Abstract
Introduction
Early detection of coronavirus disease 2019 (COVID‐19) is paramount for controlling the progression and spread of the disease. Currently, nasopharyngeal swabbing (NPS) is the standard method for collecting specimens. Saliva was recently proposed as an easy and safe option with many authorities adopting the methodology despite the limited evidence of efficacy.
Objectives
The aim of this review was to systematically evaluate the current literature on the use of saliva test for detecting severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and carry out a meta‐analysis to determine its diagnostic accuracy.
Materials and methods
Prospective studies were searched for in electronic databases, complemented by hand‐searching relevant journals. The risk of bias and applicability were assessed using the revised Quality Assessment of Studies of Diagnostic Accuracy Studies (QUADAS‐2) tool. Meta‐analyses and meta‐regression modeling were performed to calculate the diagnostic accuracy and examine sources of heterogeneity.
Results
A total of 16 studies were included with 2928 paired samples. The overall meta‐analysis showed a high sensitivity and specificity for saliva test at 0.88 (95% CI 0.82–0.92) and 0.92 (95% CI 0.75–0.98), respectively. The diagnostic odds ratio was calculated at 87 (95% CI 19–395) and area under the curve was calculated as 0.92 (95% CI 0.90–0.94) suggesting very good performance of the saliva tests in detecting SARS‐CoV‐2.
Conclusion
Saliva testing has a very good discriminative and diagnostic ability to detect of SARS‐CoV‐2. Additional large and well‐designed prospective studies are needed to further validate the diagnostic accuracy and determine a safe sample collection method prior to its recommendation for mass application.
Clinical relevance
Saliva demonstrated high sensitivity and specificity. The use of saliva will allow for self‐collection of specimens and specimen collection in outpatient and community clinics.
Keywords: coronavirus disease, detection, review, saliva, severe acute respiratory syndrome coronavirus 2
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). SARS‐CoV‐2 is considered a highly transmissible and pathogenic coronavirus which is considered more infectious when compared to SARS‐CoV and Middle‐East respiratory syndrome (Hu et al., 2021). As of the March 8, 2021, COVID‐19 has infected more than 100 million people and caused 2.6 million deaths in 223 countries and territories across the globe (WHO, 2021). Since the outbreak of the COVID‐19 pandemic, the use of oropharyngeal and/or nasopharyngeal swabs (OPS/NPS) and reverse transcription polymerase chain reaction (RT‐PCR) amplification of viral RNA was the gold standard procedure of detecting SARS‐CoV‐2. The swab collection in this technique is carried out by a trained healthcare worker who uses a synthetic fiber swab attached to a flexible plastic shaft that is introduced into one of the patient's nostrils and all the way up to the nasopharynx. Once the swab is in contact with the nasopharyngeal wall, it is rotated several times, kept in contact for few seconds to absorb secretions, and then withdrawn out in a rotating motion. The collection of such a specimen requires close contact between healthcare workers and potentially infected patients. The procedure not only causes discomfort and poses a risk of bleeding, particularly in patients with bleeding disorders, but also increases the risk of disease transmission (To et al., ,2019, 2020). Recently, saliva has been investigated as a potential specimen for the detection of SARS‐CoV‐2 (Sakanashi et al., 2021; Senok et al., 2020). The collection of the saliva sample is a practical procedure that is economical and non‐invasive and carries a low risk of disease transmission to healthcare workers. It can also be self‐collected, allowing for regular monitoring of viral load and the screening of large populations (Aita et al., 2020; Guclu et al., 2020; Lee & Wong, 2009; Sakanashi et al., 2021). Saliva has been used to detect other viruses, including coronaviruses, with high sensitivity and specificity when compared with nasopharyngeal specimens (To et al., 2019). The diagnostic potentials of saliva for COVID‐19 have been investigated in several studies with promising results (Czumbel et al., 2020; Fakheran et al., 2020). Less encouraging results or contradictory findings have been reported by others (Hanson et al., 2020; Jamal et al., 2020; Landry et al., 2020). Therefore, the purpose of the present systematic review and meta‐analysis was to determine, based on the currently available literature, the diagnostic accuracy of saliva for the detection of SARS‐CoV‐2 in comparison with the standard NPS and/or OPS methods.
2. MATERIALS AND METHODS
The preparation of the present systematic review followed standard guidelines (Deville et al., 2002; Irwig et al., 1994; Leeflang et al., 2008). The PICO framework was used to formulate a clearly focused question on the diagnostic accuracy of saliva for detecting COVID‐19:
Population: Individuals tested for COVID‐19.
Intervention: Saliva sample.
Control: OPS and/or NPS.
Outcomes: sensitivity, specificity, positive predictive value, negative predictive value, summary receiver operating characteristic (SROC) curve.
The study has been registered at the National Institute for Health Research (NHR) under the PROSPERO ID CRD2020224455. Ethical approval was not required for this systematic review.
2.1. Types of studies
Prospective and retrospective human studies that collected paired samples and compared saliva samples with OPSs and/or NPSs for the detection of SARS‐CoV‐2 were included in the analysis. Case reports, animal studies, letters to journal editors, studies that were not formally peer‐reviewed, reports on viral shedding following the first episode of infection or those that did not report sufficient information were excluded. No language restrictions were imposed.
2.2. Types of participants
Adult individuals who were 18 years of age or older and were tested for COVID‐19.
2.3. Types of diagnostic tests
Saliva sample (index test) and OPSs and/or NPSs (reference standard).
2.4. Outcome measures
2.4.1. Primary outcomes
Specificity and sensitivity.
2.4.2. Secondary outcomes
Positive predictive value.
Negative predictive value.
SROC curve.
2.5. Search strategy
The search strategy recommended by Faggion and co‐workers (Faggion et al., 2013) was used to identify studies related to the diagnostic accuracy of saliva in detecting COVID‐19. The following electronic databases were searched for published and unpublished trials up to November 12, 2020: MEDLINE, EMBASE, The Cochrane Central Register of Controlled Trials (CENTRAL), MetaRegister, ClinicalTrials.gov, and the system for information on Grey literature in Europe (http://www.opengrey.eu) (Table A1). The search was performed independently and in duplicate by two authors (M.A. and N.A.). The reference lists of all potentially eligible papers were examined for additional studies. The last 10 months of relevant journals (Clinical Infectious Diseases, Clinical Microbiology and Infection, Journal of Clinical Microbiology, Journal of Clinical Periodontology, Journal of Clinical Virology, Journal of Infectious Diseases, New England Journal of Medicine) were hand‐searched to identify any eligible papers.
2.6. Selection of studies
Two authors (M.A. and N.A.) independently screened the retrieved citations in duplicate to identify human studies that were appropriate for inclusion. The initial screening was based on the title, abstract, and keywords. After discarding non‐relevant studies, the full‐texts of the remaining studies were examined against a standardized eligibility form. Any disagreements between the two authors were resolved by consulting a third author (M.G). When a duplicate publication (i.e., multiple publications of the same study) was identified, the one with more relevant information was selected. The studies that did not meet the inclusion criteria were excluded and the reasons for exclusion were reported.
2.7. Data collection
Two authors (M.A. and N.A.) independently used a standardized data extraction form to collect the following information from eligible studies: (1) Study characteristics: title, authors' names, contact details, study location, language of publication, year of publication, published or unpublished data, source of study funding, and study design; (2) Participants: demographic characteristics, inclusion/exclusion criteria, number of participants, number of dropouts, and reasons for exclusion; (3) Interventions: number of participants tested for COVID‐19 using saliva samples; (4) Comparison: number of participants tested for COVID‐19 using OPSs and/or NPSs; and (5) Outcomes: True‐positive, false‐positive, false‐negative, and true‐negative values. Additional information was also obtained such as saliva storage, method and timing of collection and processing. All recorded data were verified by the two authors (M.A. and N.A.). Any disagreements were resolved by discussion or by seeking opinion of a third author (M.G.).
2.8. Quality assessment
The revised Quality Assessment of Diagnostic Accuracy Studies (QUADAS‐2) tool (Whiting et al., 2011) was used to assess the quality of selected studies. The risk of bias was examined in four domains: participant, selection, index test, reference standard, and flow/timing. Applicability was evaluated in the first three domains (participant selection, index test, and reference standard). Two review authors (M.A. and N.A.) graded the quality of studies as low, high, or unclear based on specific criteria of the QUADAS‐2 tool. Any disagreements were resolved by discussion or by seeking opinion of a third author (M.G.).
