Abstract
Coronavirus disease 2019 (COVID‐19) is one of the most pressing health problems of this century, but our knowledge of the disease is still limited. In this study, we aimed to examine serum‐soluble urokinase plasminogen activator receptor (suPAR) and kidney injury molecule 1 (KIM‐1) levels based on the clinical course of COVID‐19. Our study included 102 patients over the age of 18 who were diagnosed as having COVID‐19 between September 2020 and December 2020 and a control group of 50 health workers over the age of 18 whose severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) PCR results were negative. KIM‐1 was measured by ELISA and suPAR by suPARnostic™ assay. Analysis of previously identified variables of prognostic significance in COVID‐19 revealed high neutrophil to lymphocyte ratio, lactose dehydrogenase, prothrombin time, C‐reactive protein, PaO2/FiO2, D‐dimer, ferritin, and fibrinogen levels in patients with severe disease (p < 0.05 for all). KIM‐1 and suPAR levels were significantly higher in COVID‐19 patients compared to the control group (p = 0.001 for all). KIM‐1 level was higher in severe patients compared to moderate patients (p = 0.001), while suPAR level was lower (p = 0.001). KIM‐1, which is believed to play an important role in the endocytosis of SARS‐CoV‐2, was elevated in patients with severe COVID‐19 and may be a therapeutic target in the future. SuPAR may have a role in defense mechanism and fibrinolysis, and low levels in severe patients may be associated with poor prognosis in the early period.
Keywords: COVID‐19, KIM‐1, suPAR
1. INTRODUCTION
The term “pandemic” has become familiar to people worldwide over the last year as coronavirus disease 2019 (COVID‐19) has wrought sociocultural, economic, and psychological havoc on a global scale. Most people infected with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the pathogen that causes COVID‐19, experience mild symptoms, such as headache, sore throat, joint pain, and loss of taste and smell. However, the infection can also cause severe morbidity and mortality, especially in individuals over 65 and those with comorbidity. 1
The main conditions associated with severe course from the onset of COVID‐19 are acute respiratory distress syndrome (ARDS), macrophage activation syndrome (MAS), and thrombotic events secondary to endothelial dysfunction. In addition to proinflammatory cytokines, such as tumor necrosis factor‐alpha (TNF‐α), interleukin 1 (IL‐1), IL‐6, and, IL‐18, the expression of urokinase plasminogen activator (uPAR) is also markedly increased during the development and progression of ARDS and MAS. Kallikrein, which is released from lung endothelial cells, plays an important role in the conversion of uPAR into its circulating soluble form (soluble urokinase plasminogen activator receptor [suPAR]). Studies have demonstrated suPAR elevation in diabetes, coronary artery disease, cancer, kidney disease, and infections, and it has been suggested that suPAR may be a proinflammatory biomarker that can be used as an early mortality indicator in these diseases. In studies of COVID‐19 patients, it has been emphasized that suPAR elevation was correlated with clinical severity and played an important role in the development of pulmonary, renal, and cardiac complications. However, another study showed that although suPAR level increased with disease severity like in the previous study, it was even higher in asymptomatic carriers compared to symptomatic patient groups. This increased the need for more extensive studies to better understand the role of suPAR in COVID‐19.
One of the first discoveries about SARS‐CoV‐2 was that it binds to the cell surface and enters cells using the angiotensin‐converting enzyme 2 receptor. However, as we gained more information about the disease, it was found that kidney injury molecule 1 (KIM‐1) may have a greater role than ACE receptors in the severe kidney damage caused by COVID‐19. In particular, the IgV domain of the KIM‐1 molecule has been highlighted as a potential SARS‐CoV‐2 binding receptor. The discovery of the KIM‐1 receptor in the lungs has also led to speculation that it may offer a new therapeutic target that can minimize pulmonary complications due to SARS‐CoV‐2. There have been no studies on KIM‐1 in relation to the clinical course at the pulmonary level in COVID‐19 patients.
The aim of this study was to investigate the relationship between the clinical severity of COVID‐19 and patients' serum levels of suPAR, for which there are conflicting data on this disease, and serum levels of KIM‐1, which is thought to be a new viral entry pathway in the lungs.
