Abstract
Background
There is limited information about the impact of coronavirus disease (COVID‐19) on the muscular dysfunction, despite the generalized weakness and fatigue that patients report after overcoming the acute phase of the infection. This study aimed to detect impaired muscle efficiency by evaluating delta efficiency (DE) in patients with COVID‐19 compared with subjects with chronic obstructive pulmonary disease (COPD), ischaemic heart disease (IHD), and control group (CG).
Methods
A total of 60 participants were assigned to four experimental groups: COVID‐19, COPD, IHD, and CG (n = 15 each group). Incremental exercise tests in a cycle ergometer were performed to obtain peak oxygen uptake (VO2peak). DE was obtained from the end of the first workload to the power output where the respiratory exchange ratio was 1.
Results
A lower DE was detected in patients with COVID‐19 and COPD compared with those in CG (P ≤ 0.033). However, no significant differences were observed among the experimental groups with diseases (P > 0.05). Lower VO2peak, peak ventilation, peak power output, and total exercise time were observed in the groups with diseases than in the CG (P < 0.05). A higher VO2, ventilation, and power output were detected in the CG compared with those in the groups with diseases at the first and second ventilatory threshold (P < 0.05). A higher power output was detected in the IHD group compared with those in the COVID‐19 and COPD groups (P < 0.05) at the first and second ventilatory thresholds and when the respiratory exchange ratio was 1. A significant correlation (P < 0.001) was found between the VO2peak and DE and between the peak power output and DE (P < 0.001).
Conclusions
Patients with COVID‐19 showed marked mechanical inefficiency similar to that observed in COPD and IHD patients. Patients with COVID‐19 and COPD showed a significant decrease in power output compared to IHD during pedalling despite having similar response in VO2 at each intensity. Resistance training should be considered during the early phase of rehabilitation.
Keywords: SARS‐CoV‐2, Cardiopulmonary exercise test, COPD, Ischaemic heart disease, Muscular dysfunction
Introduction
Coronavirus disease 2019 (COVID‐19) is caused by severe acute respiratory syndrome coronavirus type 2 (SARS‐CoV‐2) and was first identified in Wuhan, Hubei, China, in December 2019. 1 The disease spread very rapidly to the rest of China and later worldwide. A state of alarm was declared in Spain on 16 March 2020 with the aim of managing the emergency health care situation produced by COVID‐19. Up to 6 November 2020, a total of 1 328 832 confirmed cases and 38 883 deaths by COVID‐19 have been reported in Spain. 2
As previously demonstrated by other coronaviruses, 3 SARS‐CoV‐2 can infect different systems that share the same ACE‐2 receptors present in the respiratory system. Therefore, most of the extrapulmonary manifestations occur in the organs or systems with cells that express ACE‐2 receptors (heart, central nervous system, and muscle, among others). 4 The inflammatory response induced in the airway by SARS‐CoV‐2 can also lead to multisystemic inflammation that can affect almost all organ systems, including the musculoskeletal system. 1 , 5 It has been described that the musculoskeletal system is seriously burdened in patients with moderate to severe SARS infection by causing significant skeletal muscle, bone, joint, and neurological disorders. 6 , 7 However, there is limited information about the impact of COVID‐19 on the muscular system, despite the generalized weakness and fatigue that patients report after overcoming the acute phase of the infection.
Mechanical efficiency refers to the ability of an individual to transfer energy consumed into external work. In other words, poorer efficiency will increase the percentage of maximal oxygen uptake (VO2max) required to sustain a given mechanical work. Reduced mechanical efficiency indicates that more energy is consumed at a given work output, increasing the adenosine triphosphate (ATP) cost of contraction (ATP consumed per work output). 8
Several slightly varying indicators have been proposed for the assessment of mechanical efficiency. Concretely, delta efficiency (DE) is defined as the relationship between the change in external work (ΔW ext) and the change in total energy expenditure (ΔE tot). 9 DE is considered a valid and predictive parameter of the musculoskeletal efficiency in cycling. 9 , 10 This is also due, at least partly, to the fact that the effect of various metabolic processes not contributing to work performance is removed. In this regard, the work of stabilizing muscles, and the work cost of respiratory muscles, 11 the movement cost of the lower limbs of the body, 12 and the basal metabolic rate, 9 are not considered during DE assessment. Thus, DE may be more effective for understanding the efficiency of the musculoskeletal system. The performance of patients with impaired muscle efficiency decreases and, therefore, they may be limited in terms of physical activity. 13 Consequently, analysis of mechanical efficiency could be valuable for the detection of muscle dysfunction and the evaluation of any subsequent adaptation in response to exercise. 14
Taking into consideration that COVID‐19 patients may present an important muscle dysfunction, this study aimed to detect impaired muscle efficiency by evaluating DE in COVID‐19 patients compared with subjects with chronic obstructive pulmonary disease (COPD), ischaemic heart disease (IHD), and healthy controls.
Methods
This was a cross‐sectional, observational study aiming to evaluate DE in COVID‐19 patients. All patients provided informed consent to participate in the study.
The Ethics and Research Committee of the Mataró Hospital approved the study (Codi CEIm: 89/20). The protocol was conducted in accordance with the principles of the Declaration of Helsinki, Good Clinical Practice, and the applicable and local regulatory requirements.
