Abstract
Background
Convalescent plasma (CP) is an important initial treatment in pandemics and the New York (NY) metropolitan area is likely to remain a hotspot for collection and distribution of such units. This study reports characteristics of coronavirus disease 19 CP (CCP) donors and their donations to the New York Blood Center (NYBC).
Study design and methods
All CCP data from our first day of collection on March 26th through July 7th, 2020 are included in this retrospective analysis. Donor and donation data were extracted from NYBC electronic databases. SARS‐CoV‐2 antibody testing was initially performed by the NY State Department of Health, and later by NYBC using Ortho and Abbott platforms.
Results
CCP donor age and ABO distributions were consistent with reported lower COVID‐19 susceptibility in O blood types. CCP versus whole blood donors had similar on‐site deferrals, but higher post‐donation deferral rates. CCP versus routine plasmapheresis donations had higher vasovagal reactions but similar product rejection rates. Changes in antibody (Ab) test platforms resulted in significant changes in the percent of donors regarded as antibody positive. Donor correlates with higher anti‐spike total Ig S/CO ratios were Hispanic ethnicity, overweight body mass index, and longer symptom duration; and with higher anti‐nucleocapsid IgG S/CO ratios were male gender, older age, Hispanic ethnicity, and fewer days between symptom onset and first donation.
Discussion
We identify donor characteristics not previously reported to correlate with Ab titer. Our analysis should assist with donor outreach strategies, blood center operating logistics, and recruitment of high titer donors.
Keywords: convalescent plasma, COVID‐19, COVID‐19 antibodies, SARS‐CoV‐2
List of Abbreviations
- BMI
body mass index
- COVID‐19
coronavirus disease 19
- CCP
COVID‐19 convalescent plasma
- Hct
hematocrit
- Hb
hemoglobin
- HLA
human leukocyte antigen
- MERS
Middle East Respiratory Syndrome
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus‐2
- TDT
transmissible disease testing
- WB
whole blood
1. INTRODUCTION
Convalescent plasma (CP) transfusion has been an important early therapeutic option in viral diseases ranging from measles and influenza to more recent outbreaks such as Ebola, SARS, MERS, and H1N1. 1 , 2 Given the severity of the coronavirus disease 19 (COVID‐19) pandemic and the historical safety of CP, the United States Food and Drug Administration (FDA) issued an emergency use authorization on August 23, 2020 for the use of COVID‐19 convalescent plasma (CCP) in patients hospitalized with COVID‐19. The use of high‐titer CCP early in the disease course of hospitalized patients has been shown to reduce COVID‐19 severity and mortality. 3 , 4 , 5 , 6 , 7 , 8
Although vaccines and monoclonal antibodies are now available, uncertainty about long‐term herd immunity 9 given the continuous rise of new SARS‐CoV‐2 variants highlights the importance of strategies to identify appropriate donors of CCP and CCP‐derived hyperimmune globulin, given their current advantage of broader antibody (Ab) repertoire.
Our analysis of characteristics of CCP donors and CCP donations is notable in several ways. First, the New York City (NYC) area, with its population density and international travel status, was a major epicenter of the first wave of the COVID‐19 pandemic, with 413,222 cases reported in New York State (NYS) 10 through July 7th, 2020, the end date of our study presented herein. As the major blood collection facility in the NY metropolitan area, NYBC was the first blood collection facility to implement CCP collection for nationwide distribution. 11 Thus, our experience collecting CCP donors is relevant for future pandemic planning, especially our comparison of donor Ab reactivity based on initially versus subsequently available testing platforms. Second, some of the donor characteristics collected are unique to our study; this includes measures relevant to blood center logistics, 12 such as on‐site deferral reasons and ABO type, as well as measures relevant for Ab signal to cutoff (S/CO) ratios, such as body mass index (BMI) and symptom features. Also, our analysis of correlates of Ab S/CO ratio involves a larger sample size than previous publications. 13 , 14 , 15 , 16 , 17 , 18 Lastly, some of our outcome data, such as adverse reaction rates and product rejection due to insufficient quantity, differs from and clarifies previous report, 12 likely because our comparison group was to plasmapheresis only donors, compared to also including plateletpheresis donors.
2. MATERIALS AND METHODS
2.1. Study
The study was conducted under IRB approval at New York Blood Center.
