Abstract
The kinetics of IgG antibodies after coronavirus disease 2019 (COVID‐19) remain poorly understood. We investigated factors influencing severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) IgG antibody levels and time to seronegativation during the follow‐up of severe and critically ill patients. We retrospectively reviewed serological evaluations drawn during the follow‐up of severe or critical laboratory‐proven COVID‐19 patients hospitalized at a large academic hospital. Specific IgG titers were measured using a chemiluminescent assay targeting anti‐spike and anti‐nucleocapsid protein IgG. The influence of time, demographic factors, clinical and paraclinical characteristics, and COVID‐19 therapeutics on IgG levels were assessed through linear regression using a mixed‐effect model, and delay until IgG negativation through a Weibull regression model. The cohort included 116 patients with a total of 154 IgG measurements drawn at a median of 79 days after diagnosis. IgG antibodies were increased with age (p = 0.005) and decreased significantly over time (p = 0.0002). Using elapsed time and age as covariates, we demonstrated higher IgG levels in patients with a higher body mass index (BMI) (p = 0.0026) and lower IgG levels in immunocompromised patients (p = 0.032). A high BMI was further found to delay and immunodeficiency to hasten significantly seronegativation, whereas no significant effect was observed with corticosteroids. These data highlight the waning over time of IgG antibodies after severe or critical COVID‐19. Age, BMI, and immunosuppression also appear to influence the IgG kinetics, while short‐term corticotherapy does not. Those data improve the understanding of SARS‐CoV‐2 serology while further research should determine the determinants of long‐term seroprotection.
Keywords: corticosteroids, COVID‐19, IgG, kinetics, SARS‐CoV‐2, serology
Highlights
Anti‐SARS‐CoV‐2 IgG antibody levels measured during follow‐up of severe and critical COVID‐19 were studied.
We analyzed the influence of time, demographics, clinical and paraclinical characteristics and COVID‐19 treatments on specific IgG levels and time to seronegativation.
Time and immunodepression were associated to reduced IgG levels at follow‐up, while age and BMI were associated to increased levels.
Corticosteroids given as short‐term treatment for COVID‐19 did not show influence. BMI and immunodepression were respectively associated with decreased and increased rates of seronegativation.
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has emerged as a major human pathogen. Coronavirus disease 2019 (COVID‐19) caused by SARS‐CoV‐2 is primarily diagnosed through real‐time reverse‐transcription polymerase chain reaction (RT‐PCR) testing. 1 Numerous serological tests have been developed and commercialized, relying mostly on enzyme‐linked immunosorbent assay (ELISA) and chemiluminescence assays (CLIA) to detect IgA, IgM, or IgG against SARS‐CoV‐2 antigens (spike and nucleocapsid glycoproteins). The detection of antibodies can be used as a diagnostic tool at later timepoints in the disease course, when the sensitivity of RT‐PCR decreases. 2 As IgG antibodies are expected to remain detectable after infection resolution, 3 serological testing is also used in seroprevalence studies, to diagnose past exposure to SARS‐CoV‐2, including in pauci or asymptomatic patients 4 although it has been stressed that the magnitude of the antibody response appears to be associated with disease severity. 5 Serological testing also carries the theoretical possibility to inform individuals about their immune protection against reinfections. Indeed, the presence of detectable antibodies after COVID‐19 has been shown to correlate with immune protection for at least 6 months in a large cohort of healthcare workers. 6
However, antibody kinetics remains incompletely understood. While most patients elicit a robust humoral response with detectable anti‐SARS‐CoV‐2 antibodies, those antibodies will quickly become indetectable in a significant subset of patients.7, 8 Factors influencing the persistence of high antibody titers following COVID‐19 remain poorly identified. In addition, whether treatment of COVID‐19 with immunosuppressive agents such as dexamethasone influences antibody levels and kinetic has not been assessed.
This study aims (i) to describe the kinetics of serum IgG antibodies and (ii) to investigate the determinants underlying kinetics and IgG negativation at the convalescent phase of COVID‐19 in a cohort of hospitalized patients with severe and/or critical disease.
2. MATERIALS AND METHODS
2.1. Study design and patients
Patients with RT‐PCR‐proven COVID‐19 and who reached at least four on the WHO ordinal scale (i.e., patients requiring oxygen supplementation) were identified within our database of COVID‐19 patients hospitalized in Cliniques universitaires Saint‐Luc, an academic hospital of 1000 beds in Brussels, Belgium. We further selected for analysis the patients with at least one serological assessment drawn after the 14th day of diagnosis and before November 5, the date of analysis. We excluded patients who received convalescent plasma therapy.
