Abstract
Background & Aims
Little is known about cholestasis, including its most severe variant secondary sclerosing cholangitis (SSC), in critically ill patients with coronavirus disease 19 (COVID‐19). In this study, we analysed the occurrence of cholestatic liver injury and SSC, including clinical, serological, radiological and histopathological findings.
Methods
We conducted a retrospective single‐centre analysis of all consecutive patients admitted to the intensive care unit (ICU) as a result of severe COVID‐19 at the University Hospital Zurich to describe cholestatic injury in these patients. The findings were compared to a retrospective cohort of patients with severe influenza A.
Results
A total of 34 patients with severe COVID‐19 admitted to the ICU were included. Of these, 14 patients (41%) had no cholestasis (group 0), 11 patients (32%, group 1) developed mild and 9 patients (27%, group 2) severe cholestasis. Patients in group 2 had a more complicated disease course indicated by significantly longer ICU stay (median 51 days, IQR 25‐86.5) than the other groups (group 0: median 9.5 days, IQR 3.8‐18.3, P = .001; and group 1: median 16 days, IQR 8‐30, P < .05 respectively). Four patients in group 2 developed SSC compared to none in the influenza A cohort. The available histopathological findings suggest an ischaemic damage to the perihilar bile ducts.
Conclusions
The development of SSC represents an important complication of critically ill COVID‐19 patients and needs to be considered in the diagnostic work up in prolonged cholestasis. The occurrence of SSC is of interest in the ongoing pandemic since it is associated with considerable morbidity and mortality.
Keywords: cholestasis, Coronavirus disease 19 (COVID‐19), liver injury, sclerosing cholangitis in critically ill patients, secondary sclerosing cholangitis, severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2)
Abbreviations
- AKI
acute kidney injury
- ALT
alanine aminotransferase
- ALP
alkaline phosphatase
- AST
aspartate aminotransferase
- ARDS
acute respiratory distress syndrome
- BMI
body mass index
- COVID‐19
Coronavirus disease 2019
- ECMO
extracorporeal membrane oxygenation
- GGT
gamma‐glutamyl transferase
- ICU
intensive care unit
- INR
international normalized ratio
- IQR
interquartile range
- LFT
liver function tests
- MAP
mean arterial pressure
- MRCP
magnetic resonance cholangiopancreatography
- RRT
renal replacement therapy
- SAPS II Score
Simplified Acute Physiology Score
- SARS‐Cov‐2
severe acute respiratory syndrome coronavirus 2
- SOFA Score
Sequential Organ Failure Assessment Score
- SSC
secondary sclerosing cholangitis
- SC‐CIP
sclerosing cholangitis in critically ill patients
- UDCA
Ursodeoxycholic acid
- ULN
upper limit of normal
Lay Summary.
Data on severe affection of bile ducts in COVID‐19 patients are scarce. Herein, we describe the occurrence of bile duct injury in a cohort of 34 critically ill COVID‐19 patients. In 20 patients, evidence of bile duct injury was found, thereof 4 developed a chronic disorder of the bile ducts, the so‐called secondary sclerosing cholangitis. Such bile duct injury was not detected in a comparative cohort of critically ill influenza A patients. The occurrence of this complication is of interest since the development of secondary cholangitis may be more frequently observed in COVID‐19 patients compared to other critical illnesses, leading to a significant number of patients with a chronic, difficult‐to‐treat disorder of the bile ducts.
1. INTRODUCTION
The severe acute respiratory syndrome coronavirus type 2 (SARS‐CoV‐2), a novel pathogen causing coronavirus disease 2019 (COVID‐19), has spread globally causing an ongoing pandemic with more than 1 000 000 deaths so far. 1 Most COVID‐19 patients present with mild cold‐ and flu‐like symptoms including fever (88.7%) and cough (67.8%), anticipating a good prognosis. 2 Patients with older age and/or underlying conditions, however, are at increased risk for the development of severe pneumonia, resulting in the acute respiratory distress syndrome (ARDS) and multi‐organ failure. 3 Even though the main healthcare focus lies in the severe pulmonary affections of the disease, it has become evident that COVID‐19 can affect multiple organ systems including the liver. Being still a matter of debate, the etiology of the liver injury in these patients may be multifactorial either directly as the ACE2 receptor is expressed in cholangiocytes or indirectly as a result of a severe inflammatory response/sepsis, hypoxic or drug‐induced liver injury. 4 , 5 , 6 Several studies demonstrated that elevations of liver enzymes are observed in up to 50% of the patients. 2 , 7 , 8 , 9
Within the spectrum of liver injury in COVID‐19 patients, cholestasis was considered to be a rare event. Higher gamma‐glutamyl transferase (GGT) levels as well as elevated bilirubin were reported to be associated with disease severity. 2 , 10 However, in a recent meta‐analysis, elevations of alkaline phosphatase elevation was reported in 6.1% and GGT elevation in 21.1%. 11 Bile duct injury in patients with severe COVID‐19 could be a result of hypoxia and severe SIRS or potentially by direct infection of cholangiocytes.
The development of secondary sclerosing cholangitis (SSC) has been described in ICU survivors in several case series as a late complication of different underlying diseases requiring long‐term ICU care and was designated as sclerosing cholangitis in critically ill patients (SC‐CIP). 3 , 12 , 13 SC‐CIP is a chronic cholestatic liver disease that is observed as a rare complication of critically ill patients resembling primary sclerosing cholangitis, which frequently shows rapid progress to biliary cirrhosis. 13 The etiology of SC‐CIP is incompletely understood. However, ischemic injury to the biliary tree with the formation of biliary casts, frequently infected with multiresistant bacteria, appears to be the major pathogenic determinant. 12 Since ARDS in combination with septic shock is observed in a significant proportion of patients with severe COVID‐19, SC‐CIP may be recognized as a late sequela of the COVID‐19 pandemic. 14 In fact, several case reports have described the occurrence SC‐CIP as complication of severe COVID‐19. 15 , 16 , 17
In this retrospective single‐centre cohort study, we aim to describe the pattern and severity of cholestatic liver injury including the development of SC‐CIP in critically ill COVID‐19 patients. Because little is known on the occurrence of SC‐CIP as a complication of severe viral infection with ARDS, we compare the degree of cholestasis in COVID‐19 patients to a cohort of critically ill influenza A patients.
2. METHODS
2.1. Study design
We conducted a retrospective single‐centre cohort study of all consecutive, non‐selected patients admitted to the ICU as a result of COVID‐19 at the University Hospital Zurich between March and June 2020. The study was approved by the local ethics committee (Kantonale Ethikkommission Zürich, ID 2020‐01656) and the study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki. All patients admitted to the ICU because of severe COVID‐19 during the study period were evaluated for eligibility. Only patients with existing written consent for further use of their health‐related clinical data were included. Patients with documented refusal, missing written consent and missing liver function tests were excluded from analysis. All patient characteristics, disease‐ and treatment‐related data as well as laboratory values within this period were retrieved from the patients’ medical record following a predefined protocol.
2.2. Characterization of comparative influenza A cohort
34 consecutive, critically ill influenza A patients with acute hypoxemic respiratory failure treated in the ICU of the University Hospital Zurich between February 2016 and February 2020 with existing written consent for further use of their health‐related clinical data were analysed. Patient characteristics, disease‐ and treatment‐related data as well as laboratory values within this period were also retrieved from the patients’ medical record following a predefined protocol.