2.9. Statistical analysis and data synthesis
The reported true positives/negatives and false positives/negatives were transferred to 2 × 2 contingency table to calculate sensitivity and specificity as well as positive likelihood ratio (LR+), negative likelihood ratio (LR−), and diagnostic odds ratio (DOR). In order to avoid computational issues, a 0.5 was added to each cell that contained a 0 value in the 2 × 2 table (Dinnes et al., 2005). The discriminating ability of a diagnostic test was more reliably measured using LRs as they are less dependent on the prevalence rate. A LR+ of more than 10 and a LR− of less than 0.1 indicated a satisfactory discriminating diagnostic performance (Jaeschke et al., 1994). Random effects meta‐analytic models were used to pool sensitivity, specificity, LR estimates with 95% confidence intervals (CIs). The heterogeneity between studies was evaluated visually using forest plots and statistically using Cochran Q chi‐square test and I 2 statistic. A p‐value of <0.10 and I 2 value of >50 indicated a substantial heterogeneity (Higgins et al., 2003). The potential causes of heterogeneity among studies were explained by using a meta‐regression model.
The SROC curve was used to graphically present the interaction between sensitivity and specificity. The overall diagnostic ability of saliva was quantified using the area under the curve (AUC). AUC ranges of 0.5–0.7, 0.7–0.9, and 0.9–0.99 indicate poor, moderate, and very good accuracy, respectively. A perfect accuracy is shown by an AUC of 1.0 (Akobeng, 2007). The DOR is the ratio of the odds of positive test results in participants with COVID‐19 compared with the odds of positive test results in those without COVID‐19. DOR ranges from 0 to infinity with greater values suggesting greater accuracy. The potential for publication bias was assessed using the funnel plot which is created from standard error and estimated effect size (log DOR). Statistical analysis was performed using the midas package (Deeks et al., 2005; Glas et al., 2003) in Stata/MP (version 14; StataCorp, LLC), and methodological quality was assessed using Revman 5.4 (version 5.4; The Nordic Cochrane Centre, The Cochrane Collaboration).
3. RESULTS
3.1. Characteristics of study settings
The initial search of the databases identified 49 studies (Figure A1). The titles and abstracts were assessed independently and in duplicate by two review authors (M.A. and N.A.). As a result, the full‐texts of 20 studies (Aita et al., 2020; Altawalah et al., 2020; Berenger et al., 2020; Binder et al., 2020; Chen et al., 2020; Guclu et al., 2020; Hanson et al., 2020; Iwasaki et al., 2020; Jamal et al., 2020; Kim et al., 2020; Landry et al., 2020; Moreno‐Contreras et al., 2020; Pasomsub et al., 2019; Procop et al., 2020; Rao et al., 2020; Sakanashi et al., 2021; Senok et al., 2020; Vaz et al., 2020; Williams et al., 2020; Wyllie et al., 2020) were retrieved for detailed assessment. A total of 4 studies (Berenger et al., 2020; Iwasaki et al., 2020; Kim et al., 2020; Wyllie et al., 2020) were excluded, and 16 studies (Aita et al., 2020; Altawalah et al., 2020; Binder et al., 2020; Chen et al., 2020; Guclu et al., 2020; Hanson et al., 2020; Jamal et al., 2020; Landry et al., 2020; Moreno‐Contreras et al., 2020; Pasomsub et al., 2019; Procop et al., 2020; Rao et al., 2020; Sakanashi et al., 2021; Senok et al., 2020; Vaz et al., 2020; Williams et al., 2020) were included in the present review (Table 1). The hand searching did not identify any additional studies. All studies were published in English and all together they included 2928 paired samples.
TABLE 1.
Characteristics of included studies
| Aita et al., 2020 | Altawalah et al., 2020 | Binder et al., 2020 | Chen et al., 2020 | Guclu et al., 2020 | Hanson et al., 2020 | Jamal et al., 2020 | Landry et al., 2020 | |
|---|---|---|---|---|---|---|---|---|
| Study design | Case series | Prospective cross‐sectional | Case series | Case series | Case series | Case series | Case series | Case series |
| Country | Italy | Kuwait | United States of America | China | Turkey | United States of America | Canada | United States of America |
| Number of paired samples | 43 | 848 (samples with equivocal outcomes were excluded) | 19 (only the collected NPS and saliva samples were included) | 58 | 64 | 354 (only the collected NPS and saliva samples were included) | 91 | 124 |
| Age of participants (years) |
Mean (range) Male: 64 (29–86) Female: 60 (25–94) |
Not reported |
Median (range) 50 (29–91) |
Median (IQR) 38 (31–52) |
Mean ± SD 51.04 ± 17.9 |
Mean (range) 35 (18–75) |
Median (range) 66 (23–106) |
Not reported |
| Index and reference standard | Saliva and NPS | Saliva and NPS | Saliva and NPS | Saliva and NPS | Saliva and OPS/NPS | Saliva and NPS | Saliva and NPS | Saliva and NPS |
| Methods of collecting saliva | Self‐collected saliva using cotton swab (Salivette, SARSTEDT AG & Co) | Self‐collected cough out‐saliva into sterile container | Self‐collected saliva: Passive drool into conical tube | Self‐collected cough out‐saliva into sterile container | Self‐collected saliva: in sterile dry container | Self‐collected saliva: repeated spit of a minimum of 1 ml saliva into a sterile empty tube | Self‐collected saliva: spitting 5 ml of saliva into sterile containers | Self‐collected saliva: spitting into sterile containers |
| Methods of saliva storage and transport | Cotton swabs were stored at 4°C immediately after collection and centrifuged at 4000 g for 5 min within 3 h from collection | Viscous saliva was added to 300 µl of VTM, mixed vigorously, and then 200 µl of sample was used for RNA isolation | Passive drool was diluted 2:1 with PBS (with 0.5% bovine serum albumin) to preserve potential virus contained in the sample | A 2 ml of VTM was added to the saliva immediately | The outside of the container was cleaned with 1/10 diluted bleach‐impregnated cloth and VTM was added to the samples | Saliva samples transported to the laboratory at 4°C, stored refrigerated and tested within 5 days of receipt | A 2.5 ml of PBS was added to the saliva sample and transported to the laboratory where there were aliquoted and frozen at ‐80°C within 8 h of collection | Saliva samples were frozen on day of receipt at ‐70°C. Within 2 weeks, all saliva samples were thawed and tested |
| Assay | One‐Step RT‐ddPCR Advanced Kit for Probes (Bio‐Rad) | TaqPathTM COVID‐19 multiplex real‐time RT‐PCR test (Thermo Fisher Scientific) | SuperScript III Platinium One‐Step real time RT‐PCR kit | SARS‐CoV‐2 RNA dependent RNA polymerase/Helicase (RdRp/Hel) real‐time RT‐PCR | Genesis RT‐PCR SARS‐CoV‐2 (Primer Design) kit | Hologic Aptima SARS‐CoV‐2 TMA assay (Hologic Inc.) real‐time RT‐PCR platform | Allplex 2019‐nCoV Assay (100T) (Seegene Inc) | In house RT‐PCR based on CDC assay |
| Sensitivity (%) | 93.3% | 83.4% | 90.9% | 89.1% | 85.2% | 93.8% | 68.8% | 84.8% |
| Specificity (%) | 97.2% | 99.4% | 87.5% | 14.3% | 89.2% | 97.8% | 70.4% | 97.8% |
| PPV (%) | 87.5% | 98.9% | 90.9% | 94.2% | 85.2% | 92.6% | 84.6% | 93.3% |
| NPV (%) | 98.6% | 89.8% | 87.5% | 7.7% | 89.2% | 98.2% | 48.7% | 94.7% |
|
TP: FP: TN: FN: |
7 1 35 0 |
287 3 501 57 |
10 1 7 1 |
49 3 0 6 |
23 4 33 4 |
75 6 268 5 |
44 8 19 20 |
28 2 89 5 |
| Moreno‐Contreras et al., 2020 | Pasomsub et al., 2020 | Procop et al., 2020 | Rao et al., 2020 | Sakanashi et al., 2020 | Senok et al., 2020 | Vaz et al., 2020 | Williams et al., 2020 | |
|---|---|---|---|---|---|---|---|---|
| Study design | Case series | Prospective cross‐sectional | Case series | Prospective cross‐sectional | Case series | Case series | Case series | Case series |
| Country | Mexico | Thailand | United States of America | Malaysia | Japan | United Arab Emirates | Brazil | Australia |
| Number of paired samples | 71 (only the collected NPS and saliva samples were included) | 200 | 216 | 217 | 28 | 401 | 155 | 39 with positive NPSs and subset of 50 negative NPSs |
| Age of participants (years) |
Mean ± SD 41.0 ± 14.4 |
Mean (range) 36 (28–48) |
Mean (range) 44 (18–82) |
Median (IQR) 27 (18–36) |
Not reported |
Mean (±SD) 35.5 ± 9.5 |
Median (IQR) 40 (33–48.5) |
Not reported |
| Index and reference standard | Saliva and OPS/NPS | Saliva and NPS | Saliva and NPS | Saliva and NPS | Saliva and NPS | Saliva and NPS | Saliva and NPS | Saliva and NPS |
| Methods of collecting saliva | Self‐collected saliva: spitting into sterile containers | Self‐collected saliva: spitting into sterile containers | Self‐collected cough out‐saliva into sterile container | Self‐collected deep throat saliva sample in a sterile container | Self‐collected saliva: passive drooling into sterile containers | Self‐collected saliva: spitting into sterile containers | Self‐collected saliva: spitting into sterile container | Self‐collected saliva: spitting into sterile containers |
