Abstract
This study aims to assess the efficacy and safety of convalescent plasma therapy (CPT) in COVID‐19 critically ill patients with protracted severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) RNAemia. A retrospective cohort study was conducted in intensive care unit (ICU). All patients with severe COVID‐19 pneumonia for whom RNAemia remained positive more than 14 days after onset of the infection were included and given CPT. The primary objective was to evaluate SARS‐CoV‐2 RNAemia 7 days (D7) after CPT. A total of 14 patients were included and they received a median CPT volume of 828 ml (range: 817–960). CPT was administered in a median time of 14 days after ICU admission. At D7, 13/14 patients had negative SARS‐CoV‐2 blood PCR and one patient had negative blood PCR 11 days after CPT. At D7 and at D14, the clinical status was improved in 7/14 and 11/14 patients, respectively. The 28‐day mortality rate was 14%. No CPT‐related adverse effects had been reported. CPT is safe and may be efficient in patients with protracted RNAemia admitted in ICU for severe COVID‐19 pneumonia. Randomized controlled trials are needed to confirm these results.
Keywords: combination therapy, disease control, generalized infection, pathogenesis, SARS coronavirus, virus classification
1. INTRODUCTION
Convalescent plasma transfusion (CPT) in COVID‐19 pneumonia remains controversial. Preliminary data have shown that passive transfer of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2)‐neutralizing antibodies through CPT could be efficient and safe in patients with severe humoral immunity impairment. 1 Apart from this specific situation, the encouraging results of the preliminary studies have been challenged since the publication of nonsignificant results in three recent randomized controlled studies (RCTs) showing no difference in clinical outcome in patients with moderate/severe COVID‐19 infection treated with CPT versus placebo.2, 3, 4, 5 We hypothesized that CPT should be prescribed in severe patients on primarily viro‐immunological criteria such as persistent viremia, rather than only clinical disease severity.
2. MATERIALS AND METHODS
We conducted a retrospective cohort study of patients with confirmed COVID‐19 pneumonia, consecutively admitted to the intensive care unit (ICU) between July 1 and November 30, 2020. All patients were tested for SARS‐CoV‐2 RNA in blood (RNAemia) at admission and once a week subsequently until negativization. Patients for whom RNAemia remained positive more than 14 days after onset of the symptoms were included and given CPT. Oxygen‐dependant patients received dexamethasone (6 mg/day) since ICU admission for 10 days. Among them, those who had fever associated with increased inflammatory parameters (at least two among fibrinogen >8 g/L, ferritin >1000 ng/ml, d‐dimers >3000 ng/ml, and C‐reactive protein >150 mg/L) received tocilizumab (8 mg/kg) in the first 24 h. Patients were assigned a clinical status at baseline (Day 0 [D0], date of transfusion) and evaluated at Days 7 (D7) and 14 (D14). In case of persistent positive plasma RT‐PCR at Day 7, a new RT‐PCR was performed every 48–72 h until disappearance of the viremia. The clinical status was evaluated with an adapted version of the 6‐point World Health Organization (WHO) clinical scale described elsewhere. 4
The monitoring of SARS‐CoV‐2 RNAemia was performed on plasma samples using GeneFinderTM COVID‐19 PLUS RealAmp RT‐PCR Kit (OSANG Healthcare Co., Ltd.). Briefly, viral RNA was extracted from 200 µl of plasma using MagNA Pure 96 DNA and Viral NA Small Volume Kit on MagNA Pure 96 Instrument (Roche). A total of 5 μl of RNA was mixed with 15 µl of GeneFinderTM COVID‐19 PLUS RealAmp RT‐PCR mix according to manufacturer's recommendation. The real‐time amplification was carried out using an Lc480 Roche thermocycler. This kit allows the detection of three viral targets (Nucleocapsid N, RdRp, and Envelope E) as well as a cellular control gene. Ct values less than 40 for at least two of the three target genes were considered as positive results. Viral culture on blood sample was not performed in our center. Written informed consent was obtained for all the patients. The French national authorities (Agence nationale de sécurité du médicament et des produits de santé) authorized on April 29, 2020, the COVID‐19 CPT, according to a therapeutic use protocol, that is, for severe COVID‐19‐suffering patients (WHO score ≥4). 6 Patients were informed of the anonymous use of their medical data and were included in the database, accredited by the French national data protection commission (CNIL 2009055).