2.3. Definitions
Mild cholestasis was defined as an alkaline phosphatase (ALP) level of ≥1.5× the upper limit of normal (ULN) and GGT level of ≥3× ULN (group 1) as suggested by the European Association for the Study of the Liver. 18 Patients with additional total bilirubin of ≥2× ULN, defining liver dysfunction in drug‐induced liver injury, 19 were considered to have severe cholestasis (group 2) as suggested in a previous work by de Tymowski et al evaluating burn‐associated cholestasis in critically ill patients. 20 All other patients were allocated to group 0 (without cholestasis). We defined COVID‐19 associated SC‐CIP as a cholestatic liver injury with the typical radiological findings in MRCP (as described below) in patients with severe SARS‐CoV‐2 infection admitted to the ICU, who had no evidence of a cholestatic liver disease prior to the current hospital admission.
2.4. Objectives
To describe the frequency and severity of cholestatic liver injury in critically ill COVID‐19 patients and compare it to a cohort of critically ill influenza A patients.
2.5. Radiology
Radiology reports of abdominal imaging (abdominal ultrasound, computed tomography or magnetic resonance cholangiography) were reviewed for evidence of obstructive cholestasis or SC‐CIP wherever available. All images of group 2 were additionally reviewed by an expert radiologist (CSR) in GI imaging to confirm diagnosis of SC‐CIP and exclude obstructive cholestasis. The biliary tract was preferably imaged with magnetic resonance MRCP. On MRCP, SC‐CIP was suspected in case of irregularities of the bile ducts with dilatations and strictures, if other causes of biliary dilatation were excluded. If computed tomography (CT) was performed, a non‐enhanced (in case of severe renal insufficiency) or portal venous phase of the abdomen was acquired to exclude biliary obstruction.
2.6. Histopathology
All tissue samples (2 liver biopsies as well as liver tissue from 4 autopsy cases) were processed according to standard histological methods, that is, formalin‐fixed, paraffin‐embedded and stained with haematoxylin and eosin (H&E). Additionally, Sirius red and/or elastic van Gieson (EVG) stainings to highlight the connective tissue structures as well as immunohistochemistry to highlight epithelial structures of bile ducts were performed. For immunohistochemistry, standard procedures were applied using the VentanaBenchMark automated staining system for CK7 antibody (clone OV‐TL 12/30; Dako, USA). Histological slides were evaluated by 2 experienced liver pathologists (DL and EMM).
2.7. Statistical analysis
Distribution of the data was analysed by the Kolmogorov normality test for continuous variables. Continuous variables are expressed as median and interquartile range (IQR). Categorical variables were expressed as counts and percentages. Statistical analysis was performed by Mann‐Whitney U test, Kruskal‐Wallis test, Fisher's exact test and Chi‐Square test as appropriate. All statistical analyses were performed using IBM® SPSS® Statistics Version 26 (International Business Machines Corporation – IBM) and the R environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria; Version 3.6.1.)
3. RESULTS
3.1. Patient characteristics
44 patients were admitted to the ICU because of COVID‐19 during the observation period, thereof 10 were excluded as a result of missing informed consent for further use of their health‐related clinical data (Figure 1). Finally, 34 COVID‐19 patients were eligible for analysis in this study. There was no relevant difference in comorbidities among the different cholestasis groups (Table 1) apart from diabetes mellitus. The incidence of diabetes was highest in the group with severe cholestasis but also prominent in the group without cholestasis (78% in group 2 vs 50% in group 0 and 9% in group 1; P = .228 and P = .005 respectively). A total of 20 patients (59%) developed cholestasis during hospital stay, thereof 9 patients (27%) had severe intrahepatic cholestasis (Table 1; Figure 1). None of these patients showed clinical or radiological evidence of obstructive cholestasis. Patients with severe cholestasis (group 2) had a significantly longer ICU stay than patients of groups 0 and 1, but at ICU admission disease severity was not different (measured by SAPS II Score; Table 1; Figure 2). Furthermore, patients with severe cholestasis needed higher levels of supportive care during ICU stay including renal replacement treatment, extracorporeal membrane oxygenation (ECMO) and proning compared with patients without cholestasis (Figure 2). All patients in groups 1 and 2 received Ketamine.
FIGURE 1.
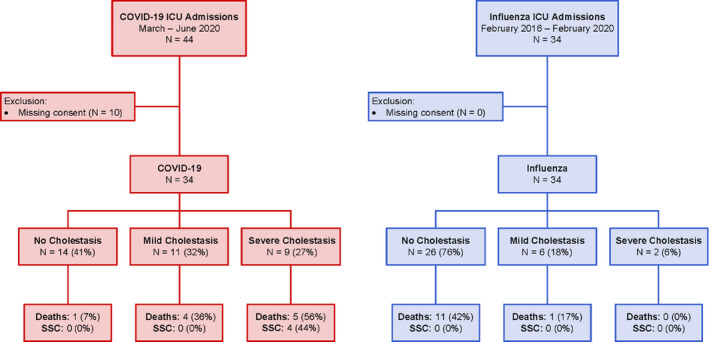
Study flow chart. Death refers to mortality during hospital stay. SSC, secondary sclerosing cholangitis
TABLE 1.
Clinical and laboratory characteristics of the COVID‐19 cohort
| Characteristics |
Overall (n = 34) |
No Cholestasis Group 0 (n = 14; 41%) |
Cholestasis Group 1 (n = 11; 32%) |
Cholestasis Group 2 (n = 9; 27%) |
p‐value |
|---|---|---|---|---|---|
| Age, years | 60 [55‐69] | 63 [55‐70] | 59 [52‐70] | 59 [53‐68] | .809 |
| Male sex | 29 (85) | 13 (93) | 9 (82) | 7 (78) | .563 |
| BMI, kg/m2 | 28.0 [25.1‐34.3] | 29.0 [25.5‐33.5] | 28.0 [26.0‐35.9] | 27.0 [24.4‐34.5] | .653 |
| Comorbidities | |||||
| Diabetes mellitus | 15 (44) | 7 (50) | 1 (9) | 7 (78) | .007 |
| Arterial hypertension | 23 (68) | 8 (57) | 8 (73) | 7 (78) | .650 |
| Adipositas | 12 (35) | 7 (50) | 4 (36) | 1 (11) | .162 |
