Abstract
Testing is an essential part of containment of the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) pandemic. This review summarizes studies for SARS‐CoV‐2 infection and testing. Nasopharyngeal samples are best at sensitivity detection, especially in early stages of disease and in asymptomatic individuals. Current swab processing involves a 100‐ to 1000‐fold dilution of the patient sample. Future optimization of testing should focus on using smaller volumes of viral transport media and swab designs to increase comfort and increased viral adhesion.
Keywords: bronchitis, COVID‐19, pharyngitis, pneumonia, respiratory infection
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) was first reported by the World Health Organization (WHO) in December 2019 in China. By January 1, 2021, the coronavirus disease 2019 (COVID‐19) amounted to 83.9 million cases and 1.8 million deaths worldwide, 1 with the United States accounting for 25% of the total cases. 2 The virus is transmitted via small liquid aerosols or larger respiratory droplets that are released by breathing, talking, sneezing, or coughing. SARS‐CoV‐2 enters the body when small, airborne viral particles contact a person's mouth, eyes, or nose.
The SARS‐CoV‐2 virus is particularly dangerous because it can persist asymptomatically in individuals for up to 14 days, providing substantial opportunity for undetected transmission. 3 Lives can be saved by identifying and isolating those infected through accurate testing at an early stage. Many different tests for SARS‐CoV‐2 have been advanced for clinical use, which vary from intranasal pharynx swab tests administered by trained healthcare providers to self‐administered saliva tests. In this review, we summarize available knowledge of the pathophysiology of this disease and data for commonly administered tests, to determine the best current practice. The goal is to highlight potential areas for improvement in testing to bring more sensitive, accurate, and comfortable tests for future use in detecting SARS‐CoV‐2 and other upper respiratory infections.
2. METHODS
PubMed, Web of Science, and Google Scholar were searched for papers published between September and November 2020. Keywords included: “COVID‐19,” “coronavirus,” “SARS‐CoV‐2”, “asymptomatic”, “saliva test”, “nasopharyngeal swab”, “COVID swab”, “ACE 2 cell infection”, “viral load,” and variations.
Papers were screened for relevance based on if they listed the relative sensitivities of various tests, dealt with symptomatic/asymptomatic patients, and provided sufficient details of the study. References in chosen studies were also searched for other relevant studies.
In addition to databases, preprints and hospital bulletins were also consulted. Documents and guidelines from international organizations, such as WHO, and national institutions, such as the Center for Disease Control (CDC) and the Food and Drug Administration (FDA) were also included. Articles published in non‐English languages were not included. News reports were used as general information about the state of the economy or stages of the pandemic.
3. PATHOPHYSIOLOGY AND TIME COURSE OF INFECTION
SARS‐CoV‐2 belongs to the coronavirus family, which are enveloped, positive‐sense, single‐stranded RNA viruses. The SARS‐CoV‐2 virus possesses unique features that allow it to bind and infect human cells. A notable feature, essential to viral function, is the spike protein protruding from the envelope of the novel coronavirus. 4 Within the spike protein is a receptor‐binding domain that helps facilitate a connection to the cellular receptor angiotensin‐converting enzyme 2 (ACE2) on human cells. After contact with a human host cell, enzymes such as TMPRSS2 break the spike protein at one or more cleavage sites, exposing fusion peptides that fuse the viral membrane with the cell membrane. This effectively creates a bridge for the viral RNA to enter and replicate within host cells.
Once viral genomic RNA enters the cell, viral replication‐transcription complexes are assembled, and viral structural proteins are translated from the RNA. These structural proteins are then inserted into the endoplasmic reticulum and move to the endoplasmic reticulum‐Golgi intermediate compartment where genomic RNA is packaged into helical structures by nucleocapsids. This forms the mature virion. The packed virus is then transported out of the cell via the constitutive exocytic pathway. 5
Cells with ACE2 expression include but are not limited to the ciliated epithelium in the nasal cavity (not including the anterior portion), ciliated epithelium in the paranasal sinuses, oral cells in the minor salivary glands, and ciliated airway epithelial cells in the lung.6, 7 The earliest infection is known to occur in the nasal passage, an area where a recent study found ACE2 expression in 20% of the cells. 7
The percentage of ciliated cells expressing ACE2 decreases along the respiratory tract. 7 The expression of ACE2 receptors in the bronchi is roughly one‐half of that in the nasopharynx. 7 This lower percentage of cells with ACE2 expression is the reason for a lower rate of infection within the distal airways.
In most respiratory cells, cilia are another factor in infection. Normally cilia beat synchronously, performing mucociliary clearance (MCC), an innate defense mechanism that removes unwanted particles and bacteria from the respiratory system. Synchronously beating cilia propel mucus from the distal airways to the nasopharynx and from the nasal cavity and paranasal sinuses towards the oropharynx. 8
Current knowledge of viral progression supports a theory of infection on the MCC system from proximal to distal airways. 9 Starting in the nasal passage or oral cavity, the infection may then proceed into the lower respiratory system through oral‐lung aspiration. For around 80% of patients, the disease will remain mild and be contained within the upper airways. 10 Studies indicate that nasal secretions are swept from the nasal surface by MCC to accumulate in the oral cavity at a rate of ∼0.5 ml/h. 7 From there, small volumes of high titer (concentrated) virus are aspirated into the deep lung, causing more severe infections.