According to these recommendations, in our study, each patient received four units (each unit = 200–220 ml) of ABO‐compatible convalescent plasma in two transfusions 24 h apart, as previously published by us and others.1, 7 Convalescent donors were eligible for plasma donation 15 days after resolution of COVID‐19. Collected apheresis plasma underwent pathogen reduction (Intercept blood system; Cerus) and standard testing as per current regulations in France. Anti‐SARS‐CoV‐2 antibody content was assessed in each donation by semiquantitative immunoglobulin G (IgG) enzyme‐linked immunosorbent assay (EUROIMMUN). A ratio of IgG patient sample/control sample of more than 5.6 was required. 1
Clinical parameters (temperature, ratio of the partial pressure of oxygen to the fraction of inspired oxygen (PaO2/FiO2), concomitant treatments and coinfections) were recorded daily. Anti‐SARS‐CoV‐2 IgG was serologically detected in recipients before CPT (cutoff value for IgG = 1.4 and immunoglobulin M [IgM] = 1 index sample/control sample). Biological parameters including inflammatory markers and hematological parameters were assessed.
The primary objective was to evaluate SARS‐CoV‐2 RNAemia at D7. Secondary objectives were to evaluate the clinical status at D7, D14, and the 28‐day mortality.
3. RESULTS
During the study period, among 64 patients admitted in the ICU for a COVID‐19 pneumonia, 14 patients had RNAemia for more than 14 days (median D0 Ct value = 37) after onset of symptoms and were included (Table 1). At D0, inflammatory parameters had improved from the ICU admission. At D0 and D7, 9 and 13 patients had anti–SARS‐CoV‐2 antibodies, respectively (Table 2). CPT was administered in a median time of 14 (range: 1–48) days after ICU admission and 25 (range: 12–55) days after onset of the illness. Median total CPT volume was 828 ml (range: 817–960), that is, 11 ml/kg (range: 9.7–14.9) body weight. No CPT‐related adverse effects had been reported.
Table 1.
Characteristics of the 14 patients at ICU admission
| Population study (N = 14) | |
|---|---|
| Median age, year (range) | 74 (53–84) |
| Age ≥65 year, n (%) | 10 (71) |
| Male sex, n (%) | 11 (79) |
| Median SAPS‐2 (range) | 37 (24–53) |
| Median SOFA (range) | 4 (0–8) |
| Median time to onset of symptoms, days (range) | 9 (5–24) |
| Median PaO2/FiO2, mmHg (range) | 117 (50–400) |
| Coexisting conditions, n (%) | |
| BMI above 30 kg/m2 | 2 (14) |
| Hypertension | 10 (71) |
| Diabetes | 4 (29) |
| Chronic obstructive pulmonary disease | 1 (7) |
| Congestive heart failure | 0 (0) |
| Chronic renal failure | 3 (21) |
| Solid tumors | 5 (36) |
| Hematologic cancer | 4 (29) |
| Chest CT‐scan parenchymal involvement | |
| <25% | 4 (29) |
| 25%–50% | 3 (21) |
| 50%–75% | 5 (36) |
| >75% | 2 (14) |
| Pulmonary embolism | 1 (7) |
| Respiratory support, n (%) | |
| Low‐flow oxygen nasal cannula | 2 (14) |
| High‐flow oxygen nasal cannula | 6 (43) |
| Noninvasive ventilation | 3 (21) |
| Invasive ventilation | 6 (43) |
| Associated treatments, n (%) | |
| Glucocorticoids | 13 (93) |
| Tocilizumab | 12 (86) |
| Heparin | 14 (100%) |
Abbreviations: BMI, body mass index; ICU, intensive care unit; SAPS‐2, simplified acute physiology score; SOFA, sequential organ failure assessment.
Table 2.
