Abstract
Coronavirus disease 2019 (COVID‐19) caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection has become a global health issue and develops into a broad range of illnesses from asymptomatic to fatal respiratory diseases. SARS‐CoV‐2 infection is associated with oxidative stress that triggers cytokine production, inflammation, and other pathophysiological processes. Glutathione‐S‐transferase (GST) is an important enzyme that catalyzes the conjugation of glutathione (GSH) with electrophiles to protect the cell from oxidative damage and participates in the antioxidant defense mechanism in the lungs. Thus, in this study, we investigated the role of GSTM1 and GSTT1 gene polymorphism with COVID‐19 susceptibility, as well as its outcome. The study included 269 RT‐PCR confirmed COVID‐19 patients with mild (n = 149) and severe (n = 120) conditions. All subjects were genotyped for GSTM1 and GSTT1 by multiplex polymerase chain reaction (mPCR) followed by statistical analysis. The frequency of GSTM1−/−, GSTT1−/− and GSTM1−/−/GSTT1−/− was higher in severe COVID‐19 patients as compared to mild patients but we did not observe a significant association. In the Cox hazard model, death was significantly 2.28‐fold higher in patients with the GSTT1−/− genotype (p = 0.047). In combination, patients having GSTM1+/+ and GSTT1−/− genotypes showed a poor survival rate (p = 0.02). Our results suggested that COVID‐19 patients with the GSTT1−/− genotype showed higher mortality.
Keywords: COVID‐19, GSTM1, GSTT1, mPCR, Oxidative Stress, SARS‐CoV‐2
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19), caused by severe acute respiratory syndrome coronavirus 2 (SARS‐COV‐2) has recently emerged as a new challenge for the medical sciences. It has been considered a pandemic by the World Health Organization (WHO) from March 11, 2020, onwards. 1 The pathogenesis of COVID‐19 and its cause of severity are still poorly understood. SARS‐CoV‐2 is associated with oxidative stress (OS) that triggers cytokine production, inflammation, and other pathophysiological activities. 2 OS is defined as the disturbance of the antioxidant and prooxidant balance in a biological system. 3 During OS, highly reactive oxygen/nitrogen species (RONS) are produced such as hydroxyl, superoxide anion, nitric oxide, and nitrosyl anion, which target various cells and damage DNA, proteins, and lipids, leading to the pathogenesis of respiratory viral infections including SARS‐CoV‐2 infections.3, 4, 5 Delgado‐Roche and Mesta, 5 suggested that OS coupled with innate immunity affects the onset of severe lung injury in COVID‐19 patients and stimulates transcription factors, such as NF‐kB, resulting in an exacerbated pro‐inflammatory host response. However, the COVID‐19 patients with pre‐existing conditions such as diabetes, hypertension, and pulmonary, cardiac, and kidney diseases are at a higher risk of developing a severe infection.6, 7, 8, 9
Glutathione S‐transferases (GSTs) are a superfamily of multifunctional isoenzymes that catalyzes glutathione conjugation with electrophilic compounds, resulting in the cellular detoxification of several endogenous and exogenous compounds. 10 GSTs play an important role in the detoxification of different carcinogens, drugs, and against various types of cellular oxidative damage. 11 The GST enzyme contributes to different interindividual activity in response to clearance of oxidative stress products. 12 In mammalian tissue, eight distinct classes of the cytosolic GST enzymes have been recognized such as alpha (α)‐GSTA, mu (μ)‐GSTM, pi (π)‐GSTP, omega (ω)‐GSTO, theta (θ)‐GSTT, sigma (σ)‐GSTS, kappa (κ)‐GSTK), and zeta (ζ)‐GSTZ. 13 The μ (GSTM1: MIM: 600436) and θ (GSTT1: MIM: 138350) members are the most common variant of GST genes, which are located on chromosome 1p13.3 and 22q11.23, respectively.14, 15 The homozygous deletion (null genotype) of the GSTM1 (GSTM1 −/− ) and GSTT1 (GSTT1 −/−) genes are associated with the loss of enzyme activity and increase the risk of several oxidative stress associated multifactorial diseases including cardiovascular and respiratory diseases.16, 17, 18, 19, 20
Thus, in this study, we investigated the association of GSTM1 and/or GSTT1 polymorphisms with COVID‐19 susceptibility as well as its outcome in the North Indian population.