The timeline of infection and list of common coronavirus symptoms provided in research studies and CDC guidelines align with this theory of infection (Figure 1). The median time from infection to symptom onset was reported to be 5 days (range of 2–14 days). 11 According to the theory of infection, viral particles replicate within the nasal and oral cavities during this presymptomatic period. In the 10 days following symptom onset, the patient will exhibit a variety of symptoms including but not limited to fever, cough, sore throat, runny nose, loss of taste or smell, and fatigue. 12 These symptoms align with the progression of infection from proximal to distal airways.
Figure 1.
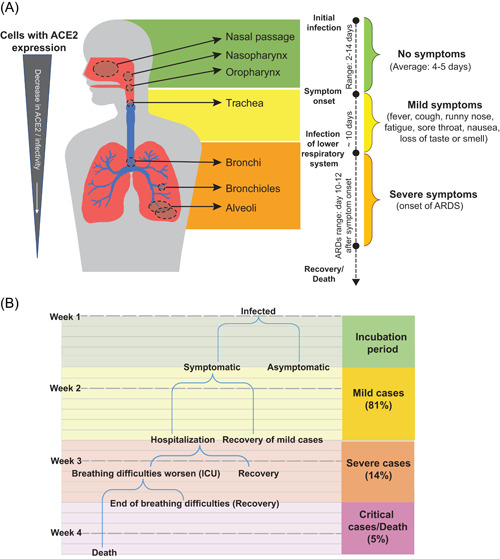
(A) A diagram showing the timeline of infection, common coronavirus symptoms, and a progression of virus for a theoretical patient with a severe case of coronavirus disease 2019 (COVID‐19). (B) A more comprehensive timeline of general COVID‐19 cases [Color figure can be viewed at wileyonlinelibrary.com]
Previous studies of SARS‐CoV, as well as more recent studies of SARS‐CoV‐2, determined that coronavirus infections cause the destruction or loss of cilia in ciliated cells. 13 Initial infection of the nasal passage leads to the cytopathic destruction of nasal epithelial cells responsible for nasal MCC, 9 symptomatic as a runny nose. There may also be an infection of sustentacular or supporting olfactory epithelial cells which can cause anosmia (loss of smell). These two commonly observed initial COVID‐19 symptoms support a model of infection beginning in the nasal passage.
The second major entry point of the virus is the stratified squamous epithelium of the oropharynx and laryngopharynx, causing laryngitis (inflammation of the voice box) and pharyngitis (inflammation of the throat). These inflammations lead to symptoms of sore throat and hoarseness of voice. Another notable infection is that of taste bud epithelial cells, which causes ageusia (loss of taste). 9
From there, serious infections proceed through the respiratory tract, infecting areas of the trachea, bronchi, bronchiole, and alveoli, in that order. Normally, inhaled particles of pathogens are eliminated in about 20 min from the end of the large bronchi to the pharynx. 9 However, due to the loss of cilia, this process is disrupted. As the infection progresses, MCC slows and more cells are infected.
Depending on the viral load and the host immune response, the progression through the lower airways may be rapid (1–2 days) or slow (7–14 days). 9 Around 10–12 days after initial symptoms, the patient can develop acute respiratory distress syndrome (ARDS) and require hospitalization.11, 14 This more severe reaction indicates that the virus has moved into the distal airways and has most likely infected the alveoli. 9
Understanding this timeline of infection and theorized progression of the virus is essential in developing tests and treatments for SARS‐CoV‐2. Tests that allow early diagnosis during the presymptomatic period allow for isolation of patients to prevent spread of disease and early treatments for best outcomes.
4. CURRENT TESTING METHODS
Numerous testing methods have been developed or adapted for detecting SARS‐CoV‐2 since the beginning of the pandemic. There are three main forms of tests (Table 1). A molecular test detects viral genetic material. An antigen test detects specific viral proteins to determine if the patient is currently infected. Finally, an antibody test detects antibodies to SARS‐CoV‐2 and determines whether someone has been previously infected.
Table 1.
A summary of the existing tests for detecting SARS‐CoV‐2
| Type of test | Sampling type | Sample method | Time until results | Use cases |
|---|---|---|---|---|
|
Molecular test: Detects the genetic material of the virus and tests to see if someone is currently infected |
Swabbing | Nasopharyngeal swab | Same day, up to 1 week | Early detection, measure of recovery |
| Mid‐turbinate swab | Early detection, measure of recovery | |||
| Anterior nasal swab | Early detection, measure of recovery | |||
| Oropharyngeal swab | Early detection, measure of recovery | |||
| Respiratory related fluid | Saliva | Early detection, measure of recovery | ||
| Sputum | Symptomatic/late | |||
| Bronchoalveolar lavage (BAL) | Symptomatic/late | |||
|
Antigen test: Detects specific proteins from the virus to see if someone is currently infected |
Swabbing | Nasal swab | Can be extremely fast (15–30 min) | Early detection |
| Nasopharyngeal swab | Early detection | |||
|
Antibody test: Looks for antibodies and tests for if someone has been previously infected |
Blood/Serology | Finger stick | Same day or 1–3 days | Asymptomatic, after infection |
| Blood draw | Asymptomatic, after infection |
This review will focus on the sampling methods for molecular and antigen tests. The most effective and common testing methods focus on one of the following three anatomic areas: the nasal cavity (specifically the nasopharynx), the oral cavity and pharynx, or the lower respiratory system (lungs). The most common ways to test these areas are to use a swab, collect saliva, or collect sputum. While different studies may report different sensitivity values, there is a relatively strong consensus regarding the effectiveness of each testing method.
