Abstract
Objective
It was aimed to evaluate long‐term radiological changes in severe coronavirus disease 2019 (COVID‐19) patients, to investigate pulmonary function, exercise capacities, and health‐related quality of life results.
Methods
Sixty‐five patients with severe COVID‐19 pneumonia were evaluated in the sixth month after discharge from the hospital. Spirometry, 6 min walking test (6MWT), and short form of health‐related quality of life scale (SF‐36) were applied in the sixth month. Chest computed tomography (CT) was performed and the findings were grouped according to lung involvement.
Results
Forty‐nine male and 16 female patients were included in the study. Forced expiratory volume in 1 s (FEV1)% values of 18 patients (30.5%), forced vital capacity (FVC)% values of 27 patients (45.8%), and 6MWT of 13 patients (23.2%) were found lower than expected in the sixth month. On the SF‐36 scale, physical function, energy‐vitality, social functionality, pain, and general health parameters were found lower than normal. Minimal interstitial changes in chest CT were seen in 26 patients. Nine patients had lung area involvement between 10% and 50% of the surface, there was a correlation between FEV1% and FVC% values in this group. There was severe pulmonary fibrosis in four patients. There was a correlation between pulmonary function and physical function and general perception of health from SF‐36 scale subparameters.
Conclusion
Functional and radiological abnormalities were detected in a significant number of patients in the sixth month after severe COVID‐19 pneumonia. A systematic monitoring plan must be established to assess and properly manage the long‐term problems that may arise.
Keywords: coronavirus, virus classification, respiratory tract, pathogenesis, psychology, social science
1. INTRODUCTION
Coronavirus disease‐19 (COVID‐19) is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). After the reporting of unexplainable cases of pneumonia in Wuhan, China in December 2019, a new coronavirus that had not been detected in humans before was identified on January 7, 2020, and was named SARS‐CoV‐2 due to the similarity of the virus to SARS‐CoV. Due to the spread and severity of the virus, the World Health Organization declared COVID‐10 a global pandemic March 11, 2020. 1 COVID‐19 may be clinically asymptomatic or may show various symptoms as a result of the involvement of multiple systems, such as respiratory, cardiovascular, gastrointestinal, and central nervous system in the disease. 2 Deaths may occur due to complications after multisystem involvement. As of February 18, 2021, 109,594,835 cases and 2,424,060 deaths were recorded worldwide. 3 The respiratory system is the most commonly affected system in patients diagnosed with COVID‐19. Most of the patients have bilateral subpleural lung involvement. Radiologically, typical COVID‐19 findings are multilobar involvement in chest‐computed tomography (CT) with frosted glass opacities and subsegmental consolidation. 4 Diffuse pulmonary parenchymal lesions, intra‐alveolar exudate, and pulmonary interstitial fibrosis can lead to poor pulmonary function in patients and affect respiratory capacity and quality of life adversely in the long term.
Negative long‐term effects on pulmonary function and health‐related quality of life have been reported after other coronavirus outbreaks, such as severe acute respiratory syndrome (SARS) in 2003 and the Middle East respiratory syndrome (MERS) in 2012,5, 6, 7 whose viral structures are similar to that of SARS‐CoV‐2. Considering that the COVID‐19 pandemic is still ongoing, it is important to investigate its effects on the long‐term pulmonary capacity and functionality with the follow‐up of patients who had the infection and to assess the morbidity associated with this disease.
The aim of this study is to search the long‐term effects of COVID‐19 on patients who have had severe COVID‐19 pneumonia history. We would like to find out the potential long‐term impacts of COVID‐19 on pulmonary capacity and health‐related quality of life.
2. METHODS
2.1. Participants and study design
Patients over 18 years old who were followed up between March 11 and July 30, 2020, in COVID inpatient clinics of our hospital; patients with SARS‐CoV‐2 PCR (+) and/or SARS‐CoV‐2 IgM/G (+); patients clinically and radiologically compatible with COVID‐19 diagnosis and patients whose cases cannot be identified by any other disease except for COVID‐19 according to the T.C. Ministry of Health, Public Health COVID‐19 Field Guide. 1 Among these patients, patients with severe pneumonia according to the Ministry's guide were included in this study.