2. MATERIALS AND METHODS
2.1. Sample collection and experimental design
This study was approved by the Ethics Committee of the Era University, India. We recruited 269 RT‐PCR confirmed COVID‐19 patients, enrolled in Eras Lucknow Medical College and Hospital (ELMC&H), Era University, Lucknow from August 2020 to September 2020, and all patients were followed up for 1 month from the date of admission. Informed consent from all participants was obtained in accordance with the ethical standards of Era University, India. All demographic and clinical data of patients were collected as per a self‐administered questionnaire and other clinical data was collected from hospital records with the help of an expert clinician. All patients with inclusion criteria (COVID‐19 patients confirmed by RT‐PCR of more than 20 years) and no exclusion criteria (Pregnant patients, patients with known malignant disease) were selected.
Patients were categorized into two groups, mild and severe as per the criteria of the Indian Council of Medical Research (ICMR), New Delhi, India. Patients with a respiratory rate less than 24 per min and SpO2 > 94% on room air were considered as mild patients while patients with a respiratory rate more than 30 per min and SpO2 < 90% on room air with pneumonia were categorized into severe patients.21, 22 2 ml of the blood sample from all patients were collected in ethylene diamine tetraacetic acid (EDTA) vials and stored at −20°C until further use.
2.2. Genotyping
Genomic DNA was extracted from peripheral blood samples by using a Commercially Available Kit (Macherey‐Nagel) and the quality/quantity of DNA was assessed by using a spectrophotometer and gel electrophoresis checked on 1% agarose gel and quantified in a biophotometer (Eppendorf). GSTM1 and GSTT1 null genotypes were detected by using multiplex polymerase chain reaction (mPCR) using specific primers: F‐5′GAACTCCCTGAAAAGCTAAAGC‐3′ and R‐5′GTTGGGCTCAAATATACGGTGG‐3′; F‐5′TCCTTACTGGTCCTCACATCTC‐3′ and R‐5′TCACCGGATCATGGCCAGCA‐3′ respectively and for positive control, angiotensin II receptor type 1 (AGTR1) gene primers were used: F‐5′GCCAAATCCCACTCAAACCTTTCAACAA‐3′ and R‐5′AAGCAGGCTAGGGAGATTGC ATTTCTGT‐3′.
PCR was performed in a 25 µl reaction mixture of 150–200 ng genomic DNA, 5 pmol of each primer, 2× master mix (Takara), and 0.5 U of Taq DNA polymerase (G‐Biosciences) per tube using a gradient Master Cycler (Bio‐Rad). The PCR products were visualized by 2.5% agarose gels in a Gel Documentation System (EZ, Bio‐Rad). The null genotypes of both genes (GSTM1 and GSTT1) were determined by the absence of gene products. The AGTR1 gene was co‐amplified and used as a positive control (Figure 1).
Figure 1.
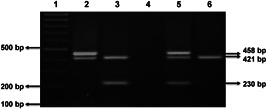
Agarose gel showing multiplex PCR products of different genotypes of GSTM1 (230 bp) and GSTT1 (458 bp). Lane 1: 100 bp ladder; Lane 2: GSTM1–/–/GSTT1+/+; Lane 3: GSTM1+/+/GSTT1–/–; Lane 4: Non template control; Lane 5: GSTM1+/+/GSTT1+/+; Lane 6: GSTM1–/–/GSTT1–/–. AGTR1 gene (421 bp) was used as a positive control. PCR, polymerase chain reaction
2.3. Statistical analysis
Demographic and clinical data were compared with genotypes using χ2 analysis and Fisher's exact test. Allele and genotype frequencies in mild and severe cases were compared using a 2 × 2 contingency table by Fisher's exact test. The odds ratio (OR) at 95% confidence interval (CI) was used to determine the strength of association. All p values were considered statistically significant if p < 0.05. Genotype effects GSTM1 and GSTT1 on overall survival were evaluated by the Kaplan–Meier function and the Cox proportional hazards model. The differences in overall survival and genotypes were compared using the log‐rank test. Hazard ratios (HRs) were estimated using a multivariate Cox hazards model/Cox regression analysis with adjustment for age, sex, hypertension, and diabetes.
3. RESULTS
3.1. Clinical characteristics of patients
The mean age of patients was 52.7 years. A total of 269 COVID‐19 patients were enrolled in this study. Out of 269, 149 patients (47.2%) were showing mild symptoms and 120 patients (38.0%) were showing severe symptoms. A total of 32 deaths (10%) were observed from 120 severe patients.