4.1. Forget sensitivity and specificity, false‐negative rate dominates testing economics
In a binary diagnostic test, there are four possible outcomes (and consequences): true‐positive (patient quarantined, transmission mitigated), false‐positive (patient quarantined, 2 weeks lost productivity), true‐negative (no change, productivity maintained), and false‐negative (no change, transmission continues). The sensitivity of a test refers to its ability to correctly identify COVID‐19 positive patients (sensitivity = ), while the specificity refers to its ability to correctly identify COVID‐19 negative patients (specificity = ). Conversely, and more informatively, one can look at the errors that the test makes either through the false‐positive rate (FPR = 1 – specificity; type I error) or the false‐negative rate (FNR = 1 – sensitivity; type II error).
When a true assessment of the patient's disease status is made, the decision to quarantine or not quarantine is justified. However, when an error in diagnosis is made there is damage, economic or physical, to the patient and society. First, consider the case of a false‐positive or type I error. The patient and perhaps close contacts unnecessarily quarantine, potentially each losing 2 weeks of productivity (~ $2000/person on average 15 ). A national lockdown is an extreme example of this outcome wherein many individuals are assumed positive and quarantined.
Next, consider the case of false‐negative or type II error. The patient continues about their daily life acting as a vector of COVID‐19 transmission for up to 2 weeks. While the number of individuals that this patient infects is highly dispersed (modeling suggests 80% of spread is driven by only 10% of those infected 16 ), at an epidemiological level, it is captured by the effective reproduction number Rt, currently estimated to be 1. 17 From the mortality data versus age and demographics of the USA, it is estimated that each COVID‐19 death is responsible for 7.6 years of life lost. 18 Utilizing current estimates for average mortality rates following infection of 0.3%–3% and a quality‐adjusted life year valuation of $100,000 results in societal damages of at least $2800–$22,800 per false‐negative result. Hospitalization costs, lost labor, etc. for those who had COVID‐19 but recovered further increases this damage. If one considers second‐order effects, the costs of FNR may be even higher. For example, does a false‐negative result lead patients to engage in riskier behavior than no test at all (e.g., deciding to attend an unmasked holiday meal with family or friends) and drive the superspreading that the high dispersion parameter indicates? Therefore, throughout this review article, we highlight the FNR of tests where possible.
4.2. Nasopharyngeal testing
Nasopharyngeal (NP) swabbing is one of the most common testing methods and involves inserting a swab into the nasal passage until the tip of the swab contacts the nasopharynx. The swab is then rotated for about 7–8 s in each nostril to collect the sample. 19 While it can be uncomfortable, the NP swab test is highly effective due to the virus being present within the area throughout the infection. According to a study of 91 inpatients at 6 different hospitals in Toronto, Canada this method has a 4‐week range of FNR ranging from 6% to 18% (sensitivities from 82% to 94%). 20 This FNR is lowest during the first week of infection and subsequently increases until the patient recovers.
Other methods that focus on testing inside the nasal cavity include anterior and mid‐turbinate nasal swabs. These methods are both commonly used due to their increased comfort over NP swabs but are less sensitive than NP swabs. According to a study of symptomatic patients in ambulatory clinics in the Puget Sound, Washington, the anterior nasal swab has an FNR ranging from 12% to 24% (6% increase compared to NP swabbing) and the mid‐turbinate has an FNR ranging from 9.8% to 21.8% (3.8% increase compared to NP swabbing). 21
4.3. Oropharyngeal testing
Oropharyngeal swabbing targets the oropharynx through the mouth. This difference in the testing location may result in a large difference in sensitivity. A study conducted by Tu et al. 21 found that compared to NP swabbing, self‐administered oral swabbing tests have an FNR ranging from 16.2% to 28.2% (10.2% increase). This method is still commonly used in testing centers throughout the country but is widely accepted to be not as effective in detecting the virus compared to the NP swab.
Another method focused on the oral cavity collects saliva. For this test, about 1 ml of saliva is collected into a sample container and taken to a lab. In addition to being a noninvasive, and therefore, more comfortable test, it also requires less PPE and interaction with healthcare professionals. Such benefits have led to a large increase in its popularity. The sensitivity of this method is still being studied for asymptomatic patients. A Canadian study of 38 patients found a 4‐week range in FNR from 12% to 34% (sensitivities from 66% to 88%) throughout the illness. 20 Like the NP method, sensitivity is highest during the first week of infection and declines subsequently.
4.4. Bronchial testing
The last method, the sputum test, targets the respiratory tract. When infected with COVID‐19, symptomatic individuals can produce sputum, mucus that forms in the respiratory tract and lungs. This test involves asking the patient to cough to produce mucus, which is collected using a sample container much like the saliva test. According to a study conducted on 205 hospitalized patients in China, the test has an average FNR of 28% (sensitivity of 72%) throughout the illness. 22 Another reason for the lack of widespread usage of this test is the difficulty for someone without symptoms to produce the necessary mucus to test. This contributes to a slightly higher FNR.
A more accurate but also more invasive test involves collecting bronchoalveolar lavage (BAL) from the lungs. 23 The test is typically performed in severe cases and has a high sensitivity. A study in China found it to have an FNR of only 7% (sensitivity of 93%) in 15 patients. 22 For patients with severe respiratory symptoms who need bronchoscopy, BAL is a highly sensitive test for diagnosis of COVID‐19, but is impractical for widespread screening.
For patients with milder cases or screening of asymptomatic patients, NP and saliva tests have proven to be the most accurate. Nasal swabs, oropharyngeal swabs, and sputum tests are all still being used, but they are deemed less effective. Consequently, further improvements should focus on perfecting the NP and saliva test methods. Public health screening programs for SARS‐CoV‐2 should place a greater emphasis on decreasing the FNR to ensure asymptomatic carriers do not continue to propagate the disease.