According to the Ministry's guide, severe pneumonia findings are shown below:
Patients who have symptoms, such as fever, muscle/joint pains, cough, sore throat, and nasal congestion, have tachypnea (≥30/min) or have SpO2 level less than 90% in room air
Poor prognostic measurement in the blood tests taken in the application (number of blood lymphocytes <800/μl or CRP > 40 mg/l or ferritin >500 ng/ml or d ‐dimer >1000 ng/ml, etc.)
Patients with bilateral diffuse pneumonia findings in pulmonary X‐ray or CT.
Spirometry and 6 min walking test (6MWT) requires good physical function and patient compliance, so among patients covering severe pneumonia criteria, we selected patients who were able to perform spirometry and 6MWT. Patients who had neurological diseases, such as advanced dementia, Alzheimer's, SVD, etc; patients who had a history of orthopedic or other surgical operations, and consequently, were not able to take the walking test; and patients over 90 years old were not included. The volunteer consent form was obtained from the patients who participated in the study. The patient participation scheme is shown in Figure 1.
Figure 1.
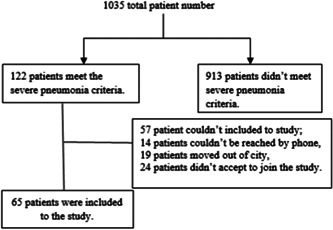
Patient participation scheme
Patients were called to the outpatient clinic between the sixth and seventh month after discharge from the hospital. The patients were given a pulmonary function test (spirometry), 6MWT. SF‐36 form was filled and chest CT was done for each patient. This study is a single‐centered, prospective observational case study. Our study has been approved by the ethics committee (April 2, 2020/1489).
2.2. Spirometry and 6MWT
The patients were subjected to a pulmonary function test (spirometry) and 6 min walking test (6MWT), associated with the daily activity of people. The tests, respectively, were aimed to evaluate respiratory functions and musculoskeletal and cardiopulmonary system functions.
6MWT provides a standardized, objective, and integrated assessment of cardiopulmonary and musculoskeletal function related to our daily activities. 8 The test (6MWT) conducted under the supervision of a physician involved a 6‐min walk along a flat corridor at a pace as quick as the patient can manage. Before and after the test, respiratory rate, heart rate per minute, and arterial blood pressure have been checked, and the walking distances of each patient within six minutes were recorded. The test was conducted in compliance with American Thoracic Society (ATS) protocols. 9
Spirometry was carried out by a spirometry technician wearing protective equipment to prevent contamination in our hospital's Pulmonary Function Testing Laboratory. Spirometry was carried out in accordance with ATS‐European Respiratory Society guidelines using MIR Spirolab II (Rome/Italy) device.10, 11 The patients were informed about maneuvers and three spirograms were performed. The best results that met the criteria for repeatability and acceptability were included in the study.
2.3. Short form 36‐point questionnaire
Health‐related quality of life is defined as the subjective sensation of the multifaceted effect of disease by patients. 12 The short form 36‐point questionnaire (SF‐36) is a popular tool for assessing health‐related quality of life. In this study, we used the Turkish version of SF‐36 to evaluate the change in health‐related quality of life of our COVID‐19 patients in tests conducted after 6 months of their recovery, which we will refer to as “sixth‐month controls” in this article. 13 This survey includes eight parameters: physical functioning, physical role difficulties, pain, general health, emotional role difficulties, energy‐vitality, mental health, and social functionality. The first four parameters are related to physical health and the others are related to mental health. Scores for each parameter vary between 0 and 100 and higher scores show a better quality of life. Normal values for parameters are different from each other. Using this scale, we planned to assess health‐related quality of life and objectively evaluate the physical and mental functionality of the patients after 6 months of recovery.
