CONFLICT OF INTEREST
None.
AUTHOR CONTRIBUTIONS
Antonella Tammaro and Gabriella De Marco: These authors are the primary physicians of the patient and supervisors of the process. The authors created the idea and reviewed the manuscript. The authors supervised the data collection process. Ganiyat Adenike Ralitsa Adebanjo, Francesca Magri, Francesca Romana Parisella, and Camilla Chello: The authors took part in the literature review, writing, and preparation of the manuscript. The authors reviewed the manuscript, photography, and literature review.
ETHICAL APPROVAL
Not applicable.
To the Editor,
Since the beginning of the COVID‐19 pandemic, strenuous efforts to create a herd immunity against this deadly disease have been made. As a matter of fact, multiple vaccines harnessing different strategies have been developed: virus‐like vaccines, protein subunit vaccines, RNA‐based vaccines, inactivated vaccines, protein subunit vaccines, and vector vaccines have been created by the international scientific community. 1
The vaccines against the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) that have been approved in Italy are the mRNA vaccines and the viral vector vaccines Ad26.COV2.S and AZD1222. 2
We report the cutaneous reaction after the AZD122 vaccine of a patient with no history of allergy.
A 35‐year‐old woman was presented because of cutaneous lesions which had appeared after the administration of the AZD122 vaccine. She reported that the day after the vaccine administration, she experienced fever, nausea, and pain on the site of injection for two consecutive days. Additionally, six days after the vaccination she noticed the appearance of a cutaneous rash on her calves in association to pruritus and warmth. After a consultation with her general practitioner, she took ebastine 10 mg and 2 tablets of betamethasone 1 mg.
Unfortunately, two days later she developed vesicular lesions on top of that rash (Figure 1). Hence, she took ebastine and betamethasone: One hour later, the vesicular lesions were substituted by white discolorations.
FIGURE 1.
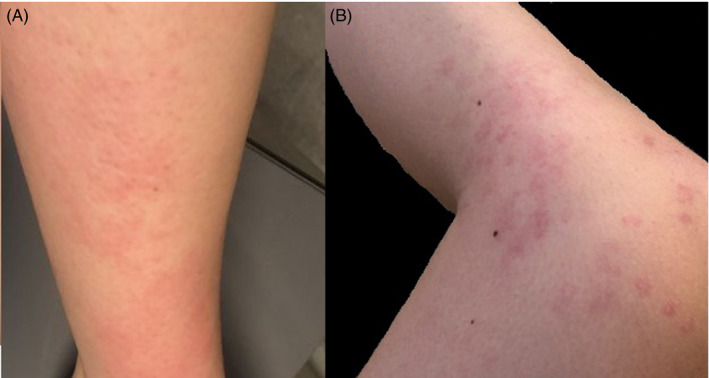
Evolution of the cutaneous lesions in a female patient after the AZD1222 vaccine. (A) Erythematous rash on the legs of the patient. (B) Erythematous lesions with vesicles on the legs of the patient
On physical examination, we detected an extended erythematous rash on the legs of the patient associated to hypopigmented areas. We decided to treat the patient by continuing the administration of ebastine and tapered betamethasone for four days. The lesions disappeared completely afterward.
Importantly, the patient had been hospitalized because of severe COVID‐19 a few months before.
We believe that the local cutaneous reaction experienced by this patient may presumptively be considered the result a nonspecific inflammatory reaction linked to the administration of the vaccine because of the temporal correlation.
Real allergic reactions to vaccines are rare events, and they may be linked to a direct response or cross‐reaction to preservatives and excipients. 3
The AZD1222 vaccine developed by AstraZeneca/Oxford University contains the wild‐type SARS‐CoV‐2 spike protein which is vectored by the attenuated adenovirus ChAdOx1 (a simian virus). 1 Notably, this vaccine against the novel coronavirus has been administered to the population since January 2021. 1 The potential excipient of this vaccine that may trigger allergic reactions is polysorbate 80. 4
A recently published article from Korea reported the adverse events following the BNT162b2 and the and AZD1222 vaccines in healthcare workers: The majority of the people receiving the AZD1222 vaccine experienced mild to moderate symptoms including pain at the injection site (77,8%). 5 Moreover, 24,9% of the AZD1222 cohort experience redness and swelling at the injection site. 5 Lymphadenopathy was reported as well. 5 Notably, the nonspecific systemic events of these patients encompassed fever (36,1%), chills (41,2%), fatigue (50,7%), nausea (23,2%), vomiting (3,8%), headache (47,4%), myalgia (60,5%), arthralgia (26,6%), and urticaria (2,9%). 5
The scientific understanding of the cutaneous manifestations in patients who had COVID‐19 and got vaccinated against the SARS‐CoV‐2 infection is still evolving.
Further studies to elucidate the mild to moderate cutaneous reactions to vaccines are warranted.
INFORMED CONSENT
Informed consent was appropriately obtained.
REFERENCES
- 1. Kyriakidis NC, López‐Cortés A, González EV, Grimaldos AB, Prado EO. SARS‐CoV‐2 vaccines strategies: a comprehensive review of phase 3 candidates. Npj Vaccines. 2021;6(1):1‐17. 10.1038/s41541-021-00292-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. COVID‐19 Vaccines | Italian Medicines Agency. Accessed April 18, 2021. https://aifa.gov.it/vaccini‐covid‐19
- 3. Caubet J‐C, Ponvert C. Vaccine allergy. Immunol Allergy Clin North Am. 2014;34(3):597‐613. 10.1016/j.iac.2014.04.004 [DOI] [PubMed] [Google Scholar]
- 4. Caballero ML, Quirce S. Excipients as Potential Agents of Anaphylaxis in Vaccines: Analyzing the Formulations of Currently Authorized COVID‐19 Vaccines. J Investig Allergol Clin Immunol. 2021;31(1):92‐93. 10.18176/jiaci.0667 [DOI] [PubMed] [Google Scholar]
- 5. Kim SH, Wi YM, Yun SY, et al. Adverse Events in Healthcare Workers after the First Dose of ChAdOx1 nCoV‐19 or BNT162b2 mRNA COVID‐19 Vaccination: a Single Center Experience. J Korean Med Sci. 2021;36(14):e107. 10.3346/jkms.2021.36.e107 [DOI] [PMC free article] [PubMed] [Google Scholar]