Participants
The study participants included adult patients who required admission to the intensive care unit (ICU) for presenting respiratory distress syndrome secondary to bilateral COVID‐19 pneumonia. Healthy volunteers (control group) and individuals with COPD or IHD were also recruited from existing databases or the outpatient Cardiac and Pulmonary Rehabilitation Unit of the Hospital de Mataró (Consorci Sanitiari del Maresme) (n = 15 each experimental group).
The adjusted morbidity groups were obtained for all the study participants. The adjusted morbidity group groups morbidities according to data of patient diagnoses encoded in the primary care and hospital medical care histories adjusted for the encoding date (acute or chronic processes). 15 , 16
To be eligible, individuals with COPD had to have a post‐bronchodilator spirometry test showing a forced expiratory volume in the first second/forced vital capacity (FEV1/FVC) <0.7 and FEV1 (% predicted) <70%. Individuals with IHD had to have angiographic evidence of disease. Patients with COPD or IHD had been clinically stable for 6 months, without any deterioration in symptoms or episodes of angina in IHD patients. Medications were taken as recommended by the participants' physicians during the study.
For COVID‐19 patients, data related to admission were collected, and the Acute Physiologic and Chronic Health Evaluation (APACHE) II was determined as a predictive system for disease severity and prognosis in patients in the ICU. 17 The assessment of patients with COVID‐19 was carried out 8 weeks after discharge from hospital.
The exclusion criteria for the four cohorts were severe neurological disease, active oncological disease, joint problems preventing the cardiopulmonary exercise test (CPET), or inability to understand or comprehend the guidelines for performing the CPET.
Cardiopulmonary exercise test
The CPET was performed on an electro‐mechanically braked bicycle ergometer (Ergoline900S, Ergoline GmbH, Bitz, Germany). The cycling position, which is known to affect energy expenditure, was standardized by adopting a top bar position. Saddle height was adjusted according to the participant's leg length, and knee flexion was between 20° or 30°. Toe‐clips were used, and the participants were instructed to stay seated during the test. The subjects were required to maintain a constant pedal cadence between 50 and 70 revolutions per minute.
An individualized exercise protocol was performed in all patients and was tailored to each patient's physical condition, with gradual increments of 5, 10, 15, or 20 W·min−1. The required exercise time was between 6 and 12 min in order to respect the proper kinetics of oxygen consumption (VO2) and maintain a linear relationship between VO2, exercise workload and heart rate during CPET. Throughout the test, the patients were kept under continuous 12‐lead electrocardiographic‐monitoring, and blood pressure was established every 3 min.
VO2 was determined breath by breath using an automated system (Ultima CardiO2, Medical Graphics Corporation, St. Paul, MN, USA). Calibration was performed prior to each test using standard gases of known oxygen and carbon dioxide concentrations as well as a calibration syringe.
The first and second ventilatory thresholds (VT1 and VT2) were determined following the method of ventilatory equivalents (VE·VO2 −1 and VE·VCO2 −1) described by Skinner et al. 18 VT1 corresponds to an increment of the VE·VO2 −1 ratio without an increased VE·VCO2 −1 ratio, and with an increased concentration of oxygen fraction (PetO2). VT2 corresponds to an increment of the VE·VCO2 −1 ratio and a fractional decrease in the concentration of CO2 (PetCO2).
Outcomes
Mechanical efficiency was calculated as the ratio of work accomplished per minute (Watts converted to kcal·min−1) and the energy expended per minute (kcal·min−1). The conversion factor 69.7 W·kcal−1·min−1 was used for estimation of the work accomplished. 19 An equation based on the thermal equivalent of oxygen for the non‐protein respiratory quotient was used to estimate the energy expended 20 : Energy expended (kcal·min−1) = VO2·(1.2341·RER + 3.8124).
The mean values measured during the last 30 s of each workload were considered for this estimation. 19 Finally, DE was calculated as the inverse of the slope in the linear regression (y = ax + b), where y is the rate of expended energy (kcal·min−1) and x is the rate of accomplished work (kcal·min−1). 9 This value was obtained from the end of the first workload, depending on the physical condition of the participants, until the power output where the respiratory exchange ratio (RER) was 1. 19 , 21
Secondary outcomes included the total exercise time, VO2peak (mL·kg−1·min−1),VE (L·min−1), peak power (W), VO2 at VT1 (mL·kg−1·min−1), VE at VT1 (L·min−1), power at VT1 (W), VO2 at VT2 (mL·kg−1·min−1), VE at VT2 (L·min−1) and power at VT2 (W).
Statistical analysis
The Shapiro–Wilk test was used to check the normal distribution of the data, which are reported as mean and standard deviation (SD), mean, and confidence intervals (95% CI). To compare the differences between the four experimental groups (healthy control group, COVID‐19, COPD, and IHD), a univariate general linear model was applied. Bonferroni adjustment was used to identify multiple comparisons among experimental groups.
The magnitude of the response to both experimental conditions was estimated by partial eta‐squared (η p 2). The scale for classification of η p 2 was 0.10 = small, 0.25 = medium, and 0.40 = large. 22 Statistical power was also calculated.
Total exercise time, VO2, VE, and power output during CPET were compared by one‐way analysis of variance (ANOVA). When significant differences emerged, Bonferroni's post hoc was applied to establish differences between experimental groups.
Significance was set at P < 0.05. All statistical procedures were applied using the software package SPSS version 25.0 for Mac (SPSS Inc., Chicago, IL, USA).