5. PCR TESTS: METHODS AND ISSUES AFFECTING SENSITIVITY
As of January 2021, the FDA has issued 320 Emergency Use Authorizations (EUA) for in vitro diagnostic products for SARS CoV‐2, which include home collection kits, assays, and collection devices, among other products. The most common products are real‐time quantitative reverse transcription polymerase chain reaction (qRT‐PCR or simply PCR) assays, which are used for processing patient samples. SARS‐CoV‐2 assays typically target some combination of at least two of the following genes: spike (S), nucleocapsid (N), envelope (E), RNA dependent RNA polymerase (RdRp), ORF1ab, or RNase P. Assays may also choose to target a subset of a particular gene (i.e., N1, N2).
Each assay differs in its limit of detection (LoD), RNA extraction methods, volumes of reagents, and samples required, but the general diagnostic procedure remains similar. A viral sample that arrives at the laboratory in 2–3 ml of viral transport medium (VTM) or equivalent must be processed before it is loaded into the PCR reaction. If an RNA extraction step is required, 400–2000 μl of buffers and reagents are typically added to 100–500 μl of the VTM sample. If the assay is a closed cartridge assay (i.e., requires no upfront extraction or has automated steps), the VTM sample is directly loaded in the PCR thermal cycler. Once 5–10 μl of the purified or VTM sample is loaded, an additional 15–20 μl of buffers and reagents are added to the PCR reaction well. As shown in Figure 2A, each step is associated with a dilution factor that is directly dependent on the volumes required by each assay. Note that most of the dilution occurs when the patient sample is placed into the transport tube with 2–3 ml of VTM.
Figure 2.
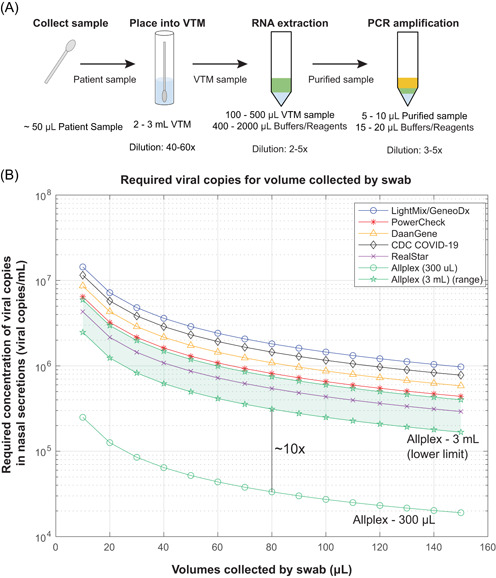
(A) Sample processing and associated dilution factors. Based on these values, a 50–100 μl swab sample undergoes an overall dilution of anywhere from 120‐ to 1500‐fold, the majority occurring when the patient sample is placed into the viral transport medium (VTM) for transport from testing site to laboratory. (B) Viral load as a function of volume collected by a swab. The volume of VTM the swab was placed into has a great influence on the required viral load, as Allplex (3 ml) requires anywhere from 2×105 to 6×106 viral copies/ml in nasal secretions for detection. Allplex (300 μl) requires 2×104 to 3×105 viral copies/ml in nasal secretions for detection. All lines represent the sample processing conditions used by the authors this paper reviews (see Table S1). Allplex (3 ml) is shown as a range, rather than a single line, as RNA extraction methods were unspecified by the authors [Color figure can be viewed at wileyonlinelibrary.com]
Figure 2B also illustrates the general capabilities of currently available SARS‐CoV‐2 assays used by some of the studies reviewed here. Using dilution factors associated with the assays (Table 1), an elution efficiency of 31.3%, 24 and assuming three viral genomes per reaction to start with (Poisson Distribution − 95% chance of having at least one viral genome copy to amplify in the PCR), the Allplex Assay has the lowest LoD (Figure 2B). Note that it is the dilution of the starting sample into different volumes of VTM that drives the difference in LoD. The Allplex assay uses only 300 μl VTM, compared to 3000 μl in other assays.
Under ideal conditions, swabs for collecting biological specimens from the nasal passage are capable of absorbing upwards of 100 μl of fluid.24, 25 As a reasonable estimate for what a swab may collect under nonideal conditions in a nasal passage, we assumed a collection volume of 50 μl. With 50 μl collected, the levels of viral load required for detection by the reviewed assays are consistent with levels found in a patient in the first few days of infection (Figures 3 and 4).
Figure 3.
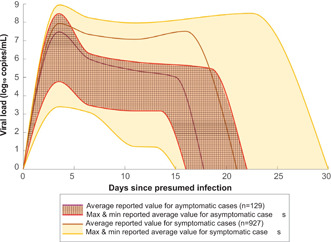
Comparison of viral load in asymptomatic (n = 129) and symptomatic (n = 927) cases of coronavirus disease 2019 (COVID‐19) at days since presumed infection. The solid darker lines represent the mean of reported averages, while the shaded regions represent the range between the maximum and minimum reported averages. The patterned areas represent viral load in asymptomatic patients and the yellow areas represent viral load in symptomatic patients. The viral load is shown in log10 RNA copies/ml. Different papers expressed viral load in different units, most commonly in C t and RNA copies/ml. Converting to log10 RNA copies/ml from RNA copies/ml was done using the log10 function. Converting to log10 RNA copies/ml from C t was done using the linear relationship presented in Vogels et al. 26 Data on viral load was then binned into days: incubation (Days 0–5), viral shedding (Days 6–7), symptomatic (Days 8–14), and recovery (Days 15+) since this is a common breakdown for COVID‐19. 27 All tabulated values used to make this figure can be found in Table S2 [Color figure can be viewed at wileyonlinelibrary.com]
Figure 4.