2.4. Radiographic assessment
The radiological lung findings of the patients were evaluated with lung chest‐CT in the sixth‐month controls. It was aimed to evaluate the course of lung involvement and the effect of COVID‐19 on the lung in the long term.
In the sixth‐month controls, 60 patients had nonenhanced chest‐CT. The patients were assigned to the protocol, with 100 kV (peak) (kV[p]) and 20 effective milli ampere‐second (eff mA‐s) during single breathold. Axial images of 2 mm slice thickness were obtained using an image matrix of 512 × 512 pixels. We used a mediatenum window setting (width, 400 HU; level, 100 HU) and a lung window setting (width, 1500 HU; level, –500 HU) for this analysis.
Nearly all studies about radiological findings of COVID‐19 show and identify the distribution of lesions while active COVID‐19 infection is present. There is no scoring that shows interstitial changes, fibrosis intensity, and distribution of lesions in patients who recovered from COVID‐19. All physicians who participated in the study have consensus on collecting CT results in five groups by prioritizing tomography findings, interstitial changes, and/or the percentage of distribution of fibrosis. The images were evaluated by two radiologists, both blind to clinical information. CT scans were taken on the same day as the spirometry, 6MWT, and SF‐36.
CT findings were grouped as shown below:
Group 1: Normal,
Group 2: Medium‐lower lobe dominant, ≤10% surface area,
Group 3: Medium‐lower lobe dominant, reticulation + traction, 10%–50% surface area,
Group 4: All lobes, reticulation + traction, >50% surface area,
Group 5: All lobes, diffuse reticulation+ traction + honeycomb, >50% surface area.
2.5. Analysis of data
Statistical analysis of the data was conducted in IBM SSPS Statics Version 22 program. Pearson χ 2 and Fisher's exact test was used for the comparison of categorical data between groups. However, as the continuous data did not show normal distribution (Kolmogorov–Smirnov, p < 0.05), Mann–Whitney U statistical analyses were used to compare continuous variables between two groups. p < 0.05 was considered statistically significant.
3. RESULTS
Of the 65 patients who participated in the study, 49 (75.4%) were male and 16 (24.6%) were female. The average hospitalization time for the group was 11.7 days (min 4–max 42 days). The demographic data of patients are shown in Table 1. The most common symptoms of patients were weakness‐malaise seen in 51 of the patients (78.5%), fever in 41 of the patients (63.1%), widespread muscle pains in 38 of the patients (58.5%), cough in 37 of the patients (56.9%), and shortness of breath in 33 of the patients (50.8%).
Table 1.
Demographic findings of patients
| N | % | ||
|---|---|---|---|
| Gender | Male | 49 | 75·4 |
| Woman | 16 | 24·6 | |
| Profession | Working | 39 | 60·0 |
| Unemployed | 26 | 40·0 | |
| BMI | Normal | 8 | 12·3 |
| Overweight | 28 | 43·1 | |
| Obese | 29 | 44·6 | |
| ICU hospitalization story | Yes | 8 | 12·3 |
| No | 57 | 87·7 | |
| Comorbid diseases | Asthma/chronic obstructive pulmonary disease (COPD) | 4 | 6·2 |
| Heart diseases | 9 | 13·8 | |
| Hypertension | 30 | 46·2 | |
| Metabolic diseases | 16 | 24·6 | |
| Chronic renal failure/chronic liver disease | 3 | 4·6 | |
| Cigarette | Smoker | 5 | 7·7 |
| Ex‐smoker | 25 | 38·5 | |
| Nonsmoker | 35 | 53·8 | |
| Patient with COVID‐19 in the family | Yes | 29 | 44·6 |
| No | 36 | 55·4 |
Abbreviations: BMI, body mass index; COVID‐19, coronavirus disease 2019; ICU, intensive care unit.
3.1. Spirometry and 6MWD
In the sixth‐month controls, spirometry was applied to 59 patients. Eighteen of the patients (30.5%) were found to have a low FEV1% value, 27 of the patients (45.8%) had lower FVC% values. 6MWD was also measured as low in 13 of the patients (23.2%), (p < 0.001). Four of our patients were known to have a history of obstructive lung disease (chronic obstructive pulmonary disease/asthma), but none of them had respiratory distress at the time of the tests and FEV1/FVC ratio measurements were greater than 70%.