2. MATERIALS AND METHODS
2.1. Study design
As standard procedure, high‐resolution computed tomography (HRCT) was performed for high‐risk patients with COVID‐19. Patients with typical HRCT findings (bilateral ground‐glass opacity with primarily peripheral distribution, subsegmental consolidation or linear opacities, cobblestone pattern, and inverse halo sign) and patients with atypical radiological findings but consistent clinical presentation were hospitalized. Hematological parameters; biochemical parameters, including liver and kidney function tests; coagulation parameters, such as ferritin, D‐dimer, troponin‐I, C‐reactive protein (CRP); and arterial blood gas parameters were analyzed at admission and daily thereafter.
2.2. Study group
The 152 people included in our study were divided into 3 groups: Group 1, asymptomatic health workers whose PCR results were negative for SARS‐CoV‐2 (n = 50); Group 2, moderately ill patients with nonsevere pneumonia (severe pneumonia was defined as meeting any of the following criteria: respiratory rate ≥30 breaths/min, SpO2 ≤92%, and >50% lung infiltration) (n = 62); and Group 3, severely ill patients who were admitted with severe pneumonia and developed MAS during follow‐up (n = 40).
2.3. Exclusion criteria
Patients with chronic or clinically significant infectious or inflammatory conditions in the last month, asthma, chronic obstructive pulmonary disease (COPD), malignancy, invasive surgical intervention in the last month, uncontrolled hypertension, high fasting blood glucose, diabetes, cerebrovascular disease, kidney disease, and coronary artery disease were excluded. History and laboratory parameters obtained at admission were used to evaluate patients in terms of the exclusion criteria. The presence of coronary artery disease, asthma, COPD, and diabetes was determined based on consultation with the cardiology, chest diseases, and internal medicine departments.
2.4. Definitions and treatment
Axillary body temperature higher than 37.3°C was defined as fever. For patients with a high fever, while receiving treatment for COVID‐19, blood, urine, and sputum cultures were performed for possible bacterial and fungal superinfections. Empiric antibiotherapy was initiated and revised according to culture results. Diagnosis and grading of acute respiratory failure were done according to the Berlin 2015 diagnostic criteria. 2 If cardiac‐specific troponin level was above normal, patients were evaluated with echocardiography for newly developed cardiologic pathologies. Coagulopathy was defined as prothrombin time 3 s above normal and partial thromboplastin time 5 s above normal.
The treatment strategy was determined based on the patient's disease severity and the COVID‐19 adult diagnosis and treatment guide from the Turkish Ministry of Health. Patients with symptoms, such as refractory fever, persistent CRP and ferritin elevation, lymphopenia and thrombocytopenia, impaired liver function tests, hypofibrinogenemia, or elevated triglyceride and D‐dimer values were monitored for MAS. If daily serial measurements of these parameters showed progression that could not be explained by secondary bacterial infection, the patient was administered 400 mg of tocilizumab for MAS unless contraindicated. The treatment was not repeated if patients showed appropriate clinical and laboratory response after 24 h; however, patients with no response were treated again at the same dose.
3. MEASUREMENT OF BIOCHEMICAL MARKERS
After 15 min of semi‐supine rest, blood samples were obtained from an antecubital vein into tubes containing ethylenediaminetetraacetic acid to prevent coagulation. Troponin‐I concentrations were measured by chemiluminescent immunoassay using an Immulite 2500 (Siemens Medical Solutions). KIM‐1 concentration was measured by enzyme‐linked immunosorbent assay (Elabscience human ELISA Kit) and serum suPAR was measured using the suPARnostic™ assay (Virogates) following the manufacturer's protocol.