2.2. Donors
All CCP data from March 26th, our first day of CCP collection, through July 7th were included in this retrospective analysis, a time period in which SARS‐CoV‐2 is not thought to have undergone mutations affecting its infectivity or clinical disease severity. To be eligible to donate, CCP donors were required to show laboratory confirmation of a positive SARS‐CoV‐2 test, be 14 days symptom‐free, and meet all regular blood donor eligibility criteria. NYBC does not require repeat nucleic acid testing prior to donation. Collection was by standard donor plasmapheresis with weight‐adjusted collection volume up to a maximum of 600 ml. CCP donors were allowed to donate once per week up to 8 weeks and then monthly until their Ab level no longer met requirements. Donor zip code, donation site by state county, residence, gender, age, BMI, ABO type, ethnicity, symptom duration in days, and days from symptom onset to first donation were tabulated by unique donor from donor registration data obtained with their first donation. Optional ethnicity data were missing for five CCP donors. On‐site deferrals, post‐donation deferrals, and donor adverse events were tabulated by unique donation, as these characteristics can vary between donations from the same donor. We do not actively monitor for post‐donation adverse events, and the small number of adverse events called in post‐donation were not included in our analysis. For gender, age, ABO type, ethnicity, BMI, on‐site deferrals, and post‐donation deferrals, CCP data were compared to whole blood (WB) donor data from the same time period in the year prior (2019). For donor adverse events, CCP data were compared to standard plasmapheresis only donation data from the same time period in the year prior (2019).
2.3. Antibody tests
SARS‐CoV‐2 Ab testing on each donation was initially performed by the NYS Department of Health (DOH) (New York SARS‐CoV Microsphere Immunoassay for Ab Detection, Wadsworth Center at the NYS DOH, New York, NY); results were provided as positive, negative, or indeterminate. Of donors initially tested by NY State, all negative and indeterminate donors and a subset of reactive donors were later tested by NYBC laboratories using tests providing an Ab S/CO ratio: VITROS Immunodiagnostic Products Anti‐SARS‐CoV‐2 Total Reagent Pack (Ortho‐Clinical Diagnostics, Rochester, NY) and/or Architect SARS‐CoV‐2 IgG (Abbott Laboratories, Abbott Park, IL).
2.4. Statistics
Descriptive statistics were generated for ethnicity, gender, ABO group, BMI category, symptom duration, and time since symptom onset. For comparison of CCP to WB and plasmapheresis donor and donation data, one‐sample tests of proportions and one‐sample t‐tests were conducted. In addition, for two‐group comparisons, Student's t‐tests were used to test mean differences for continuous measures and Chi‐square tests were used to test for categorical associations. Antibody platform analyses were conducted stratified by gender and parallel analyses were run for each of the two immunoassay tests. For donor data, invalid dates were set to missing and listwise deletion was implemented for missing data.
Bivariate statistical tests were conducted to test associations between characteristics and Ab S/CO ratio levels. Linear regression models were generated to further explore the relation between measures examined in the stratified and bivariate analyses. Statistical analyses were conducted with RStudio (R Foundation for Statistical Computing, 2019, https://www.R-project.org), Tableau, Microsoft Office 365 Excel (version: 2002), and Python.
3. RESULTS
3.1. Donor characteristics
Over the ~4 month period of analysis, a total of 8875 unique CCP donors contributed to a total of 11,794 successful donations. Figure 1 shows, by zip code (NYC) and county (NYS) residence, the differences in the percent of CCP donors subtracted from the percent of COVID‐19 cases (as tabulated by NYC 19 and NYS 10 ). Within NYC, the CCP donor distribution generally mirrored the COVID‐19 infection distribution, with the highest over‐representation in parts of Manhattan, Brooklyn, and Staten Island. On the NYS level, CCP donor percentages were higher than COVID‐19 case percentages in NYC, Long Island, Rockland, and Westchester counties. Counties with the highest proportion of donors were Nassau (17%), New York (14%), Suffolk (10%), Queens (10%), Westchester (10%), Kings (9%), and Rockland (6%).
FIGURE 1.