3.2. Genotyping
The distribution of GSTM1 and GSTT1 genotypes in mild and severe patients is shown in Table 1. The frequency of GSTM1 −/− and GSTT1 −/− (null genotypes) was higher among severe patients than in mild (13.3% vs. 10.7%; 14.2% vs. 10.7%, respectively) that showed the corresponding marginal increased risk of severity with null genotypes (Table 1). Individuals with a combination of two null genotypes (GSTM1 −/−/GSTT1 −/−) showed a 3.91‐folds higher risk of severity due to COVID‐19 infection when adjusted with age, sex, hypertension, and diabetes (Table 1). However, GSTM1/GSTT1 polymorphism was not shown to have a significant association with the severity of the COVID‐19 (p > 0.05, Table 1). We have also shown the distribution of GSTM1 and GSTT1 genotypes with demographic and clinical data in COVID‐19 severe patients. But none of the demographic and clinical parameters showed a significant association with GSTM1/GSTT1 polymorphism (p > 0.05, Table 2).
Table 1.
Genotype frequencies of GSTM1 and GSTT1 and their association with severity of COVID‐19
| Genes | Mild, n (%) | Severe, n (%) | Unadjusted OR (95% CI) | p | Adjusteda OR (95% CI) | p |
|---|---|---|---|---|---|---|
| GSTM1 | 149 | 120 | ||||
| M1+/+ | 133 (89.3) | 104 (86.7) | 1.0 (Ref.) | 1.0 (Ref.) | ||
| M1−/− | 16 (10.7) | 16 (13.3) | 1.28 (0.611–2.677) | 0.514 | 1.47 (0.638–3.384) | 0.367 |
| GSTT1 | ||||||
| T1+/+ | 133 (89.3) | 103 (85.8) | 1.0 (Ref.) | 1.0 (Ref.) | ||
| T1−/− | 16 (10.7) | 17 (14.2) | 1.37 (0.661–2.846) | 0.396 | 1.33 (0.574–3.059) | 0.51 |
| GSTM1/GSTT1 | ||||||
| M1+/+/T1+/+ | 120 (80.6) | 90 (75.0) | 1.0 (Ref.) | 1.0 (Ref.) | ||
| M1+/+/T1−/− | 13 (8.7) | 14 (11.7) | 1.44 (0.643–3.205) | 0.377 | 1.08 (0.436–2.694) | 0.863 |
| M1−/−/T1+/+ | 13 (8.7) | 13 (10.8) | 1.33 (0.590–3.015) | 0.49 | 1.22 (0.493–3.009) | 0.67 |
| M1−/−/T1−/− | 3 (2.0) | 3 (2.5) | 1.33 (0.263–6.761) | 0.728 | 3.91 (0.587–26.062) | 0.159 |
Note: n, number; %, percentage; Significance association (p < 0.05); CI, confidence interval; OR, odds ratio; 1.0 (Reference); (+/+), present; (−/−), null.
Adjusted for age, sex, hypertension, and diabetes.
Table 2.
Distribution of GSTM1 and GSTT1 genotypes with demographic and clinical data in COVID‐19 severe patients
| GSTM1 | GSTT1 | |||||
|---|---|---|---|---|---|---|
| Patients | M1+/+, n (%) | M1−/−, n (%) | p | T1+/+, n (%) | T1−/−, n (%) | p |
| Age | ||||||
| ≤45 | 11 (78.6) | 93 (87.7) | 0.343 | 12 (85.7) | 91 (85.8) | 0.989 |
| ≥46 | 3 (21.4) | 13 (12.3) | 2 (14.3) | 15 (14.2) | ||
| Gender | ||||||
| Male | 63 (86.3) | 41 (87.2) | 0.883 | 60 (82.2) | 43 (91.5) | 0.154 |
| Female | 10 (13.7) | 6 (12.8) | 13 (17.8) | 4 (8.5) | ||
| Diabetes | ||||||
| No | 74 (86.0) | 30 (88.2) | 0.751 | 76 (88.4) | 27 (79.4) | 0.205 |
| Yes | 12 (14.0) | 4 (11.8) | 10 (11.6) | 7 (20.6) | ||
| Hypertension | ||||||
| No | 85 (85.0) | 19 (95.0) | 0.23 | 87 (87.0) | 16 (80.0) | 0.412 |
| Yes | 15 (15.0) | 1 (5.0) | 13 (13.0) | 4 (20.0) | ||
Note: n, number; %, percentage; Significant association (p < 0.05); (+/+), Present; (−/−), null.