| Methods of saliva storage and transport | No VTM or stabilizing agents were added. Saliva samples were stored at 4°C immediately after collection and were analyzed within 24 to 36 h | Saliva samples were treated with lysis buffer to inactivate the SARS‐CoV‐2. Viral RNA was extracted within 26 min | Saliva samples refrigerated at the collection site and transported to the laboratory in insulated coolers with ice packs. All samples were tested on the day of collection | Samples were stored at room temperature and transported to the laboratory within 5 h of collection for further processing | A 0.5 ml of saliva was resuspended in a sterile 15 ml tube containing 3 ml of PBS. The resuspended saliva was centrifuged at 500 g for 1 min and the supernatant fluid was used for the assay | Samples were stored at room temperature and transported to the laboratory within 3 h of collection for further processing | Samples were transported to the laboratory in a thermal box at 2–8°C and stored at −80°C until nucleic acid extraction. Whenever possible, RNA was isolated from fresh saliva within 6 h of collection | Saliva samples were transported to the laboratory within 3 h of collection. An approximate 1:1 ratio of liquid Amies media was then added prior to further processing |
| Assay | QIAamp viral RNA minikit (Qiagen) | SARS‐CoV‐2 Nucleic Acid Diagnostic Kit (Sansure) | CDC 2019 noval coronavirus Real‐Time RT‐PCR Diagnostic Panel | RT‐PCR of Real‐Q 2019 nCoV detection kit (Biosewoom, Inc) | BD MAX open system (Japan Becton Dickinson and Company) | NeoPlex COVID‐19 kit (GeneMatrix) | BIOMOL OneStep/COVID‐19 Kit (Parana Molecular Biology Institute) protocol | Coronavirus Typing (8–35 well) assay, AusDiagnostics |
| Sensitivity (%) | 67.9% | 84.2% | 98.7% | 86.9% | 96.8% | 73.1% | 94.4% | 84.6% |
| Specificity (%) | 86.0% | 98.9% | 99.4% | 0.65% | 69.2% | 97.6% | 97.6% | 98.0% |
| PPV (%) | 76.0% | 88.9% | 97.4% | 49.0% | 78.9% | 67.9% | 97.1% | 97.1% |
| NPV (%) | 80.4% | 98.4% | 99.7% | 4.3% | 94.7% | 98.1% | 95.3% | 89.1% |
|
TP: FP: TN: FN: |
19 6 37 9 |
16 2 179 3 |
38 1 177 0 |
73 76 0 11 |
15 4 9 0 |
19 9 366 7 |
67 2 82 4 |
33 1 49 6 |
Abbreviations: CDC, complement dependent cytotoxicity; COVID‐19, coronavirus disease; FN, false negative; FP, false positive; IQR, interquartile range; NPS, nasopharyngeal swab; NPV, negative predictive value; OPS, oropharyngeal swab; PBS, phosphate buffered saline; PPV, positive predictive value; RT‐PCR, reverse transcription‐polymerase chain reaction; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; SD, standard deviation; TMA, transcription mediated amplification; TN, true negative; TP, true positive; VTM, viral transport medium.
Of the 16 included studies, eight Studies (Altawalah et al., 2020; Binder et al., 2020; Chen et al., 2020; Hanson et al., 2020; Jamal et al., 2020; Moreno‐Contreras et al., 2020; Pasomsub et al., 2019; Rao et al., 2020) were funded or supported by university or research institutes, while five studies (Aita et al., 2020; Guclu et al., 2020; Procop et al., 2020; Vaz et al., 2020; Williams et al., 2020) did not provide any information on funding. Three studies (Landry et al., 2020; Sakanashi et al., 2021; Senok et al., 2020) did not receive any funding, and their sampling was part of routine laboratory investigations.
3.2. Characteristics of participants
All participants were aged ≥18 years old. Five studies (Hanson et al., 2020; Landry et al., 2020; Pasomsub et al., 2019; Procop et al., 2020; Senok et al., 2020; Williams et al., 2020) collected samples from outpatients, with or without symptoms suggestive of COVID‐19, attending test centers or screening clinics, while one study (Rao et al., 2020) collected samples from individuals staying in quarantine centers. Three studies (Altawalah et al., 2020; Binder et al., 2020; Guclu et al., 2020) included in‐patients with confirmed or suspected COVID‐19. Three studies (Aita et al., 2020; Chen et al., 2020; Jamal et al., 2020) included only confirmed COVID‐19 in‐patients with fever, dyspnea, pneumonia, anosmia, or gastrointestinal symptoms. In one of these studies (Jamal et al., 2020), 77% of the participants had at least one comorbidity. Three studies (Moreno‐Contreras et al., 2020; Sakanashi et al., 2021; Vaz et al., 2020) collected samples from both in‐ and out‐patients.
3.3. Characteristics of index test and reference standard
The NPS was collected in the standard way of passing the swab through the nostril and up to the posterior nasopharynx and then removing the swab while rotating. NPS was considered the reference standard in all included studies but two (Guclu et al., 2020; Moreno‐Contreras et al., 2020), which included both OPS and NPS. In those two studies, the swab was passed into the posterior oropharynx prior to inserting it into one nostril. NPS were collected by trained healthcare workers in all studies except for one (Hanson et al., 2020), where patients were instructed to self‐collect under the supervision of healthcare workers. Differences in the commercial kits and laboratory protocols to detect SARS‐CoV‐2 were noticed (Table 1). Fourteen studies (Aita et al., 2020; Altawalah et al., 2020; Binder et al., 2020; Chen et al., 2020; Guclu et al., 2020; Jamal et al., 2020; Landry et al., 2020; Moreno‐Contreras et al., 2020; Pasomsub et al., 2019; Rao et al., 2020; Sakanashi et al., 2021; Senok et al., 2020; Vaz et al., 2020; Williams et al., 2020) used RT‐PCR, while two studies used both RT‐PCR and transcription mediated amplification (TMA) (Hanson et al., 2020; Procop et al., 2020).
With regard to saliva sampling, three studies (Altawalah et al., 2020; Chen et al., 2020; Procop et al., 2020) collected saliva by asking patients to “cough up,” while other studies collected saliva by passive drooling into a sterile container (Binder et al., 2020; Sakanashi et al., 2021) or spitting (Guclu et al., 2020; Jamal et al., 2020; Moreno‐Contreras et al., 2020; Pasomsub et al., 2019; Rao et al., 2020; Senok et al., 2020; Vaz et al., 2020; Williams et al., 2020). Two studies (Hanson et al., 2020; Landry et al., 2020) used both drooling and spitting to collect saliva specimen. Only one study (Aita et al., 2020) used chewing on an absorbent material to encourage salivation.
3.4. Methodological quality
Two QUADAS‐2 domains, the index test and reference standard, were associated with unclear concerns in all studies, as it was not clear whether the interpretation of the saliva test results was influenced by the knowledge of the outcome of OPS and/or NPS. For the domain of patient selection, three studies (Aita et al., 2020; Chen et al., 2020; Jamal et al., 2020) enrolled only patients with confirmed COVID‐19 and therefore were judged to be at high risk of bias and concerns regarding applicability. The domain of flow and timing was associated with a low risk of bias in all studies except for five (Altawalah et al., 2020; Binder et al., 2020; Hanson et al., 2020; Moreno‐Contreras et al., 2020; Williams et al., 2020), where some samples were not included in the analysis (Figure A2).
Only one study (Senok et al., 2020) reported a priori‐power analysis to calculate the required sample size to examine the diagnostic accuracy of the saliva test.
3.5. Results of meta‐analyses
All of the 16 studies were included in the analysis. The pooled sensitivity and specificity for saliva test were relatively high at 0.88 (95% CI 0.82–0.92) and 0.92 (95% CI 0.75–0.98), respectively (Figure 1). Consequently, the pooled LR+ and LR− were 11.6 (95% CI 3.2–42.5) and 0.13 (95% CI 0.09–0.20), respectively, indicating adequate diagnostic information. The DOR was calculated at 87 (95% CI 19–395) which indicates good diagnostic value. However, wide 95% CI indicates significant heterogeneity among the included studies. The AUC was calculated as 0.92 (95% CI 0.90–0.94; Figure 2) suggesting very good performance of the saliva tests in detecting SARS‐CoV‐2. The differences in clinical utility between saliva and OPS/NPS for diagnosis of COVID‐19 were evaluated using Fagan plot analysis. In terms of detecting SARS‐CoV‐2, the probability of COVID‐19 increased from 20% to 74% when the saliva test was positive and decreased to 3% when the results were negative (Figure 3). A funnel graph analysis showed no evidence of publication bias (Figure A3).