All patients were treated according to Belgian and local guidelines. Besides best supportive care, and in accordance with ongoing Belgian guidelines at that time, 9 most patients were treated with hydroxychloroquine until the end of May 2020. Although not recommended before the publication of Recovery trial results, 10 corticosteroids had been used earlier by some clinicians in case of progressing respiratory failure.
2.2. Data collection
The following data were studied: demographics, clinical characteristics (signs and symptoms, comorbidities, and usual treatment), biological and imaging results, treatments administered, and outcome.
The local ethics committee approved our database (registration number 2020/06AVR/201) and waived the requirement for informed consent based on the retrospective observational design of the study.
2.3. Definitions
With the aim of providing the most objective assessment of disease onset, the date of diagnosis was defined as the date of the first positive SARS‐CoV‐2 RT‐PCR.
Immunosuppression was defined as any condition or treatment known to interfere with humoral or cellular immunity. Before running statistical analysis, we decided not to consider as immunosuppressed two HIV‐positive patients with high CD4 cells count (650 and 1120 cells/mm3, respectively) and long‐term viral replication control.
2.4. SARS‐CoV‐2 serological testing
The detection of SARS‐CoV‐2 antibodies in human serum was performed using the MAGLUMI‐800 CLIA system (Snibe Diagnostic). This system allows the detection of both IgM and IgG binding SARS‐CoV‐2 spike and nucleocapsid proteins. MAGLUMI assay has received CE marking and Food and Drug Administration's Emergency Use Authorization.
According to the manufacturer, the calculated sensitivities and specificities of IgG were respectively 91.2% and 97.3%. This high sensitivity was recently confirmed by Soleimani et al. 11 who reported a sensitivity of more than 95% by the 18th day after symptom onset. A result was considered positive if the index is more than or equal to 1.00 AU/ml, according to the manufacturer's recommendations.
2.5. Statistical analysis
Results are presented as means ± SD or median (interquartile range [IQR]) for continuous variables and as numbers and proportions for categorical variables.
Mixed effect model linear regressions were performed to assess the effect of independent variables on IgG titers. After evaluation of their effect on IgG titers, the time lapse from diagnosis to IgG measurements (Model 1) and the time lapse in combination to the subject's age (Model 2) were added as model covariates. To account for non‐normal IgG titer distribution, IgG titers were normalized by Box‐Cox transformation. As we cannot a priori assume a linear relationship between IgG titers and time, time was expressed as a categorical variable by dividing the sample into five successive categories with equivalent numbers of patients: Day 14–64 (Period 1), Day 65–75 (Period 2), Day 76–85 (Period 3), Day 86–105 (Period 4), and Day 106–221 (Period 5).
For therapeutic interventions (corticosteroids, high‐flow nasal cannula [HFNC], and mechanical ventilation [MV]), we completed the analysis with a propensity score: probability of receiving the intervention was calculated by logistic regression as a function of date of hospitalization, sex, age, BMI, renal function (inverse of serum creatinine), presence of fever, cough, dyspnea, hypertension, diabetes and immunodeficiency; the relationship between IgG titer and therapeutic intervention was studied and regressions on therapeutic interventions were weighted by the inverse of the probability of receiving the intervention.
We studied the delay until IgG negativation using a Weibull regression model; when the last available IgG measurement remained positive, this test date was considered as a censor; when the first available test was already negative, we assumed that the last positive date was Day 21. Hazard ratios (HR) were calculated with age as a covariate.
To minimize the bias related to the asymmetry of the independent variables, we used a sandwich‐variance for regressions and calculated degrees of freedom according to Bell and McCaffrey. 12 All p values were two‐sided and p values ≤ 0.05 were considered significant. Statistical analyses were performed using SPSS v27 (IBM).
3. RESULTS
We included a total of 154 IgG measurements performed on 116 patients (Figure 1). The main baseline characteristics of our cohort are shown in Table 1. Briefly, the median age (IQR) was 58 (51–65) years, with a majority (70/116, 60%) of males. About 88 patients (76%) had at least one comorbidity, with 55 (47%) suffering from cardiovascular disease, 39 (34%) from obesity, and 22 (19%) being immunocompromised.
Figure 1.
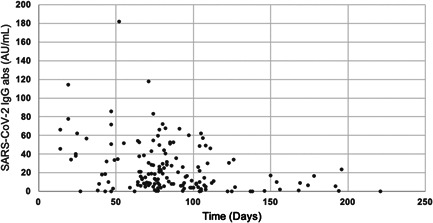
Distribution of IgG antibodies over time after COVID‐19. abs, antibodies; COVID‐19, coronavirus disease 2019; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
Table 1.