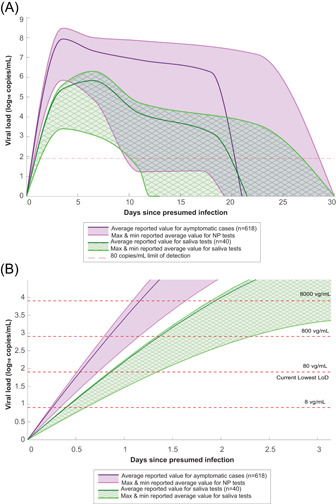
(A) Comparison of viral load of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) collected by nasopharyngeal (NP) swabs (n = 618) and saliva samples (n = 40) at days since presumed infection. (B) Magnified view on the first 3 days with varying LoD lines. These values were converted to log10 RNA copies/ml, using methods of Vogels et al. 26 to convert from C t. Full tabulated values used to make this figure can be found in Table S2 [Color figure can be viewed at wileyonlinelibrary.com]
In the first few days of infection, SARS‐CoV‐2 symptoms are similar to those of other respiratory pathogens. For example, fever, cough, and runny nose are symptoms of both influenza and SARS‐CoV‐2 infection. 28 To distinguish SARS‐CoV‐2 from other respiratory pathogens early in the infection, the diagnosis can be made using multiplex respiratory panels (MRP). MRP are assays that can identify and differentiate between multiple respiratory pathogens. Two such MRP are the Luminex xTAG Respiratory Viral Panels and the BioFire Diagnostics FilmArray Respiratory Panel, each of which can detect 20+ pathogens. They typically have varying limits of detection for each pathogen. For example, the BioFire RP has an LoD of 3.0×103 copies/ml for adenovirus, which is on par with the LoD for some of the more restrictive SARS CoV‐2 assays (Table S1). While the specificity, sensitivity, and cross‐reactivity must be taken into consideration, using a MRP can help public health experts quickly determine which patients are infected with SARS‐CoV‐2, and which are infected with other respiratory pathogens. From a practical standpoint, patients with respiratory symptoms are likely to have symptoms like the flu and be tested by a respiratory pathogen panel.
6. CLINICAL DATA ON TESTING IN PATIENTS INFECTED WITH SARS‐CoV‐2
Figures 3 and 4 were created to streamline, visualize, and compare data from 15 papers. These figures focus on comparing viral load in asymptomatic versus symptomatic patients and viral load collected from NP swabs versus saliva tests, respectively. The data sets used included sources that provided quantitative viral load data throughout infections. Papers in preprint and letters to the editor were omitted from the data set.
6.1. Viral load in asymptomatic (presymptomatic) vs symptomatic patients
Of the studies reviewed, four included information on asymptomatic and presymptomatic cases. Two studies reported that there was no statistically significant difference in viral load between asymptomatic and symptomatic cases.29, 30 Ra et al. tested 39 asymptomatic and 144 symptomatic patients using the RT‐PCR process that targeted the E, RdRp, and N genes of SARS‐CoV‐2. The cycle threshold (C t) values reported in mean ± standard deviation of the asymptomatic and symptomatic groups, respectively, were 31.15 ± 2.72 and 31.43 ± 2.80 for the E gene, 32.26 ± 2.86 and 32.93 ± 2.87 for the RdRp gene, and 33.05 ± 2.52 and 33.28 ± 2.48 for the N gene. 30 Alternatively, a study comparing 13 asymptomatic and 17 symptomatic cases by Chau et al. and a study with 27 asymptomatic and 19 symptomatic cases by Gniazdowski et al. reported lower viral load in asymptomatic cases than in symptomatic cases in the first 14–19 days post presumed infection.31, 32 After 14–19 days, the viral load in asymptomatic and symptomatic cases was not statistically different.
As shown in Figure 3, there is a very wide range in viral load across COVID‐19 cases. This is confirmed by multiple studies that were reviewed, most notably by Arnaout et al. who reported viral load in COVID‐19 cases to be evenly distributed between 2.5 and 8.5 log10 copies/ml. 33 In symptomatic patients, this wide range can be explained by the theory of infection as cases with more severe symptoms have a viral load of 10 vg/ml for longer than those with less severe symptoms, though both severe and mild cases are included in the symptomatic grouping. 34 Asymptomatic patients have, on average, a lower viral load of 4.05e7 vg/ml at the peak loading compared to 1.12e8 vg/ml in symptomatic patients. Asymptomatic patients also test negative earlier than symptomatic patients—on average asymptomatic patients first test negative 16 days after presumed infection while symptomatic patients first test negative 21 days after presumed infection (Figure 3). However, since the range of symptomatic case viral load encompasses the range for asymptomatic cases, testing methods and protocols that are used on symptomatic cases will also capture asymptomatic cases. The most worrisome conclusion from these data is that asymptomatic patients can still be infectious for many days. Finding these patients, who are mobile and don't appear to be ill, must be a goal of any public health screening program to slow the spread of disease and stop the pandemic.
