Abstract
This study is to estimate in‐hospital mortality in severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) patients stratified by hemoglobin (Hb) level. Patients were stratified according to hemoglobin level into two groups, that is, Hb <100 g/L and Hb >100 g/L. A total of 6931 patients were included. Of these, 6377 (92%) patients had hemoglobin levels >100 g/L. The mean age was 44 ± 17 years, and 66% of the patients were males. The median length of overall hospital stay was 13 days [2; 31]. The remaining 554 (8%) patients had a hemoglobin level <100 g/L. Overall mortality was 176 patients (2.54%) but was significantly higher in the group with hemoglobin levels <100 g/L (124, 22.4%) than in the group with hemoglobin levels >100 g/L (52, 0.82%). Risk factors associated with increased mortality were determined by multivariate analysis. The Kaplan‐Meier survival analysis showed hemoglobin as a predictor of mortality. Cox proportional hazards regression coefficients for hemoglobin for the HB ≤ 100 category of hemoglobin were significant, B = 2.79, SE = 0.17, and HR = 16.34, p < 0.001. Multivariate logistic regression showed Hb < 100 g/L had a higher cumulative all‐cause in‐hospital mortality (22.4% vs. 0.8%; adjusted odds ratio [aOR], 0.33; 95% [CI]: [0.20–0.55]; p < 0.001). In this study, hemoglobin levels <100 g/L were found to be an independent predictor of in‐hospital mortality.
Keywords: anemia, COVID‐19, hemoglobin, in‐hospital mortality, SARS‐CoV‐2
1. INTRODUCTION
In severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection, the level of serum hemoglobin remains a predictor of adverse events. Low hemoglobin (Hb) levels in SARS‐CoV‐2 are associated with the development of respiratory failure. The requirement for mechanical ventilation was observed to be higher with low Hb levels [1]. Low hemoglobin levels in the setting of SARS‐CoV‐2 pneumonia may exacerbate O2 desaturation and are a predictor of poor prognosis [2]. Disseminated intravascular coagulation‐related low Hb levels are seen in SARS‐CoV‐2 [3]. Many studies have shown that ventilator‐dependent critically ill SARS‐CoV‐2 patients have low Hb levels [4]. Anemia is an independent predictor of severe SARS‐CoV‐2 infection as well as overall mortality [5].
2. METHODS
Participants and the study design comprised a total of 6931 confirmed COVID‐19 patients, both Kuwaitis and non‐Kuwaitis above the age of 18, who were enrolled in this retrospective cohort study between February 26 and September 8, 2020. The data were collected from electronic medical records from two tertiary care hospitals in Kuwait, Jaber Al‐Ahmed Hospital and Al Adan General Hospital.
SARS‐CoV‐2 infection was confirmed by a positive Reverse Transcription Polymerase Chain Reaction (RT‐PCR) swab from the nasopharynx. The treatment and care of all patients were standardized according to protocol by the Ministry of Health in Kuwait. The standing committee for coordination of health and medical research at the Ministry of Health in Kuwait approved the protocol and waived the requirement of informed consent (Institutional review board number 2020/1422). Patients were stratified according to hemoglobin levels into two groups, that is, Hb <100 g/L and Hb >100 g/L. “The intended outcome” was a COVID‐19‐related death based on ICD 10 code U07.1. The following variables were assessed: sociodemographic characteristics, underlying comorbidities, clinical presentations, laboratory results, and duration of ICU and in‐hospital stays. An electronic case‐record form (CRF) was used for data entry. The data from medical records from each participating site were submitted to an electronic CRF by qualified doctors.
3. STATISTICAL ANALYSIS
Descriptive statistics are used to present the data. Categorical variables are summarized as frequencies and percentages and were analyzed using Pearson's χ2 test. Continuous variables are summarized using the mean and standard deviation. To evaluate the impact of Hb level (Hb <100 g/L and Hb >100 g/L) on all‐cause mortality, we used multivariable logistic regression. The odds ratios (ORs) for in‐hospital all‐cause mortality status were adjusted for sex, age, white blood cells, platelet counts, neutrophils, and hemoglobin levels.