Characteristics of the 116 patients with serological assessment included in the cohort
| Demographics and comorbidities | Whole cohort (n = 116) |
|---|---|
| Age, mean (SD), years | 58.5 (11.9) |
| Male gender – no. (%) | 70 (60.3) |
| Ethnicity – no. (%) | |
| Caucasian | 68 (58.6) |
| Sub‐Saharan African | 21 (18.1) |
| Other | 23 (19.8) |
| Unknown | 4 (3.4) |
| BMI, mean (SD), kg/m2 | 28.6 (5.0) |
| Obesity (BMI ≥ 30) – no. (%) | 39 (33.6) |
| Cardiovascular disease – no. (%) | 55 (47.4) |
| Chronic pulmonary disease – no. (%) | 18 (15.5) |
| Hypertension – no. (%) | 56 (48.3) |
| Diabetes – no. (%) | 19 (16.4) |
| Chronic kidney disease, Stage 4 or 5 – no. (%) | 7 (6.0) |
| Immunosuppression – no. (%) | 22 (18.9) |
| Solid organ transplant recipient | 8 (6.9) |
| Auto‐immune diseasea | 7 (5.2) |
| Ongoing treatment for cancerb | 6 (6.0) |
| Hypogammaglobulinemiac | 2 (1.7) |
| COVID‐19 severity | |
| Highest grade of respiratory support | |
| HFNC | 11 (9.5) |
| Mechanical ventilation | 9 (7.8) |
| ECMO | 5 (4.3) |
| COVID‐19 treatment | |
| Chloroquine – no. (%) | 2 (1.7) |
| Hydroxychlorquine – no. (%) | 101 (87.1) |
| Corticosteroids – no. (%) | 27 (23.3) |
Abbreviations: BMI, body mass index; COVID‐19, coronavirus disease 2019; ECMO, extracorporeal membrane oxygenation; HFNC, high flow nasal cannula.
1 receiving high‐dose corticosteroids, 1 tocilizumab, 1 ocrelizumab, 1 corticosteroids and tocilizumab, 1 corticosteroids and mycophenolate mofetil, 1 dimethyl fumarate, and 1 etanercept.
3 under chemotherapy, 1 chemoradiotherapy, 1 high‐dose corticosteroids, and 1 durvalumab.
One transplant patient had hypogammaglobulinemia.
Of the 116 patients, 30 patients had 2 measurements while four patients had 3. IgG measurements were performed at a median of 79 days after COVID diagnosis (IQR 67–103), 63 measurements were performed more than 12 weeks and 8 more than 24 weeks after diagnosis. Ten patients had a negative IgG measurement, drawn at a median of 77 days (IQR 51–98), of which three patients had had a previous positive IgG serology.
3.1. Influence of time‐lapse on IgG titer
We found a significant decrease of specific IgG titers over time (p = 0.0002). Mean IgG titers decreased when comparing every time period to the next one, with the exception of the second to third period: mean IgG titers of 29.82 (95% confidence interval [CI], 19.31–44.39) for Period 1, 17.15 (95% CI, 13.60–21.39) for Period 2, 18.22 (95% CI, 13.50–24.13) for Period 3, 10.04 (95% CI, 6.98–19.99) for Period 4, and 6.85 (95% CI, 4.56–9.86) for Period 5. The distribution of IgG titers over time is further described with medians and CI in Figure S1.
3.2. Influence of demographics and comorbidities on IgG antibody levels
We found a trend towards an increase of IgG with increased age in univariate analysis (p = 0.077), which became significant (p = 0.005) when considering time elapsed between diagnosis and IgG measurements as a covariate. We found increasing IgG titers with higher BMI (p = 0.012 and 0.0026, respectively for Model 1 and 2) and lower IgG levels in the group of immunocompromised patients (two patients with hypogammaglobulinemia excluded) (p = 0.063 and 0.032, respectively for Model 1 and 2). We found no significant effect for other studied variables, including gender, ethnicity, and main co‐morbidities (cardio‐vascular disease, hypertension, pulmonary disease, diabetes, and chronic kidney disease) (Table 2).
Table 2.