FIGURE 1.
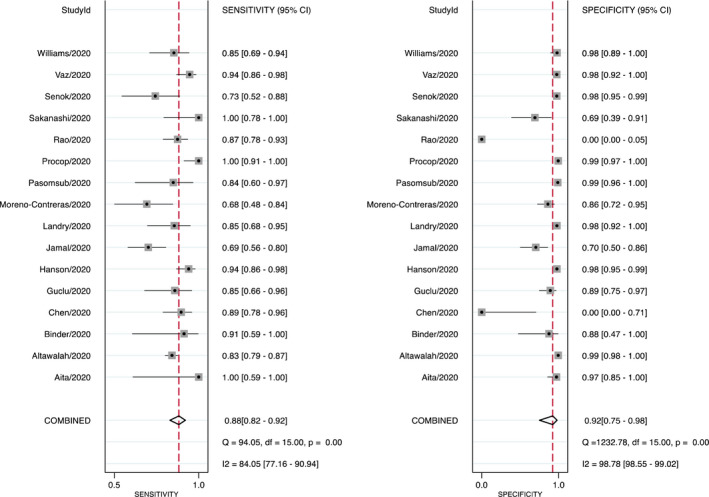
Sensitivity and specificity of saliva tests for detecting SARS‐CoV‐2. Forest plots of individual/pooled sensitivity and specificity of the included studies (CI: confidence interval; Q: Cochran chi‐square test)
FIGURE 2.
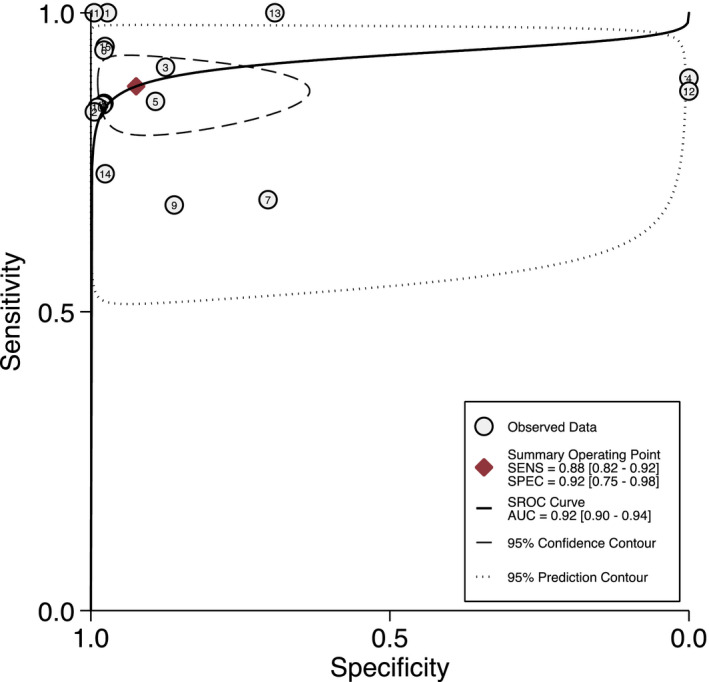
Diagnostic test accuracy of saliva test for detecting SARS‐CoV‐2 (SROC: summary receiver operating characteristic; SENS: sensitivity; SPEC: specificity; AUC: area under the curve; O: observed data; ♦: Summary Oberating Point; ➖: SORC curve; ‐‐‐: 95% confidence contour; …… 95% prediction contour; 1: Aita et al., 2020; 2: Altawalah et al., 2020; 3: Binder et al., 2020; 4: Chen et al., 2020; 5: Guclu et al., 2020; 6: Hanson et al., 2020; 7: Jamal et al., 2020; 8: Landry et al., 2020; 9: Moreno‐Contreras et al., 2020; 10: Pasomsub et al., 2020; 11: Procop et al., 2020; 12: Rao et al., 2020; 13: Sakanashi et al., 2020; 14: Senok et al., 2020; 15: Vaz et al., 2020; 16: Williams et al., 2020).
FIGURE 3.
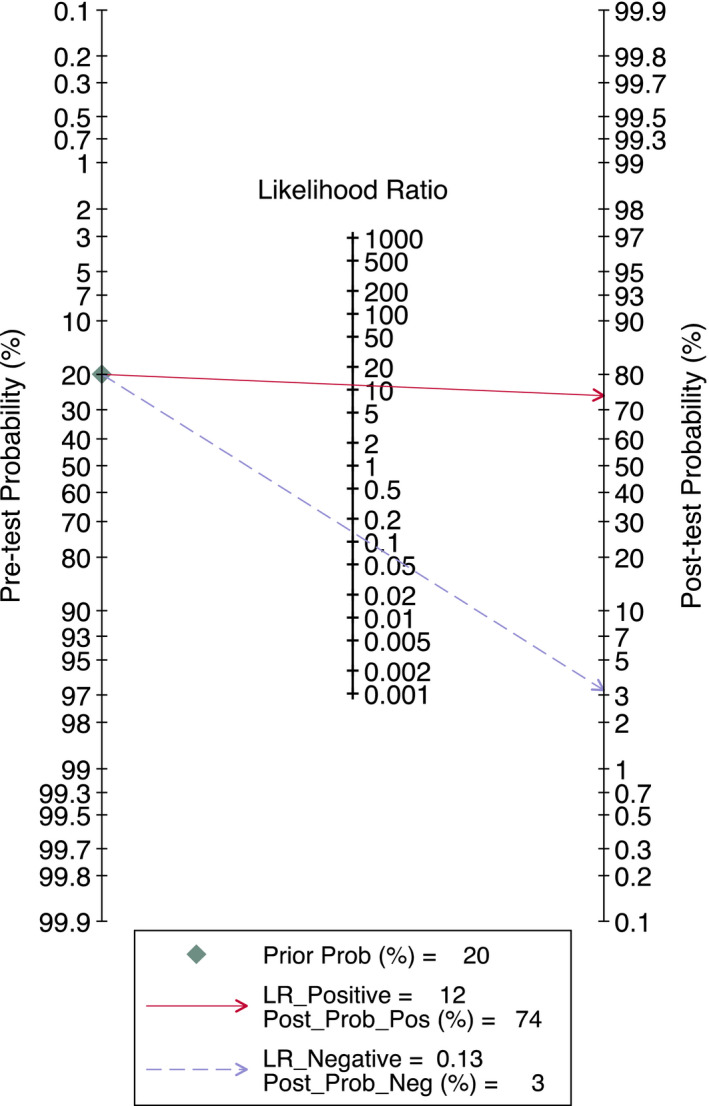
Fagan nomogram (LR: likelihood ratio)
Six studies (Aita et al., 2020; Altawalah et al., 2020; Binder et al., 2020; Chen et al., 2020; Guclu et al., 2020; Jamal et al., 2020) examined the use of saliva tests among in‐patients with confirmed or suspected diagnosis of COVID‐19 without including healthy individuals or out‐patients. Moreover, half of the studies (Aita et al., 2020; Binder et al., 2020; Chen et al., 2020; Guclu et al., 2020; Jamal et al., 2020; Moreno‐Contreras et al., 2020; Sakanashi et al., 2021; Williams et al., 2020) had a sample size less than 100, and three studies (Altawalah et al., 2020; Chen et al., 2020; Procop et al., 2020) collected coughed‐out saliva without using any measure to stimulate saliva. The meta‐regression analysis was used to assess these potential sources of heterogeneity. Studies were divided into groups as follows: sample size (≥100 vs. <100 patients), characteristics of patients (in‐patients vs. in‐/out‐patients), and method of saliva collection (coughed out vs. other methods). Higher pooled sensitivity was observed when the study included a sample size of ≥100 in‐and out‐patients compared to sample size of <100 in‐patients only (p < 0.05). The method of saliva collection did not significantly alter the performance of saliva test (Table 2, Figure A4).
TABLE 2.