| Chronic liver disease | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1.000 |
| Chronic lung disease | 6 (18) | 3 (21) | 3 (27) | 0 (0) | .278 |
| Chronic kidney disease | 7 (21) | 3 (21) | 3 (27) | 1 (11) | .766 |
| Immunosuppressiona | 6 (18) | 1 (7) | 4 (36) | 1 (11) | .166 |
| Severity Scores | |||||
| SOFA (ICU admission) | 8 [3‐10] | 4.5 [2.0‐8.25] | 10 [7‐11] | 8 [6‐13.5] | .021 |
| SAPS II | 33 [24‐50] | 25 [22‐35] | 42 [26‐59] | 44.0 [26‐60] | .035 |
| LFT at admission | |||||
| AST, × ULN | 0.9 [0.8‐1.4] | 0.9 [0.6‐1.1] | 1.9 [0.9‐2.1] | 0.9 [0.7‐1.0] | .012 |
| ALT, × ULN | 0.8 [0.5‐1.2] | 0.6 [0.5‐1.0] | 1.1 [0.9‐2.1] | 0.6 [0.5‐0.9] | .102 |
| GGT, × ULN | 1.2 [0.6‐1.5] | 0.9 [0.6‐1.6] | 1.3 [1.0‐2.0] | 1.2 [0.6‐1.5] | .402 |
| ALP, × ULN | 0.6 [0.4‐0.7] | 0.5 [0.4‐0.7] | 0.7 [0.4‐0.7] | 0.4 [0.4‐0.7] | .235 |
| Total bilirubin, × ULN | 0.4 [0.3‐0.6] | 0.5 [0.2‐0.6] | 0.5 [0.2‐0.6] | 0.3 [0.3‐0.4] | .392 |
| INR | 1.0 [1.0‐1.0] | 1.0 [1.0‐1.0] | 1.0 [1.0‐1.0] | 1.0 [1.0‐1.0] | .181 |
| Peak LFT | |||||
| AST, × ULN | 4.0 [2.5‐11.0] | 2.8 [1.5‐3.6] | 4.1 [2.5‐16] | 13.4 [8.3‐100.5] | <.001 |
| ALT, × ULN | 3.7 [2.3‐8.1] | 2.4 [2.0‐3.6] | 5.5 [1.7‐8.1] | 12.4 [7.3‐55.6] | .001 |
| GGT, × ULN | 7.9 [4.9‐26.5] | 4.5 [2.0‐6.5] | 9.4 [6.0‐14.5] | 36.9 [26.6‐54.9] | <.001 |
| ALP, × ULN | 1.8 [1.0‐3.5] | 0.9 [0.5‐1.0] | 2.1 [1.8‐2.9] | 9.1 [3.8‐18.1] | <.001 |
| Total bilirubin, × ULN | 1.0 [0.5‐2.6] | 0.5 [0.4‐0.8] | 1.0 [0.6‐1.6] | 8.0 [3.5‐13.5] | <.001 |
| INR | 1.0 [1.0‐1.0] | 1.0 [1.0‐1.0] | 1.0 [1.0‐1.0] | 1.1 [1‐2.2] | .378 |
| LFT at discharge / death | |||||
| AST, × ULN | 0.9 [0.6‐2.1] | 0.7 [0.5‐0.9] | 1.0 [0.9‐1.6] | 2.5 [0.7‐3.7] | .032 |
| ALT, × ULN | 1.2 [0.8‐2.3] | 1.0 [0.7‐1.6] | 1.2 [0.8‐3.2] | 2.0 [1.0‐2.8] | .302 |
| GGT, × ULN | 4.8 [2.2‐11.2] | 1.7 [1.2‐3.7] | 5.5 [3.0‐10.0] | 11.9 [5.4‐30.4] | .001 |
| ALP, × ULN | 1.4 [0.9‐3.4] | 0.7 [0.4‐0.9] | 1.7 [1.2‐2.0] | 5.4 [2.5‐7.4] | <.001 |
| Total bilirubin, × ULN | 0.4 [0.2‐1.6] | 0.3 [0.2‐0.4] | 0.3 [0.1‐1.2] | 6.0 [0.4‐9.5] | .012 |
| INR | 1.0 [1.0‐1.0] | 1.0 [1.0‐1.0] | 1.0 [1.0‐1.0] | 1.0 [1.0‐1.0] | .734 |
| Treatments at ICU | |||||
| Vasopressors | 27 (79) | 8 (57) | 11 (100) | 8 (89) | .018 |
| Mechanical ventilation | 31 (91) | 11 (79) | 11 (100) | 9 (100) | .102 |
| Proning | 23 (68) | 6 (43) | 8 (73) | 9 (100) | .012 |
| Nitric Oxide | 5 (15) | 0 (0) | 1 (9) | 4 (44) | .007 |
| ECMO | 7 (21) | 0 (0) | 3 (27) | 4 (44) | .028 |
| RRT | 15 (44) | 2 (14) | 7 (64) | 6 (55) | .013 |
| Parenteral nutrition | 5 (14) | 0 (0) | 2 (18) | 3 (27) | .065 |
| Outcomes | |||||
| Nosocomial Infection | 18 (53) | 6 (43) | 6 (55) | 6 (67) | .516 |
| Bacteraemia | 9 (27) | 1 (7) | 3 (27) | 5 (56) | .045 |
| Sepsis | 10 (29) | 3 (21) | 3 (27) | 4 (44) | .565 |
| Septic shock | 12 (35) | 2 (14) | 6 (55) | 4 (44) | .128 |
| AKI | 18 (53) | 4 (29) | 8 (73) | 6 (67) | .059 |
| ARDS | 28 (82) | 9 (64) | 10 (91) | 9 (100) | .080 |
| ICU days | 17 [8‐36] | 9.5 [3.8‐18.3] | 16 [8‐30] | 51 [25‐86.5] | .002 |
| Overall Mortality | 10 (29) | 1 (7) | 4 (36) | 5 (56) | .043 |
| COVID‐19‐specific medication | |||||
| Hydroxychloroquine | 21 (62) | 6 (43) | 9 (82) | 3 (75) | .065 |
| Remdesivir | 6 (18) | 2 (14) | 2 (18) | 1 (11) | 1.000 |
| Lopinavir/Ritonavir | 5 (15) | 1 (7) | 2 (18) | 2 (22) | .579 |
| Tocilizumab | 1 (3) | 1 (7) | 0 (0) | 0 (0) | 1.000 |
Abbreviation: AKI, acute kidney injury; ALT, alanine aminotransferase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; ARDS, acute respiratory distress syndrome; BMI, body mass index; GGT; gamma‐glutamyl transferase; ICU, intensive care unit; INR, international normalized ratio; LFT, liver function tests; RRT, renal replacement therapy; SAPS II Score, Simplified Acute Physiology Score; SOFA Score, Sequential Organ Failure Assessment Score; ULN, upper limit of normal. †Immunosuppression was defined as any of the following: haematological malignancy, human immunodeficiency virus or prescribed immunosuppressive medication.
Continuous variables are expressed as median and interquartile range [IQR]. Categorical variables are expressed as n (%). Statistical Analysis: Kruskal‐Wallis and Fisher's exact test as appropriate.
FIGURE 2.
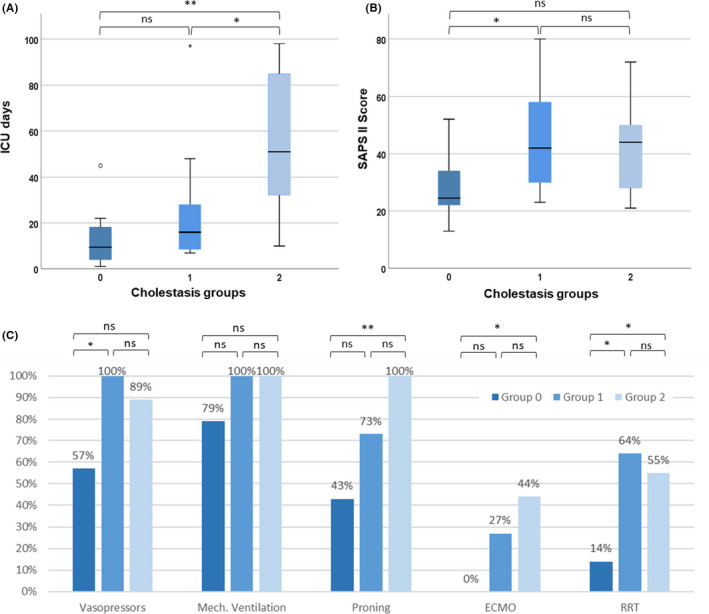
ICU characteristics according to cholestasis groups of patients with COVID‐19 infection. A: ICU days among different groups. B: SAPS II score among different groups. Median (black bar), interquartile range (blue box) and 95% confidence interval (whiskers) are indicated. C: ICU treatments among different groups (percentages of patients receiving in each group). Statistical analysis: A/B: Mann‐Whitney U test. C: Fisher's exact. *P <.05, **P <.01, ***P <.001. ICU, Intensive care unit; SAPS II Score, Simplified Acute Physiology Score; ns, not significant
3.2. Comparison with the influenza A cohort
All COVID‐19 patients were compared to 34 consecutive, critically ill influenza A patients (Figure 1). In the COVID‐19 cohort, there were significantly more male patients (Table 2). According to SAPS II and SOFA score, patients with influenza A were significantly sicker compared to the patients with COVID‐19. However, length of hospital stay was significantly longer in the COVID‐19 cohort. With respect to comorbidities, arterial hypertension was more prevalent in the COVID‐19 group. COVID‐19 patients received significantly more often respiratory support using prone positioning. The 2 cohorts did not differ in frequency of vasopressor use (Table 2). No ketamine was used in the influenza A cohort.