Influence of demographic parameters, comorbidities, and clinical course on SARS‐CoV‐2 IgG titersa
| p value, considering time‐lapse as covariate (Model 1)b | Standardized beta coefficient (95% CI) | p value, considering time lapse and age as covariates (Model 2)b | Standardized beta coefficient (95% CI) | |
|---|---|---|---|---|
| Age | 0.0050 ** | +0.228 (+0.071 to +0.385) | / | |
| BMI | 0.012* | +0.212 (+0.049 to +0.375) | 0.0026** | +0.233 (+0.084 to +0.382) |
| Comorbidities | ||||
| Diabetes | 0.074 | +0.175 (−0.021 to +0.37) | 0.079 | +0.167 (−0.024 to +0.358) |
| Chronic kidney disease | 0.73 | +0.040 (−0.211 to +0.290) | 0.96 | +0.008 (−0.263 to +0.280) |
| Immunodeficiency | 0.063 | −0.178 (−0.374 to +0.018) | 0.032* | −0.200 (−0.388 to −0.013) |
| Clinical course | ||||
| Need for HFNC | 0.92 | −0.010 (−0.197 to +0.177) | 0.66 | +0.041 (−0.147 to +0.229) |
| Need for HFNC after propensity score weightingc | 0.93 | +0.012 (−0.228 to +0.252) | / | |
| Need for MV | 0.32 | −0.090 (−0.271 to +0.091) | 0.44 | −0.073 (−0.264 to +0.119) |
| Need for MV after propensity score weightingc | 0.62 | −0.058 (−0.175 to +0.058) | / | |
| Need for either HFNC or MV | 0.52 | −0.062 (−0.254 to +0.129) | 0.85 | −0.019 (−0.215 to +0.177) |
| Need for either HFNC or MV after propensity score weightingc | 0.83 | −0.092 (−0.302 to +0.119) | / | |
| Corticoid treatment | 0.12 | +0.120 (−0.034 to +0.275) | 0.072 | +0.137 (−0.014 to +0.288) |
| Corticoid treatment after propensity score weightingc | 0.13 | +0.140 (−0.042 to +0.322) | / |
Abbreviations: BMI, body mass index; CI, confidence interval; HFNC, high flow nasal cannula; MV, mechanical ventilation; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Effect was evaluated on transformed values of IgG.
Time lapse between diagnosis and IgG measurement is analyzed as a categorical covariable, measurements being divided in five consecutive time periods.
The probability of receiving the intervention was calculated by logistic regression as a function of date of hospitalization, sex, age, BMI, renal function (inverse of creatinine), presence of fever, cough, dyspnea, hypertension, diabetes, and immunodeficiency.
3.3. Association between clinical course, paraclinical findings, and IgG antibody levels
We did not find association between symptoms presented by the patients at admission and the IgG titers, with the exception of cough (p value respectively 0.078 and 0.035 for Model 1 and 2). Laboratory tests on admission known as associated with unfavorable outcome such as C‐reactive protein, lactate dehydrogenase, creatinine, or low lymphocyte count did not show any influence on IgG levels (Model 1 and 2), with the exception of liver function test upon admission: high aspartate aminotransferase (ASAT) levels were significantly associated with higher IgG titers (p = 0.047 and 0.036) while the association was not significant for alanine aminotransferase (Table S1). Concerning the severity of infection, the extent of lung involvement on the admission computed tomography scan showed no significant association with IgG levels. Moreover, we did not find an association between the severity of infection and IgG titers (Model 1 ad 2), as assessed by the need for HFNC (p = 0.92 and 0.66), MV (p = 0.32 and 0.44), or either respiratory support (p = 0.52 and 0.85). Results were confirmed after weighting with a propensity score for the use of respiratory support (Table 2).
3.4. Influence of corticosteroid treatment on IgG antibody levels
We did not find the influence of the administration of corticosteroids as COVID‐19 treatment on IgG titers (p = 0.12 and 0.072) using both models. Those results were confirmed after weighting with a propensity score for the use of corticosteroids (Table 2).
3.5. Variables influencing time to seronegativation
We further assessed the association between the different variables and the occurrence of IgG seronegativity during follow‐up. Age was not shown to affect the time to seronegativation (p = 0.34). A higher BMI (HR = 0.843; p = 0.020) and the presence of cough (HR = 0.203; p = 0.026) were associated with delayed seronegativation. On the opposite, immunodeficiency was found significantly associated with shorter time to seronegativation (HR = 6.170; p = 0.0042). Other co‐morbidities and severity of infection (assessed by the need for HFNC, MV, or either respiratory support) showed no significant effect. No patient treated with corticosteroids was found seronegative at follow‐up. All results were confirmed in a bivariate analysis using age as a covariate (Table 3).
Table 3.
Influence of BMI and comorbidities on time to seronegativation of SARS‐CoV‐2 IgG
| Univariate analysis | Bivariate analysis considering age as a covariate | |||
|---|---|---|---|---|
| p value | Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) | |
| BMI | 0.020* | 0.843 (0.730–0.973) | 0.018* | 0.844 (0.733–0.791) |
| Cardio‐vascular disease | 0.23 | 2.328 (0.601–9.017) | 0.13 | 2.990 (0.729–12.262) |
| Hypertension | 0.23 | 2.301 (0.594–8.906) | 0.15 | 2.836 (0.695–11.573) |
| Pulmonary disease | 0.62 | 1.490 (0.316–7.018) | 0.50 | 1.724 (0.355–8.365) |
| Diabetes | 0.58 | 0.553 (0.070–4.364) | 0.56 | 0.537 (0.068–4.248) |
| Immunodeficiency | 0.0042** | 6.170 (1.777–21.430) | 0.0030** | 6.703 (1.911–23.512) |
Abbreviations: BMI, body mass index; CI, confidence interval; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
4. DISCUSSION
In this study, we retrospectively analyzed IgG titers drawn during the follow‐up of severe and critical COVID‐19 patients. We found a significant decrease of IgG titers over time. We showed a negative association between immunosuppression and IgG levels at follow‐up while the use of corticosteroids to treat COVID‐19 did not show an influence within this timeframe. In contrast, we found that age, a high BMI and high ASAT at admission were associated with raised follow‐up IgG antibody titers. Lastly, reinforcing our previous findings, immunosuppression was associated with reduced time to seronegativation while it appeared delayed by high BMI.