A Cox proportional hazards model was performed to determine whether hemoglobin had a significant effect on the hazard of mortality. The live category of mortality was used to indicate survival while the dead category was used to represent a hazard event. The level of significance was set at p < 0.05 a priori. Statistical analyses were conducted using R statistical packages [6] and SPSS version 27 (SPSS, Chicago, IL, USA).
4. RESULTS
A total of 6931 SARS‐CoV‐2‐positive patients were included in the study; the mean patient age was 44 ± 17 years, and 66% of the patients were male. We observed that 6377 (92%) of the patients had hemoglobin levels >100 g/L. The median length of overall hospital stay was 13 days [2; 31]. In this study, 554 (8%) patients had a hemoglobin level <100 g/L. The overall mortality was 176 patients (2.54%), and the mortality rate was higher in the group with hemoglobin levels <100 g/L (124, 22.4%) than in the group with hemoglobin levels >100 g/L (52, 0.82%). Risk factors associated with increased mortality were established by multivariate logistic regression. The median duration of hospitalization was 13.0 (2.00, 31.0) days. The average length of hospital stay was higher in the group with lower levels of hemoglobin (<100 g/L), 16.5 (2.00, 39.5) days, while in the group with higher levels of hemoglobin (greater than or equal to 100 g/L), this figure was 13.0 (2.00, 29.0) days (p < 0.001). The overall cumulative all‐cause in‐hospital mortality was 2.54% (n = 176). The mortality rate was higher in the group with lower hemoglobin levels (124, 22.4%) than in the group with higher hemoglobin levels 100 g/L (52, 0.82%), (p < 0.001) (Table 1). Individuals with low hemoglobin levels < 100 g/L had a higher cumulative all‐cause in‐hospital mortality than those with high hemoglobin levels > 100 g/L (22.4% vs. 0.8%; adjusted OR (aOR), 0.33; 95% confidence interval (CI): [0.20–0.55]; p < 0.001). Age had no significant impact among the groups with regard to the all‐cause cumulative in‐hospital mortality (p = 0.809). Male sex had a significant impact on cumulative all‐cause in‐hospital mortality (6.8% vs. 2.3%; aOR, 2.63; 95% confidence interval (CI): [1.49, 4.80]; p < 0.001) (Table 2).
TABLE 1.
Demographics and clinical characteristics of the cohort stratified by hemoglobin (HB) levels among patients admitted with SARS‐CoV 2
| Demographics and clinical characteristics | [ALL] N = 6931 | HB < = 100 N = 554 | HB > 100 N = 6377 | p‐value | N |
|---|---|---|---|---|---|
| Age, mean (SD), years | 44.1 ± 17.2 | 55.1 ± 14.6 | 42.6 ± 16.9 | <0.001 | 3360 |
| Male, n (%) | 2221 (66.1%) | 239 (61.0%) | 1982 (66.8%) | 0.026 | 3360 |
| ICU admission (days), IQR | 0.00 (0.00; 4.00) | 1.00 (0.00; 12.0) | 0.00 (0.00; 3.00) | <0.001 | 3360 |
| Admission to discharge (number of days in hospital), IQR | 13.0 (2.00; 31.0) | 16.5 (2.00; 39.5) | 13.0 (2.00; 29.0) | <0.001 | 2900 |
| ICU to discharge (days), IQR | 9.00 (0.00; 38.6) | 8.00 (0.00; 39.0) | 9.00 (0.00; 35.8) | 0.459 | 416 |
| Mortality, n (%) | 176 (2.54%) | 124 (22.4%) | 52 (0.82%) | <0.001 | 6931 |
Abbreviations: IQR, interquartile range; SD, standard deviation.
Percentages might not add up to 100% due to rounding off.
TABLE 2.