Meta‐regression analyses
| Covariate | Number of studies | Pooled sensitivity | p‐Value | Pooled specificity | p‐Value |
|---|---|---|---|---|---|
| Sample size ≥100 | |||||
| Yes | 8 | 0.89 (0.84–0.94) | 0.04 | 0.96 (0.90–1.00) | 0.05 |
| No | 8 | 0.85 (0.77–0.93) | 0.85 (0.61–1.00) | ||
| Characteristics of patients | |||||
| In‐patients | 6 | 0.85 (0.76–0.93) | <0.001 | 0.89 (0.66–1.00) | 0.07 |
| In‐/out‐patients | 10 | 0.89 (0.84–0.94) | 0.94 (0.84–1.00) | ||
| Method of saliva collection | |||||
| Coughed out | 3 | 0.91 (0.83–0.99) | 0.23 | 0.95 (0.78–1.00) | 0.11 |
| Other methods | 13 | 0.87 (0.81–0.92) | 0.92 (0.80–1.00) | ||
4. DISCUSSION
Saliva tests have been granted clearance for detecting SARA‐CoV‐2 by several health authorities including the Food and Drug Administration (Czumbel et al., 2020). However, the scientific evidence supporting their use has not been systematically reviewed in the current published literature. The present review followed a standardized approach to evaluate the best available evidence for the use of saliva in detecting SARS‐CoV‐2 by RT‐PCR. The results of the meta‐analysis showed high sensitivity (0.88 [95% CI 0.82–0.92]) and specificity (0.92 [95% CI 0.75–0.98]) when compared with current standards of collecting OPS or NPS samples.
Potential sources of heterogeneity among the studies included in this review were identified. For example, a meta‐regression model showed that studies including more than 100 in‐ and out‐patients had a better sensitivity than those including only in‐patients of less than 100 participants (p < 0.05). This finding, however, is in fact supportive of the diagnostic accuracy of the saliva test when considering that these studies that included a wide variety of healthy, symptomatic, and asymptomatic patients. Interestingly, using coughed‐out saliva did not affect the sensitivity or the specificity of saliva in detecting SARS‐CoV‐2 when compared with other methods of saliva collection, such as drooling and spitting. The latter techniques have been previously used in other analyses (Golatowski et al., 2013) and proved to be simple, safe, self‐collectable and do not pose any risk of disease transmission compared to OPS/NPS, and coughing out saliva without a mask could increase the risk of transmission unless collected in appropriate setting. Other potential sources of heterogeneity included the accuracy of healthcare workers in collecting OPS/NPS or supervising the self‐collection of saliva samples but there is insufficient information on this confounding factor to be included in the meta‐regression model.
4.1. Agreement and disagreements with other systematic reviews
The question of whether saliva is a reliable sample for detecting SARS‐CoV‐2 has been addressed in other systematic reviews (Czumbel et al., 2020; Fakheran et al., 2020; Fernandes et al., 2020; Torretta et al., 2020). Common limitations across all these reviews were the limited number of included studies, the small sample sizes within studies and the lack of stringent selection criteria allowing the inclusion of non‐peer‐reviewed studies. In addition, while the reliability of saliva as a diagnostic specimen was cited in the previous reviews, the conclusions were less robust due to their acknowledged serious limitations. By contrast, the findings of the present review were based on a comprehensive search strategy and meta‐analyses of 16 peer‐reviewed studies with each having a control group of the standard NPS/OPS sample as an a priori criterion for inclusion in the review.
4.2. Strengths and weaknesses
Despite the limited number of included studies, this up‐to‐date systematic review gives an evidence‐based appraisal on the performance of the saliva test as an alternative diagnostic tool to the standard reference NPS/OPS for the detection of the SARS‐CoV‐2. It comes at a very appropriate time considering the staggering number of infected cases across the globe and the ongoing need for a simple and effective screening and diagnostic tool. The findings from the meta‐analyses in this review support the use of saliva in detecting SARS‐CoV‐2. We should, however, bear in mind that detection of a virus by RT‐PCR does not indicate infectivity. Although viral cultures are not feasible as a general screening test, they are still required to confirm whether the virus is in fact infectious. In this context, there remains a need to validate the RT‐PCR results, whether from NPS or saliva, against a viral culture before recommending NPS or saliva as the “gold” standard in the detection of SARS‐CoV‐2. Another element of relevance within this context is the need to identify the source of SARS‐CoV‐2 in saliva. Potential sources such as draining debris from nasopharyngeal epithelium, gingival crevicular fluid, secretions from infected salivary glands, and oral mucosal endothelial cells have all been proposed but remain inconclusive (Liu et al., 2011; Silva‐Boghossian et al., 2013; To et al., 2020; Xu et al., 2020).
Nevertheless, the use of saliva for detecting other RNA viruses, including Zika and Ebola viruses, is well‐documented (Gorchakov et al., 2019; Khurshid et al., 2019; Niedrig et al., 2018), and in our findings, saliva demonstrated good diagnostic accuracy with considerable similarities to the results obtained with the standard OPS/NPS.
The use of saliva will allow for self‐collection of specimens and specimen collection in outpatient and community clinics. These possibilities will help reduce the overall cost of testing, including healthcare worker time and personal protective equipment (PPE) requirement, and reducing the healthcare workers' risk of infection. In addition, the effectiveness of self‐collected saliva was shown to be in moderate agreement with trained healthcare worker‐collected NPS samples for detecting SARS‐CoV‐2 (Ku et al., 2021). On the other hand, the ability of the patient to understand the safe sampling instructions and the ability to collect sufficient quantity of saliva could be challenging (Torretta et al., 2020). The risks of disease spread may not be completely eliminated with the use of saliva sample as spitting or coughing is required to collect the saliva specimens. This in itself could provide a route for aerosol transmission (Sullivan et al., 2020), and the need for a standardized safe method for the saliva sample collection, therefore, remains an essential requirement.
Further research looking specifically at different confounding factors such as the method and timing of sample collection, the transport medium, storage, timing of RNA isolation, and detection is needed prior to mass application of the saliva sample test as a standard method for the detection of the SARS‐CoV‐2.
5. CONCLUSIONS
Saliva is a fluid that can enable very good discriminative and sensitive detection of SARS‐CoV‐2. Its recommendation for mass application as an alternative method to the current NPS/OPS sampling requires further support from large and well‐designed prospective studies. These studies should further substantiate the diagnostic accuracy of saliva in detecting SARS‐CoV‐2 and determine appropriate, safe, sample collection techniques to reduce potentials for cross infection.
CONFLICT OF INTEREST
Momen Atieh declares that he has no conflict of interest. Marina Guirguis declares that she has no conflict of interest. Nabeel Alsabeeha declares that he has no conflict of interest. Richard Cannon declares that he has no conflict of interest.
AUTHOR CONTRIBUTIONS
Momen Atieh: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Software; Writing‐original draft; Writing‐review & editing. Marina Guirguis: Data curation; Methodology; Writing‐original draft; Writing‐review & editing. Nabeel Alsabeeha: Formal analysis; Investigation; Methodology; Writing‐original draft; Writing‐review & editing. Richard D Cannon: Investigation; Resources; Software; Writing‐original draft; Writing‐review & editing.
ETHICAL APPROVAL
All procedures performed in studies included in this review were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
INFORMED CONSENT
For this type of study, formal consent is not required.
ACKNOWLEDGMENTS
None.
APPENDIX A1.
FIGURE A1.
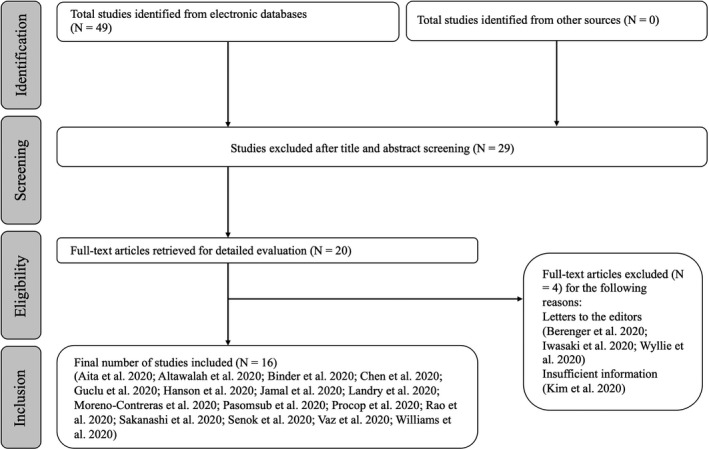
Flowchart of the search process
FIGURE A2.