TABLE 2.
Patient characteristics of the 2 cohorts at admission to ICU
| Characteristics | Overall (n = 68) | COVID‐19 (n = 34) | Influenza A (n = 34) | p‐value |
|---|---|---|---|---|
| Age, years | 59 [53‐68] | 60 [55‐69] | 58 [49‐67] | .164 |
| Male sex | 48 (71) | 29 (85) | 19 (56) | .017 |
| BMI, kg/m2 | 27.3 [23.9‐31.1] | 28.0 [25.1‐34.3] | 25.9 [23.0‐28.9] | .050 |
| Comorbidities | ||||
| Arterial hypertension | 35 (51) | 23 (68) | 12 (35) | .015 |
| Diabetes mellitus | 23 (34) | 15 (44) | 8 (24) | .124 |
| Chronic liver disease | 4 (6) | 0 (0) | 4 (12) | .122 |
| Chronic lung disease | 20 (29) | 6 (18) | 14 (41) | .062 |
| Chronic kidney disease | 15 (22) | 7 (21) | 8 (24) | 1.000 |
| Immunosuppression a | 14 (21) | 6 (18) | 8 (24) | .764 |
| Severity Scores | ||||
| SOFA (ICU admission) | 9 [6‐11] | 8 [3‐10] | 9 [8‐12] | .025 |
| SAPS II | 44 [30‐57] | 33 [24‐50] | 53 [40‐60] | .002 |
| PaO2/FiO2, mmHg | 99 [75‐165] | 105 [84‐170] | 93 [68‐140] | .130 |
| LFT at admission | ||||
| AST, × ULN | 1.1 [0.8‐1.9] | 0.9 [0.8‐1.4] | 1.5 [1.0‐2.9] | .008 |
| ALT, × ULN | 0.9 [0.5‐1.4] | 0.8 [0.5‐1.2] | 1.0 [0.6‐1.6] | .213 |
| GGT, × ULN | 1.2 [0.7‐1.6] | 1.2 [0.6‐1.5] | 1.3 [0.8‐2.0] | .582 |
| ALP, × ULN | 0.6 [0.4‐0.8] | 0.6 [0.4‐0.7] | 0.7 [0.5‐1.2] | .004 |
| Total bilirubin, × ULN | 0.4 [0.2‐0.6] | 0.4 [0.3‐0.6] | 0.4 [0.2‐0.9] | .610 |
| INR | 1.1 [1.0‐1.2] | 1.0 [1.0‐1.0] | 1.2 [1.1‐1.4] | <.001 |
| Treatments at ICU | ||||
| Vasopressors | 59 (87) | 27 (79) | 32 (94) | .152 |
| Mechanical ventilation | 60 (88) | 31 (91) | 29 (85) | .707 |
| Proning | 30 (44) | 23 (68) | 7 (21) | <.001 |
| Parenteral nutrition | 10 (15) | 5 (15) | 5 (15) | 1.000 |
| ECMO | 17 (25) | 7 (21) | 10 (29) | .575 |
| Complications | ||||
| Septic shock | 30 (44) | 12 (35) | 15 (53) | .222 |
| ARDS | 61 (90) | 28 (81) | 33 (97) | .110 |
| Acute Kidney Injury | 39 (57) | 18 (53) | 21 (62) | .624 |
| Cholestasis | .009 | |||
| No | 40 (59) | 14 (41) | 26 (76) | |
| Mild | 17 (25) | 11 (32) | 6 (18) | |
| Severe | 11 (16) | 9 (27) | 2 (6) | |
| SSC | 4 (6) | 4 (12) | 0 (0) | |
| Outcome | ||||
| ICU days | 15 [7‐30] | 17 [8‐36] | 13 [6‐24] | .414 |
| Hospital mortality | 19 (28) | 10 (29) | 12 (35) | .280 |
Abbreviation: ALT, alanine aminotransferase; ALP, alkaline phosphatase; AST, aspartate aminotransferase; ARDS, acute respiratory distress syndrome; BMI, body mass index; COVID‐19, Coronavirus disease 2019; ECMO, extracorporeal membrane oxygenation; GGT; gamma‐glutamyl transferase; ICU, intensive care unit; INR, international normalized ratio; BMI, body mass index; RRT, renal replacement therapy; SAPS II, Simplified Acute Physiology Score; SOFA, Sequential Organ Failure Assessment Score; SSC, secondary sclerosing cholangitis.
Data are presented as median (interquartile range) or counts (percentages) as appropriate. Statistical analysis: Kruskal‐Wallis and Chi‐square test as appropriate.
Immunosuppression was defined as any of the following: haematological malignancy, human immunodeficiency virus or prescribed immunosuppressive medication.
3.3. Liver injury in the COVID‐19 cohort
None of the included individuals had a history of pre‐existing chronic liver disease. There was no difference in liver function tests at admission among different cholestasis subgroups, apart from significantly increased AST in group 1 compared to groups 0 and 2 (1.9 [0.9‐2.1] × ULN vs 0.9 [0.6‐1.1] and 0.9 [0.7‐1.0], respectively; P = .007 and 0.039, respectively, Table 1). Of interest, 4 patients with severe cholestasis (group 2) revealing persistently elevated cholestasis parameters developed SC‐CIP (diagnosed by MRCP, 2 of them additionally received a liver biopsy – see below and Table 3). One of these patients was evaluated and is currently listed for liver transplantation as a result of persistent jaundice and pruritus; 2 died after ICU discharge. Regardless of the degree of cholestasis, most patients (6 of 7 patients; 86%) with markedly elevated ALT (>10x ULN) during ICU stay showed an unfavourable outcome either developing SC‐CIP and/or dying (Figure 3).
TABLE 3.