Evolution of the 14 patients' laboratory values
| ICU admission | D0 | D7 | |
|---|---|---|---|
| CRP, mg/L (range) | 106 (17–318) | 6 (1–258) | 7 (1–115) |
| normal range: <4 mg/L | |||
| Fibrinogen, g/L (range) | 8 (7–10) | 4 (1–10) | 4 (2–9) |
| normal range: 2–5 | |||
| Ferritin, ng/ml (range) | 873 (181–8100) | 622 (170–5759) | 545 (116–3275) |
| normal range: 20–260 | |||
| D‐Dimer, ng/ml (range) | 1041 (448–3301) | 1568 (215–5820) | 1447 (327–4542) |
| normal range: 0–500 | |||
| Lymphocytes, G/L (range) | 0.49 (0.16–1.00) | 0.73 (0.37–1.63) | 0.80 (0.22–1.62) |
| normal range: 1.20–3.60 | |||
| Anti‐SARS‐CoV‐2 IgM, ratio versus control sample (range) | – | 4.4 (0.0–80.6) | 15.7 (0.0–59.3) |
| Cutoff value ≥1.0 | |||
| Anti‐SARS‐CoV‐2 IgG, ratio versus control sample (range) | – | 3.0 (0.0–6.5) | 4.7 (0.1–8.6) |
| Cutoff value ≥1.4 |
Abbreviations: CRP, C‐reactive protein; D0, date of convalescent plasma transfusion (CPT); D7, 7 days after CPT; ICU, intensive care unit; IgG, immunoglobulin G; IgM, immunoglobulin M; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
At D7, 13/14 patients had negative SARS‐CoV‐2 plasma PCR and one patient had negative plasma PCR 11 days after CPT. As expected due to CPT, all patients displayed Anti‐SARS‐CoV‐2 IgG and IgM at D7 (Table 2). Compared with D0, all seronegative patients (n = 5) seroconverted: IgM = 15.7 and IgG = 3.5. The level of specific antibodies in seropositive patients (n = 9) did not increase significantly: IgM = 20.6 and IgG = 5.5 versus IgM = 18.5 and IgG = 5.9 at D0 and D7, respectively.
At D7 and at D14, the 6‐point scale was improved in 7/14 and 11/14 patients, respectively. The 28‐day mortality rate after CPT was 14%. Two male patients (aged 76 and 81 years) died 11 and 22 days after CPT, respectively, due to nosocomial bacterial pneumonia unrelated with CPT.
4. DISCUSSION
The first report of CPT efficacy was reported in an uncontrolled case series in five critically ill patients from China. 8 Since this preliminary study, many noncomparative studies have reported concordant positive results in severe COVID‐19, especially when CPT was administred before 14 days after onset of illness.2, 9, 10, 11, 12 Unfortunately, three recent controlled studies failed to confirm these encouraging results.3, 4 Two of them compared CPT and a placebo in severe respiratory COVID‐19 patients and the third compared CPT with usual care in patients hospitalized with COVID‐19 without severity criteria. Transfusion doses of CPT varied from to 4 to 13 ml/kg of recipient body weight in the first study, from 415 to 600 ml in the second, and 550 ml in the third, compared with more than 800 ml or 11 ml/kg in the present study. Conversely, three others controlled studies showed a benefit in terms of clinical severity especialy in older patients when plasma is transfused early during the course of COVID‐19.3, 13, 14
Our study shows that 92% of patients with protracted SARS‐CoV‐2 infection had negative plasma PCR within 7 days after CPT. Oxygen requirement decreased significantly for 50% and 78% of them at D7 and D14, respectively, with excellent tolerance of CPT. Concordantly, Hueso et al. 1 showed that CPT with the same regimen could be a promising therapy in seronegative patients with profound B‐cell lymphopenia with protracted RNAemia.
SARS‐CoV‐2 viral load appeared to peak in the upper respiratory tract within the first week after symptom onset. 15 Numerous studies identified an association between older age (>60 years) and prolonged viral RNA shedding. Male sex and corticosteroids therapy seem to be two other factors associated with delayed viral clearance. 15 Faster clearance was frequently observed in asymptomatic individuals. Median duration of shedding in serum samples varies from 16.6 days (3.6–29.7) to 21 days (9–39) with a maximum of RNA shedding of 60 days.15, 16
The clinical significance of SARS‐CoV‐2 RNA in blood circulation is unknown. It could be associated with free viral particles or altered RNA. Less than 15% of symptomatic COVID‐19 patients have RNAemia and no correlation between upper respiratory Ct values and RNAemia frequency or plasma RNA loads, as evaluated by Ct comparison, could be established. 17 Moreover, a very small fraction of blood donors, even those who remain asymptomatic after donation, can be RNAemic for SARS‐CoV‐2 with low viral loads, as suggested by high Ct, and infectivity of positive plasma was not evidenced in cell culture experiments.18, 19, 20
Literature data cannot determine yet whether RNAemia represents direct viral involvement in causing extrapulmonary pathology or is merely spill‐over from an intense pulmonary infection. 17
However, numerous concordant studies showed that RNAemia is a strong indicator for clinical severity, mainly ARDS, and is detected more frequently in critical patients who were admitted to the ICU and/or died.11, 17, 20, 21, 22
The individuals with RNAemia were older than those with undetectable SARS‐CoV‐2 RNA in plasma. 20 In a recent study, Hagman et al. 9 showed that SARS‐CoV‐2 RNA in serum at admission was associated with a sevenfold increased risk of critical disease and an eightfold increased risk of death in a cohort of 167 patients hospitalized for COVID‐19. These apparently conflicting results probably reflect the very variable severity of SARS‐CoV‐2 infection.