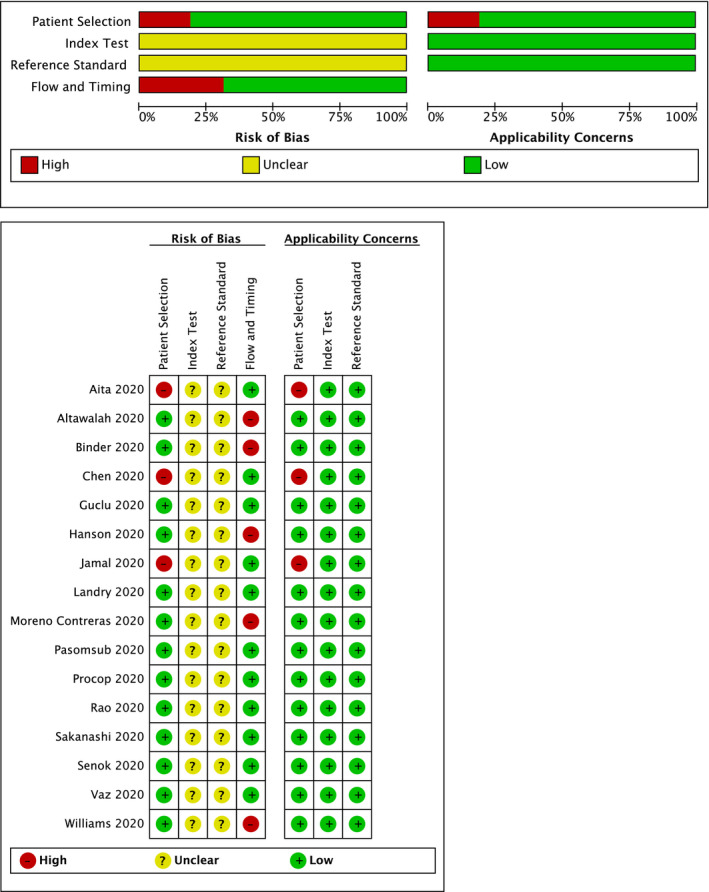
Assessment of applicability concerns and risk of bias of the included studies presented with low (green), unclear (yellow) and high (red) risk of bias
FIGURE A3.
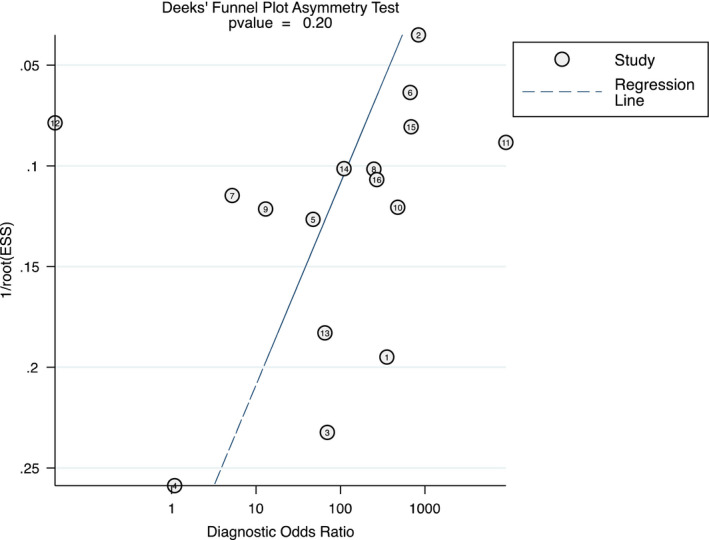
Funnel plot for estimating publication bias (ESS: effective sample size; 1: Aita et al., 2020; 2: Altawalah et al., 2020; 3: Binder et al., 2020; 4: Chen et al., 2020; 5: Guclu et al., 2020; 6: Hanson et al., 2020; 7: Jamal et al., 2020; 8: Landry et al., 2020; 9: Moreno‐Contreras et al., 2020; 10: Pasomsub et al., 2020; 11: Procop et al., 2020; 12: Rao et al., 2020; 13: Sakanashi et al., 2020; 14: Senok et al., 2020; 15: Vaz et al., 2020; 16: Williams et al., 2020)
FIGURE A4.
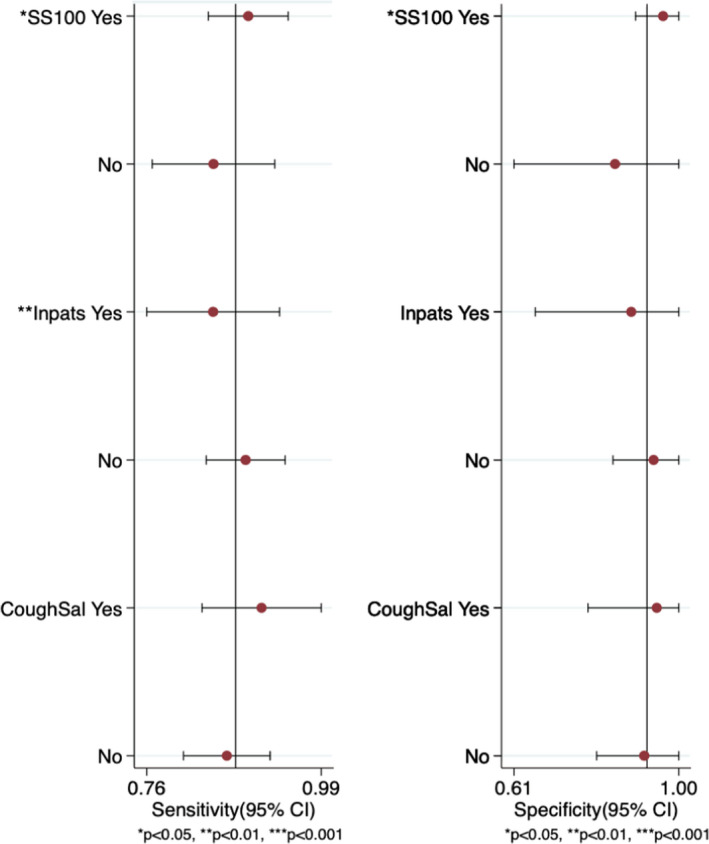
Meta‐regression and subgroup analyses (SS100: sample size ≥100; Inpats: in‐patients; CoughSal: coughed out saliva)
TABLE A1.
Databases and search terms
| Databases | Keywords |
|---|---|
| Published studies | |
|
PubMed (1965–November 14, 2020) |
(coronavirus infection* OR SARS‐CoV‐2) AND (saliva OR oral fluid*) AND (nasopharyngeal swab OR oropharyngeal swab) |
|
EMBASE via Ovid (1947–November 14, 2020) |
(coronavirus adj infection$).mp OR (SARS‐CoV‐2$).mp AND (saliva OR oral adj fluid$).mp. AND (nasopharyngeal adj swab OR oropharyngeal adj swab) |
|
Cochrane Central Register of Controlled Trials (CENTRAL) via Ovid (November 14, 2020) |
(coronavirus adj infection$).mp OR (SARS‐CoV‐2$).mp AND (saliva OR oral adj fluid$).mp. AND (nasopharyngeal adj swab OR oropharyngeal adj swab) |
| Unpublished studies | |
|
MetaRegister of controlled studies OpenGrey (www.opengrey.eu) (November 14, 2020) |
(coronavirus infection OR SARS‐CoV‐2) AND (saliva OR oral fluid) AND (nasopharyngeal swab OR oropharyngeal swab) |
Atieh, M. A. , Guirguis, M. , Alsabeeha, N. H. M. , & Cannon, R. D. (2021). The diagnostic accuracy of saliva testing for SARS‐CoV‐2: A systematic review and meta‐analysis. Oral Diseases, 00, 1–15. 10.1111/odi.13934
REFERENCES
- Aita, A. , Basso, D. , Cattelan, A. M. , Fioretto, P. , Navaglia, F. , Barbaro, F. , Stoppa, A. , Coccorullo, E. , Farella, A. , Socal, A. , Vettor, R. , & Plebani, M. (2020). SARS‐CoV‐2 identification and IgA antibodies in saliva: One sample two tests approach for diagnosis. Clinica Chimica Acta, 510, 717–722. 10.1016/j.cca.2020.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akobeng, A. K. (2007). Understanding diagnostic tests 3: Receiver operating characteristic curves. Acta Paediatrica, 96(5), 644–647. 10.1111/j.1651-2227.2006.00178.x [DOI] [PubMed] [Google Scholar]
- Altawalah, H. , AlHuraish, F. , Alkandari, W. A. , & Ezzikouri, S. (2020). Saliva specimens for detection of severe acute respiratory syndrome coronavirus 2 in Kuwait: A cross‐sectional study. Journal of Clinical Virology, 132, 104652. 10.1016/j.jcv.2020.104652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenger, B. M. , Conly, J. M. , Fonseca, K. , Hu, J. , Louie, T. , Schneider, A. R. , Singh, T. , Stokes, W. , Ward, L. , & Zelyas, N. (2021). Saliva collected in universal transport media is an effective, simple and high‐volume amenable method to detect SARS‐CoV‐2. Clinical Microbiology and Infection, 27(4), 656–657. 10.1016/j.cmi.2020.10.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder, R. A. , Alarja, N. A. , Robie, E. R. , Kochek, K. E. , Xiu, L. , Rocha‐Melogno, L. , Abdelgadir, A. , Goli, S. V. , Farrell, A. S. , Coleman, K. K. , Turner, A. L. , Lautredou, C. C. , Lednicky, J. A. , Lee, M. J. , Polage, C. R. , Simmons, R. A. , Deshusses, M. A. , Anderson, B. D. , & Gray, G. C. (2020). Environmental and aerosolized severe acute respiratory syndrome coronavirus 2 among hospitalized coronavirus disease 2019 patients. Journal of Infectious Diseases, 222(11), 1798–1806. 10.1093/infdis/jiaa575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. H. K. , Yip, C. C. Y. , Poon, R. W. S. , Chan, K. H. , Cheng, V. C. C. , Hung, I. F. N. , Chan, J. F. W. , Yuen, K. Y. , & To, K. K. W. (2020). Evaluating the use of posterior oropharyngeal saliva in a point‐of‐care assay for the detection of SARS‐CoV‐2. Emerging Microbes & Infections, 9(1), 1356–1359. 10.1080/22221751.2020.1775133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czumbel, L. M. , Kiss, S. , Farkas, N. , Mandel, I. , Hegyi, A. , Nagy, Á. , Lohinai, Z. , Szakács, Z. , Hegyi, P. , Steward, M. C. , & Varga, G. (2020). Saliva as a candidate for COVID‐19 diagnostic testing: a meta‐analysis. Frontiers in Medicine, 7, 465. 10.3389/fmed.2020.00465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks, J. J. , Macaskill, P. , & Irwig, L. (2005). The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. Journal of Clinical Epidemiology, 58(9), 882–893. 10.1016/j.jclinepi.2005.01.016 [DOI] [PubMed] [Google Scholar]
- Devillé, W. L. , Buntinx, F. , Bouter, L. M. , Montori, V. M. , de Vet, H. C. W. , van der Windt, D. A. W. M. , & Bezemer, P. D. (2002). Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Medical Research Methodology, 2(1), 9. 10.1186/1471-2288-2-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinnes, J. , Deeks, J. , Kirby, J. , & Roderick, P. (2005). A methodological review of how heterogeneity has been examined in systematic reviews of diagnostic test accuracy. Health Technology Assessment, 9(12), 1–113. 10.3310/hta9120 [DOI] [PubMed] [Google Scholar]
- Faggion, C. M. Jr , Atieh, M. A. , & Park, S. (2013). Search strategies in systematic reviews in periodontology and implant dentistry. Journal of Clinical Periodontology, 40(9), 883–888. 10.1111/jcpe.12132 [DOI] [PubMed] [Google Scholar]
- Fakheran, O. , Dehghannejad, M. , & Khademi, A. (2020). Saliva as a diagnostic specimen for detection of SARS‐CoV‐2 in suspected patients: a scoping review. Infectious Diseases of Poverty, 9(1), 100. 10.1186/s40249-020-00728-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes, L. L. , Pacheco, V. B. , Borges, L. , Athwal, H. K. , de Paula Eduardo, F. , Bezinelli, L. , Correa, L. , Jimenez, M. , Dame‐Teixeira, N. , Lombaert, I. , & Heller, D. (2020). Saliva in the diagnosis of COVID‐19: A review and new research directions. Journal of Dental Research, 99(13), 1435–1443. 10.1177/0022034520960070 [DOI] [PubMed] [Google Scholar]