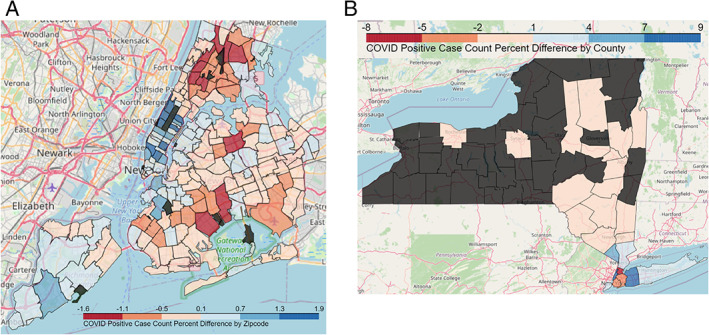
Difference in the percent of coronavirus disease 19 (COVID‐19) convalescent plasma donors subtracted from the percent of COVID‐19 case infections. (A) By donor home zip code (B) by donor center county. Dark gray areas are those from which no donors were collected [Color figure can be viewed at wileyonlinelibrary.com]
Figure 2 shows characteristics of CCP donors, totaling 8875 unique donors, compared to our 2019 WB donors, totaling 74,368 unique donors. The gender distribution of CCP donors at 51.6% female and 48.4% male was significantly (p < 0.002) although mildly different from that of our WB donors of 49.8% female and 50.2% male. Most CCP donors were in their 30s through 50s, with the mean being 45 ± 14 years, statistically (p < 0.001) but again mildly different from our WB donor age mean of 43 ± 17 years. Although mostly of O and A blood types, fewer CCP donors than WB donors had O blood type and more had A blood type. NYBC, however, specifically recruits WB donors with O blood type, whereas specific ABO types were not targeted in CCP donors. More CCP compared to WB donors had the AB type, which is desirable as the universal plasma donor. CCP compared to WB donors were more likely to be white and less likely to be Latino, Asian, or black. During this time period, however, NYBC suspended its mobile drives, which provide outreach to communities of color. The BMI distribution of CCP donors was 30% obese, 36% overweight, 32% normal, and less than 1% underweight; distribution compared to that of WB donors significantly differed only in the underweight category.
FIGURE 2.
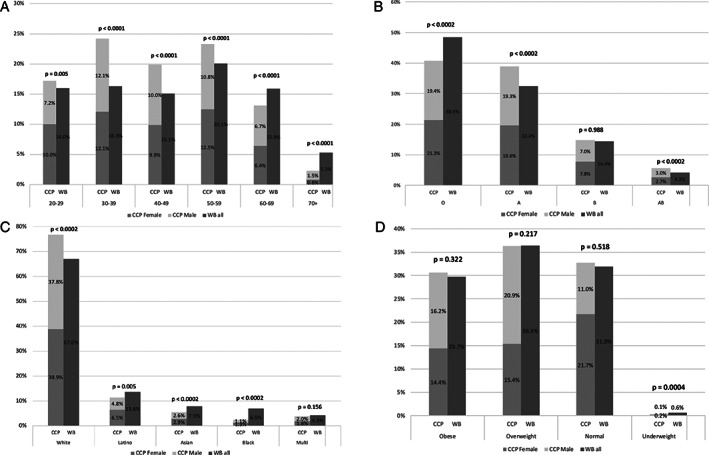
Characteristics of coronavirus disease 19 convalescent plasma donors, with comparisons to whole blood donors. (A) Age (B) ABO type (C) ethnicity (D) body mass index class
3.2. Donation deferrals
Of 13,633 presentations for CCP donation and 82,468 presentations for WB, 1502 (11%) of CCP compared to 8875 (11%) of WB presentations were deferred at the time of their onsite visit. For onsite deferrals (Figure 3(A)), prospective CCP compared to WB donors had higher percentages deferred for Hgb/Hct, medications, weight, and not feeling well, and lower percentages deferred for vital signs and travel/residence. Of 11,794 successful CCP donations and 78,342 successful WB donations, 1086 (9.2%) of CCP donations versus 1142 (1.5%) of WB donations (p < 0.0001) were discarded or diverted based on post‐donation testing. For post‐donation deferrals (Figure 3(B)), CCP donors had a higher percentage of deferrals from plasma and platelet donation for HLA Abs and a lower percentage of deferrals for transmissible disease testing (TDT) markers. Given the lower number of successful CCP donations, however, the absolute TDT deferral rate was 0.63% of all successful CCP donations versus 0.27% of all successful WB donations. Of note, first‐time donors made up 64% of CCP donors compared to 19% of WB donors during their respective time periods.
FIGURE 3.
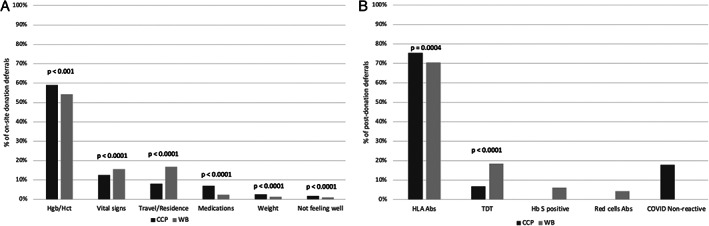
Main reasons for on‐site and post‐donation deferrals for coronavirus disease 19 convalescent plasma (CCP) compared to whole blood (WB) donations. (A) On‐site deferrals (N = 1502 CCP and 8875 WB). (B) Post‐donation deferrals (N = 1086 CCP and 1142 WB)
3.3. Donation adverse events
In order to compare the same type of collection procedure, adverse events incurred while donating CCP were compared to those with standard plasmapheresis, for which 598 unique donors contributed a total of 754 successful donations in 2019. Of the three types of adverse events (Figure 4), CCP donors had a higher rate of vasovagal reactions (1.1% versus 0.5%, p < 0.0001) and lower rate of bruising (4% versus 4.6%, p < 0.001), but there was no difference in the rate of citrate toxicity (0.14% versus 0.13%). Within the CCP donor group, first‐time blood donors had higher rates of all reactions compared to repeat blood donors. Overall, collected products were rejected for insufficient quantity in 1.4% of CCP donations compared to 1.6% of plasmapheresis only donations.
