national prospective cohort study. Lancet. 2021;397(10285):1646‐1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS‐CoV‐2 infections and COVID‐19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397(10287):1819‐1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Washington State Department of Health . COVID‐19 Vaccine Prioritization Guidance and Allocation Framework. https://www.doh.wa.gov/Portals/1/Documents/1600/coronavirus/820-112-InterimVaccineAllocationPrioritization.pdf. Accessed May 22, 2021.
- 9. Adadie A, Diamond A, Hainmueller J. Synthetic control methods for comparative case studies: estimating the effect of California's Tobacco Control Program. J Am Stat Assoc. 2010;105(490):493‐505. [Google Scholar]
- 10. Brodersen K, Gallusser F, Koehler J, Remy N, Scott S. Inferring causal impact using Bayesian structural time‐series models. Ann Appl Stat. 2015;9:247‐274. [Google Scholar]