Results
Patients
Table 1 describes the characteristics of the groups. Differences were observed in respiratory function tests among the four study groups, except between the COVID‐19 and IHD groups (FVC, L, P = 0.361; FVC%, P = 0.840; FEV1, L, P = 0.805; FEV1, %, P = 0.676; FEV1/FVC%, P = 0.086). Likewise, and given the study pathologies, we observed statistically significant differences in morbidity among the four study groups (Table 1). Table 2 describes the most relevant clinical characteristics of the COVID‐19 patients during hospitalization.
Table 1.
Patients' characteristics
| HG | COVIDG | COPDG | HDG | P value | |
|---|---|---|---|---|---|
| Age (years) | 52.2 (4.9) | 54.6 (9.1) | 56.9 (7.1) | 54.4 (8.5) | 0.369 |
| Men (%) | 100 | 100 | 100 | 100 | — |
| Body mass Index (kg/m2) | 24.2 (3.5) | 29.1 (4.4) | 28.3 (6.51) | 28.2 (4.0) | 0.552 |
| Adjusted morbidity groups (%) | — | — | — | — | <0.001 |
| Basal risk | 100% | 33.3% | 0% | 0% | — |
| Low risk | 0% | 46.6% | 13.3% | 6.6% | — |
| Moderate risk | 0% | 13.4% | 53.3% | 46.7% | — |
| High risk | 0% | 6.7% | 26.7% | 46.7% | — |
| Very high risk | 0% | 0% | 6.7% | 0% | — |
| FVC (L) | 5.1 (1.1) | 3.8 (1.1) | 2.7 (0.7) | 4.1 (0.8) | 0.007 |
| FVC (%) | 103.2 (15.8) | 87.3 (17.1) | 68.3 (15.2) | 87.2 (9.7) | 0.002 |
| FEV1 (L) | 3.9 (0.8) | 3.1 (0.8) | 1.4 (0.6) | 3.2 (0.8) | <0.001 |
| FEV1 (%) | 102.2 (17.7) | 88.1 (24.1) | 48.1 (21.1) | 87.9 (15.1) | <0.001 |
| FEV1/FVC, % | 79.9 (7.4) | 82 (6.3) | 53.3 (16.6) | 77.2 (9.1) | <0.001 |
COPDG, chronic obstructive pulmonary disease group; COVIDG, COVID‐19 group; FEV1, forced expiratory volume in the first second; FVC, forced vital capacity; HDG, heart disease group; HG, healthy group; SD, standard deviation.
Table 2.
Patients' characteristics COVID‐19
| ICU admission days* | 11.6 (5.8) |
| Days of hospital admission* | 23.2 (3.7) |
| Days mechanical ventilation* | 10.1 (5.1) |
| Tracheostomy (no n, %) | 100% |
| Prone positioning (yes, %) | 86.6% |
| APACHE II* | 11.6 (4) |
| Pa/Fi 24 h* post VM | 168.4 (87.4) |
| D‐dimer ICU admission* | 673.6 (457.8) |
| PCR admission* | 16.9 (12.4) |
| Lymphocytes admission* | 687.3 (212.4) |
| Azithromycin (yes, %) | 100% |
| Hydroxychloroquine (yes, %) | 100% |
| Lopinavir (yes, %) | 100% |
| Tocilizumab (yes, %) | 53.3% |
| Interferon beta 1β (yes, %) | 40% |
| Corticosteroid bolus (yes, %) | 80% |
APACHE, Acute Physiologic and Chronic Health Evaluation; ICU, intensive care unit.
Data are provided as mean and standard deviation (SD).
Delta efficiency
There were significant differences between experimental groups in DE (F = 7.92; P < 0.001, η p 2 = 0.30, SP = 0.99). Bonferroni's multiple comparisons showed a greater DE in the healthy control group than in the COVID‐19 and COPD groups (P < 0.001 and P = 0.033, respectively). No significant differences were found between the control group and the IHD group (P = 0.052). No significant differences were detected between the pathologies (P > 0.05) (Figure 1).
Figure 1.