Multivariate logistic regression analysis of in‐hospital death in the overall study cohort
| In‐hospital mortality | Alive | Dead | Univariate a OR (95% CI, a p‐value) | Multivariate logistic regression a OR (95% CI, a p‐value) | |
|---|---|---|---|---|---|
| Gender | Female | 1113 (97.7%) | 26 (2.3%) | – | – |
| Male | 2071 (93.2%) | 150 (6.8%) | 3.10 (2.07‐4.83, p < 0.001) | 2.63 (1.49‐4.80, p = 0.001) | |
| Age | Mean (SD) | 43.4 ± 17.1 | 56.6 ± 12.1 | 1.05 (1.04‐1.06, p < 0.001) | 1.00 (0.99‐1.02, p = 0.809) |
| WBC | Mean (SD) | 7.4 ± 3.2 | 18.2 ± 10.0 | 1.28 (1.25‐1.32, p < 0.001) | 1.08 (1.04‐1.11, p < 0.001) |
| NEU | Mean (SD) | 55.2 ± 13.6 | 86.8 ± 8.4 | 1.23 (1.20‐1.26, p < 0.001) | 1.14 (1.11‐1.17, p < 0.001) |
| HB | HB < = 100 | 430 (77.6%) | 124 (22.4%) | – | – |
| HB > 100 | 6325 (99.2%) | 52 (0.8%) | 0.03 (0.02‐0.04, p < 0.001) | 0.33 (0.20‐0.55, p < 0.001) | |
| PLT | Mean (SD) | 288.3 ± 102.5 | 217.2 ± 151.1 | 0.99 (0.99‐0.99, p < 0.001) | 1.00 (0.99‐1.00, p < 0.001) |
Number in data frame = 6931, number in model = 3344, missing = 3587, AIC = 534.7, C‐statistic = 0.982, H&L = chi‐sq(8) 6.30 (p = 0.614).
Abbreviation: CI, confidence interval.
OR, adjusted odds ratio; a P‐value, adjusted p‐value.
Multivariable analyses were conducted using logistic regression models utilizing the simultaneous method. The models were adjusted for gender, age, WBC (white blood cells); NEU (neutrophils); HB (heamoglobin); PLT (platelet count).
Percents are row percentages.
The Kaplan‐Meier survival probability plot shows that lower hemoglobin levels were associated with higher mortality levels. The results of the model were significant based on an alpha of 0.05, LL = 295.50, and df = 1, p < 0.001, indicating that hemoglobin was able to predict the hazard of mortality adequately. Kaplan‐Meier survival probability plots are included for hemoglobin. Each plot represents the survival probabilities for different groups over time. Cox proportional hazards regression coefficients for hemoglobin for the HB ≤ 100 category of hemoglobin were significant, B = 2.79, SE = 0.17, and HR = 16.34, p < 0.001, indicating that at any particular time, an observation in the HB ≤ 100 category will have a hazard that is 16.34 times as high as the HB > 100 category. The event B is HB <100 g/dL (Figure 1).
FIGURE 1.
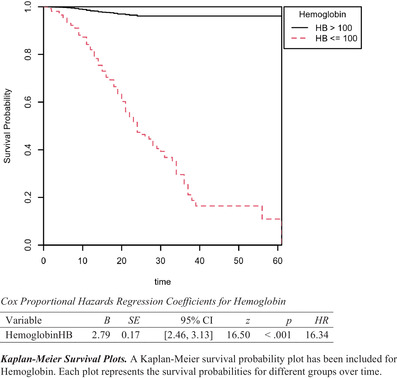
Kaplan‐Meier survival plot of mortality grouped by hemoglobin
5. DISCUSSION
The observations from this multicenter observational study showed that SARS‐CoV‐2 patients with lower hemoglobin levels are at higher risk of in‐hospital mortality than those with higher levels of >100 g/L. In this study, lower hemoglobin levels were found to be an independent predictor of in‐hospital mortality. Male sex had a significant impact on cumulative all‐cause in‐hospital mortality among the group with low hemoglobin levels. Among the two groups, age showed no significant impact on all‐cause cumulative in‐hospital mortality.
The incidence of anemia is reported in all types of pneumonia and ranges between 7% and 15% [7, 8, 9]. In SARS and SARS‐CoV‐2, the incidence of anemia ranges between 16% and 53% [5, 10]. Lower hemoglobin levels can lead to a decrease in tissue oxygen delivery, as the Hb concentration is interrelated with arterial oxygen content [11, 12]. SARS‐CoV‐2‐related infection may also alter iron metabolism and reduced iron availability [13]. Virus‐induced intestinal mucosal erosion in SARS‐CoV‐2 and associated bleeding were reported [14, 15]. Hospitalized elderly SARS‐CoV‐2 patients were found to have lower levels of hemoglobin [16]. Additionally, the incidence of anemia was found to be markedly higher in critically ill SARS‐CoV‐2 patients admitted to the intensive care unit [17].