3.3. Survival of patients
The follow‐up duration for all patients was 1 month. During the study period, 11.9% of patients succumbed to death. The median survival had not been reached and the overall mean survival time was 27.79 days. The association of GSTM1 and GSTT1 genotypes with overall survival were analyzed by Cox proportional hazards model, adjusted for age, sex, hypertension, and diabetes are shown in Table 3. There was a significant increase in the hazard of death to 2.28 among patients with GSTT1−/− when compared with patients having the GSTT1+/+ genotype (95% CI = 1.013–5.141; p = 0.047). However, there was no significant association with GSTM1 genotypes (p = 0.853). In the combined effect of GSTM1 and GSTT1, individuals with GSTM1+/+ and GSTT1−/−genotypes showed a significantly 2.72‐folds higher risk of death due to COVID‐19 (95% CI = 1.172–6.295; p = 0.02). The Kaplan–Meier function for survival in cases with GSTM1 and GSTT1 genotypes is shown in Figure 2. In the Kaplan–Meier curve, GSTT1−/− was associated with poor overall survival (log‐rank, p = 0.020, Figure 1B). In addition, the combined effect showed that both genes have an impact on survival. Patients with GSTM1+/+/GSTT1−/− genotype showed significantly poor overall survival as compared to patients having GSTM1+/+/GSTT1+/+ genotypes (log‐rank, p = 0.015, Figure 1C).
Table 3.
Associations between GSTM1 and GSTT1 genetic polymorphisms and survival of COVID‐19 patients
| Genotypes | No. of cases, n (%) | Deaths, n (%) | HRa (95% CI) | p |
|---|---|---|---|---|
| GSTM1 | ||||
| M1+/+ | 209 (88.2) | 28 (87.5) | 1.0 (Ref.) | |
| M1−/− | 28 (11.8) | 4 (12.5) | 1.11 (0.386–3.165) | 0.853 |
| GSTT1 | ||||
| T1+/+ | 212 (89.5) | 24 (75.0) | 1.0 (Ref.) | |
| T1−/− | 25 (10.5) | 8 (25.0) | 2.28 (1.013–5.141) | 0.047 |
| GSTM1/GSTT1 | ||||
| M1+/+/T1+/+ | 190 (80.2) | 20 (62.5) | 1.0 (Ref.) | |
| M1+/+/T1−/− | 19 (8.0) | 8 (25.0) | 2.72 (1.172–6.295) | 0.02 |
| M1−/−/T1+/+ | 22 (9.3) | 4 (12.5) | 1.52 (0.515–4.468) | 0.449 |
| M1−/−/T1−/− | 6 (2.5) | 0 |
Note: n, number; %, percentage; Significant association (p < 0.05); CI, confidence interval; HR, hazard ratio; 1.0 (Reference); (+/+), present; (−/−), Null.
Adjusted for age, sex, hypertension, and diabetes.
Figure 2.