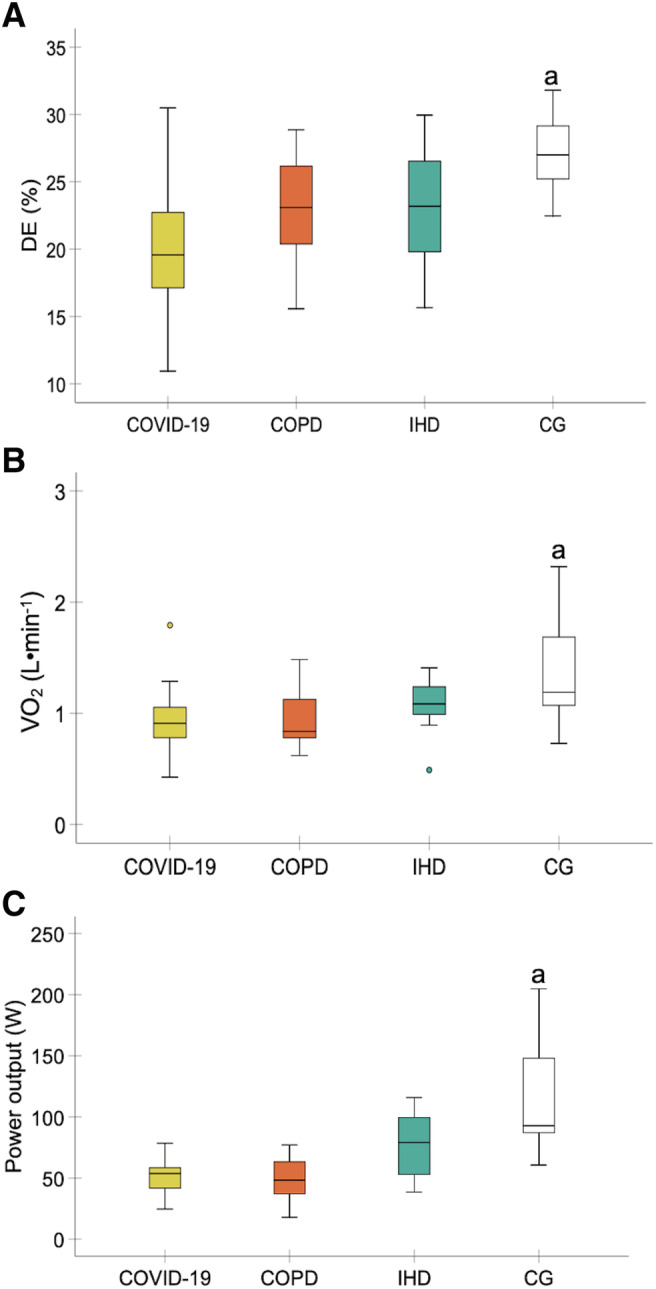
Comparisons in delta efficiency (A), VO2 when the RER was 1 (B), and peak power when the RER was 1 (C) between the experimental groups. Abbreviations: CG, healthy control group; COPD, chronic obstructive pulmonary disease; DE, delta efficiency; IHD, ischaemic heart disease; RER, respiratory exchange ratio; VO2, oxygen uptake. aHigher DE, VO2, and peak power were detected in the healthy control group compared with the COVID‐19 and COPD groups (P < 0.05). (n = 15 each experimental group).
Secondary outcomes
To determine DE, VO2, and power output were obtained where the RER was 1. VO2 and power output were lower in patients with COVID‐19 and COPD compared with the control group (P < 0.01). Power output was higher in patients with IHD compared with patients con COVID‐19 and COPD (P < 0.05). No significant differences were detected between patients with COVID‐19 and COPD in both variables (P > 0.05). No significant differences were found between patients with IHD and control group in both variables (P > 0.05) (Figure 1).
The data corresponding to VO2, VE, power output, and total test time during CPET at VT1, VT2, and peak intensity are presented in Table 3. VO2, VE, power output, and total exercise time were lower in the three groups with diseases than in the healthy group (P < 0.05) at VT1, VT2, and peak intensities. When comparing the study groups with pathologies, a higher peak power was found in IHD than in COPD patients (P < 0.05). Power output was greater in the IHD than in the COVID‐19 and COPD groups (P < 0.05) at VT1 and VT2. No other significant differences were detected among the experimental groups (P > 0.05).
Table 3.
Differences in total exercise time, VO2, VE, and power output at VT1 and VT2, and peak intensities between experimental groups
| HG | COVIDG | COPDG | IHDG | P value | |
|---|---|---|---|---|---|
| Total exercise time (min:s) | 11:20 a (10:14–12:25) | 8:14 (7:01–9:28) | 7:22 (6:25–8:20) | 9:04 (7:36–10:32) | <0.001 |
| VO2peak (mL·kg−1·min−1) | 32.31 a (28.32–36.31) | 17.30 (14.82–19.78) | 14.35 (12.97–15.73) | 18.82 (15.64–22) | <0.001 |
| VE (L·min−1) | 83.35 a (67.33–99.36) | 55.05 (45.94–64.15) | 38.19 (33.11–43.28) | 58.79 c (49.09–68.50) | <0.001 |
| Peak power (W) | 215.60 a (181.84–249.37) | 89.67 (69.74–109.60) | 74.67 (62.79–86.54) | 130.93 c (102.25–159.62) | <0.001 |
| VO2 at VT1 (mL·kg−1·min−1) | 14.40 a (12.33–16.47) | 8.94 (7.89–9.99) | 9.25 (8.19–10.30) | 10.58 (9.03–12.13) | <0.001 |
| VE at VT1 (L·min−1) | 24.75 (19.81–29.70) | 21.42 (17.61–25.19) | 21.57 (18.73–24.42) | 24.54 (20.84–28.24) | 0.394 |
| Power at VT1 (W) | 84.47 a (68.74–100.19) | 29.67 (17.51–41.83) | 28.20 (15.39–41.01) | 61.60 b (45.73–77.47) | <0.001 |
| VO2 at VT2 (mL·kg−1·min−1) | 25.18 a (21.78–28.58) | 12.80 (11.34–14.26) | 12.14 (10.56–13.72) | 14.83 (12.53–17.13) | <0.001 |
| VE at VT2 (L·min−1) | 53.21 a (42.02–64.41) | 35.81 (29.71–41.92) | 29.92 (25.11–34.72) | 39.08 (33.11–45.04) | <0.001 |
| Power at VT2 (W) | 176.53 a (147.48–205.58) | 69.20 (52.53–85.87) | 62.21 (49.36–75.04) | 108.41 b (87.11–129.69) | <0.001 |
COPDG, chronic obstructive pulmonary disease group; COVIDG, COVID‐19 group; HG, healthy group; IHDG, ischaemic heart disease group; VE, minute ventilation; VO2, oxygen uptake; VT1, first ventilatory threshold; VT2, second ventilatory threshold.
Data are provided as mean and 95% confidence intervals (95% CIs).
Significantly different from COVIDG, COPDG, and IHDG (P < 0.05).