- Glas, A. S. , Lijmer, J. G. , Prins, M. H. , Bonsel, G. J. , & Bossuyt, P. M. (2003). The diagnostic odds ratio: a single indicator of test performance. Journal of Clinical Epidemiology, 56(11), 1129–1135. 10.1016/s0895-4356(03)00177-x [DOI] [PubMed] [Google Scholar]
- Golatowski, C. , Gesell Salazar, M. , Dhople, V. M. , Hammer, E. , Kocher, T. , Jehmlich, N. , & Völker, U. (2013). Comparative evaluation of saliva collection methods for proteome analysis. Clinica Chimica Acta, 419, 42–46. 10.1016/j.cca.2013.01.013 [DOI] [PubMed] [Google Scholar]
- Gorchakov, R. , Berry, R. M. , Patel, S. M. , El Sahly, H. M. , Ronca, S. E. , & Murray, K. O. (2019). Optimizing PCR detection of Zika virus from various body fluids. American Journal of Tropical Medicine and Hygiene, 100(2), 427–433. 10.4269/ajtmh.18-0755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güçlü, E. , Koroglu, M. , Yürümez, Y. , Toptan, H. , Kose, E. , Güneysu, F. , & Karabay, O. (2020). Comparison of saliva and oro‐nasopharyngeal swab sample in the molecular diagnosis of COVID‐19. Revista da Associação Médica Brasileira, 66(8), 1116–1121. 10.1590/1806-9282.66.8.1116 [DOI] [PubMed] [Google Scholar]
- Hanson, K. E. , Barker, A. P. , Hillyard, D. R. , Gilmore, N. , Barrett, J. W. , Orlandi, R. R. , & Shakir, S. M. (2020). Self‐collected anterior nasal and saliva specimens versus health care worker‐collected nasopharyngeal swabs for the molecular detection of SARS‐CoV‐2. Journal of Clinical Microbiology, 58(11), e01824–20. 10.1128/JCM.01824-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins, J. P. , Thompson, S. G. , Deeks, J. J. , & Altman, D. G. (2003). Measuring inconsistency in meta‐analyses. BMJ, 327(7414), 557–560. 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, B. , Guo, H. , Zhou, P. , & Shi, Z. L. (2021). Characteristics of SARS‐CoV‐2 and COVID‐19. Nature Reviews Microbiology, 19(3), 141–154. 10.1038/s41579-020-00459-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwig, L. (1994). Guidelines for meta‐analyses evaluating diagnostic tests. Annals of Internal Medicine, 120(8), 667–676. 10.7326/0003-4819-120-8-199404150-00008 [DOI] [PubMed] [Google Scholar]
- Iwasaki, S. , Fujisawa, S. , Nakakubo, S. , Kamada, K. , Yamashita, Y. , Fukumoto, T. , Sato, K. , Oguri, S. , Taki, K. , Senjo, H. , Sugita, J. , Hayasaka, K. , Konno, S. , Nishida, M. , & Teshima, T. (2020). Comparison of SARS‐CoV‐2 detection in nasopharyngeal swab and saliva. Journal of Infection, 81(2), e145–e147. 10.1016/j.jinf.2020.05.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke, R. , Guyatt, G. H. , & Sackett, D. L. (1994). Users' guides to the medical literature. III. How to use an article about a diagnostic test. B. What are the results and will they help me in caring for my patients? The Evidence‐Based Medicine Working Group. JAMA, 271(9), 703–707. 10.1001/jama.271.9.703. [DOI] [PubMed] [Google Scholar]
- Jamal, A. J. , Mozafarihashjin, M. , Coomes, E. , Powis, J. , Li, A. X. , Paterson, A. , Anceva‐Sami, S. , Barati, S. , Crowl, G. , Faheem, A. , Farooqi, L. , Khan, S. , Prost, K. , Poutanen, S. , Taylor, M. , Yip, L. , Zhong, X. Z. , McGeer, A. J. , Mubareka, S. , … Walmsley, S. (2021). Sensitivity of nasopharyngeal swabs and saliva for the detection of severe acute respiratory syndrome coronavirus 2. Clinical Infectious Diseases, 72(6), 1064–1066. 10.1093/cid/ciaa848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurshid, Z. , Zafar, M. , Khan, E. , Mali, M. , & Latif, M. (2019). Human saliva can be a diagnostic tool for Zika virus detection. Journal of Infection and Public Health, 12(5), 601–604. 10.1016/j.jiph.2019.05.004 [DOI] [PubMed] [Google Scholar]
- Kim, S. E. , Lee, J. Y. , Lee, A. , Kim, S. , Park, K. H. , Jung, S. I. , Kang, S. J. , Oh, T. H. , Kim, U. J. , Lee, S. Y. , Kee, S. J. , & Jang, H. C. (2020). Viral load kinetics of SARS‐CoV‐2 infection in saliva in Korean patients: a prospective multi‐center comparative study. Journal of Korean Medical Science, 35(31), e287. 10.3346/jkms.2020.35.e287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku, C. W. , Shivani, D. , Kwan, J. Q. T. , Loy, S. L. , Erwin, C. , Ko, K. K. K. , Ng, X. W. , Oon, L. , Thoon, K. C. , Kalimuddin, S. , & Chan, J. K. Y. (2021). Validation of self‐collected buccal swab and saliva as a diagnostic tool for COVID‐19. International Journal of Infectious Diseases, 104, 255–261. 10.1016/j.ijid.2020.12.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry, M. L. , Criscuolo, J. , & Peaper, D. R. (2020). Challenges in use of saliva for detection of SARS CoV‐2 RNA in symptomatic outpatients. Journal of Clinical Virology, 130, e104567. 10.1016/j.jcv.2020.104567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, Y. H. , & Wong, D. T. Saliva: an emerging biofluid for early detection of diseases. American Journal of Dentistry, 22(4), 241–248. Available: https://www.ncbi.nlm.nih.gov/pubmed/19824562 [PMC free article] [PubMed] [Google Scholar]
- Leeflang, M. M. , Deeks, J. J. , Gatsonis, C. , & Bossuyt, P. M. , Cochrane Diagnostic Test Accuracy Working Group . Systematic reviews of diagnostic test accuracy, Annals of Internal Medicine, 149(12), 889. 10.7326/0003-4819-149-12-200812160-00008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, L. , Wei, Q. , Alvarez, X. , Wang, H. , Du, Y. , Zhu, H. , Jiang, H. , Zhou, J. , Lam, P. , Zhang, L. , Lackner, A. , Qin, C. , & Chen, Z. (2011). Epithelial cells lining salivary gland ducts are early target cells of severe acute respiratory syndrome coronavirus infection in the upper respiratory tracts of rhesus macaques. Journal of Virology, 85(8), 4025–4030. 10.1128/JVI.02292-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno‐Contreras, J. , Espinoza, M. A. , Sandoval‐Jaime, C. , Cantú‐Cuevas, M. A. , Barón‐Olivares, H. , Ortiz‐Orozco, O. D. , Muñoz‐Rangel, A. V. , de la Hernández Cruz, M. , Eroza‐Osorio, C. M. , Arias, C. F. , & López, S. (2020). Saliva sampling and its direct lysis, an excellent option to increase the number of SARS‐CoV‐2 diagnostic tests in settings with supply shortages. Journal of Clinical Microbiology, 58(10), e01659–20. 