Kaplan–Meier estimates of 30‐day survival of the 269 COVID‐19 patients, by GSTM1 (A) and GSTT1 (B) genotypes, in combination (C). Survival difference by log‐rank test
4. DISCUSSION
GST‐mediated GSH conjugations have been well recognized for the detoxification of several exogenous xenobiotics and/or their Phase I metabolites.17, 23 However, the null genotype of the GSTM1 and GSTT1 genes raise the risk of several oxidative stress‐associated multifactorial diseases, including COVID19.18, 24, 25 GST polymorphisms are associated with a higher risk of oxidative stress, which may play an important role in susceptibility to infection with SARS‐CoV‐2 and/or its outcome. 25 SARS‐CoV‐2 induced reactive oxygen species (ROS) production disturbs the antioxidant defense system that triggers a pro‐inflammatory environment and severe tissue damage, contributing to the fatal outcomes of COVID‐19 patients. 26 However, the mechanisms of virus‐induced OS and its subsequent effects in cells, tissue, and the organism are not well known. There are indeed many contradictory data on antioxidants and the role of ROS in viral replication. 27 Melatonin treated animals showed significantly enhanced activity of the GST enzyme that may reduce COVID‐19 infection‐associated OS.28, 29
The current study found that COVID‐19 patients with GSTT1−/− have a higher risk of mortality and lower overall survival. These findings support the theory that oxidative stress is more prevalent in patients with low or no GST activity. Saadat 25 reported that individuals with GSTT1−/− had a higher risk of COVID‐19 infection as compared to an individual with GSTT1+/+, however, the population with a low prevalence of GSTT1−/− genotype showed the higher numbers of COVID‐19 cases and deaths in East‐Asian countries. Another study reported that individual with GSTT1−/− alone or in combination with GSTM1−/− genotype had an excess decrease in forced expiratory volume in the first second (FEV1) in men, regardless of the smoking status. 30 Ding et al. 31 reported that individual with GSTT1−/− and/or GSTM1−/− had a higher risk for the development of pulmonary fibrosis in chronic obstructive pulmonary disease which is also one of the most important complications of COVID‐19 and characterized by long‐term breathing problems. The main observations of the present study are that GSTT1−/− was positively associated with COVID‐19 mortality in our population but does not have a correlation with the prevalence of COVID‐19. The present findings suggest that the GSTT1−/− could have a clinical impact on the COVID‐19 treatment and help to identify the individuals who are at high risk of COVID‐19 severity in the North Indian population. However, the present study is preliminary with limited sample size. Thus, further experiments are currently ongoing in our laboratory to identify the role of GSTT1 polymorphisms for the cause‐effect on COVID‐19 severity in a larger patient population.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Farzana Mahdi, Mohammad Abbas, Faizan H. Khan, and Sushma Verma conceived and designed the experiments. Shrikant Verma and Mohammad Abbas carried out the practicability study. Shrikant Verma and Ale Eba performed the experiments. Mohammad Abbas, Sahabjada Siddiqui, Zeba Siddiqi, Syed T. Raza analyzed the data, and Mohammad Abbas, Faizan H. Khan, and Sushma Verma wrote the paper.
ACKNOWLEDGMENT
This study was supported by intramural research grants (ELMC&H/R_Cell/EC/2020/272 dated 31/12/2020) from Era University, Lucknow, India.
Abbas M, Verma S, Verma S, et al. Association of GSTM1 and GSTT1 gene polymorphisms with COVID‐19 susceptibility and its outcome. J Med Virol. 2021;93:5446–5451. 10.1002/jmv.27076
Mohammad Abbas and Sushma Verma contributed equally to this study as first authors.
REFERENCES
- 1. Adhikari SP, Meng S, Wu YJ, et al. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID‐19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li X, Geng M, Peng Y, Meng L, Lu S. Molecular immune pathogenesis and diagnosis of COVID‐19. J Pharm Anal. 2020;10(2):102‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Yoshikawa T, Naito Y. What is oxidative stress? Jpn Med Assoc J. 2000;124(11):1549‐1553. [Google Scholar]
- 4. Ntyonga‐Pono MP. COVID‐19 infection and oxidative stress: an under‐explored approach for prevention and treatment. Pan Afr Med J. 2020;35(suppl 2):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Delgado‐Roche L, Mesta F. Oxidative stress as key player in severe acute respiratory syndrome coronavirus (SARS‐CoV) infection. Arch Med Res. 2020;51(5):384‐387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. ESGO . Cervical Cancer Guidelines. The European Society of Gynaecological Oncology (ESGO). 2018.