Significantly different from COVIDG and COPDG at VT1 and VT2 intensities (P < 0.05).
Significantly different from COPDG (P < 0.05).
A significant correlation (r = 64, P < 0.001) was found between the VO2peak and DE and between the peak power output and DE (r = 61, P < 0.001) (Figure 2). No other correlations were detected (P > 0.05).
Figure 2.
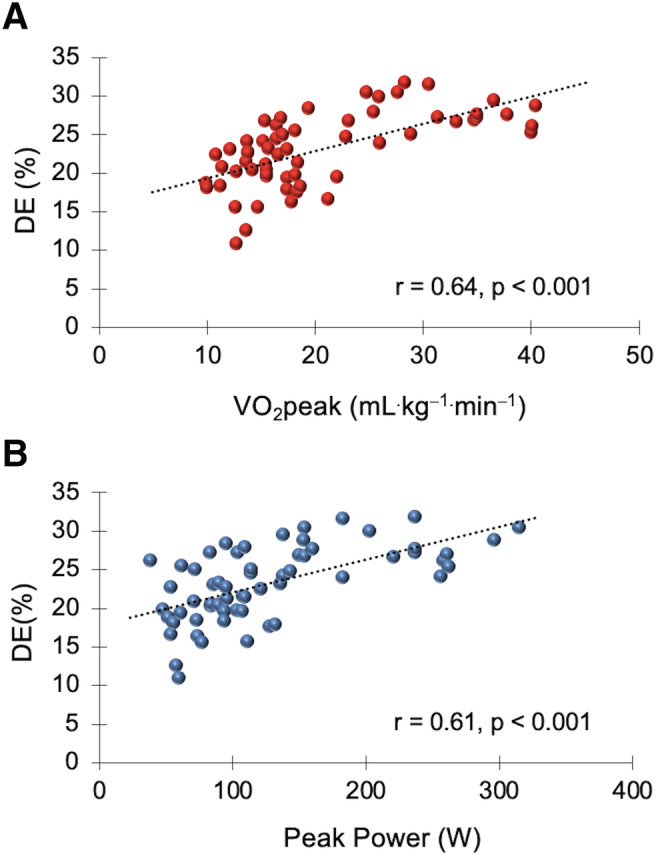
Correlations between delta efficiency (DE) and peak oxygen uptake (VO2peak) (r = 0.64, P < 0.001) (A); peak power output in watts (W) (r = 0.61, P < 0.001) (B) (all participants, n = 60).
Discussion
To our knowledge, this is the first study to examine mechanical efficiency during exercise in patients who have had COVID‐19 infection and required admission to the ICU due to adult respiratory distress. The most relevant finding was that patients with COVID‐19 and COPD presented reduced DE compared with healthy adult volunteers (control group). It was noteworthy that DE did not change among the experimental groups with cardiorespiratory diseases. Furthermore, no differences were observed between patients with IHD and the control group.
Other relevant findings showed a lower total exercise time, VO2, VE (not at VT1), and power output in the three disease groups compared with the control group after CPET. When the three pathologies were compared, the power output in patients with COVID‐19 and COPD was lower than that of patients with IHD at intensities of VT1 and VT2 and when the RER was 1.
Unfortunately, at present, the DE results of our study cannot be reinforced by previous studies in patients with COVID‐19 and, furthermore, research comparing patients with COPD and IHD are limited. The DE observed in patients with COVID‐19 (19.9%) were slightly lower (no significant) than those obtained in patients with COPD and IHD (~23%), and significantly lower compared to the control group (~27%). Other studies have reported mean DE values of ~25–26% in patients with COPD and with heart failure with reduced ejection fraction during submaximal cycling. 23 , 24 Perrault et al. did not find significant differences between patients with COPD (26.3%) and a control group (24.8%). 25 According to a review article, 26 the mean DE of 14 studies was 23.8 ± 2.6%. Similar DE (23.8%) was reported in another study with sport science students. 27 Given the reported results in these studies, it appears that COVID‐19 patients may have decreased mechanical efficiency compared with healthy people and about 20% could be a reference value for DE. More studies with COVID‐19 patients are needed to corroborate such claims.
Several clinical sequelae should be considered to understand a decrease in mechanical efficiency in patients with COVID‐19. Survivors of SARS‐CoV‐2 are highly prone to developing severe clinical respiratory, cardiovascular, and psychosocial sequelae. These sequelae are linked to important physical decline and significant fatigue. 28 In addition, muscle soreness, muscle fatigue, and weakness are stated symptoms in COVID‐19 patients. 29
Similarly, patients with COPD also present symptoms associated with impaired respiratory capacity, 30 discomfort in the legs and dysfunction of the peripheral muscles (muscle atrophy and weakness, fatigue) that limit exercise capacity. 31 , 32 , 33 Given the significant muscle pain, fatigue, and weakness that patients with COVID‐19 present, it is plausible to propose a poor mechanical efficiency in COVID‐19 patients, which may, in turn affect the exercise tolerance as in patients with COPD occur. 8
Several physiological mechanisms have been proposed to explain deficiencies in mechanical efficiency associated with the peripheral skeletal muscle. Leg discomfort and peripheral muscle dysfunction could affect exercise tolerance by alter muscle energy production during exercise and rest. 8 This increase is usually associated with a rise in the proportion of Type II muscle fibres during exercise in patients with COPD. 34 , 35 This recruitment of Type II fibres is three to four times greater than in Type I fibres. 36
During CPET, different metabolic moments took place from the start of exercise until the value of the RER was 1, and thus, the recruitment pattern of motor units was likely modified from Type I to Types IIa and IIb as the work rate increased and the fibres became progressively fatigued. 37 The similar behaviour observed between patients with COPD and COVID‐19 suggests that a premature recruitment of less efficient Type II motor units occurred, leading to an increase in energy cost during skeletal muscle contraction. The decreased oxidative capacity of Type II fibres, 38 the attenuated activity of some enzymes involved in the Krebs cycle, 39 , 40 and the greater energy cost of muscle contraction induced an unusual ATP consumption and, consequently, a reduction in DE in these patients, 8 contributing to the early onset of muscle fatigue. Inefficiency in humans is associated with a decrease in mitochondrial efficiency. 41 Probably, these biochemical and physiological mechanisms were, at least partly, a key factor to detect a reduced DE in COVID‐19 patients. In this study, we did not analyse the increase in energy cost during muscle contraction and its association with the recruitment of motor units, and therefore, our arguments were based on the findings of others. 8 , 34 , 35 , 36 , 37 Unfortunately, the absence of muscle biopsies is a methodological limitation for this study. More research is warranted to clarify these arguments in patients with COVID‐19 disease.