4. STATISTICAL ANALYSIS
The data were analyzed using IBM SPSS Statistics for Windows version 20.0 (IBM Corp). Pearson's χ 2 test and Mann–Whitney U test were used for intergroup comparisons of parametric data and nonnormally distributed numerical data, respectively. Independent‐samples t test was used to compare demographic data and laboratory parameters between the groups. Wilcoxon analysis was used for intragroup comparisons of laboratory values during follow‐up. Multivariate analysis of KIM‐1 and suPAR levels in moderate and severe COVID‐19 patients was performed. Pearson correlation analysis was used to evaluate relationships between KIM‐1 and suPAR levels and CRP, ferritin, D‐dimer, lymphocyte count, neutrophil to lymphocyte ratio (NLR), troponin‐I, and PaO2/FiO2. The p values of less than 0.05 were considered statistically significant.
5. RESULTS
The mean age of the 102 patients (59 men, 43 women) included in the study was 56.1 ± 14.9 years. The mean age of the control group was 53.1 ± 18.1 years. There was no statistically significant difference in age between the patient and control groups (p = 0.34). The mean age of the male patients was 56.3 ± 15.8 years and that of the female patients was 55.9 ± 13.8 years (p = 0.9).
None of the patients died during follow‐up, and none of the patients with severe disease required mechanical ventilation. Two patients with severe pneumonia developed massive pulmonary thromboembolism despite receiving enoxaparin sodium at 12‐h intervals in accordance with their disease severity.
The laboratory parameters of the COVID‐19 patients are presented according to disease severity in Table 1. Patients with severe disease had significantly higher NLR, lactose dehydrogenase (LDH), prothrombin time, CRP, PaO2/FiO2, D‐dimer, ferritin, and fibrinogen levels (p = 0.001, 0.001, 0.05, 0.001, 0.001, 0.005, 0.001, and 0.001, respectively), which have been stated in previous studies to have prognostic significance in COVID‐19.
Table 1.
Comparison of laboratory parameters at admission in patients with moderate and severe COVID‐19
| Moderate Illness (n = 62) | Severe Illness (n = 40) | p | ||
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| Age (year) | 56.5 ± 15.8 | 55.5 ± 13.6 | 0.753 | |
| WBC (/µl) | 9597.7 ± 4246.6 | 13769.3 ± 5799.2 | 0.001 | |
| Lymphocytes (/µl) | 934.2 ± 504.5 | 553.1 ± 332.1 | 0.001 | |
| Neutrophils (/µl) | 7829.6 ± 4076.6 | 8909.2 ± 4218.6 | 0.205 | |
| NLR | 10.5 ± 8.1 | 25.7 ± 28.4 | 0.001 | |
| AST (U/L) | 58.7 ± 41.7 | 60.5 ± 41.6 | 0.836 | |
| ALT (U/L) | 71.9 ± 70.1 | 80.2 ± 78.9 | 0.582 | |
| LDH (U/L) | 434.2 ± 139.8 | 620.9 ± 201.2 | 0.001 | |
| GGT (U/L) | 74.5 ± 40.4 | 134.6 ± 258.3 | 0.075 | |
| ALP (U/L) | 98.6 ± 50.8 | 103.7 ± 59.1 | 0.655 | |
| Creatine (mg/dl) | 1.1 ± 0.9 | 0.9 ± 1 | 0.543 | |
| Prothrombin time (s) | 12.6 ± 2.3 | 14.5 ± 5.4 | 0.05 | |
| CRP (mg/dl) | 59.3 ± 67.2 | 174.6 ± 89.9 | 0.001 | |
| Troponin‐I (ng/dl) | 27.5 ± 63.3 | 44.5 ± 47.3 | 0.127 | |
| PaO2/FiO2 | 270.8 ± 71.9 | 174.1 ± 26.2 | 0.001 | |
| D‐Dimer (ng/ml) | 1271.5 ± 1807.2 | 3374.5 ± 5246.8 | 0.005 | |
| Ferritin (ng/ml) | 633.3 ± 286.4 | 1433.3 ± 305.6 | 0.001 | |
| Fibrinogen (ng/ml) | 417.6 ± 141.9 | 524.9 ± 158.1 | 0.001 | |
Note: Bold values highlight statistically significant parameters. p = Comparison of parameters between groups.
Abbreviations: ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; COVID‐19, coronavirus disease 2019; CRP, C‐reactive protein; GGT, gamma glutamyl transferase; LDH, lactate dehydrogenase; NLR, neutrophil to lymphocyte ratio; PaO2/FiO2, ratio of arterial oxygen partial pressure to fractional inspired oxygen; WBC, white blood cells.