Antibodies waning after SARS‐CoV‐2 infection has been repeatedly reported, especially in mild/moderate COVID‐19,13, 14 a feature raising concerns about similarities with immunity against other coronaviruses, where declining antibodies at 1 year do not fully protect against reinfection. 15 Long et al. 7 found that the IgG levels in 93% (28/30) of the asymptomatic patients and 97% (30/31) of the symptomatic patients declined during the early convalescent phase. Contrarily, a recent study of humoral response to SARS‐CoV‐2 in Iceland by Gudbjartsson et al. 4 using two pan‐immunoglobulin assays found that among 1215 persons who had recovered from SARS‐CoV‐2 infection, 1107 (91%) were seropositive and antiviral antibody titers increased during 2 months after diagnosis by RT‐PCR and remained on a plateau for 4 months.
Limited follow‐up data is available after severe infection. Wang et al. 16 showed in a cohort of hospitalized patients that antibody levels peaked between day 31‐40, followed by a slight decrease. In a small subset of 8 patients, they found diverse kinetics of neutralizing antibody, with some patients seeing increased and others decreased titers. Zhang et al. 17 showed in 112 COVID‐19 hospitalized patients that IgG persisted for over 194 days after symptom onset, although patients showed a 46% reduction in antibodies titers against SARS‐CoV‐2 nucleocapsid protein compared with the acute phase.
A striking finding of our analysis is the association of age and obesity with higher IgG titers. This may appear counterintuitive, as both factors are usually thought to associate with poor adaptive immune response,18, 19 but confirms similar findings for age4, 16, 20, 21, 22, 23 and obesity4, 21, 22 after COVID‐19. Although absolute IgG levels have been shown to positively correlate with age, a decreased capacity of humoral response to novel antigens has been shown in elderlies, linked to a decreased naïve B cell repertoire. 24 Illustrating this reduced B‐cell responsiveness, overall lower IgG levels after vaccination have been demonstrated in elderlies. 25 Moreover, a decreased antibody quality in old age has also been shown. 26 The impact of obesity on humoral immunity appears more complex. A reduced level of immunoglobulin levels after hepatitis B and tetanus vaccination has been shown in this population, 27 but Sheridan et al. 28 found a significant correlation between BMI and IgG levels one month after influenza vaccination, but a greater decline at 12 months in obese patients. Further follow‐up of the obese COVID‐19 patient population is warranted to draw a better picture of long‐term immunoglobulin kinetics.
In their seroprevalence study of the Icelandic population, Gudbjartsson et al. 4 found an association in recovered persons between higher antibody levels and older age, a higher BMI, but also disease severity. The latter finding corroborates other results showing that levels of anti‐SARS‐CoV‐2 antibodies correlate with disease severity.23, 29 As age and obesity constitute major risk factors for severe COVID‐19 and its dysregulated immune response, 30 higher antibody levels at follow‐up might mirror the intensity of the immune response during infection. Interestingly, we also showed higher IgG titers in patients with elevated ASAT at admission, a feature that has been associated to COVID‐19 severity. 31 One might argue that our analysis did not show any correlation between IgG levels and infection severity. However, one should keep in mind that our analysis focused on hospitalized patient with severe COVID‐19, thus limiting its ability to detect an effect when considering severity as a spectrum ranging from asymptomatic to critical disease. This ability was further decreased by the fact that we could not include the sickest patients in our analysis, as death at the acute stage of infection precluded the drawing of follow‐up blood samples.