As expected, functional capacity was significantly reduced in patients with diseases compared with the control group. Similar values of VO2peak have been observed in other studies in patients with COPD (12.8 mL·kg−1·min−1, 42 heart diseases (16–18 mL·kg−1·min−1) 43 and COVID‐19 (17.2 mL·kg−1·min−1). 44 Interestingly, Carvalho‐Jr et al. found that patients with COPD who had lower FEV1 had lower VO2peak. 42 They concluded that FEV1 was a predictor of VO2peak to determine risk and severity in patients with COPD. In our study, patients with COVID‐19 presented a VO2peak similar to that of patients with COPD and IHD, however, FEV1 was much higher in patients with COVID‐19 and IHD compared with patients with COPD. The ventilatory response at rest (spirometry) in patients with COVID‐19 and IHD was similar to that of the control group and higher compared with those in patients with COPD; however, cardiorespiratory response was impaired in all experimental groups with diseases during CPET. Perhaps, FEV1 is not a differential factor to predict VO2peak determining risk and severity in COVID‐19 patients.
No differences were detected in VO2peak between the pathologies while, conversely, variances were found in the power output developed between the patients with respiratory diseases (COVID‐19 and COPD) and IHD. Under these premises, patients with IHD should probably have a higher DE (ΔW ext/ΔVO2) due to increased power output compared with patients with COVID‐19 and COPD. However, DE remained unchanged among the experimental groups with diseases, which represents a similar proportional raise in the increase in power output and in VO2 from the end of the first workload until where the respiratory exchange ratio was 1. What seems evident is that patients with COVID‐19 and COPD develop less force when pedalling during incremental CPET.
To conclude this discussion, we would like to emphasize the relationship between VO2peak and peak power and DE. Several studies have demonstrated an inverse correlation between DE, gross efficiency, and VO2max in world‐class cyclists. 19 , 45 The participants with the highest DE and gross efficiency had the lowest VO2max. The authors concluded that a low VO2max could be offset by increased muscle efficiency. This could be due to a physiological adaptation to training that would allow world‐class cyclists to continue at a highly competitive level. However, we found a positive correlation between DE and VO2peak and peak power in the experimental groups. The participants with the highest DE had the highest VO2peak and peak power. The participants in this study suffered from various diseases (except the control group) and were not trained. This probably suggests that the cardiorespiratory fitness and muscular fitness of the lower extremities are a determining factor for improving mechanical efficiency. The physical condition of COVID‐19 patients could be a key factor in achieving a faster recovery. It would be interesting to propose a rehabilitation programme to know the evolution of cardiorespiratory and muscular fitness and mechanical efficiency in COVID‐19 patients.
Conclusions
Patients with COVID‐19 infection showed marked mechanical inefficiency similar to that observed in patients with COPD and IHD. The limiting factor was the muscle power developed during pedalling, which showed muscle dysfunction in patients with COVID‐19 as a determining symptomatic factor. Strength development programmes should be considered during the early phase of rehabilitation.
Funding
None.
Conflict of interest
All authors declare no competing interests.