Comparisons of suPAR and KIM‐1 levels between the patient groups and with the control group are shown in Table 2. KIM‐1 and suPAR levels were significantly higher in the patient groups than in the control group (p = 0.001 for all). When compared between patients with moderate and severe COVID‐19, the KIM‐1 level was higher in the severe group (p = 0.001), while the suPAR level was higher in the moderate group (p = 0.034).
Table 2.
Comparison of admitting suPAR and KIM‐1 levels between COVID‐19 patients with moderate and severe disease and between patients and controls
| COVID‐19 severity | Control (mean ± SD) (n = 50) | *p/**p | ||
|---|---|---|---|---|
| Moderate (mean ± SD) (n = 62) | Severe (mean ± SD) (n = 40) | |||
| suPAR (ng/ml) | 8.4 ± 4.2 | 5.5 ± 3.1 | 1.3 ± 1.1 | 0.001/0.001 |
| KIM‐1 (pg/ml) | 84.5 ± 62.3 | 134.9 ± 66.5 | 34.6 ± 57.4 | 0.001/0.001 |
Note: *p = Comparison of moderate and severe patients, **p = Comparison of patients and controls.
Abbreviations: COVID‐19, coronavirus disease 2019; KIM‐1, kidney injury molecule‐1; suPAR, soluble urokinase plasminogen activator receptor.
A very weak negative correlation was observed between suPAR and age (r = −0.197; p = 0.05), while a weak positive correlation was observed between suPAR and PaO2/FiO2 (r = 0.336; p = 0.01) (Figure 1). There was no significant relationship between suPAR level and D‐dimer (r = −0.114; p = 0.255). A weak negative correlation was also observed between KIM‐1 level and PaO2/FiO2 (r = −0.319; p = 0.01; Figure 1), while weak to moderate positive correlations were detected between KIM‐1 and other prognostic parameters, such as NLR (r = 0.336; p = 0.01) and LDH (r = 0.466; p = 0.01).
Figure 1.
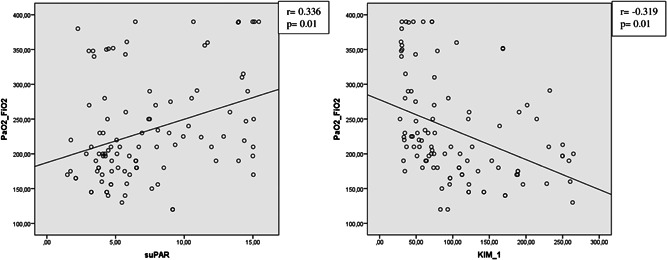
Correlation analysis of PaO2/FiO2 and serum suPAR and KIM‐1 levels of patients with COVID‐19. COVID‐19, coronavirus disease 2019; KIM‐1, kidney injury molecule‐1; suPAR, soluble urokinase plasminogen activator receptor
The results of multivariate analysis between moderate and severe COVID‐19 patients are shown in Table 3. The results demonstrated significant differences in both suPAR and KIM‐1 levels (F = 13.637, p = 0.001; Wilk's λ = 0.219; F = 15.151, p = 0.001, Wilk's λ = 0.219, respectively).
Table 3.
Multivariate analysis of suPAR and KIM‐1 levels in patients with moderate and severe COVID‐19
| Variable | Moderate (mean ± SD) (n = 62) | Severe (mean ± SD) (n = 40) | Type III sum of squares | F | p | Wilk's λ |
|---|---|---|---|---|---|---|
| suPAR (ng/ml) | 8.4 ± 4.2 | 5.5 ± 3.1 | 198.942 | 13.637 | 0.001 | 0.219 |
| KIM‐1 (pg/ml) | 84.5 ± 62.3 | 134.9 ± 66.5 | 61989.318 | 15.151 | 0.001 |
Abbreviations: COVID‐19, coronavirus disease 2019; KIM‐1, kidney injury molecule‐1; suPAR, soluble urokinase plasminogen activator receptor.