Patient characteristics of the 4 individuals developing SC‐CIP (sclerosing cholangitis in critically ill patients)
| Characteristics | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
|---|---|---|---|---|
| Age, years | 59 | 67 | 54 | 64 |
| Sex | M | M | F | M |
| BMI, kg/m2 | 28.0 | 24.0 | 24.7 | 24.7 |
| Comorbidities | ||||
| Diabetes mellitus | Yes | Yes | Yes | No |
| Adipositas | No | No | No | No |
| Chronic liver disease | No | No | No | No |
| Chronic lung disease | No | No | No | No |
| Chronic kidney disease | No | No | No | No |
| Severity Scores | ||||
| SOFA (ICU admission) | 15 | 12 | 7 | 14 |
| SAPS II | 72 | 45 | 28 | 70 |
| LFT at admission | ||||
| AST, × ULN | 0.96 | 0.62 | 0.77 | 0.92 |
| ALT, × ULN | 0.54 | 0.36 | 0.86 | 0.62 |
| GGT, × ULN | 0.60 | 0.25 | 1.55 | 0.62 |
| ALP, × ULN | 0.25 | 0.58 | 0.58 | 0.39 |
| Total bilirubin, × ULN | 0.38 | 0.24 | 0.29 | 0.57 |
| INR | 1.0 | 1.0 | 1.0 | 1.0 |
| Peak LFT | ||||
| AST, × ULN | 28.70 | 13.44 | 9.26 | 33.14 |
| ALT, × ULN | 19.84 | 7.26 | 12.37 | 23.04 |
| GGT, × ULN | 55.97 | 28.15 | 85.13 | 50.52 |
| ALP, × ULN | 21.26 | 18.81 | 17.30 | 12.85 |
| Total bilirubin, × ULN | 26.05 | 8.05 | 3.81 | 17.43 |
| INR | 1.0 | 1.0 | 1.0 | 2.2 |
| Time form ICU admission to first rise of ALP (days) | 20 | 10 | 10 | 6 |
| Time from ICU admission to diagnosis of SSC (days) | 70 | 74 | 153 | 75 |
| Treatments at ICU | ||||
| Vasopressors | Yes | Yes | Yes | Yes |
| Mechanical ventilation | Yes (43 days) | Yes (20 days) | Yes (55 days) | Yes (28 days) |
| Proning | Yes (10 days) | Yes (6 days) | Yes (10 days) | Yes (11 days) |
| Nitric Oxide | No | No | Yes | Yes |
| ECMO | No | Yes | Yes | No |
| RRT | Yes | Yes | No | Yes |
| Parenteral nutrition | Yes | No | No | No |
| Ketamine Sedation | Yes (16 days) | Yes (5 days) | Yes (23 days) | Yes (14 days) |
| Anticoagulation | Heparin | Heparin | Heparin | Heparin |
| Outcomes | ||||
| Nosocomial Infection | Yes | Yes | No | No |
| Septic shock | No | No | Yes | Yes |
| AKI | Yes | Yes | No | Yes |
| ARDS | Yes | Yes | Yes | Yes |
| ICU days | 51 | 98 | 78 | 88 |
| ICU mortality | No | No | No | No |
| Listed for liver transplant | Yes | n.a. | No | n.a. |
| COVID‐19‐specific medication | Hydroxychloroquine | Hydroxychloroquine | Hydroxychloroquine, Lopinavir / Ritonavir | None |
| Other Medication | ||||
| UDCA | Yes | Yes | Yes | Yes |
| Steroids | No | No | No | No |
| MRCP with typical findings of SC‐CIP | Yes | Yes | Yes | Yes |
| Follow up after hospital discharge | Last follow up, 7 month 3 weeks after discharge from ICU; cirrhosis Child B, MELD 17, deeply jaundiced (Bilirubin 217 ymol/l) and on the transplant waiting list | Exitus letalis 55 days after ICU discharge (80 days after SSC diagnosis) as a result of respiratory failure (pulmonary infection) | Last follow up, 9 month and 2 weeks after discharge from ICU; compensated liver function, persistently marked increased ALP and GGT | Exitus letalis 14 days after ICU discharge (26 days after SSC diagnosis) caused by sepsis / pulmonary infection |
Abbreviation: AKI, acute kidney injury; ALT, alanine aminotransferase; ALP, alkaline phosphatase, AST, aspartate aminotransferase; ARDS, acute respiratory distress syndrome; BMI, body mass index; GGT; gamma‐glutamyl transferase; ICU, intensive care unit; INR, international normalized ratio; LFT, liver function tests; MRCP, magnetic resonance cholangiopancreatography; RRT, renal replacement therapy; SAPS II Score, Simplified Acute Physiology Score; SOFA Score, Sequential Organ Failure Assessment Score; SSC, secondary sclerosing cholangitis; UDCA, Ursodeoxycholic acid; ULN, upper limit of normal.
FIGURE 3.
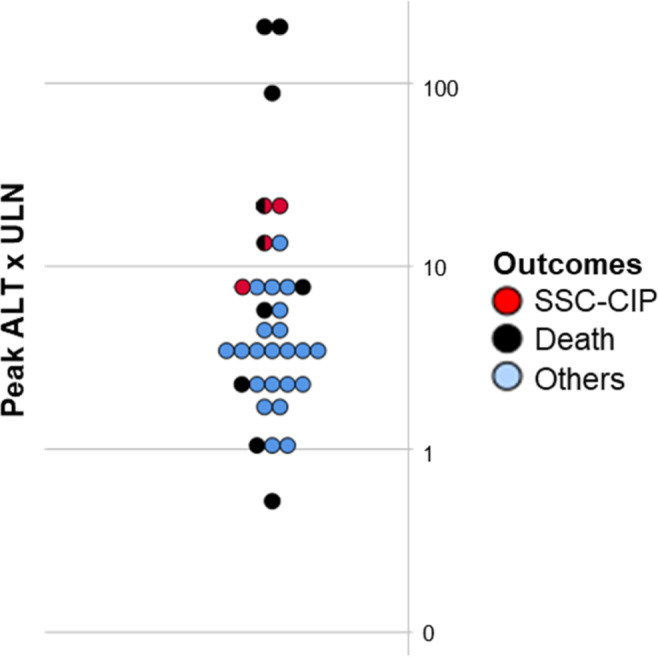
Peak ALT x ULN and outcome of each individual. Death refers to mortality during follow‐up time. ALT, alanine aminotransferase; ULN, upper limit of normal, SSC, secondary sclerosing cholangitis
3.4. Liver injury in the Influenza A cohort
Four patients in this comparative cohort had a history of chronic liver disease. Furthermore, patients with influenza A presented with significantly higher AST and AP levels at admission to ICU compared to the COVID‐19 patients (Table 2). In the influenza cohort, only 2 patients developed severe cholestasis but none of these patients was diagnosed with SC‐CIP during follow up (Figure 1; Table 2). Cholestasis parameters spontaneously declined in both patients.
3.5. UDCA treatment
All 4 patients with SC‐CIP received UDCA treatment (Table 3). Of the 2 patients who died, 1 patient had decreasing serological parameter of cholestasis before death, and the other patient did not show any improvement. Both of the 2 patients with SC‐CIP who are alive reported a significant improvement of pruritus, the patient on the waiting list for liver transplantation showed a decline in laboratory markers of cholestasis but is still deeply jaundiced. In the other patient, no effect on laboratory markers was detectable, moreover transient elastography increased over time.
3.6. Follow up of patients with COVID‐19 infection
Follow‐up data were available up to 10 months after diagnosis of COVID‐19 (median 4.7, range 1‐11). Ten patients (29%) died during the follow‐up time (Table 1). Mortality was significantly higher in the group 2 compared to group 0 (65% vs 7%, P = .018). However, there was no difference between the other groups.
One patient (group 2) deceased 6.5 months after COVID‐19 diagnosis owing to several complications after prolonged and repetitive stays in the ICU. This patient had moderate cholestasis (GGT 626 U/l with normal bilirubin levels and unremarkable liver imaging). Three patients of group 1 presented with mild cholestasis during follow‐up consultations, whereas the remaining showed normal LFTs. One patient was lost to follow up. Taken together, there is no evidence that additional patients developed SSC during the follow‐up period.
3.7. Radiological findings
All patients of group 2 and most patients of group 1 (9 of 12, 78%) had at least 1 abdominal imaging study to exclude obstruction of the biliary tract. In group 1, 8 patients had at least 1 CT scan and 1 patient had an abdominal ultrasound, all negative for biliary obstruction. In group 2, 4 patients had a MRCP, 2 patients an abdominal ultrasound and CT, 2 additional patients an abdominal ultrasound. In all 4 patients undergoing MRCP, imaging confirmed diagnosis of SC‐CIP (Figure 4). In none of the 9 patients of group 2 there was any evidence for biliary obstruction.
FIGURE 4.