Anemia in SARS‐CoV‐2 infection may be related to cytokine‐induced inhibition of erythropoietin formation and can lead to an increased requirement for mechanical ventilation in critically ill patients [18, 19]. A systematic review of 63 studies showed that severe SARS‐CoV‐2 infection is associated with lower hemoglobin levels [20]. The association of anemia with respiratory diseases was previously proven to be a predictor of poor outcome and increased mortality [8, 21, 22]. A study by Fan et al showed that 1.6% of SARS‐CoV‐2 patients admitted to the intensive care unit underwent blood transfusion for anemia correction [23]. The majority of the studies proved anemia as an independent predictor of mortality [24, 25].
Our study was focused on mortality, and therefore we did not report other outcome variables. We did not use the cutoff values as defined for anemia. Mild, moderate, and severe anemia classifications were not followed. There were many missing values when analyzing the data.
In conclusion of our study, SARS‐CoV‐2 patients with hemoglobin <100 g/L have a 67% higher risk of in‐hospital mortality than those with hemoglobin >100 g/L. In this study, hemoglobin levels <100 g/L were found to be an independent predictor of in‐hospital mortality. The length of hospital stay was longer with hemoglobin <100 g/L. Male sex had a significant impact on cumulative all‐cause in‐hospital mortality.
AKNOWLEDGMENTS
Authors would like to thank Prof. Dr. Peter A Brady.
CONFLICT OF INTEREST
No conflict of interest exists for any author on this manuscript.
AUTHOR CONTRIBUTIONS
Mohammed Al‐Jarallah participated in analysis and manuscript preparation. Rajesh Rajan participated in data analysis and manuscript preparation. Ahmad Al Saber and Jiazhu Pan did the statistical analysis as well as manuscript review. All authors had access to data and take responsibility for the integrity of data and the accuracy of data analysis. All authors have read and approved the manuscript.
ETHICS APPROVAL STATEMENT
This study was approved by the ethics committee and Ministry of Health Kuwait.
PATIENT CONSENT STATEMENT
Patient consented was not mandated for this retrospective observational study. Permission to reproduce material from other sources: No material from other sources is included in this study.
CLINICAL TRIAL REGISTRATION
This study was not a clinical trial.
NOVELTY STATEMENT
This study mainly focused on the clinical significance of hemoglobin levels while treating SAR‐CoV‐2 infection. These results will help the clinicians to categorize the patients as the low hemoglobin levels were proven to be an independent predictor in‐hospital mortality. This will help the clinicians for early initiation of critical care strategies for such patients and thereby may reduce mortality and related complications to an extent.