6. DISCUSSION
Consistent with previous studies, laboratory parameters reported having prognostic significance in COVID‐19 patients increased in correlation with clinical severity in this study. In addition, our evaluation of suPAR and KIM‐1 levels showed that suPAR level was lower in patients with severe disease compared to those with moderate disease, while KIM‐1 level increased in correlation with disease severity. PaO2/FiO2 was positively correlated with suPAR level and negatively correlated with KIM‐1 level.
SARS‐CoV‐2 is closely related to SARS‐CoV and MERS‐CoV, other coronaviruses that have caused past epidemics with significant morbidity and mortality. However, neither reached the scale of the current pandemic, which has infected over a hundred million people to date and continues to spread. 3
Lymphopenia is detected in most COVID‐19 patients, suggesting that SARS‐CoV‐2 may affect lymphocytes, particularly T lymphocytes, like SARS‐CoV does. T lymphocyte damage is also important in the development of the cytokine storm, 4 which occurs as a result of virus particles released from the respiratory mucosa and other infected cells cause abnormal cytokine discharge. Many proinflammatory cytokines are released during the cytokine storm, especially TNF‐α, IL‐1, IL‐2, IL‐6, and nitric oxide. These cytokines can cause increased vascular permeability, resulting in impaired tissue perfusion, endothelial damage, and microthrombus formation. Increased vascular permeability causes fluid accumulation in lung tissue and the interstitial area, leading to acute respiratory failure. It has been reported that IL‐1 and IL‐6 antagonists can be used to control this.5, 6
The dense presence of the KIM‐1 molecule in T cells led to a different name: T cell/transmembrane, immunoglobulin, and mucin (TIM‐1). The interaction of KIM‐1/TIM‐1 with T cells plays an important role in immune response, allergy, asthma, autoimmune diseases, and response to viral infections. 7 Initial evaluations of KIM‐1/TIM‐1 in COVID‐19 patients primarily focused on its relationship with acute kidney damage. 8 Like ACE‐2 receptor, KIM‐1/TIM‐1 was found to facilitate the viral entry into cells via the IgV domain. Higher proinflammatory cytokine levels due to increased viremia may cause further progression of kidney damage. 9 In studies with SARS‐CoV and MERS‐CoV, it was also determined that the IgV unit facilitates entry for other members of the family. 10 In another publication, it was suggested that KIM‐1/TIM‐1 receptors are abundant in the lungs and kidneys and that TW‐37, a molecule that can inhibit anti‐KIM‐1/TIM‐1 antibody and endocytosis, may be used as a therapeutic target. 7
Humoral immunity plays an important role in controlling infection after the development of viremia. One of the chemotactic agents that plays an important role in the migration of these cells is uPAR. uPAR and its serum‐soluble form, suPAR, mediate the conversion of plasminogen into plasmin.
By enabling a number of proteolytic activities to occur in the extracellular matrix, suPAR facilitates the migration of cells involved in the immune response. 11 Studies investigating the relationship between suPAR level and inflammatory diseases found that levels were high in diabetes mellitus, coronary artery disease, community‐acquired and ventilator‐associated pneumonia, smoking, acute exacerbations of COPD, and sepsis when compared with healthy controls.12, 14, 15, 16, 17, 18, 19, 20 Studies in patients with sepsis also showed that suPAR may have an important place in the prediction of early mortality and surveillance, and in a study of COVID‐19 patients, high suPAR level was evaluated as a potential early biomarker indicating the need for intensive care admission. 21 In another study, it was observed that although suPAR level increased in correlation with disease severity, asymptomatic COVID‐19 patients had higher suPAR levels than symptomatic patient groups. This was interpreted as possibly being due to increased production or increased shedding from the cell surface.
In this study, it was observed that laboratory parameters examined at admissions, such as NLR, LDH, prothrombin time, CRP, PaO2/FiO2, D‐dimer, ferritin, and fibrinogen levels, were higher in severe patients, consistent with previous COVID‐19 studies. It was also observed that the KIM‐1 level at admission was higher in patients with severe disease compared to those with moderate disease. This can be interpreted as a result of KIM‐1 mediating endocytosis of the virus into the cell and increasing viremia. In addition, increased viremia may have led to later development of MAS due to abnormal cytokine discharge in these patients.