6.2. Viral load in NP swabs compared to saliva tests
Of the papers reviewed, nine studies provided data on viral load collected with NP swabs as a function of days since presumed infection, totaling 682 cases, and four studies provided similar data on saliva tests, totaling 40 cases. Figure 4 visually compares the viral load collected on NP swabs to the viral load in saliva tests. The solid darker lines represent the mean of reported average values for particular days while the shaded regions represent the range between the maximum and minimum reported averages at particular days. The purple areas represent viral load collected with NP swabs and the green patterned areas represent viral load in saliva tests.
Based on Figure 4A, NP swabs carry, on average, a higher viral load than saliva. NP swabs collect an average of 3.02e7 vg/ml during peak viral load (Days 0–5) whereas saliva tests collect an average of 5.16e5 vg/ml during peak viral load (Days 6–7). However, the difference between NP swabs and saliva tests may not matter depending on the LoD of the assay being used. For an LoD of 80 copies/ml (lowest reported in this paper, see Table S1), the ranges of detectable viral load for NP swabs and saliva tests intersect with the LoD line around the same day—on Days 10 or 11 at the earliest, on Days 20 or 21 on average, and between Days 27 and 29 at the latest. This supports literature such as Wyllie et al. 35 and Pasomsub et al. 36 that claim saliva tests are suitable substitutes for NP swabs however, Figure 4A also supports that an NP swab is more likely to carry a higher viral load and therefore be more likely to test positive than a saliva test.
Perhaps a more important distinction between NP swabs and saliva tests is their ability to detect SARS‐CoV‐2 early in a patient's infection. Figure 4B shows a magnified image of Figure 4A in the first 3 days of infection with LoD detection lines varying from 8 to 8000 vg/ml. At an LoD of 8 vg/ml, both tests are likely to detect the virus within one day. However, at LoD of 80 vg/ml, only NP swabs are likely to detect the virus consistently within the first day, whereas saliva tests may only detect it on day two of infection. A difference in one day may not seem significant, but knowing a day earlier that a person is positive may increase their chances of seeking appropriate care sooner, and more importantly, keep them from spreading the virus to others.
7. SOCIAL AND ECONOMIC BURDEN OF DELAYED DIAGNOSIS
Accurately detecting the virus at an early stage can deter further spread, help prevent deaths, and reduce economic burden. Figure 5 shows the economic burden of different testing and sheltering scenarios. The left branch of the tree in Figure 5, highlighted in red, shows the effects of a 30% FNR. In a scenario where all 30,000 infected asymptomatic individuals shelter at home, there is an economic burden of about $89,631,000 per 100,000 tests. This value is calculated using the following assumptions: the infected asymptomatic individual shelters at home and only exposes 3 others to a high‐level of the virus, the infection rate for those high‐level exposure cases is 5% for those that shelter at home, 15% of infections lead to hospitalization, 1.6% of infections lead to death, and the costs associated with sheltering at home, hospitalization, and death are approximately $2000, 15 $15,000, 37 and $1,000,000, respectively.
Figure 5.
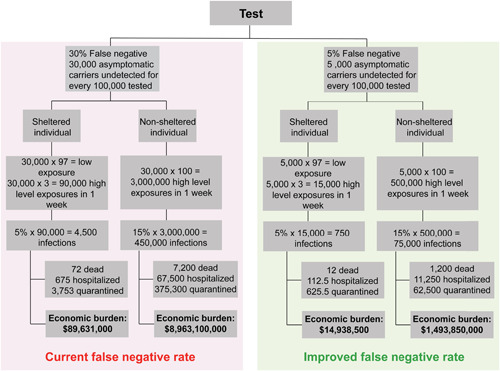
Potential testing scenarios and their associated costs calculated for a population of 100,000 (assuming that the entire population is tested). A lower false‐negative rate along with individuals who shelter at home leads to the lowest economic burden, whereas a higher false‐negative rate with individuals who do not shelter at home leads to the highest economic burden. This analysis highlights why the false‐negative rate is the salient metric when comparing test efficacy. The majority of the economic burden comes from asymptomatic patients not being detected and quarantined to break the chain of infection (type II error). The personal and societal costs of false‐negative rate (FNR) are significant [Color figure can be viewed at wileyonlinelibrary.com]
The branch for non‐sheltering individuals with 30% FNR presents an economic burden of $8,963,100,000 and uses the same assumptions as above but with two significant changes. The number of high‐level exposures in a week is now assumed to increase to 100, as these infected asymptomatic individuals are not sheltering at home. Subsequently, the infection rate is estimated to increase to 15% due to the lack of social distancing and other safety precautions. 38 This branch shows the cost burden that results from individuals failing to practice recommended sheltering in place measures.
The right branch of Figure 5, highlighted in green, projects a more ideal case where testing has an improved FNR of 5%. Using this new FNR with the same assumptions for sheltered and unsheltered individuals yields a much lower economic burden, as can be observed in the bottom row of boxes in Figure 5. This comparison demonstrates the importance of developing tests that can accurately detect the virus as there is immense value in ensuring that infected individuals are aware of their situation and do their best to mitigate spread.
8. DISCUSSION
The COVID‐19 pathophysiology and time course suggest that most infected individuals do not experience serious symptoms in the first week after infection with SARS‐CoV‐2. However, these infected people also have the highest level of viral shedding around Day 5 after exposure. 39 The ease with which unknown community spread occurs has been a significant challenge that governments and communities have faced in controlling the spread of SARS‐CoV‐2. This problem is only exacerbated by long incubation periods and asymptomatic cases.