Al‐Jarallah M, Rajan R, Saber AAl, Pan J, Al‐Sultan AT, Abdelnaby H, et al. In‐hospital mortality in SARS‐CoV‐2 stratified by hemoglobin levels: A retrospective study. eJHaem. 2021;2:335–339. 10.1002/jha2.195
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1. Anai M, Akaike K, Iwagoe H, Akasaka T, Higuchi T, Miyazaki A, et al., Decrease in hemoglobin level predicts increased risk for severe respiratory failure in COVID‐19 patients with pneumonia, Respir Investig. 2021;59:187–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Algassim AA, Elghazaly AA, Alnahdi AS, Mohammed‐Rahim OM, Alanazi AG, Aldhuwayhi NA, et al. Prognostic significance of hemoglobin level and autoimmune hemolytic anemia in SARS‐CoV‐2 infection. Ann Hematol. 2021;100(1):37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guan WJ, Ni Z‐Y, Hu Y, Liang W‐H, Ou C‐Q, He J‐X, et al. China medical treatment expert group for Covid‐19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tao Z, Xu J, Chen W, Yang Z, Xu X, Liu L, et al. Anemia is associated with severe illness in COVID‐19: A retrospective cohort study. J Med Virol. 2021;93:1478–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thomas L, Reyes EM. Tutorial: survival estimation for Cox regression models with time‐varying coefficients using SAS and R. J Stat Softw. 2014;61.c1:1–23. [Google Scholar]
- 7. Zhang JJ, Dong X, Cao YY, Yuan Y‐D, Yang Y‐B, Yan Y‐Q, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020;75(7):1730–41. [DOI] [PubMed] [Google Scholar]
- 8. Reade MC, Weissfeld L, Angus DC, Kellum JA, Milbrandt EB. The prevalence of anemia and its association with 90‐day mortality in hospitalized community‐acquired pneumonia. BMC Pulm Med. 2010;10:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhou F, Yu T, Du RH, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Choi KW, Chau TN, Tsang O, Tso E, Chiu MC, Tong WL, et al. Outcomes and prognostic factors in 267 patients with severe acute respiratory syndrome in Hong Kong. Ann Intern Med. 2003;139:715–23. [DOI] [PubMed] [Google Scholar]
- 11. Dunn JO, Mythen MG, Grocott MP. Physiology of oxygen transport. BJA Educ. 2016;16(10):341–8 [Google Scholar]
- 12. Mistry N, Mazer CD, Sled JG, Lazarus AH, Cahill LS, Solish M, et al. Red blood cell antibody‐induced anemia causes differential degrees of tissue hypoxia in kidney and brain. Am J Physiol‐Reg I. 2018;314:R611–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wessling‐Resnick M. Crossing the iron gate: why and how transferrin receptors mediate viral entry. Annu Rev Nutr. 2018;38(1):431–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lin L, Jiang X, Zhang Z, Huang S, Zhang Z, Fang Z, et al. Gastrointestinal symptoms of 95 cases with SARS‐CoV‐2 infection. Gut. 2020;69(6):997–1001. [DOI] [PubMed] [Google Scholar]
- 15. Melazzini F, Lenti MV, Mauro A, De Grazia F, Di Sabatino A. Peptic ulcer disease as a common cause of bleeding in patients with coronavirus disease 2019. Am J Gastroenterol. 2020;115:1139–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang L, He W, Yu X, Hu D, Bao M, Liu H, et al. Coronavirus disease 2019 in elderly patients: Characteristics and prognostic factors based on 4‐week follow‐up. J Infect. 2020;80(6):639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fan BE, Chong VCL, Chan SSW, Lim GH, Lim KGE, Tan GB, et al. Hematologic parameters in patients with COVID‐19 infection. Am J Hematol. 2020;95(6):E131–4. [DOI] [PubMed] [Google Scholar]
- 18. Weiss G, Ganz T, Goodnough LT. Anemia of inflammation. Blood. 2019;133(1):40–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taneri PE, Gómez‐Ochoa SA, Llanaj E, Raguindin PF, Rojas LZ, Roa‐Díaz ZM, et al. Anemia and iron metabolism in COVID‐19: a systematic review and meta‐analysis. Eur J Epidemiol. 2020;35(8):763–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Doshi SM, Rueda AM, Corrales‐Medina VF, Musher DM. Anemia and community‐acquired pneumococcal pneumonia. Infection. 2011;39:379–83. [DOI] [PubMed] [Google Scholar]
- 22. Lazarian G, Quinquenel A, Bellal M, Siavellis J, Jacquy C, Re D, et al. Autoimmune haemolytic anaemia associated with COVID‐19 infection. Br J Haematol. 2020;190:29–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fan BE, Ong KH, Chan SS, Young BE, Chong VC, Chen SP, et al. Blood and blood product use during COVID‐19 infection. Am J Hematol. 2020;95(7):E158–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cen Y, Chen X, Shen Y, Zhang X‐H, Lei Y, Xu C, et al. Risk factors for disease progression in patients with mild to moderate coronavirus disease 2019‐a multi‐centre observational study. Clin Microbiol Infect. 2020;26:P1242–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu Z, Sun R, Li J, Cheng W, Li L. Relations of anemia with the all‐cause mortality and cardiovascular mortality in general population: a meta‐analysis. Am J Med Sci. 2019;358(3):191–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