3.2. SF‐36
SF‐36 was applied to all 65 patients. This test could be separated into eight parameters. Of the 55 patients (84.6%), the parameter of physical function was lower than expected, energy‐vitality parameter in 46 patients (70.8%), social functionality parameter in 53 patients (81.5%), pain parameter in 50 patients (76.9%), and general health perception parameter in 60 patients (92.3%) were found to be lower than expected. Changes in these parameters are statistically significant (p < 0.05).
Comparison of SF‐36 scores of participants with those of the Turkish population is also shown in Figure 2.
Figure 2.
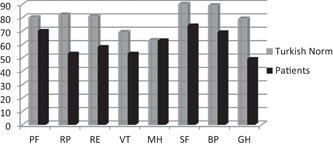
Comparison of SF‐36 scores of COVID‐19 patients with Turkish population. The vertical axis represents mean (SD) SF‐36 domain scores from 0 to 100. The horizontal axis shows SF‐36 subparameters. BP, pain; COVID‐19, coronavirus disease 2019; GH, general health perception; MH, mental health; PF, physical function; RE, emotional role difficulties; RP, physical role difficulties; SF, social functionality; VT, energy vitality
3.3. Chest‐CT
Sixty patients were given chest‐CT in the sixth‐month controls. In this study, while chest CT was normal in 21 patients (35%), 39 of the patient (65%) had chest CT findings with a spectrum from minimal changes to advanced changes including honeycombs. CT changes were minimal in 26 of the 39 patients with abnormal CT findings. Considerable fibrotic changes were seen in four patients. The distribution of the sixth‐month controls of CT findings is shown in Table 2. As a result of the sixth‐month controls, in chest‐CT findings of the 60 patients revealed a statistically significant difference in terms of observed and expected values (p < 0.05).
Table 2.
Sixth‐month chest‐CT findings distribution of patients
| Sixth‐month chest‐CT | Observed N | Expected N | Residual | χ 2 | p |
|---|---|---|---|---|---|
| Normal | 21 | 12.0 | 9.0 | 40.667 | <0.001 |
| Medium‐lower lobe dominant, ≤10% surface area | 26 | 12.0 | 14.0 | ||
| Medium‐lower lobe dominant, reticulation + traction, 10%–‐50% surface area | 9 | 12.0 | −3.0 | ||
| All lobes, reticulation + traction, more than 50% surface area | 3 | 12.0 | −9.0 | ||
| Common in all lobes, reticulation + traction + honeycomb more than 50% surface area | 1 | 12.0 | −11.0 |
Abbreviation: CT, computed tomography.
3.4. Comparison of parameters
Compared to the cases with and without deterioration in chest‐CT in the sixth‐month controls, FEV1% and FVC% values of the cases with deterioration in chest‐CT were significantly lower (p < 0.05). Group 3 (Middle‐lower lobe dominant, reticulation + traction, 10%–50% surface area) FVC% and FEV1% values were found to be low (p < 0.05). There was no statistically significant difference between the groups in terms of other variables (p > 0.05) (Table 3).
Table 3.