Ethical guidelines statement
The Ethics and Research Committee of the Mataró Hospital approved the study (Codi CEIm: 89/20). The protocol was conducted in accordance with the principles of the Declaration of Helsinki, Good Clinical Practice, and the applicable and local regulatory requirements. The authors of this manuscript certify that they comply with the ethical guidelines for authorship and publishing in the Journal of Cachexia, Sarcopenia and Muscle. 46
Pleguezuelos E., Del Carmen A., Llorensi G., Carcole J., Casarramona P., Moreno E., Ortega P., Serra‐Prat M., Palomera E., Miravitlles M. M., Yebenes J. C., Boixeda R., Campins L., Villelabeitia‐Jaureguizar K., and Garnacho‐Castaño M. V. (2021) Severe loss of mechanical efficiency in COVID‐19 patients, Journal of Cachexia, Sarcopenia and Muscle, 12, 1056–1063, 10.1002/jcsm.12739
References
- 1. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Actualización no 245. Enfermedad por el coronavirus (COVID‐19) https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/Actualizacion_245_COVID‐19.pdf Accessed November 7th 2020)
- 3. Desforges M, Le Coupanec A, Dubeau P, Bourgouin A, Lajoie L, Dubé M, et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses 2020;12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baig AM. Neurological manifestations in COVID‐19 caused by SARS‐CoV‐2. CNS Neurosci Ther 2020;26:499–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cheng H, Wang Y, Wang GQ. Organ‐protective effect of angiotensin‐converting enzyme 2 and its effect on the prognosis of COVID‐19. J Med Virol 2020;92:726–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Leung TW, Wong KS, Hui AC, To KF, Lai ST, Ng WF, et al. Myopathic changes associated with severe acute respiratory syndrome: a postmortem case series. Arch Neurol 2005;62:1113–1117. [DOI] [PubMed] [Google Scholar]
- 7. Griffith JF. Musculoskeletal complications of severe acute respiratory syndrome. Semin Musculoskelet Radiol 2011;15:554–560. [DOI] [PubMed] [Google Scholar]
- 8. Layec G, Haseler LJ, Hoff J, Richardson RS. Evidence that a higher ATP cost of muscular contraction contributes to the lower mechanical efficiency associated with COPD: preliminary findings. Am J Physiol Regul Integr Comp Physiol 2011;300:R1142–R1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Coyle EF, Sidossis LS, Horowitz JF, Beltz JD. Cycling efficiency is related to the percentage of type I muscle fibers. Med Sci Sports Exerc 1992;24:782–788. [PubMed] [Google Scholar]
- 10. Horowitz JF, Sidossis LR, Coyle EF. High efficiency of type I muscle fibres improves performance. Int J Sports Med 1994;15:152–157. [DOI] [PubMed] [Google Scholar]
- 11. Xu F, Rhodes EC. Oxygen uptake kinetics during exercise. Sports Med 1999;27:313–327. [DOI] [PubMed] [Google Scholar]
- 12. Sidossis LS, Horowitz JF, Coyle EF. Load and velocity of contraction influence gross and delta mechanical efficiency. Int J Sports Med 1992;13:407–411. [DOI] [PubMed] [Google Scholar]
- 13. Jabbour G, Iancu HD. Mechanical efficiency improvement in relation to metabolic changes in sedentary obese adults. BMJ Open Sport Exerc Med 2015;1:e000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jabbour G, Iancu HD, Mauriège P, Joanisse DR, Martin LJ. High‐intensity interval training improves performance in young and older individuals by increasing mechanical efficiency. Physiol Rep 2017;5:e13232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Monterde D, Vela E, Clèries M, Garcia‐Eroles L, Roca J, Pérez‐Sust P. Multimorbidity as a predictor of health service utilization in primary care: a registry‐based study of the Catalan population. BMC Fam Pract 2020;21:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Arias‐López C, Rodrigo Val MP, Casaña Fernández L, Salvador Sánchez L, Dorado Díaz A, Estupiñán RM. Validity of predictive power of the adjusted morbidity groups (AMG) with respect to others population stratification tools. Rev Esp Salud Publica 2020;94:e202007079. [PMC free article] [PubMed] [Google Scholar]
- 17. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med 1985;13:818–829. [PubMed] [Google Scholar]
- 18. Skinner JS, McLellan MT, McLellan TH. The transition from aerobic to anaerobic metabolism. Res Q Exerc Sport 1980;51:234–248. [DOI] [PubMed] [Google Scholar]
- 19. Santalla A, Naranjo J, Terrados N. Muscle efficiency improves over time in world‐class cyclists. Med Sci Sports Exerc 2009;41:1096–1101. [DOI] [PubMed] [Google Scholar]
- 20. McDaniel J, Durstine JL, Hand GA, Martin JC. Determinants of metabolic cost during submaximal cycling. J Appl Physiol 2002;93:823–828. [DOI] [PubMed] [Google Scholar]
- 21. Moseley L, Jeukendrup AE. The reliability of cycling efficiency. Med Sci Sports Exerc 2001;33:621–627. [DOI] [PubMed] [Google Scholar]
- 22. Cohen J. Quantitative methods in psychology: a power primer. Psychol Bull 1992;112:1155–1159. [Google Scholar]
- 23. Beijers RJ, Huysmans SM, van de Bool C, Kingma BRM, Verdijk LB, van Loon LJC, et al. The effect of acute and 7‐days dietary nitrate on mechanical efficiency, exercise performance and cardiac biomarkers in patients with chronic obstructive pulmonary disease. Clin Nutr 2018;37:1852–1861. [DOI] [PubMed] [Google Scholar]
- 24. Coggan AR, Broadstreet SR, Mahmood K, Mikhalkova D, Madigan M, Bole I, et al. Dietary Nitrate increases VO2peak and performance but does not alter ventilation or efficiency in patients with heart failure with reduced ejection fraction. J Card Fail 2018. Feb;24:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Perrault H, Gravel G, Ofir D, Rittmaster D, Aguilaniu B, Bourbeau J. Cycling efficiency is not compromised for moderate exercise in moderately severe COPD. Med Sci Sports Exerc 2007. Jun;39:918–925. [DOI] [PubMed] [Google Scholar]