The usefulness and yield of serological evaluation in immunocompromised patients remain unknown. Small case series32, 33 have found seroconversion of transplant recipients but reduced seroconversion has been shown in hematological malignancy patients. 34 Inflammatory bowel disease patients treated with infliximab showed reduced seropositivity and reduced antibody reactivity, compared with controls treated with the non‐immunosuppressive vedolizumab. 35
We found no correlation between the administration of corticosteroids as COVID‐19 treatment and IgG titers. In the context of SARS‐CoV‐1, Woo et al. 36 described a biphasic IgG antibody response after infection and speculated this was the result of high‐dose corticosteroid treatment. Glucocorticoids are potent immunosuppressive drugs with pleiotropic effects, including blocking the synthesis of numerous cytokines, inhibiting the expression of cellular adhesion molecules, and promoting lymphocyte apoptosis. 37 Short‐course corticosteroid treatments have been shown to induce IgG as well as IgA level depression that may persist for several weeks. 38 More worryingly, a decreased neutralizing antibody response to naturally acquired herpes virus infection has been shown on volunteers exposed to short‐term corticosteroids. However, in line with our findings, antibody response to antigens appeared unaffected. 39 Although limited by its sample size, our data provide some reassurance on the indirect effects of COVID‐19 corticosteroid treatment on humoral response.
The strengths of this study include the rigorous selection of severe and critical patients with proven infection, the length of follow‐up and serial serological assessments enabling evaluation of the effect of time on IgG levels. Moreover, the originality of this study is the in‐depth characterization of patients and of COVID‐19 clinical and paraclinical phenotype, and the study of their impact on IgG titers. Most published studies so far focused on overall antibody kinetics without taking into account individual factors to explain the variability in serological course.
The first limitation of this study is its retrospective design. Data were retrieved from electronic health records, and incomplete data reporting cannot be excluded, particularly regarding the reported symptoms, as other collected data are by nature less prone to errors.
The second limitation is that we did not assess the neutralizing activity of included serums. Valvidia et al. 40 studied the correlation between spike‐binding neutralizing antibody and different commercial serology assays, including the MAGLUMI assay. Overall, all tested assays correlated with neutralizing activity, albeit at varying levels. 40 For the MAGLUMI test used in our study, they found an overall Rho value for correlation of 0.48 (p < 0.001). Differences between assays regarding recognized epitopes may play an important role, as antibodies against immunodominant epitopes elicit the highest neutralizing activity. Interestingly, after the fourteenth day of symptoms, they found only one over 49 samples being discordant with negative MAGLUMI and positive neutralizing assay. As such, our results only provide a partial overview of humoral response to SARS‐CoV‐2. However, as stated, IgG values measured with MAGLUMI correlate with neutralizing activity. Moreover, we provided concordant results when studying determinants of IgG kinetics and seronegativity. Eventually, the fact that our main results corroborate results of other groups offers reassurement regarding their robustness. In any case, our results can help clinicians in their interpretation of serological tests.
Another limitation is that our work only assessed humoral immunity. Seronegativity should not be interpreted as the absence of protecting immunity, as cellular immunity is a key component of response and protection against COVID‐19. 41 Indeed, using interferon‐γ ELISPOT, Schwarzkopf et al. 42 observed that 78% of RT‐PCR‐positive volunteers with undetectable antibodies showed T‐cell immunity against SARS‐CoV‐2. However, cellular immunity assessment is not routinely available, and serology is often used to determine past exposure. Different compartments of memory immunity have been shown to follow independent kinetics after COVID‐19, 43 and all warrant specific interest. Determining predictors of IgG decay after severe COVID‐19 is highly relevant in the context of the selection of plasma therapy donors, as hospitalized patients have been suggested as an interesting donor population. 44
Lastly, the possibility of reexposure to SARS‐CoV‐2 during follow‐up cannot be excluded. However, all but four long‐term serological assessments had been drawn before October, when the second COVID‐19 wave started in Belgium.
In summary, we measured specific IgG antibodies after severe or critical COVID‐19 and found that time and immunodeficiency are associated with reduced IgG levels at follow‐up, while age and BMI were associated to increased levels. Corticosteroids given as short‐term treatment for COVID‐19 did not show influence, and BMI and immunodepression were respectively associated with decreased and increased rates of seronegativation.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Julien De Greef and Jean Cyr Yombi conceptualized the article. Julien De Greef and Leïla Belkhir undertook the data collection. Jean Cyr Yombi and Leïla Belkhir contributed equally to the article. Anaïs Scohy conducted the serological testing. Julien De Greef, Leïla Belkhir, Jean Cyr Yombi, Frank Aboubakar, Charles Pilette, Ludovic Gerard, Lucie Pothen, and Halil Yildiz took care of the patients during acute infection and follow‐up. Francis Zech realized the statistical analysis. Julien De Greef wrote the initial draft with all authors providing critical feedback and edits to subsequent revisions. All authors approved the final draft of the manuscript.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/jmv.27059
Supporting information
Supporting information.
Supporting information.