One method to increase the sensitivity of tests and thus decrease the FNR is to focus on the volume of VTM used in assays. Currently, CDC guidelines recommend using 2–3 ml of VTM per sample. 40 Unfortunately, the usage of VTM introduces a dilution factor that may severely impact the effectiveness of assays. For example, if the average NP swab collects and releases 50 μl of nasal secretions and is stored in a tube of 3 ml VTM, the original sample would be diluted 60:1 (Figure 2A). Such a large dilution factor can be the difference between a positive and a false‐negative result by pushing a sample below the LoD. As seen in Figure 2B with the Allplex example, a 10‐fold difference in VTM volume to 300 µl can drastically change the number of viral copies required for detection. Few published sources justify the need for 2–3 ml of VTM per sample. On ResearchGate, it was claimed that some laboratories require 3 ml of the sample to be centrifuged at low RPMs for sedimenting heavy debris. 41 Furthermore, assay manuals, such as the CDC 2019‐novel coronavirus (2019‐nCoV) Real‐Time RT‐PCR Diagnostic Panel, highlight the importance of having enough sample solution to repeat an assay, should something go wrong. In these cases, it may be possible to vary the volume of VTM needed.
The existence of several research studies using less than the recommended VTM volumes have shown promising results, although none of the studies focused exclusively on the variation of VTM volume.30, 31 The effectiveness of existing tests will likely improve greatly should there be a research study that proves the viability of smaller volumes of VTM usage.
The need for placing the current sampling swabs in each nostril for prolonged periods has been an obstacle for compliance to testing. Comfort may be increased by designing an NP swab with more surface area on the tip and made out of a material that binds to the virus better than currently used materials, such that the swab requires less time inside of the patient's nose. The redesigned swab should meet or exceed current NP performance in the collection of patient samples, comfort, and required insertion time.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
SYNOPSIS
Testing is an essential part of containment of the SARS‐CoV‐2 pandemic. Nasopharyngeal samples are best at sensitivity detection, especially in the early stages of disease and in asymptomatic individuals. Future optimization of testing should focus on using smaller volumes of viral transport media and swab designs to increase comfort and increased viral adhesion.
Supporting information
Supporting information.
ACKNOWLEDGMENTS
The authors would like to acknowledge and thank several individuals whose contributions have made this paper possible. The authors thank Tyler Seto, Vice President of Enterprise Quality and Patient Safety at City of Hope, for explaining how healthcare professionals are trained and how COVID‐19 tests are administered at a testing site. We would also like to express our gratitude toward Gilbert Morales, supervisor of Clinical Pathology from City of Hope, for providing essential information on how testing samples are processed within the lab. Finally, we would like to thank Vivian Lam, Kaitlyn Paulsen, and Supriya Deshpande for providing editing assistance.
Roque M, Proudfoot K, Mathys V, et al. A review of nasopharyngeal swab and saliva tests for SARS‐CoV‐2 infection: Disease timelines, relative sensitivities, and test optimization. J Surg Oncol. 2021;124:465–475. 10.1002/jso.26561
DATA AVAILABILITY STATEMENT
All data have been presented in the manuscript.
REFERENCES
- 1. Allen JAS, Aufrichtig A, Barnard A, et al. Coronavirus World Map: Tracking the Global Outbreak. 2020. https://www.nytimes.com/interactive/2020/world/coronavirus-maps.html
- 2. CDC COVID Data Tracker 2020. https://covid.cdc.gov/covid-data-tracker/#cases_casesper100klast7days
- 3. Coronavirus disease (COVID‐19): World Health Organization . 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-and-answers-hub/q-a-detail/coronavirus-disease-covid-19
- 4. Cyranoski D. Profile of a killer: the complex biology powering the coronavirus pandemic. Nature. 2020;581(7806):22‐26. [DOI] [PubMed] [Google Scholar]
- 5. Burmer G, Burmer M, Pabuwal V. SARS‐CoV‐2 and COVID‐19 Pathogenesis: A Review. Seattle, WA: LifeSpan BioSciences, Inc.; 2020. [Google Scholar]
- 6. Schröder I. COVID‐19: A risk assessment perspective. ACS Chem Health Saf. 2020;27(3):160‐169. [DOI] [PubMed] [Google Scholar]
- 7. Hou YJ, Okuda K, Edwards CE, et al. Sars‐CoV‐2 reverse genetics reveals a variable infection gradient in the respiratory tra. Cell. 2020;182(2):429‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Howard P. Pediatric Pulmonolgy. 1st ed. Elsevier Mosby; 2005. [Google Scholar]
- 9. Gentzsch M, Rossier BC. A pathophysiological model for COVID‐19: critical importance of transepithelial sodium transport upon airway infection. Function. 2020;1(2):zqaa024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mason RJ. Pathogenesis of COVID‐19 from a cell biology perspective. Eur Respir J. 2020;55(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lei S, Jiang F, Su W, et al. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID‐19 infection. EClinicalMedicine. 2020;21:100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duration of Isolation and Precautions for Adults with COVID‐19: Centers for Disease Control. October 19, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/duration-isolation.html