- 7. Mihalopoulos M, Dogra N, Mohamed N, Badani K, Kyprianou N. COVID‐19 and kidney disease: molecular determinants and clinical implications in renal cancer. Eur Urol Focus. 2020;6(5):1086‐1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee LYW, Cazier JB, Starkey T, et al. COVID‐19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol. 2020;21(10):1309‐1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu S, Zhi Y, Ying S. COVID‐19 and asthma: reflection during the pandemic. Clin Rev Allergy Immunol. 2020;59(1):78‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ginsberg G, Smolenski S, Hattis D, Guyton KZ, Johns DO, Sonawane B. Genetic polymorphism in glutathione transferases (GST): population distribution of GSTM1, T1, and P1 conjugating activity. J Toxicol Environ Health B Crit Rev. 2009;12(5–6):389‐439. [DOI] [PubMed] [Google Scholar]
- 11. Hayes JD, Strange RC. Glutathione S‐transferase polymorphisms and their biological consequences. Pharmacology. 2000;61(3):154‐166. [DOI] [PubMed] [Google Scholar]
- 12. Dasari S, Ganjayi MS, Meriga B. Glutathione S‐transferase is a good biomarker in acrylamide induced neurotoxicity and genotoxicity. Interdiscip Toxicol. 2018;11(2):115‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sheehan D, Meade G, Foley VM, Dowd CA. Structure, function and evolution of glutathione transferases: implications for classification of non‐mammalian members of an ancient enzyme superfamily. Biochem J. 2001;360(Pt 1):1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Daniel V. Glutathione S‐transferases: gene structure and regulation of expression. Crit Rev Biochem Mol Biol. 1993;28(3):173‐207. [DOI] [PubMed] [Google Scholar]
- 15. Okcu MF, Selvan M, Wang LE, et al. Glutathione S‐transferase polymorphisms and survival in primary malignant glioma. Clin Cancer Res. 2004;10(8):2618‐2625. [DOI] [PubMed] [Google Scholar]
- 16. Allocati N, Masulli M, Di Ilio C, Federici L. Glutathione transferases: substrates, inihibitors and pro‐drugs in cancer and neurodegenerative diseases. Oncogenesis. 2018;7(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51‐88. [DOI] [PubMed] [Google Scholar]
- 18. Bolt HM, Thier R. Relevance of the deletion polymorphisms of the glutathione S‐transferases GSTT1 and GSTM1 in pharmacology and toxicology. Curr Drug Metab. 2006;7(6):613‐628. [DOI] [PubMed] [Google Scholar]
- 19. McIlwain CC, Townsend DM, Tew KD. Glutathione S‐transferase polymorphisms: cancer incidence and therapy. Oncogene. 2006;25(11):1639‐1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bowatte G, Lodge CJ, Perret JL, Matheson MC, Dharmage SC. Interactions of GST polymorphisms in air pollution exposure and respiratory diseases and allergies. Curr Allergy Asthma Rep. 2016;16(12):85. [DOI] [PubMed] [Google Scholar]
- 21. ICMR . Clinical Management Protocol: COVID‐19. Government of India, Ministry of Health and Family Welfare, Directorate General of Health Services (EMR Division). 2020.
- 22. Verma S, Abbas M, Verma S, et al. Impact of I/D polymorphism of angiotensin‐converting enzyme 1 (ACE1) gene on the severity of COVID‐19 patients. Infect Genet Evol. 2021;91:104801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li Y, Cao Z, Zhu H, Trush MA. Differential roles of 3H‐1,2‐dithiole‐3‐thione‐induced glutathione, glutathione S‐transferase and aldose reductase in protecting against 4‐hydroxy‐2‐nonenal toxicity in cultured cardiomyocytes. Arch Biochem Biophys. 2005;439(1):80‐90. [DOI] [PubMed] [Google Scholar]
- 24. Saadat M. The morbidity and mortality of COVID‐19 are correlated with the Ile105Val glutathione S‐transferase P1 polymorphism. Egypt J Med Hum Genet. 2020;21(52):52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saadat M. An evidence for correlation between the glutathione S‐transferase T1 (GSTT1) polymorphism and outcome of COVID‐19. Clin Chim Acta. 2020;508:213‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Khomich OA, Kochetkov SN, Bartosch B, Ivanov AV. Redox biology of respiratory viral infections. Viruses. 2018;10(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Siew‐Keah L, Sundaram A, Sirajudeen KN, Zakaria R, Singh HJ. Effect of melatonin supplementation and cross‐fostering on renal glutathione system and development of hypertension in spontaneously hypertensive rats. J Physiol Biochem. 2014;70(1):73‐79. [DOI] [PubMed] [Google Scholar]
- 29. Zhang R, Wang X, Ni L, et al. COVID‐19: melatonin as a potential adjuvant treatment. Life Sci. 2020;250:117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Imboden M, Downs SH, Senn O, et al. Glutathione S‐transferase genotypes modify lung function decline in the general population: SAPALDIA cohort study. Respir Res. 2007;8:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ding Z, Wang K, Li J, Tan Q, Tan W, Guo G. Association between glutathione S‐transferase gene M1 and T1 polymorphisms and chronic obstructive pulmonary disease risk: a meta‐analysis. Clin Genet. 2019;95(1):53‐62. [DOI] [PubMed] [Google Scholar]