Average distribution of 6MWD, FVC%, and FEV% values in the sixth month according to chest‐CT findings in patients
| Yes | No | Z | p | |
|---|---|---|---|---|
| Median (25–75) | Median (25–75) | |||
| Group 1: Normal, n: 21 | ||||
| Sixth month 6MWD m | 490 (421–573) | 492 (395–554) | −0.465 | 0.642 |
| Sixth month FVC% | 89 (78–99) | 78.5 (75–86) | −2.323 | 0.020 |
| Sixth month FEV1% | 91 (80‐100) | 82 (77–90) | −2.201 | 0.028 |
| Group 2: Medium‐lower lobe dominant, reticulation 10% surface area, n: 26 | ||||
| Sixth month 6MWD m | 527.5 (–575) | 472.5 (391–557) | −0.637 | 0.524 |
| Sixth month FVC% | 83 (77–87) | 80 (73–91) | −0.069 | 0.945 |
| Sixth month FEV1% | 86 (81–91) | 88 (77–95) | −0.176 | 0.860 |
| Group 3: Medium‐lower lobe dominant, reticulation + traction 10%–50% surface area, n: 9 | ||||
| Sixth month 6MWD m | 443 (380.5–495.5) | 502 (410–575) | −1.522 | 0.128 |
| Sixth month FVC% | 75 (72–76) | 84 (75–91) | −2.449 | 0.014 |
| Sixth month FEV1% | 79 (77–80) | 86.5 (79–95) | −1.979 | 0.048 |
| Group 4: All lobes, reticulation + traction more than 50% surface area n: 3 | ||||
| Sixth month 6MWD m | 461 (413–558) | 492 (403–561) | −0.036 | 0.971 |
| Sixth month FVC% | 64 (64–77.5) | 83 (75–90.5) | −1.210 | 0.226 |
| Sixth month FEV1% | 77 (67.5–84) | 86 (78.5–95) | −1.161 | 0.246 |
Abbreviations:6MWD, 6 min walk distance; CT, computed tomography; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity.
When spirometry and 6MWD correlation with SF‐36 scores were examined in the sixth‐month control; a positive and statistically significant correlation was found between sixth‐month 6MWD values and SF‐36 physical function, physical role difficulties, vitality, pain, overall health, and health change size scores (p < 0.05). Statistically, a significant correlation was found between FVC% values and SF‐36 physical function and overall health perception size scores (p < 0.05). Statistically, a significant correlation was also found between the sixth month FEV1% values and SF‐36 physical function, physical role difficulties, vitality, social functionality, and general health perception parameters (p < 0.05) (Table 4). When the average distribution of SF‐36 parameters was examined, according to the chest‐CT finding in cases, there was no statistically significant difference between the groups (p > 0.05)
Table 4.
Correlating SF‐36 scores with spirometry and 6MWD in sixth month
| 6th month 6MWD m | 6th month FVC % | 6th month FEV1% | ||
|---|---|---|---|---|
| Physical function | r | 0.688 | 0.397 | 0.474 |
| p | <0.001 | 0.002 | <0.001 | |
| Physical role difficulties | r | 0.291 | 0.197 | 0.291 |
| p | 0.030 | 0.134 | 0.026 | |
| Emotional role difficulties | r | 0.229 | 0.150 | 0.208 |
| p | 0.090 | 0.256 | 0.115 | |
| Energy–vitality | r | 0.526 | 0.242 | 0.290 |
| p | <0.001 | 0.064 | 0.026 | |
| Mental health | r | 0.153 | 0.083 | 0.127 |
| p | 0.261 | 0.532 | 0.339 | |
| Social functionality | r | 0.186 | 0.227 | 0.285 |
| p | 0.169 | 0.084 | 0.029 | |
| Pain | r | 0.338 | 0.172 | 0.155 |
| p | 0.011 | 0.194 | 0.241 | |
| General health perception | r | 0.651 | 0.308 | 0.397 |
| p | <0.001 | 0.017 | 0.002 |
Abbreviations: 6MWD, 6 min walk distance; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; SF‐36, short form of health‐related quality of life scale.
4. DISCUSSION
Etiological and clinical features of COVID‐19 are similar to SARS and MERS, 14 so the course and long‐term effects of COVID‐19 can be expected to be similar to those of SARS and MERS. The follow‐up data from recovered SARS patients showed radiological and functional changes including pulmonary interstitial fibrosis. 5 Although many studies have been carried out about early findings of COVID‐19, the potential problems that patients with severe clinics may face in the future are not clearly known. There are few studies showing the medium and long‐term consequences of the disease. In this study, patients who recovered from COVID‐19 severe pneumonia were evaluated 6 months after discharge from the hospital. When we examined the chest CT of patients, from minimal changes to advanced changes including honeycombs were observed. Deterioration in respiratory function of patients and statistically significant deterioration in health‐related quality of life were detected.