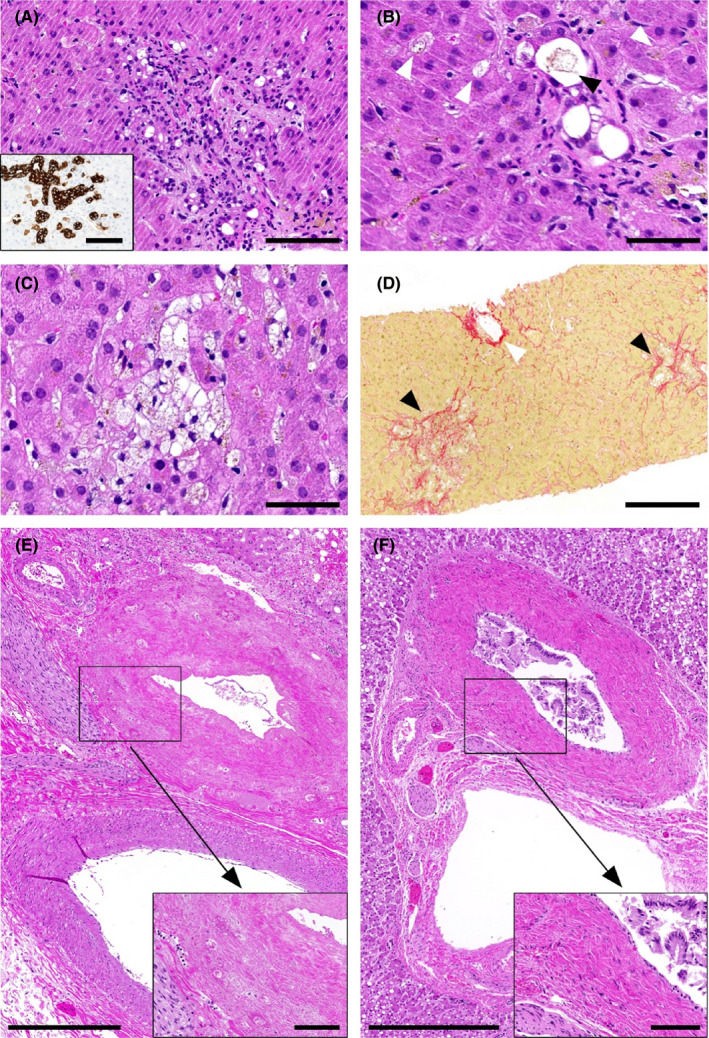
Representative MRCP image of a SC‐CIP patient in our cohort showing diffuse irregularities of the bile ducts with dilatations and strictures. MRCP, magnetic resonance cholangiopancreatography; SC‐CIP, sclerosing cholangitis in critically ill patients
3.8. Histopathological findings
In 2 of the 4 patients who developed SC‐CIP, a liver biopsy was performed (55 and 67 days after COVID‐19 was diagnosed). Histopathological findings in both patients were almost identical and included portal oedema, mixed portal inflammation and pronounced bile duct damage with ductular reaction as well as lobular bile infarcts and severe hepatocellular, canalicular and focally ductular cholestasis (Figure 5A‐C). There was some degree of pericellular fibrosis around portal tracts and central veins, but no fibrotic septa or cirrhosis (Figure 5D).
FIGURE 5.
Representative findings in liver biopsies from patients who developed SC‐CIP (A)‐(D) and in autopsy livers (E)‐(F). (A) Portal tract with oedema, mixed portal inflammation and severe bile duct damage with ductular reaction (H&E; scale bar 100 μm; insert: CK7‐immunohistochemistry; scale bar 100 μm). (B) Ductular (black arrowhead) and canalicular (white arrowheads) cholestasis (H&E; scale bar 50 μm). (C) Lobular bile infarcts and hepatocellular cholestasis (H&E; scale bar 50 μm). (D) Pericellular fibrosis around portal tracts (black arrowheads) and central vein (white arrowhead) (Sirius red; scale bar 200 μm). (E) Transmural necrosis of perihilar bile ducts in patients with preterminally high bilirubin levels (H&E; scale bar 500 μm; insert scale bar 100 μm). (F) Perihilar bile ducts with damage and detachment of the epithelium but viable walls in patients with mild cholestasis (H&E; scale bar 500 μm; insert scale bar 100 μm). In (E) and (F) viable blood vessel walls, viable peripheral nerves and viable hepatocytes are visible
In 4 of the 7 deceased patients, an autopsy was performed (1 patient from cholestasis group 2 and 3 patients from cholestasis group 1), and thus, liver tissues – including hilar structures – were available for histopathological evaluation. Time between death and autopsy was 22 hours in cholestasis group 2 and 1 day 5 hours, 2 days 6 hours and 2 days 8 hours in cholestasis group 1. In the patient in cholestasis group 2, transmural necrosis was present in some large‐ and medium‐sized perihilar bile ducts (Figure 5E), whereas in 2 of the 3 patients in cholestasis group 1 those bile ducts showed mild‐to‐moderate epithelial damage and detachment but viable walls (Figure 5F). The third patient in group 1 presented with acute abdomen and rising bilirubin levels 1 day before death. The autopsy indeed revealed not only mesenteric ischaemia but also complete transmural necrosis of perihilar bile ducts like the patient in cholestasis group 2. While mild damage and inflammation of the small peripheral bile ducts was observed in both groups, hepatocellular, canalicular and ductular cholestasis as well as necrosis of peribiliary arterioles were detected only in the patients with transmural bile duct necrosis. In none of the analysed samples, unequivocal endothelialitis and/or thrombi were evident in the peribiliary vessels. However, in the patient with mesenteric ischaemia, focal endothelialitis was found in a peripheral portal vein branch, associated with intraluminal thrombotic material. In the patient in cholestasis group 2, portal vein thrombi were observed even without evident endothelialitis. A further important histopathological finding in the patients with transmural bile duct necrosis was hepatocyte necrosis. Whereas the patient with mesenteric ischaemia had only recent pericentral necrosis, the patient in cholestasis group 2 additionally showed several, up to a few centimetres large, fresh parenchymal infarcts.
4. DISCUSSION
In this retrospective cohort study of 34 consecutive unselected patients admitted to the ICU because of severe COVID‐19, a high proportion developed cholestatic liver injury. Of note, 4 patients (12%) in the COVID‐19 cohort developed SC‐CIP compared to none in the influenza A cohort with severe ARDS.
Up to date, SC‐CIP has been described in several cohort studies from different centers. 3 , 12 However, the prevalence of SC‐CIP is unknown, yet indirect evidence suggests that it is rare sequelae of critical illness. A recent review summarizing published case series of SC‐CIP since 2001 indicated approximately 200 cases in total. 21 In contrast, in our cohort a relatively high proportion of COVID‐19 patients (12%) developed SC‐CIP.