De Greef J, Scohy A, Zech F, et al. Determinants of IgG antibodies kinetics after severe and critical COVID‐19. J Med Virol. 2021;93:5416‐5424. 10.1002/jmv.27059
DATA AVAILABILITY STATEMENT
The anonymized data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Mustafa Hellou M, Górska A, Mazzaferri F, et al. Nucleic acid amplification tests on respiratory samples for the diagnosis of coronavirus infections: a systematic review and meta‐analysis. Clin Microbiol Infect. 2021;27(3):341‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kweon OJ, Lim YK, Kim HR, et al. Antibody kinetics and serologic profiles of SARS‐CoV‐2 infection using two serologic assays. PLoS One. 2020;15(10):e0240395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357(19):1903‐1915. [DOI] [PubMed] [Google Scholar]
- 4. Gudbjartsson DF, Norddahl GL, Melsted P, et al. Humoral immune response to SARS‐CoV‐2 in Iceland. N Engl J Med. 2020;383(18):1724‐1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Seow J, Graham C, Merrick B, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS‐CoV‐2 infection in humans. Nat Microbiol. 2020;5(12):1598‐1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lumley SF, O'Donnell D, Stoesser NE, et al. Antibodies to SARS‐CoV‐2 are associated with protection against reinfection [published online ahead of print November 19, 2020]. medRxiv. 2020. [Google Scholar]
- 7. Long QX, Tang XJ, Shi QL, et al. Clinical and immunological assessment of asymptomatic SARS‐CoV‐2 infections. Nat Med. 2020;26(8):1200‐1204. [DOI] [PubMed] [Google Scholar]
- 8. Marot S, Malet I, Leducq V, et al. Rapid decline of neutralizing antibodies against SARS‐CoV‐2 among infected healthcare workers. Nat Commun. 2021;12(1):844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Catteau L, Dauby N, Montourcy M, et al. Low‐dose hydroxychloroquine therapy and mortality in hospitalised patients with COVID‐19: a nationwide observational study of 8075 participants. Int J Antimicrob Agents. 2020;56(4):106144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Horby P, Lim WS, Emberson JR, et al, RECOVERY Collaborative Group . Dexamethasone in Hospitalized Patients with COVID‐19 — Preliminary Report. N Engl J Med. 2021;384:693‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Soleimani R, Khourssaji M, Gruson D, et al. Clinical usefulness of fully automated chemiluminescent immunoassay for quantitative antibody measurements in COVID‐19 patients. J Med Virol. 2020;93:1465‐1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bell RM, McCaffrey D. Bias reduction in standard errors for linear regression with multi‐stage samples. Surv Methodol. 2002;28:169‐181. [Google Scholar]
- 13. Choe PG, Kang CK, Suh HJ, et al. Waning antibody responses in asymptomatic and symptomatic SARS‐CoV‐2 infection. Emerg Infect Dis J. 2021;27(1):327‐329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ibarrondo FJ, Fulcher JA, Goodman‐Meza D, et al. Rapid decay of anti–SARS‐CoV‐2 antibodies in persons with mild COVID‐19. N Engl J Med. 2020;383(11):1085‐1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Callow KA, Parry HF, Sergeant M, Tyrrell DA. The time course of the immune response to experimental coronavirus infection of man. Epidemiol Infect. 1990;105(2):435‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang X, Guo X, Xin Q, et al. Neutralizing antibody responses to severe acute respiratory syndrome coronavirus 2 in coronavirus disease 2019 inpatients and convalescent patients. Clin Infect Dis. 2020;71(10):2688‐2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang X, Lu S, Li H, et al. Viral and antibody kinetics of COVID‐19 Patients With Different Disease Severities In Acute And Convalescent Phases: A 6‐month Follow‐up Study. Virol Sin. 2020;35(6):820‐829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Milner JJ, Beck MA. The impact of obesity on the immune response to infection. Proc Nutr Soc. 2012;71(2):298‐306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nikolich‐Žugich J. The twilight of immunity: emerging concepts in aging of the immune system. Nat Immunol. 2018;19(1):10‐19. [DOI] [PubMed] [Google Scholar]
- 20. Lumley SF, Wei J, O'Donnell D, et al. The duration, dynamics and determinants of SARS‐CoV‐2 antibody responses in individual healthcare workers [published online ahead of print January 06, 2021]. Clin Infect Dis. 2021:ciab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shields AM, Faustini SE, Perez‐Toledo M, et al. Serological responses to SARS‐CoV‐2 following non‐hospitalised infection: clinical and ethnodemographic features associated with the magnitude of the antibody response [published online ahead of print November 16, 2020]. medRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grzelak L, Velay A, Madec Y, et al. Sex differences in the decline of neutralizing antibodies to SARS‐CoV‐2 [published online ahead of print November 15, 2020]. medRxiv. 2020. [Google Scholar]