In the evaluation of suPAR levels, our results contradicted those of previous studies. Compared to the healthy control group, both moderate and severe COVID‐19 patients had higher suPAR levels, but severely ill patients had a lower level than moderate COVID‐19 patients. SuPAR plays an important role in the migration of cells involved in immune defenses; therefore, low levels in patients with severe disease suggest that inability to launch an adequate immune response may be responsible for the increased clinical severity. It also plays a role in fibrinolytic activity; therefore, reduced levels may have led to a hypofibrinolytic state, resulting in acute respiratory distress. In addition, as suggested in previous studies, cell shedding may cause higher serum levels in patients with a high tissue regeneration rate. When the data obtained in the present study are evaluated in line with this interpretation, cell surface shedding may be higher in patients with moderate COVID‐19, who have better tissue regeneration, thereby increasing serum suPAR levels compared to patients with severe COVID‐19.
The most important limitation of this study was that the number of patients with severe disease was small compared to those with moderate disease. The small number of patients was a result of our exclusion of patients with comorbidities due to concern about their effect on the study parameters.
In conclusion, KIM‐1 has been identified as a new entry pathway for SARS‐CoV‐2 and its increase in correlation with disease severity as shown in our study indicates that it may be used as a therapeutic target in the future. Although suPAR has been evaluated as an early marker of poor prognosis in many diseases, more extensive studies are needed to determine if the same is true in COVID‐19.
7. CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Conceptualization, methodology, software, validation, formal analysis: Ferhan Kerget, Buğra Kerget, Alperen Aksakal. Investigation, resources, data curation: Ferhan Kerget, Buğra Kerget, Seda Aşkın. Writing–Original Draft, Writing—Review and Editing: Elif Yılmazel Uçar. Visualization, supervision, project administration: Leyla Sağlam.
Kerget B, Kerget F, Aksakal A, Aşkın S, Uçar EY, Sağlam L. Evaluation of the relationship between KIM‐1 and suPAR levels and clinical severity in COVID‐19 patients: A different perspective on suPAR. J Med Virol. 2021;93:5568‐5573. 10.1002/jmv.27099
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Yuki K, Fujiogi M, Koutsogiannaki S. COVID‐19 pathophysiology: a review. Clin Immunol. 2020;215:108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sjoding MW, Hofer TP, Co I, Courey A, Cooke CR, Iwashyna TJ. Interobserver reliability of the Berlin ARDS definition and strategies to improve the reliability of ARDS diagnosis. Chest. 2018;153(2):361‐367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peeri NC, Shrestha N, Rahman MS, et al. The SARS, MERS and novel coronavirus (COVID‐19) epidemics, the newest and biggest global health threats: what lessons have we learned? Int J Epidemiol. 2020;49(3):717‐726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. He R, Lu Z, Zhang L, et al. The clinical course and its correlated immune status in COVID‐19 pneumonia. J Clin Virol. 2020;127:104361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kerget B, Kerget F, Koçak AO, et al. Are serum interleukin 6 and surfactant protein D levels associated with the clinical course of COVID‐19? Lung. 2020;198(5):777‐784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kerget B, Kerget F, Aksakal A, Aşkın S, Sağlam L, Akgün M. Evaluation of alpha defensin, IL‐1 receptor antagonist, and IL‐18 levels in COVID‐19 patients with macrophage activation syndrome and acute respiratory distress syndrome. J Med Virol. 2020;93:2090‐2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ichimura T, Mori Y, Aschauer P, et al. KIM‐1/TIM‐1 is a receptor for SARS‐CoV‐2 in lung and kidney. medRxiv. 2020. 10.1101/2020.09.16.20190694 [DOI] [Google Scholar]