- 13. Li W, Li M, Ou G. COVID‐19, cilia, and smell. FEBS J. 2020;287(17):3672‐3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Larry W. A Timeline of COVID‐19 Symptoms: Blue Cross and Blue Shield of North Carolina. July 13, 2020. https://blog.bcbsnc.com/2020/07/a-timeline-of-covid-19-symptoms/
- 15.Usual Weekly Earnings of Wage and Salary Workers. October 2020. https://www.bls.gov/news.release/wkyeng.toc.htm
- 16. Endo A, Abbott S, Kucharski AJ, Funk S. Estimating the overdispersion in COVID‐19 transmission using outbreak sizes outside China. Wellcome Open Res. 2020;5:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Systrom K, Krieger M, Vladeck T. Changing Metrics and Additional Resources. https://rt.live/
- 18.United States COVID‐19 Cases and Deaths by State over Time: Centers for Disease Control and Prevention. January 26, 2021. https://data.cdc.gov/Case-Surveillance/United-States-COVID-19-Cases-and-Deaths-by-State-o/9mfq-cb36
- 19.Coronavirus (COVID‐19) testing: What you should know. November 23, 2020. https://health.ucdavis.edu/coronavirus/coronavirus-testing.html
- 20. Jamal AJ, Mozafarihashjin M, Coomes E, et al. Sensitivity of nasopharyngeal swabs and saliva for the detection of severe acute respiratory syndrome coronavirus 2. Clin Infect Dis. 2021;72(6):1064‐1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tu YP, Jennings R, Hart B, et al. Swabs collected by patients or health care workers for SARS‐CoV‐2 testing. N Engl J Med. 2020;383(5):494‐496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang W, Xu Y, Gao R, et al. Detection of SARS‐CoV‐2 in Different Types of Clinical Specimens. JAMA. 2020;323(18):1843‐1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bronchoscopy and Bronchoalveolar Lavage (BAL): National Library of Medicine. September 29, 2020. https://medlineplus.gov/lab-tests/bronchoscopy-and-bronchoalveolar-lavage-bal/
- 24. Bruijns BB, Tiggelaar RM, Gardeniers H. The extraction and recovery efficiency of pure DNA for different types of swabs. J Forensic Sci. 2018;63(5):1492‐1499. [DOI] [PubMed] [Google Scholar]
- 25. Zasada AA, Zacharczuk K, Woznica K, Glowka M, Ziolkowski R, Malinowska E. The influence of a swab type on the results of point‐of‐care tests. AMB Express. 2020;10(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vogels CBF, Brito AF, Wyllie AL, et al. Analytical sensitivity and efficiency comparisons of SARS‐CoV‐2 RT‐qPCR primer‐probe sets. Nat Microbiol. 2020;5(10):1299‐1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Viral cultures for COVID‐19 infectivity assessment. medRxiv; 2020. 10.1101/2020.08.04.20167932. Accessed March 24, 2021. [DOI]
- 28.Similarities and differences between flu and COVID‐19. December 29, 2020. https://www.cdc.gov/flu/symptoms/flu-vs-covid19.htm
- 29. Lee S, Kim T, Lee E, et al. Clinical course and molecular viral shedding among asymptomatic and symptomatic patients with SARS‐CoV‐2 infection in a community treatment center in the Republic of Korea. JAMA Intern Med. 2020;180:1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ra SH, Lim JS, Kim GU, Kim MJ, Jung J, Kim SH. Upper respiratory viral load in asymptomatic individuals and mildly symptomatic patients with SARS‐CoV‐2 infection. Thorax. 2021;76(1):61‐63. [DOI] [PubMed] [Google Scholar]
- 31. Van Vinh Chau N, Lam VT, Dung NT, et al. The natural history and transmission potential of asymptomatic severe acute respiratory syndrome coronavirus 2 infection. Clin Infect Dis. 2020;71(10):2679‐2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gniazdowski V, Morris CP, Wohl S, et al. Repeat COVID‐19 molecular testing: correlation of SARS‐CoV‐2 culture with molecular assays and cycle thresholds [published online ahead of print October 27, 2020]. Clin Infect Dis. 2020.ciaa1616. 10.1093/cid/ciaa1616 [DOI] [Google Scholar]
- 33. Arnaout R, Lee RA, Lee GR, et al. SARS‐CoV2 testing: the limit of detection matters. bioRxiv. 2020. [Google Scholar]
- 34. To KK, Tsang OT, Leung WS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS‐CoV‐2: an observational cohort study. Lancet Infect Dis. 2020;20(5):565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wyllie AL, Fournier J, Casanovas‐Massana A, et al. Saliva or nasopharyngeal swab specimens for detection of SARS‐CoV‐2. N Engl J Med. 2020;383(13):1283‐1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pasomsub E, Watcharananan SP, Boonyawat K, et al. Saliva sample as a non‐invasive specimen for the diagnosis of coronavirus disease 2019: a cross‐sectional study. Clin Microbiol Infect. 2021;27(2):285 e281‐285 e284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bartsch SM, Ferguson MC, McKinnell JA, et al. The potential health care costs and resource use associated with COVID‐19 In the United States. Health Aff. 2020;39(6):927‐935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Transmission of COVID‐19: European Centre for Disease Prevention and Control. 2020. https://www.ecdc.europa.eu/en/covid-19/latest-evidence/transmission
- 39. Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID‐2019. Nature. 2020;581(7809):465‐469. [DOI] [PubMed] [Google Scholar]
- 40.Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens for COVID‐19: Centers for Disease Control and Prevention. 2020. https://www.cdc.gov/coronavirus/2019-ncov/lab/guidelines-clinical-specimens.html
- 41.Shanmuganathan A. Does the reduction of volume of the viral transport medium (VTM) during sampling increase the concentration of viral load in the sample? May 25, 2020. https://www.researchgate.net/post/Does_the_reduction_of_volume_of_the_viral_transport_medium_VTM_during_sampling_increase_the_concentration_of_viral_load_in_the_sample
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
All data have been presented in the manuscript.