- 23. Huang M, Lu QB, Zhao H, et al. Temporal antibody responses to SARS‐CoV‐2 in patients of coronavirus disease 2019. Cell Discov. 2020;6(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Listì F, Candore G, Modica MA, et al. A study of serum immunoglobulin levels in elderly persons that provides new insights into B cell immunosenescence. Ann N Y Acad Sci. 2006;1089:487‐495. [DOI] [PubMed] [Google Scholar]
- 25. Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006;24(8):1159‐1169. [DOI] [PubMed] [Google Scholar]
- 26. Howard WA, Gibson KL, Dunn‐Walters DK. Antibody quality in old age. Rejuvenation Res. 2006;9(1):117‐125. [DOI] [PubMed] [Google Scholar]
- 27. Weber DJ, Rutala WA, Samsa GP, Bradshaw SE, Lemon SM. Impaired immunogenicity of hepatitis B vaccine in obese persons. N Engl J Med. 1986;314(21):1393. [DOI] [PubMed] [Google Scholar]
- 28. Sheridan PA, Paich HA, Handy J, et al. Obesity is associated with impaired immune response to influenza vaccination in humans. Int J Obes. 2012;36(8):1072‐1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhao J, Yuan Q, Wang H, et al. Antibody responses to SARS‐CoV‐2 in patients with novel coronavirus disease 2019. Clin Infect Dis. 2020;71(16):2027‐2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID‐19‐related death using OpenSAFELY. Nature. 2020;584(7821):430‐436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Weber S, Hellmuth JC, Scherer C, Muenchhoff M, Mayerle J, Gerbes AL. Liver function test abnormalities at hospital admission are associated with severe course of SARS‐CoV‐2 infection: a prospective cohort study [published online ahead of print January 29, 2021]. Gut. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang AX, Quintero Cardona O, Ho DY, Busque S, Lenihan CR. Influence of immunosuppression on seroconversion against SARS‐CoV‐2 in two kidney transplant recipients. Transpl Infect Dis. 2020;23:e13423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fung M, Chiu CY, DeVoe C, et al. Clinical outcomes and serologic response in solid organ transplant recipients with COVID‐19: a case series from the United States. Am J Transplant. 2020;20(11):3225‐3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thakkar A, Pradhan K, Jindal S, et al. Patterns of seroconversion for SARS‐CoV‐2 IgG in patients with malignant disease and association with anticancer therapy. Nature Cancer. 2021;2:392‐399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kennedy NA, Goodhand JR, Bewshea C, et al. Anti‐SARS‐CoV‐2 antibody responses are attenuated in patients with IBD treated with infliximab. Gut. 2021;70(5):865‐875. [DOI] [PubMed] [Google Scholar]
- 36. Woo PC, Lau SK, Wong BH, et al. Longitudinal profile of immunoglobulin G (IgG), IgM, and IgA antibodies against the severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein in patients with pneumonia due to the SARS coronavirus. Clin Diagn Lab Immunol. 2004;11(4):665‐668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cain DW, Cidlowski JA. Immune regulation by glucocorticoids. Nat Rev Immunol. 2017;17(4):233‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Posey WC, Nelson HS, Branch B, Pearlman DS. The effects of acute corticosteroid therapy for asthma on serum immunoglobulin levels. J Allergy Clin Immunol. 1978;62(6):340‐348. [DOI] [PubMed] [Google Scholar]
- 39. Butler WT. Corticosteroids and immunoglobulin synthesis. Transplant Proc. 1975;7(1):49‐53. [PubMed] [Google Scholar]
- 40. Valdivia A, Torres I, Latorre V, et al. Inference of SARS‐CoV‐2 spike‐binding neutralizing antibody titers in sera from hospitalized COVID‐19 patients by using commercial enzyme and chemiluminescent immunoassays. Eur J Clin Microbiol Infect Dis. 2021;40(3):485‐494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sekine T, Perez‐Potti A, Rivera‐Ballesteros O, et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID‐19. Cell. 2020;183(1):158‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schwarzkopf S, Krawczyk A, Knop D, et al. Cellular immunity in COVID‐19 convalescents with PCR‐confirmed infection but with undetectable SARS‐CoV‐2‐specific IgG. Emerg Infect Dis. 2021;27(1). [DOI] [PubMed] [Google Scholar]
- 43. Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS‐CoV‐2 assessed for greater than six months after infection [published online ahead of print November 16, 2020]. bioRxiv. 2020. [Google Scholar]
- 44. Libster R, Pérez Marc G, Wappner D, et al. Early high‐titer plasma therapy to prevent severe COVID‐19 in older adults. N Engl J Med. 2021;384(7):610‐618. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Supporting information.
Data Availability Statement
The anonymized data that support the findings of this study are available from the corresponding author upon reasonable request.