- 26. Ettema G, Lorås HW. Efficiency in cycling: a review. Eur J Appl Physiol 2009. May;106:1–14, Epub 2009 Feb 20. PMID: 19229554. [DOI] [PubMed] [Google Scholar]
- 27. Matomäki P, Linnamo V, Kyröläinen H. A comparison of methodological approaches to measuring cycling mechanical efficiency. Sports Med Open 2019. Jun 10;5:23, PMID: 31183594; PMCID: PMC6557926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xiong Q, Xu M, Li J, Liu Y, Zhang J, Xu Y, et al. Clinical sequelae of COVID‐19 survivors in Wuhan, China: a single‐centre longitudinal study. Clin Microbiol Infect 2021. Jan;27:89–95, Epub 2020 Sep 23. PMID: 32979574; PMCID: PMC7510771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, et al. COVID‐19 patients' clinical characteristics, discharge rate, and fatality rate of meta‐analysis. J Med Virol 2020. Jun;92:577–583, Epub 2020 Mar 23. PMID: 32162702; PMCID: PMC7228329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Broekhuizen R, Wouters EF, Creutzberg EC, Schols AM. Raised CRP levels mark metabolic and functional impairment in advanced COPD. Thorax 2006. Jan;61:17–22, Epub 2005 Jul 29. PMID: 16055618; PMCID: PMC2080712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Debigaré R, Maltais F. The major limitation to exercise performance in COPD is lower limb muscle dysfunction. J Appl Physiol (1985 2008. Aug;105:751–753; discussion 755‐7, PMID: 18678623. [DOI] [PubMed] [Google Scholar]
- 32. Killian KJ, Leblanc P, Martin DH, Summers E, Jones NL, Campbell EJ. Exercise capacity and ventilatory, circulatory, and symptom limitation in patients with chronic airflow limitation. Am Rev Respir Dis 1992. Oct;146:935–940. [DOI] [PubMed] [Google Scholar]
- 33. Maltais F, Simard AA, Simard C, Jobin J, Desgagnés P, LeBlanc P. Oxidative capacity of the skeletal muscle and lactic acid kinetics during exercise in normal subjects and in patients with COPD. Am J Respir Crit Care Med 1996. Jan;153:288–293. [DOI] [PubMed] [Google Scholar]
- 34. Richardson RS, Leek BT, Gavin TP, Haseler LJ, Mudaliar SR, Henry R, et al. Reduced mechanical efficiency in chronic obstructive pulmonary disease but normal peak VO2 with small muscle mass exercise. Am J Respir Crit Care Med 2004;169:89–96. [DOI] [PubMed] [Google Scholar]
- 35. Whittom F, Jobin J, Simard PM, Leblanc P, Simard C, Bernard S, et al. Histochemical and morphological characteristics of the vastus lateralis muscle in patients with chronic obstructive pulmonary disease. Med Sci Sports Exer 1998;30:1467–1474. [DOI] [PubMed] [Google Scholar]
- 36. He ZH, Bottinelli R, Pellegrino MA, Ferenczi MA, Reggiani C. ATP consumption and efficiency of human single muscle fibers with different myosin isoform composition. Biophys J 2000;79:945–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lucía A, Sánchez O, Carvajal A, Chicharro JL. Analysis of the aerobic–anaerobic transition in elite cyclists during incremental exercise with the use of electromyography. Br J Sports Med 1999;33:178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kushmerick MJ, Meyer RA, Brown TR. Regulation of oxygen consumption in fast‐ and slow‐twitch muscle. Am J Physiol 1992;263:C598–C606. [DOI] [PubMed] [Google Scholar]
- 39. Jakobsson P, Jorfeldt L, Henriksson J. Metabolic enzyme activity in the quadriceps femoris muscle in patients with severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1995;151:374–377. [DOI] [PubMed] [Google Scholar]
- 40. Maltais F, LeBlanc P, Whittom F, Simard C, Marquis K, Bélanger M, et al. Oxidative enzyme activities of the vastus lateralis muscle and the functional status in patients with COPD. Thorax 2000;55:848–853, PMID: 10992537; PMCID: PMC1745616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gore CJ, Clark SA, Saunders PU. Nonhematological mechanisms of improved sea‐level performance after hypoxic exposure. Med Sci Sports Exerc 2007;39:1600–1609. [DOI] [PubMed] [Google Scholar]
- 42. Carvalho‐Jr LCS, Trimer R, Arêas GP, Caruso FC, Zangrando KT, Jürgensen SP, et al. COPD assessment test and FEV1: do they predict oxygen uptake in COPD? Int J Chron Obstruct Pulmon Dis 2018;13:3149–3156, PMID: 30349223; PMCID: PMC6183695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Szmedra L, Bacharach DW, Buckenmeyer PJ, Hermann DT, Ehrich DA. Response of patients with coronary artery disease stratified by ejection fraction following short‐term training. Int J Cardiol 1994. Oct;46:209–222. [DOI] [PubMed] [Google Scholar]
- 44. Debeaumont D, Boujibar F, Ferrand‐Devouge E, Artaud‐Macari E, Tamion F, Gravier FE, et al. Cardiopulmonary exercise testing to assess persistent symptoms at 6 months in people with COVID‐19 who survived hospitalization—a pilot study. Phys Ther 2021. Mar 18;pzab099, 10.1093/ptj/pzab099 Epub ahead of print. PMID: 33735374; PMCID: PMC7989156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lucía A, Hoyos J, Pérez M, Santalla A, Chicharro JL. Inverse relationship between VO2max and economy/efficiency in world‐class cyclists. Med Sci Sports Exerc 2002;34:2079–2084. [DOI] [PubMed] [Google Scholar]
- 46. von Haehling S, Morley JE, Coats AJS, Anker SD. Ethical guidelines for publishing in the Journal of Cachexia, Sarcopenia and Muscle: update 2019. J Cachexia Sarcopenia Muscle 2019;10:1143–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]