In the present study, eligible patients remained symptomatic with persistent viremia, whatever the serological results. Consequently, we hypothesize that these disappointing results obtained by controlled studies could be partially linked to the inclusion criteria and suggest that the viral load or the host's humoral response should be taken into account to define patients who could benefit from CPT in symptomatic/severe COVID‐19 pneumonia, especially in older patients.
These results underline the key role of neutralizing humoral immunity. Nevertheless, our study confirms that some patients failed to clear the virus despite apparent humoral response. In these patients, as other authors have already reported, IgG and IgM titers were much higher than they were in less symptomatic individuals.23, 24
At first glance, the efficacy of CPT is of course more difficult to understand in seropositive than in seronegative patients. Nevertheless, the possibility of reinfection by the virus in convalescent individuals should inspire caution regarding the antiviral protection associated with the detected antibodies. Antibodies may not be efficient enough for several reasons: for instance, due to their isotypes or their lack of affinity, in line with the clinical context, especially age, cancer, previous chemotherapy (Figure 1).
Figure 1.
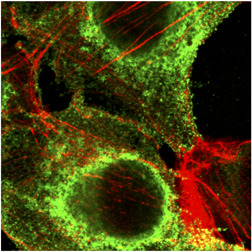
SARS‐CoV‐2 attached on epithelial cell surface analyzed by confocal microscopy (green, antinucleocapsid antibodies and red, actin in cytosqueleton) (Kindly provided by Harald Wodrich from Bordeaux University). SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
The main limitation of our study lies in its retrospective and noncomparative nature: we cannot exclude that viral clearance is a natural evolution of the infection. Appropriate control groups are missing. Moreover, it is possible that an earlier CPT administration (i.e., in the first 14 days of disease) could have had a better clinical efficacy as suggested by recent data.14, 25 To the best of our knowledge, this is the first report about CPT use in a cohort of viremic critically ill patients. Prospective controlled trials are warranted to specify optimal CPT regimen in terms of volume and timing for transfusion from onset of illness and to target the most eligible patients.
5. CONCLUSION
Our study suggests that passive transfer of SARS‐CoV‐2‐neutralizing antibodies through CPT is safe and may be efficient in severe COVID‐19 patients with protracted RNAemia beyond 14 days despite positive serology, especially in older patients. RCTs are needed to confirm this therapeutic option in viremic critically ill patients.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Fabrice Camou and Nahéma Issa: designed the study, collected and analyzed data, and wrote the manuscript. Camille Tumiotto, Olivier Guisset, and Gaëlle Mourissoux: participated in study enrollment and data collection. Mathilde Beguet‐Yachine, Diana Ratiarison, and Xavier Lafarge: contributed for making convalescent plasma available at local levels. Pantxika Bellecave, Camille Tumiotto, and Marie‐Edith Lafon: performed SARS‐CoV‐2 PCR and serology. Fabrice Bonnet: participated in study enrollment. All authors critically revised and approved the manuscript.
Camou F, Tinevez C, Beguet‐Yachine M, et al. Feasibility of convalescent plasma therapy in severe COVID‐19 patients with persistent SARS‐CoV‐2 viremia. J Med Virol. 2021;93:5594–5598. 10.1002/jmv.27032
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Hueso T, Pouderoux C, Péré H, et al. Convalescent plasma therapy for B‐cell‐depleted patients with protracted COVID‐19. Blood. 2020;136(20):2290‐2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rajendran K, Krishnasamy N, Rangarajan J, Rathinam J, Natarajan M, Ramachandran A. Convalescent plasma transfusion for the treatment of COVID‐19: Systematic review. J Med Virol. 2020;92(9):1475‐1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life‐threatening COVID‐19: a randomized clinical trial. JAMA. 2020;324(5):460‐470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Simonovich VA, Burgos Pratx LD, Scibona P, et al. A randomized trial of convalescent plasma in Covid‐19 severe pneumonia. N Engl J Med. 2021;384(7):619‐629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Horby P, Estcourt L, Peto L, Emberson J. Convalescent plasma in patients admitted to hospital with COVID‐19 (RECOVERY): a randomized, controlled, open‐label, platform trial. medRxiv. 2021. 030921252736.2021. https://www.medrxiv.org/content/10.1101/2021.03.09.21252736v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. ANSM . Décision du 29/04/2020 autorisant la collecte, la préparation, la conservation, la distribution et la délivrance du produit sanguin labile « plasma convalescent Covid‐19 ». In: 2020.