COVID‐19 primarily affects the lungs and respiratory tract and can cause respiratory failure. 15 It was observed that 6MWT measurements were low in the long‐term follow‐up of patients with severe SARS and MERS infections and in the examinations of patients who survived ARDS 5 years after the illness.16, 17 Guler et al. and Anastasio et al. have worked with patients with COVID‐19 in the fourth month of their discharge. They found 6MWD lower in groups that suffered the disease more severely.18, 19 In the other study, in which 6‐month results in China were published, researchers grouped patients and found lower 6MWD in groups receiving oxygen support and/or respiratory support. 20 We measured 6MWD in the sixth‐month controls of our patients. Our results were similar to other study results.
The number of studies assessing the respiratory function of patients with COVID‐19 is increasing. In the studies carried out by Van der Sar‐van der Brugge et al., 21 You et al., 22 and Fumagalli et al. 23 spirometric values were found lower than expected. These three studies were carried out 6 weeks after acute infection. Although the 6‐week results are valuable for the follow‐up of patients, we think 6 weeks is not enough to show the long‐term consequences of COVID‐19. In the third‐month study of Zhao et al., 24 which included 55 patients, respiratory function abnormalities in 24,45% of the patients were detected. The medium‐term study of Anastasio et al. 19 revealed lower spirometry results in patients who suffered the disease severity. This study had 379 patients. In our study, spirometry was applied in the sixth‐month controls and 18 of the 59 patients (30.5%) had lower FEV1% values and 27 of the 59 patients (45.8%) had lower FVC % values.
The uncertainty about the disease, the possibility of being re‐infected at any time, the fear of spreading the virus around, and prolonged isolations made it necessary for us to use health‐related quality of life scales in COVID‐19 patients. Chen et al. 25 evaluated 361 patients with the SF‐36 test in the first month of discharge. physical role difficulties (RP), SF, and RE values in patients were lower than those of the normal population and these values were lower in women than in men. Sar et al. used the SF‐36 test in their studies and found physical function (PF), energy vitality (VT), and RP parameters low. 21 Truffaut et al. 26 performed SF‐36 tests at the end of the third month of the patients' recovery after treatment in the intensive care unit and recorded that scores were lower than expected. They also stated that the longer the patients' time in intensive care is, the lower the SF‐36 scores are. A recently published study showed that there is no deterioration in mental health parameters in patients recovering from COVID‐19 but in parameters associated with physical health, there is regression. 19 We used the SF‐36 scale in our study. PF, VT, SF, pain, and general health perception parameters were found lower. These results show us that despite long‐term isolation, there is no significant deterioration in the mental health of patients although decreased physical health is at the forefront of our study. The SF‐36 parameters of our patients, exercise capacity, and spirometric measurements were studied to see if there was a correlation between the scores. Physical health parameters were also found lower in those with low 6MWD and low spirometry measurements.
As a result of our past and present experiences with coronavirus infections, pulmonary fibrosis findings of different shapes and proportions can be expected in chest CT after COVID‐19. We performed Chest‐CT in the sixth‐month control. Han et al., 27 showed the development of pulmonary fibrosis‐like changes in the chest CT of approximately one‐third of severe COVID‐19 patients after 6 months of their discharge. Guler et al. identified lesions of different proportions from weak mosaic patterns to honeycombs in their study in which they included 113 people. They rarely observed pulmonary fibrosis and stated that lesions were more pronounced in those who suffered the disease more severely. 18 Zhao et al. 24 and Truffaut et al. 26 studied patients 3 months after discharge. Both studies showed some distortions in CT findings and the researchers recommended long‐term follow‐up. In our study, while chest CT was normal in 21 patients (35%), 39 of the patients (65%) had chest CT findings with a spectrum from minimal changes to advanced changes including honeycombs. CT changes were minimal in 26 of the 39 patients with abnormal CT findings (Group 1). In Group 2 patients (nine out of 39 patients), interstitial changes were predominant in the middle and lower lobes and involvement was in the range of 10%–50% of the total lung area. Three patients with more than 50% surface involvement and one patient with honeycomb were detected. Considerable fibrotic changes were seen in Groups 3 and 4 patients (in four patients). When the CT findings of the patients were correlated with spirometry and 6MWD findings, and FVC% and FEV1% values of Groups 3 and 4 were found lower than expected.