SC‐CIP is typically observed after sepsis in the setting of polytrauma, complicated surgery and other life‐threatening diseases. 22 The potential role of severe viral diseases in the development of SC‐CIP is less well characterized; however, SC‐CIP was described in a case series as a complication of influenza A(H1N1)‐associated ARDS in 5 of 21 patients (24%). 23
The pathophysiology of SC‐CIP is not fully understood; however, bile duct ischaemia plays a critical role. 12 Unlike the hepatic parenchyma with its redundant blood supply from the hepatic artery and the portal vein, the biliary tree obtains its blood supply exclusively from the hepatic arterial branches, the so‐called peribiliary vascular plexus. Therefore, the biliary epithelium is more susceptible to disturbances in the arterial blood flow compared to the hepatic parenchyma. 13 , 23 , 24 , 25 Supporting the hypothesis of bile duct ischaemia, several predisposing factors for the development of SC‐CIP have been elaborated. Severe hypotension with prolonged decrease in mean arterial pressure (MAP), 22 initial need of large amounts of transfusions or colloids 22 and longer duration spent in prone position in patients with ARDS 23 have been suggested as trigger mechanisms of SC‐CIP. By contrast, the role of high‐dose catecholamine remains unclear, as Leonhardt and colleagues could not find any evidence that vasopressors play a crucial role in the pathogenesis of SC‐CIP. 22 In addition to ischaemic cholangiopathy, the concept of ‘toxic bile’ has been suggested as another driver of SC‐CIP. The disturbance of the finely tuned proportion between bile acid and protective mechanisms as consequence of alterations of the hepatobiliary transporter systems has been shown to result in sclerosing cholangitis. 22 Interestingly, in the group of severe cholestasis, 7 of 9 patients were diabetics, which was significantly higher than in the group with mild cholestasis and 3 of 4 patients who developed SC‐CIP had diabetes, which may suggest diabetes mellitus as a risk factor for a more severe cholestatic course in our cohort. However, the presence of diabetes was not different between the group of severe cholestasis and without cholestasis, therefore, this finding has to be interpreted with caution. Also, the prevalence of diabetes was not significantly different between the COVID‐19 cohort compared to the influenza cohort.
As mentioned above, none of our influenza patients developed SC‐CIP even though these patients were even more critically ill reflected by higher SAPS II scores. Vasopressor use in turn was not significantly different between the 2 cohorts. Furthermore, it is mentionable that ketamine was used in all patients of group 1 and 2 of the COVID‐19 cohort. However, the potential role of ketamine as contributing factor in den development of SC‐CIP as suggested in a recent report 26 cannot additionally be elucidated since no clear association between the occurrence of SC‐CIP and ketamine use is demonstrable. Lastly, it is noteworthy that all 4 SC‐CIP patients had been treated using prone positioning, whereas proning was not used for the ventilation of our influenza patients. However, because of the fact that proning for the treatment of severe ARDS has become standard of care 27 , 28 within the last decade, it cannot sufficiently explain the striking occurrence of SC‐CIP in our COVID‐19 cohort.
Taken together, clinical characteristics cannot readily explain the occurrence of SC‐CIP among COVID‐19 patients but not in influenza patients. It is, therefore, tempting to speculate that there are COVID‐19‐associated factors that promote the development of SC‐CIP such as COVID‐19‐associated vascular damage to the peribiliary plexus or direct damage to cholangiocytes. However, the limited number of patients precludes any firm conclusions to be drawn with respect to the incidence and pathogenesis of this complication in the context of COVID‐19. Hepatic involvement in COVID‐19 patients has been shown in several cohorts from China comprising mostly less severe infections of non‐ICU patients. 8 , 10 , 29 , 30 Hepatocellular injury pattern among COVID‐19 patients is found in up to 41% and ALT elevations are generally mild, defined as less than 5 times upper reference limit. 10 , 29 , 31 Cholestatic liver disease in COVID‐19 patients appears to be less prevalent in these cohorts. A recently published review suggested that elevated ALP occurs in 2%‐5% of patients, whereas increased GGT is found in 13%‐54%. 31 In contrast, our cohort study reveals a high prevalence of cholestatic injury in critically ill COVID‐19 patients. Fifty‐nine per cent had intrahepatic cholestasis during ICU stay including 9 patients (27%) with severe cholestasis and jaundice.
Cholestasis in critically ill patients is mostly intrahepatic and occurs as a result of a variety of causes including SIRS, hypoxic hepatitis, drug‐induced liver injury and pertinent ICU therapy such as ventilatory support and total parenteral nutrition. 32 In line, patients with severe intrahepatic cholestasis in our study had significantly longer ICU stay compared to the other groups. Furthermore, therapeutic interventions such as vasopressors use, proning, ECMO and RRT were significantly more frequent in these patients.
The post‐mortem histopathological findings in 4 patients impressively reflect the preterminal biochemical severity of cholestasis. The most striking finding in the 2 patients with preterminal high or rising bilirubin levels is the transmural necrosis of perihilar bile ducts. According to previous reports on bile duct lesions in the setting of liver transplantation, biliary casts, which presumably derive from necrotic bile ducts, and the subsequent formation of bile duct strictures have been reported as serious complications 33 , 34 and have been also identified histologically as ischaemic‐type biliary lesions. 35 The transmural necrosis in the 2 COVID‐19 patients in our study, therefore, presumably represents the initial lesion that could lead to SC‐CIP. It is tempting to speculate that these patients could also have developed SC‐CIP if they had survived. Indeed, early histopathological findings in the liver biopsies of 2 COVID‐19 survivors in our study who developed SC‐CIP in the follow up were highly suggestive of larger perihilar bile duct obstruction. This is in line with previously described histological changes in patients who develop SC‐CIP. 36 , 37
Additionally to the well‐known causes for ischaemic bile duct damage in critically ill patients, there may be further harmful factors in COVID‐19 patients. The fact that there were no unequivocal thrombi and/or endothelialitis in the small peribiliary vessels does not exclude them to occur on another level of the vasculature. In fact, several thrombi were detected in peripheral portal veins in both patients with transmural bile duct necrosis as well as in the patient with mesenteric ischaemia. Interestingly, in the latter patient, thrombi were even associated with endothelialitis – as it was described previously in COVID‐19 patients. 6 Furthermore, also a mechanical component of vessel and/or bile duct kinking during prone positioning cannot be excluded. Even though we did not perform additional analysis for the detection of viral particles, a direct viral damage to endothelial cells of the peribiliary plexus and/or cholangiocytes cannot be excluded and may lead to further damage. 5 , 38 Overall, these data suggest that multiple factors might damage the biliary tree (or the bile ducts) and may contribute to the surprisingly high prevalence of SC‐CIP in severely ill COVID‐19 patients.
The natural history of SC‐CIP as sequelae of severe COVID‐19 may be comparable to the dismal prognosis of SC‐CIP in patients with severe burns or other causes 13 , 20 since 2 patients died after discharge from ICU and another SC‐CIP patient had already been evaluated for liver transplantation because of deteriorating liver function.
In conclusion, we demonstrate the development of SC‐CIP in 4 of 34 patients with severe COVID‐19 but not in a comparative cohort of influenza A patients with severe ARDS. This entity, therefore, needs to be considered in the diagnostic work up of prolonged cholestasis. Whether disease‐specific factors of COVID‐19 constitutes an additional risk factor for the development of SC‐CIP cannot be proven and warrants further studies.
5. ETHICAL APPROVAL STATEMENT
The study was approved by the local ethics committee (Kantontale Ethikkomisssion Zürich, ID 2020‐01656).
CONFLICT OF INTEREST
BMo: none. BMü: has received speaking and/or consulting fees from Merck/MSD, AbbVie, Intercept, Astra, Bayer and Gilead and research support from Gilead. CJ: none. CG: none. CSR: none. DL: none. EMM: none. GB: none. KPB: none. MH: none. PDWG: none. SB: none.
AUTHORS CONTRIBUTIONS
Conception and design: BMo, BMü, CJ, CG, KPB and SB. Data acquisition: BMo, CJ, DL, EMM, GB, MH, PDWG and SB. Analysis and interpretation of the data: BMo, BMü, CJ, CSR, DL, EMM, KPB, PDWG and SB. Drafting of the article: BMo, CJ, DL and SB. Critical revision of the article for important intellectual content: BMü, CSR, EMM, GB and MH. Final approval of the article: all authors.
