Funding source
None.
Conflict of interest
The authors have no financial obligations or conflict of interest to declare.
Dear Editor,
We have recently observed a 41‐year‐old woman who developed chilblain‐like lesions (CLL) soon after the second administration of Pfizer (New York, NY, USA) BNT162b2 mRNA COVID‐19 Vaccine. The lesions occurred exclusively on the volar aspects of the second and the third fingertip of right hand (Fig. 1). The latency between the administration of the second dose of vaccine and the occurrence of CLL was 24 h. The lesions were extremely painful. History for similar lesions was negative. The patient was otherwise healthy, and her blood examination was within normal ranges except for high levels of IgG anti‐spike antibodies, thus determining the positive response to the vaccine. Molecular swab for SARS‐CoV‐2 showed a negative result.
Figure 1.
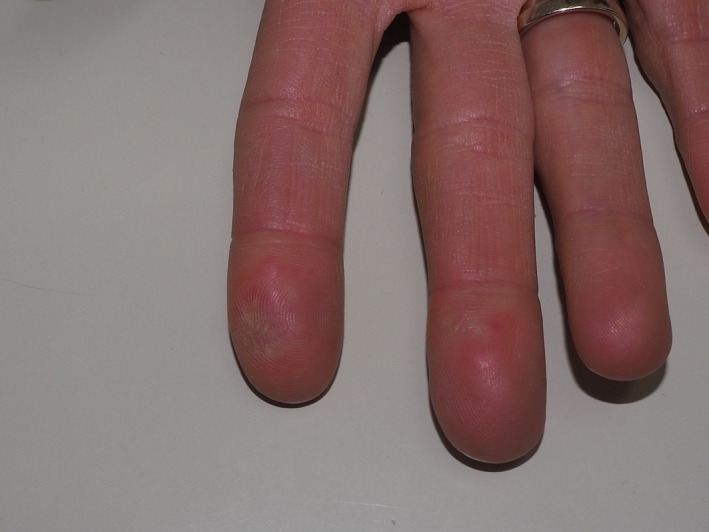
Erythematous lesions with tendency to skin detachment affecting the second and the third fingertip of the right hand.
After an initial phase of confusion and disagree among researchers, CLL are nowadays considered a highly likely immune‐mediated reaction to SARS‐CoV‐2 usually observed in healthy asymptomatic young people, whose COVID status is often unremarkable. 1 , 2 , 3 , 4 , 5 , 6 It has been postulated that this apparent contradiction could be explained as virus‐induced interferonopathy associated with a strong activation of innate immune system and fast clearance of antibodies. 7 , 8
Recently, Davido et al. 9 reported a similar case in a 41‐year‐old woman developing ‘blue toes’ after BNT162b2 mRNA COVID‐19 vaccine. Differently from our case, the patient developed CLL 4 days after the first dose of the vaccine, leading to avoid the second dose for safety reasons. Another minor difference was related to the location (toes vs. fingers). Pain was as impactful as in our patient, in contrast to what was seen in adolescents, whose CLL were often asymptomatic or poorly symptomatic (itch or mild pain), although sometimes patients complain about intense pain.
This is so the second European case of COVID vaccine‐induced CLL and the first in Italy. As the number of vaccinated people is still limited, the amount of similar cases is expected to increase over time.
A clinical overlap does exist with the non‐vaccine‐associated CLL, and it seems obvious to think that a relationship with the vaccine is actually present.
Chilblain‐like lesions are still considered an enigmatic sign, whose association with COVID‐19 is a matter of debate. However, the parallel ‘epidemic’ of CLL contemporary to COVID‐19 pandemic is one of the major proofs of their correlation.
Basically, it is very important to report postmarketing reactions to vaccines. In the specific case, Pfizer BNT162b2 mRNA COVID‐19 vaccine was not associated with the occurrence of CLL in the premarketing registration study 10 and this is an important information to add to the plethora of data we are collecting about COVID and vaccine as well. We think that it cannot be casual to observe CLL after COVID vaccine and the reason for that could be the high immune response secondary to the vaccine in some subjects then developing CLL, as a result of strong immune activation against the virus. Although two cases are not enough to establish the cause‐effect relationship, we consider the appearance of CLL in patients receiving COVID vaccine as a further unassailable proof of the dependence of CLL from COVID‐19.
During a period of health uncertainty like the one we are living now, we should be cautious with definitive assertion, but in this specific case, we think that our observation reinforces the hypothesis of a true association between CLL and immune response against SARS‐CoV‐2. Further larger studies are desirable to confirm our data and to support our hypothesis.
Acknowledgments
The patients in this manuscript have given written informed consent to the publication of their case details.
References
- 1. Piccolo V, Neri I, Filippeschi C et al. Chilblain‐like lesions during COVID‐19 epidemic: a preliminary study on 63 patients. J Eur Acad Dermatol Venereol 2020; 34: e291–e293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Piccolo V, Neri I, Manunza F, Mazzatenta C, Bassi A. Chilblain‐like lesions during the COVID‐19 pandemic: should we really worry? Int J Dermatol 2020; 59: 1026–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Piccolo V, Bassi A, Argenziano G et al. Dermoscopy of chilblain‐like lesions during the COVID‐19 outbreak: a multicenter study on 10 patients. J Am Acad Dermatol 2020; 83: 1749–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Piccolo V, Bassi A. Acral findings during the COVID‐19 outbreak: Chilblain‐like lesions should be preferred to acroischemic lesions. J Am Acad Dermatol 2020; 83: e231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bassi A, Russo T, Argenziano G et al. Chilblain‐like lesions during COVID‐19 pandemic: the state of the art. Life 2021; 11: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Piccolo V, Bassi A, Russo T et al. Chilblain‐like lesions and COVID‐19: second wave, second outbreak. J Eur Acad Dermatol Venereol 2021; 35: e316–e318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hubiche T, Cardot‐Leccia N, Le Duff F et al. Clinical, laboratory, and interferon‐alpha response characteristics of patients with chilblain‐like lesions during the COVID‐19 pandemic. JAMA Dermatol 2020; 25: e204324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sekine T, Perez‐Potti A, Rivera‐Ballesteros O et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID‐19. Cell 2020; 183: 158–168.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Davido B, Mascitti H, Fortier‐Beaulieu M, Jaffal K, de Truchis P. 'Blue toes' following vaccination with the BNT162b2 mRNA COVID‐19 vaccine. J Travel Med 2021: taab024. 10.1093/jtm/taab024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Polack FP, Thomas SJ, Kitchin N et al. Safety and efficacy of the BNT162b2 mRNA Covid‐19 vaccine. N Engl J Med 2020; 31: 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]