5. LIMITATIONS
This study has some limitations. Firstly, spirometric measurements, predisease data of the 6MWD, and SF‐36 scales of patients included in the study were not available. Since the basal data results are unknown, the lower results detected may not be directly attributed to COVID‐19. Previously undiagnosed pulmonary and other system diseases may affect our results. The other limitation is that this study could not cover all the severe COVID‐19 pneumonia cases we followed. Due to the continuing pandemic, some of the patients refused to come to health facilities due to the risk of transmission and isolation rules. A group of patients also had temporarily migrated to rural areas from Istanbul, the country's most crowded city.
6. CONCLUSION
The results of our sixth‐month controls obtained from spirometric measurements, 6MWD, SF‐36, and radiological evaluations of patients recovering from severe COVID‐19 are in accordance with the studies that have assessed medium‐term effects of COVID‐19. A decrease in spirometric measurements of participants in the sixth month was detected and these measurements were correlated with physical health parameters of SF‐36. Although radiological changes were detected in 39 of the patients, the lesions found in 13 of them are more prominent. Considerable fibrotic changes were detected in 4 of the patients while 26 of the patients had minimal changes. It is yet unknown whether or not the changes detected in the spirometry and observed in chest CT can be fully recovered. Because of that, we need to continue to work with the first‐year controls and continue to search for answers to these questions.
CONFLICT OF INTERESTS
The authors declare that there is no conflict of interest that could be perceived as damaging to the impartiality of the reported research.
AUTHOR CONTRIBUTIONS
Design of the study: Bardakci Mustafa Ilteris, Ozturk Esin Nagihan, and Yildiz Sevgi Dilek. Data collection: Ozturk Esin Nagihan, Bardakci Mustafa Ilteris, and Ozkarafakılı Mufide Arzu. Interpretation and analysis of the data: Ozturk Esin Nagihan, Bardakci Mustafa Ilteris, Ozkarafakılı Mufide Arzu, Ozkurt Huseyin, Yildiz Sevgi Dilek, and Yanç Ugur. All authors reviewed the results and approved the final version of the manuscript.
Bardakci MI, Ozturk EN, Ozkarafakili MA, Ozkurt H, Yanc U, Yildiz Sevgi D. Evaluation of long‐term radiological findings, pulmonary functions, and health‐related quality of life in survivors of severe COVID‐19. J Med Virol. 2021;93:5574‐5581. 10.1002/jmv.27101
Contributor Information
Mustafa Ilteris Bardakci, Email: milterisbar@hotmail.com.
Esin Nagihan Ozturk, Email: esin_n_66@hotmail.com.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data is not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Republic of Turkey Ministry of Health General Directorate of Public Health . Covid‐19 (SARS‐CoV‐2 infection) Guideline. 2020.
- 2. Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID‐19). JAMA. 2020;324(8):782‐793. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization . Coronavirus disease 2019 (COVID‐19) situation report. 2021. https://covid19.who.int/. Accessed February 18, 2021.