- 8. Luther T, Bülow‐Anderberg S, Larsson A, et al. COVID‐19 patients in intensive care develop predominantly oliguric acute kidney injury. Acta Anaesthesiol Scand. 2020;65:364‐372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang C, Zhang Y, Chen H, et al. Kidney injury molecule‐1 is a potential receptor for SARS‐CoV‐2. bioRxiv. 2020. 10.1101/2020.10.09.334052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rabaan AA, Al‐Ahmed SH, Haque S, et al. SARS‐CoV‐2, SARS‐CoV, and MERS‐COV: a comparative overview. Infez Med. 2020;28(2):174‐184. [PubMed] [Google Scholar]
- 11. D'Alonzo D, De Fenza M, Pavone V. COVID‐19 and pneumonia: a role for the uPA/uPAR system. Drug Discovery Today. 2020;25(8):1528‐1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu C‐Z, Chang L‐C, Lin Y‐F, et al. Urokinase plasminogen activator receptor and its soluble form in common biopsy‐proven kidney diseases and in staging of diabetic nephropathy. Clin Biochem. 2015;48(18):1324‐1329. [DOI] [PubMed] [Google Scholar]
- 13. Van Oort PM, Bos LD, Póvoa P, et al. Soluble urokinase plasminogen activator receptor for the prediction of ventilator‐associated pneumonia. ERJ Open Res. 2019;5(1):00212‐02018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tsai P‐K, Tsao S‐M, Yang W‐E, Yeh C‐B, Wang H‐L, Yang S‐F. Plasma soluble urokinase‐type plasminogen activator receptor level as a predictor of the severity of community‐acquired pneumonia. Int J Environ Res Public Health. 2019;16(6):1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gumus A, Altintas N, Cinarka H, et al. Soluble urokinase‐type plasminogen activator receptor is a novel biomarker predicting acute exacerbation in COPD. Int J Chronic Obstruct Pulm Dis. 2015;10:357‐365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guthoff M, Wagner R, Randrianarisoa E, et al. Soluble urokinase receptor (suPAR) predicts microalbuminuria in patients at risk for type 2 diabetes mellitus. Sci Rep. 2017;7(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Theilade S, Lyngbaek S, Hansen TW, et al. Soluble urokinase plasminogen activator receptor levels are elevated and associated with complications in patients with type 1 diabetes. J Intern Med. 2015;277(3):362‐371. [DOI] [PubMed] [Google Scholar]
- 18. Eugen‐Olsen J, Ladelund S, Sørensen LT. Plasma su PAR is lowered by smoking cessation: a randomized controlled study. Eur J Clin Invest. 2016;46(4):305‐311. [DOI] [PubMed] [Google Scholar]
- 19. Okulu E, Arsan S, Akin IM, et al. Serum levels of soluble urokinase plasminogen activator receptor in infants with late‐onset sepsis. J Clin Lab Anal. 2015;29(5):347‐352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hoenigl M, Raggam RB, Wagner J, et al. Diagnostic accuracy of soluble urokinase plasminogen activator receptor (suPAR) for prediction of bacteremia in patients with systemic inflammatory response syndrome. Clin Biochem. 2013;46(3):225‐229. [DOI] [PubMed] [Google Scholar]
- 21. Donadello K, Scolletta S, Covajes C, Vincent J‐L. suPAR as a prognostic biomarker in sepsis. BMC Med. 2012;10(1):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Poyiadji N, Cormier P, Patel PY, et al. Acute pulmonary embolism and COVID‐19. Radiology. 2020;297(3):E335‐E338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kerget B, Akgun M, Dogan N. Atypical presentation of COVID‐19: acute renal failure. Eurasian J Med. 2020;52(2):224‐226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Asselta R, Paraboschi EM, Mantovani A, Duga S. ACE2 and TMPRSS2 variants and expression as candidates to sex and country differences in COVID‐19 severity in Italy. 2020. [DOI] [PMC free article] [PubMed]
- 25. Azam TU, Shadid HR, Blakely P, et al. Soluble urokinase receptor (SuPAR) in COVID‐19–related AKI. J Am Soc Nephrol. 2020;31(11):2725‐2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hamie L, Daoud G, Nemer G, et al. SuPAR, an emerging biomarker in kidney and inflammatory diseases. Postgrad Med J. 2018;94(1115):517‐524. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