Bütikofer S, Lenggenhager D, Wendel Garcia PD, et al. Secondary sclerosing cholangitis as cause of persistent jaundice in patients with severe COVID‐19. Liver Int. 2021;41:2404–2417. 10.1111/liv.14971
Christoph Jüngst and Bernhard Morell contributed equally to this work.
Funding information
The study was founded by internal grants.
Handling Editor: Jian Sun
REFERENCES
- 1. WHO . Coronavirus disease 2019 (COVID‐19) situation report. [Website]. https://www.who.int/publications/m/item/weekly‐epidemiological‐update‐‐‐10‐november‐2020
- 2. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lin T, Qu K, Xu X, et al. Sclerosing cholangitis in critically ill patients: an important and easily ignored problem based on a German experience. Front Med. 2014;8(1):118‐126. [DOI] [PubMed] [Google Scholar]
- 4. Morgan K, Samuel K, Vandeputte M, Hayes PC, Plevris JN. SARS‐CoV‐2 Infection and the Liver. Pathogens. 2020;9(6):430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet. 2020;395(10234):1417‐1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Becker RC. COVID‐19‐associated vasculitis and vasculopathy. J Thromb Thrombolysis. 2020;50(3):499‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xu XW, Wu XX, Jiang XG, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS‐Cov‐2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang Y, Liu S, Liu H, et al. SARS‐CoV‐2 infection of the liver directly contributes to hepatic impairment in patients with COVID‐19. J Hepatol. 2020;73(4):807‐816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kulkarni AV, Kumar P, Tevethia HV, et al. Systematic review with meta‐analysis: liver manifestations and outcomes in COVID‐19. Aliment Pharmacol Ther. 2020;52(4):584‐599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gelbmann CM, Rummele P, Wimmer M, et al. Ischemic‐like cholangiopathy with secondary sclerosing cholangitis in critically ill patients. Am J Gastroenterol. 2007;102(6):1221‐1229. [DOI] [PubMed] [Google Scholar]
- 13. Ruemmele P, Hofstaedter F, Gelbmann CM. Secondary sclerosing cholangitis. Nat Rev Gastroenterol Hepatol. 2009;6(5):287‐295. [DOI] [PubMed] [Google Scholar]
- 14. Nardo AD, Schneeweiss‐Gleixner M, Bakail M, Dixon ED, Lax SF, Trauner M. Pathophysiological mechanisms of liver injury in COVID‐19. Liver Int. 2021;41(1):20‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Durazo FA, Nicholas AA, Mahaffey JJ, et al. Post‐Covid‐19 cholangiopathy‐a new indication for liver transplantation: a case report. Transplant Proc. 2021;53(4):1132–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Edwards K, Allison M, Ghuman S. Secondary sclerosing cholangitis in critically ill patients: a rare disease precipitated by severe SARS‐CoV‐2 infection. BMJ Case Rep. 2020;13(11):e237984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roth NC, Kim A, Vitkovski T, et al. Post‐COVID‐19 Cholangiopathy: a novel entity. Am J Gastroenterol. 2021;116(5):1077‐1082. [DOI] [PubMed] [Google Scholar]
- 18. European Association for the Study of the L . EASL clinical practice guidelines: management of cholestatic liver diseases. J Hepatol. 2009;51(2):237‐267. [DOI] [PubMed] [Google Scholar]
- 19. Benichou C. Criteria of drug‐induced liver disorders. Report of an international consensus meeting. J Hepatol. 1990;11(2):272‐276. [DOI] [PubMed] [Google Scholar]
- 20. de Tymowski C, Depret F, Soussi S, et al. Contributing factors and outcomes of burn‐associated cholestasis. J Hepatol. 2019;71(3):563‐572. [DOI] [PubMed] [Google Scholar]
- 21. Hentschel F, Bornscheuer T, Luth S. Secondary cholangitis of the critically ill. Z Gastroenterol. 2019;57(8):977‐982. [DOI] [PubMed] [Google Scholar]
- 22. Leonhardt S, Veltzke‐Schlieker W, Adler A, et al. Trigger mechanisms of secondary sclerosing cholangitis in critically ill patients. Crit Care. 2015;19:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Weig T, Schubert MI, Gruener N, et al. Abdominal obesity and prolonged prone positioning increase risk of developing sclerosing cholangitis in critically ill patients with influenza A‐associated ARDS. Eur J Med Res. 2012;17:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Batts KP. Ischemic cholangitis. Mayo Clin Proc. 1998;73(4):380‐385. [DOI] [PubMed] [Google Scholar]
- 25. Kobayashi S, Nakanuma Y, Matsui O. Intrahepatic peribiliary vascular plexus in various hepatobiliary diseases: a histological survey. Hum Pathol. 1994;25(9):940‐946. [DOI] [PubMed] [Google Scholar]
- 26. Knooihuizen SAI, Aday A, Lee WM. Ketamine‐Induced Sclerosing Cholangitis (KISC) in a Critically Ill Patient with COVID‐19. Hepatology. 2020. 10.1002/hep.31650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guerin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159‐2168. [DOI] [PubMed] [Google Scholar]
- 28. Guerin C, Albert RK, Beitler J, et al. Prone position in ARDS patients: why, when, how and for whom. Intensive Care Med. 2020;46(12):2385‐2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yip TC, Lui GC, Wong VW, et al. Liver injury is independently associated with adverse clinical outcomes in patients with COVID‐19. Gut. 2021;70(4):733‐742. [DOI] [PubMed] [Google Scholar]
- 30. Cai Q, Huang D, Yu H, et al. COVID‐19: abnormal liver function tests. J Hepatol. 2020;73(3):566‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bertolini A, van de Peppel IP, Bodewes F, et al. Abnormal liver function tests in COVID‐19 patients: relevance and potential pathogenesis. Hepatology. 2020;72(5):1864–1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Horvatits T, Trauner M, Fuhrmann V. Hypoxic liver injury and cholestasis in critically ill patients. Curr Opin Crit Care. 2013;19(2):128‐132. [DOI] [PubMed] [Google Scholar]
- 33. Waldram R, Williams R, Calne RY. Bile composition and bile cast formation after transplantation of the liver in man. Transplantation. 1975;19(5):382‐387. [DOI] [PubMed] [Google Scholar]
- 34. Parry SD, Muiesan P. Cholangiopathy and the biliary cast syndrome. Eur J Gastroenterol Hepatol. 2003;15(4):341‐343. [DOI] [PubMed] [Google Scholar]
- 35. Hansen T, Hollemann D, Pitton MB, et al. Histological examination and evaluation of donor bile ducts received during orthotopic liver transplantation–a morphological clue to ischemic‐type biliary lesion? Virchows Arch. 2012;461(1):41‐48. [DOI] [PubMed] [Google Scholar]
- 36. Esposito I, Kubisova A, Stiehl A, Kulaksiz H, Schirmacher P. Secondary sclerosing cholangitis after intensive care unit treatment: clues to the histopathological differential diagnosis. Virchows Arch. 2008;453(4):339‐345. [DOI] [PubMed] [Google Scholar]
- 37. Benninger J, Grobholz R, Oeztuerk Y, et al. Sclerosing cholangitis following severe trauma: description of a remarkable disease entity with emphasis on possible pathophysiologic mechanisms. World J Gastroenterol. 2005;11(27):4199‐4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhao B, Ni C, Gao R, et al. Recapitulation of SARS‐CoV‐2 infection and cholangiocyte damage with human liver ductal organoids. Protein Cell. 2020;11(10):771–775. [DOI] [PMC free article] [PubMed] [Google Scholar]