- 7. London J, Boutboul D, Lacombe K, et al. Severe COVID‐19 in patients with B cell alymphocytosis and response to convalescent plasma therapy. J Clin Immunol. 2021;41(2):356‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shen C, Wang Z, Zhao F, et al. Treatment of 5 critically ill patients with COVID‐19 with convalescent plasma. JAMA. 2020;323(16):1582‐1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hagman K, Hedenstierna M, Gille‐Johnson P, et al. Severe acute respiratory syndrome coronavirus 2 RNA in serum as predictor of severe outcome in coronavirus disease 2019: a retrospective cohort study. Clin Infect Dis. 2020. 10.1093/cid/ciaa1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID‐19 patients. Proc Natl Acad Sci USA. 2020;117(17):9490‐9496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Salazar E, Kuchipudi SV, Christensen PA, et al. Convalescent plasma anti‐SARS‐CoV‐2 spike protein ectodomain and receptor‐binding domain IgG correlate with virus neutralization. J Clin Invest. 2020;130(12):6728‐6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hegerova L, Gooley TA, Sweerus KA, et al. Use of convalescent plasma in hospitalized patients with COVID‐19: case series. Blood. 2020;136(6):759‐762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Salazar E, Christensen PA, Graviss EA, et al. Treatment of coronavirus disease 2019 patients with convalescent plasma reveals a signal of significantly decreased mortality. Am J Pathol. 2020;190(11):2290‐2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Libster R, Pérez Marc G, Wappner D, et al. Early high‐titer plasma therapy to prevent severe Covid‐19 in older adults. N Engl J Med. 2021;384(7):610‐618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cevik M, Tate M, Lloyd O, Maraolo AE, Schafers J, Ho A. SARS‐CoV‐2, SARS‐CoV, and MERS‐CoV viral load dynamics, duration of viral shedding, and infectiousness: a systematic review and meta‐analysis. Lancet Microbe. 2021;2(1):e13‐e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen L, Wang G, Long X, et al. Dynamics of blood viral load is strongly associated with clinical outcomes in coronavirus disease 2019 (COVID‐19) patients: a prospective cohort study. J Mol Diagn. 2021;23(1):10‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Prebensen C, Myhre PL, Jonassen C, et al. Severe acute respiratory syndrome coronavirus 2 RNA in plasma is associated with intensive care unit admission and mortality in patients hospitalized with coronavirus disease 2019. Clin Infect Dis. 2020. 10.1093/cid/ciaa1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chang L, Zhao L, Gong H, Wang L, Wang L. Severe acute respiratory syndrome coronavirus 2 RNA detected in blood donations. Emerging Infect Dis. 2020;26(7):1631‐1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cappy P, Candotti D, Sauvage V, et al. No evidence of SARS‐CoV‐2 transfusion transmission despite RNA detection in blood donors showing symptoms after donation. Blood. 2020;136(16):1888‐1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hogan CA, Stevens BA, Sahoo MK, et al. High frequency of SARS‐CoV‐2 RNAemia and association with severe disease. Clin Infect Dis. 2020. 10.1093/cid/ciaa1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen X, Zhao B, Qu Y, et al. Detectable serum severe acute respiratory syndrome coronavirus 2 viral load (RNAemia) is closely correlated with drastically elevated interleukin 6 level in critically ill patients with coronavirus disease 2019. Clin Infect Dis. 2020;71(8):1937‐1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fajnzylber J, Regan J, Coxen K, et al. SARS‐CoV‐2 viral load is associated with increased disease severity and mortality. Nat Commun. 2020;11(1):5493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Poland GA, Ovsyannikova IG, Kennedy RB. SARS‐CoV‐2 immunity: review and applications to phase 3 vaccine candidates. Lancet. 2020;396(10262):1595‐1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Eberhardt KA, Meyer‐Schwickerath C, Heger E, et al. RNAemia corresponds to disease severity and antibody response in hospitalized COVID‐19 patients. Viruses. 2020;12(9):1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wooding DJ, Bach H. Treatment of COVID‐19 with convalescent plasma: lessons from past coronavirus outbreaks. Clin Microbiol Infect. 2020;26(10):1436‐1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