- 4. Cömert SŞ, Kıral N. COVID‐19 Pnömonisinin Radyolojik Bulguları. Southern Clinics of Istanbul Eurasia. 2020. [Google Scholar]
- 5. Hui DS, Joynt GM, Wong KT, et al. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax. 2005;60(5):401‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ong KC, Ng AK, Lee LU, et al. Pulmonary function and exercise capacity in survivors of severe acute respiratory syndrome. Eur Respir J. 2004;24(3):436‐442. [DOI] [PubMed] [Google Scholar]
- 7. Lau HMC, Lee EWC, Wong CNC, Ng GYF, Jones AYM, Hui DSC. The impact of severe acute respiratory syndrome on the physical profile and quality of life. Arch Phys Med Rehabil. 2005;86(6):1134‐1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Weisman IM, Zeballos RJ. Clinical exercise testing. Clin Chest Med. 2001;22:679‐701. [DOI] [PubMed] [Google Scholar]
- 9. American Thoracic Society . Statement: guidelines for the six‐minute walk test. Am J Respir Crit Care Med. 2002;166:111‐117. [DOI] [PubMed] [Google Scholar]
- 10. American Thoracic Society . Standardization of spirometry: 1994 update. Am J Respir Crit Care Med. 1995;152:1107‐1136. [DOI] [PubMed] [Google Scholar]
- 11. Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26:511‐522. [DOI] [PubMed] [Google Scholar]
- 12. Caballero T, Prior N. Burden of illness and quality‐of‐life measures in angioedema conditions. Immunol Allergy Clin North Am. 2017;37:597‐616. [DOI] [PubMed] [Google Scholar]
- 13. Kocyigit H, Aydemir O, Fisek G, Olmez N, Memis A. Validity and reliability of Turkish version of Short form 36: a study of a patients with romatoid disorder. İlaç ve Tedavi Dergisi. 1999;12:102‐106. [Google Scholar]
- 14. Hosseiny M, Kooraki S, Gholamrezanezhad A, Reddy S, Myers L. Radiology perspective of coronavirus disease 2019 (COVID‐19): lessons from severe acute respiratory syndrome and Middle East respiratory syndrome. AJR. 2020;214:1078‐1082. [DOI] [PubMed] [Google Scholar]
- 15. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ahmed H, Patel K, Greenwood DC, et al. Long term clinical outcames in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: a systematic rewiew and meta‐analysis. J Rehebil Med. 2020;52:jrm00063. [DOI] [PubMed] [Google Scholar]
- 17. Herridge MS, Tansey CM, Matté A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293‐1304. [DOI] [PubMed] [Google Scholar]
- 18. Guler SA, Ebner L, Aubry‐Beigelman C, et al. Pulmonary function and radiological features four months after COVID‐19: first results from the national prospective observational Swiss COVID‐19 lung study. Eur Respir J. 2021;8:2003690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Anastasio F, Barbuto S, Scarnecchia E, et al. Medium‐term impact of COVID‐19 on pulmonary function, functional capacity and quality of life. Eur Respir J. 2021;11:2004015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang C, Huang L, Wang Y, et al. 6‐month consequences of COVID‐19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Van der Sar‐van der Brugge S, Talman S, Boonman‐de Winter L, et al. Pulmonary function and health‐related quality of life after COVID‐19 pneumonia. Respir Med. 2021;176:106272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. You J, Zhang L, Ni‐Jia‐Ti M, et al. Anormal pulmonary function and residual CT abnormalities in rehabilitating COVID‐19 patients after discharge. Infection. 2020;81:81‐e152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fumagalli A, Misuraca C, Bianchi A, et al. Pulmonary function in patients surviving to COVID‐19 pneumonia. Infection. 2020:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhao YM, Shang YM, Song WB, et al. Follow‐up study of the pulmonary function and related physiological characteristics of COVID‐19 survivor three months after recovery. Eclin Med. 2020;25:100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen KY, Li T, Gong FH, Zhang JS, Xk Li. Predictors of health related quality of life and influencing factors for COVID‐19 patients, a follow‐up at one month. Front Psychiatr. 2020;11:668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Truffaut L, Demey L, Bruyneel AV, et al. Post‐discharge critical COVID‐19 lung function related to severity of radiologic lung involvement at admission. Respiratory Res. 2021;29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Han X, Fan Y, Alwalid O, et al. Six‐month follow‐up chest CT findings after sever COVID‐19 pneumonia. Radiology. 2021:radiol2021203153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data is not publicly available due to privacy or ethical restrictions.