10.1128/JCM.01659-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedrig, M. , Patel, P. , El Wahed, A. A. , Schädler, R. , & Yactayo, S. (2018). Find the right sample: A study on the versatility of saliva and urine samples for the diagnosis of emerging viruses. BMC Infectious Diseases, 18(1), 707. 10.1186/s12879-018-3611-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasomsub, E. , Watcharananan, S. P. , Boonyawat, K. , Janchompoo, P. , Wongtabtim, G. , Suksuwan, W. , Sungkanuparph, S. , & Phuphuakrat, A. (2021). Saliva sample as a non‐invasive specimen for the diagnosis of coronavirus disease 2019: A cross‐sectional study. Clinical Microbiology and Infection, 27(2), 285.e1–285.e4. 10.1016/j.cmi.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procop, G. W. , Shrestha, N. K. , Vogel, S. , Van Sickle, K. , Harrington, S. , Rhoads, D. D. , Rubin, B. P. , & Terpeluk, P. (2020). A direct comparison of enhanced saliva to nasopharyngeal swab for the detection of SARS‐CoV‐2 in symptomatic patients. Journal of Clinical Microbiology, 58(11), e01946–20. 10.1128/JCM.01946-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, M. , Rashid, F. A. , Sabri, F. S. A. H. , Jamil, N. N. , Zain, R. , Hashim, R. , Amran, F. , Kok, H. T. , Samad, M. A. A. , & Ahmad, N. Comparing nasopharyngeal swab and early morning saliva for the identification of SARS‐CoV‐2, Clinical Infectious Diseases, 72(9), e352–e356. 10.1093/cid/ciaa1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakanashi, D. , Asai, N. , Nakamura, A. , Miyazaki, N. , Kawamoto, Y. , Ohno, T. , Yamada, A. , Koita, I. , Suematsu, H. , Hagihara, M. , Shiota, A. , Kurumiya, A. , Sakata, M. , Kato, S. , Muramatsu, Y. , Koizumi, Y. , Kishino, T. , Ohashi, W. , Yamagishi, Y. , & Mikamo, H. (2021). Comparative evaluation of nasopharyngeal swab and saliva specimens for the molecular detection of SARS‐CoV‐2 RNA in Japanese patients with COVID‐19. Journal of Infection and Chemotherapy, 27(1), 126–129. 10.1016/j.jiac.2020.09.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senok, A. , Alsuwaidi, H. , Atrah, Y. , Al Ayedi, O. , Al Zahid, J. , Han, A. , Al Marzooqi, A. , Al Heialy, S. , Altrabulsi, B. , AbdelWareth, L. , Idaghdour, Y. , Ali, R. , Loney, T. , & Alsheikh‐Ali, A. (2020). Saliva as an alternative specimen for molecular COVID‐19 testing in community settings and population‐based screening. Infect Drug Resist, 13, 3393–3399. 10.2147/IDR.S275152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva‐Boghossian, C. M. , Colombo, A. P. , Tanaka, M. , Rayo, C. , Xiao, Y. , & Siqueira, W. L. (2013). Quantitative proteomic analysis of gingival crevicular fluid in different periodontal conditions. PLoS One, 8(10), e75898. 10.1371/journal.pone.0075898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan, P. S. , Sailey, C. , Guest, J. L. , Guarner, J. , Kelley, C. , Siegler, A. J. , Valentine‐Graves, M. , Gravens, L. , del Rio, C. , & Sanchez, T. H. (2020). Detection of SARS‐CoV‐2 RNA and antibodies in diverse samples: Protocol to validate the sufficiency of provider‐observed, home‐collected blood, saliva, and oropharyngeal samples. JMIR Public Health and Surveillance, 6(2), e19054. 10.2196/19054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- To, K. K. , Tsang, O. T. , Leung, W. S. , Tam, A. R. , Wu, T. C. , Lung, D. C. , Yip, C. C. , Cai, J. P. , Chan, J. M. , Chik, T. S. , Lau, D. P. , Choi, C. Y. , Chen, L. L. , Chan, W. M. , Chan, K. H. , Ip, J. D. , Ng, A. C. , Poon, R. W. , Luo, C. T. , & Yuen, K. Y. (2020). Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS‐CoV‐2: an observational cohort study. The Lancet Infectious Diseases, 20(5), 565–574. 10.1016/S1473-3099(20)30196-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- To, K. K. W. , Yip, C. C. Y. , Lai, C. Y. W. , Wong, C. K. H. , Ho, D. T. Y. , Pang, P. K. P. , Ng, A. C. K. , Leung, K. H. , Poon, R. W. S. , Chan, K. H. , Cheng, V. C. C. , Hung, I. F. N. , & Yuen, K. Y. (2019). Saliva as a diagnostic specimen for testing respiratory virus by a point‐of‐care molecular assay: A diagnostic validity study. Clinical Microbiology & Infection, 25(3), 372–378. 10.1016/j.cmi.2018.06.009 [DOI] [PubMed] [Google Scholar]
- Torretta, S. , Zuccotti, G. , Cristofaro, V. , Ettori, J. , Solimeno, L. , Battilocchi, L. , D'Onghia, A. , Bonsembiante, A. , Pignataro, L. , Marchisio, P. , & Capaccio, P. (2021). Diagnosis of SARS‐CoV‐2 by RT‐PCR using different sample sources: Review of the literature. Ear, Nose & Throat Journal, 100(2_Suppl), 131S–138S. 10.1177/0145561320953231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz, S. N. , Santana, D. S. , Netto, E. M. , Pedroso, C. , Wang, W. K. , Santos, F. D. A. , & Brites, C. (2020). Saliva is a reliable, non‐invasive specimen for SARS‐CoV‐2 detection. The Brazilian Journal of Infectious Diseases, 24(5), 422–427. 10.1016/j.bjid.2020.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting, P. F. (2011). QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine, 155(8), 529–536. 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- WHO . (2021). World Health Organization Coronavirus disease (COVID‐19). http://www.who.int/emergencies/diseases/novel‐coronavirus‐2019. Accessed 08 March 2021.
- Williams, E. , Bond, K. , Zhang, B. , Putland, M. , & Williamson, D. A. (2020). Saliva as a noninvasive specimen for detection of SARS‐CoV‐2. Journal of Clinical Microbiology, 58(8), e00776–20. 10.1128/JCM.00776-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie, A. L. , Fournier, J. , Casanovas‐Massana, A. , Campbell, M. , Tokuyama, M. , Vijayakumar, P. , Warren, J. L. , Geng, B. , Muenker, M. C. , Moore, A. J. , Vogels, C. B. F. , Petrone, M. E. , Ott, I. M. , Lu, P. , Venkataraman, A. , Lu‐Culligan, A. , Klein, J. , Earnest, R. , Simonov, M. , … Ko, A. I. (2020). Saliva or nasopharyngeal swab specimens for detection of SARS‐CoV‐2. New England Journal of Medicine, 383(13), 1283–1286. 10.1056/NEJMc2016359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, H. , Zhong, L. , Deng, J. , Peng, J. , Dan, H. , Zeng, X. , Li, T. , & Chen, Q. (2020). High expression of ACE2 receptor of 2019‐nCoV on the epithelial cells of oral mucosa. International Journal of Oral Science, 12(1), 8. 10.1038/s41368-020-0074-x [DOI] [PMC free article] [PubMed] [Google Scholar]