FIGURE 4.
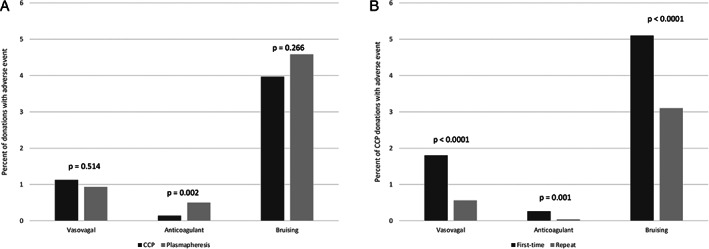
On‐site adverse events for coronavirus disease 19 convalescent plasma (CCP) donations. (A) CCP versus plasmapheresis donations. (B) First‐time CCP versus repeat CCP donations
3.4. Utility of various Ab testing platforms
Table S1 summarizes the Ab platforms used to test NYBC CCP donor samples during the time period studied. Initially, CCP plasma was tested with the NYS DOH anti‐nucleocapsid total Ig test, for which results were reported as reactive, non‐reactive, or indeterminate. Figure 5(A) shows the percentage of each NYS DOH result by gender. Of 8340 donors tested with the NYS DOH platform, 92% were reactive, 4% were non‐reactive, and 4% were indeterminate. Because of its conservative criteria for reactivity (6 SDs above cutoff) and nucleocapsid specificity, donors were re‐tested once NYBC initiated in‐house Ortho platform anti‐spike total Ig testing. Figure 5(B) shows results of anti‐spike total Ig testing in individuals who were indeterminate or negative by NYS DOH testing; 95% of indeterminate and 40% of non‐reactive donors tested reactive for anti‐spike total Ig. Figure 5(C) displays the anti‐spike total Ig S/CO ratios for the three groups of NYS DOH results. The median S/CO ratio for the entire sample was 55, while the median S/CO ratios for the reactive, indeterminate, and non‐reactive groups were 148, 44, and 0.1, respectively.
FIGURE 5.
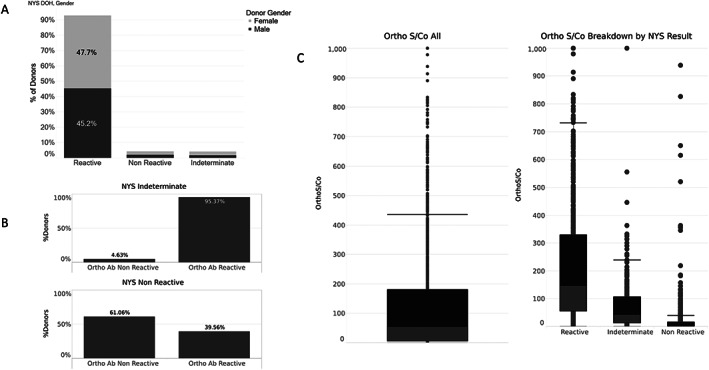
Comparison of donor Ab reactivity with New York State (NYS) Department of Health (DOH) compared to Ortho anti‐spike total Ig test. (A) NYS DOH Ab reactivity. (B) Ortho reactivity for NYS DOH reactive and indeterminate result categories. (C) Ortho Ab titers for each NYS DOH result category
3.5. Correlates of Ab S/CO ratios
Table 1 shows the results of univariate regression analysis for the outcome of anti‐spike total Ig and anti‐nucleocapsid IgG S/CO ratios (on first donation) and the following independent variables: age, gender, BMI, ABO type, ethnicity, symptom duration, and days from symptom onset to first donation. The mean symptom duration was 14.1 ± 9.4 days, with a median 13 (range 0–103) days. The mean days from symptom onset to first donation was 56.9 ± 24.7 days, with a median 53 (range 14–120) days. On univariate analysis, significant correlates for anti‐spike total Ig S/CO were male gender, Hispanic ethnicity, obese BMI, and symptom duration. Significant correlates for anti‐nucleocapsid IgG S/CO were increasing age, male gender, overweight or obese BMI, and days between symptom onset and first donation.
TABLE 1.
Univariate and multivariable analyses for correlates of Ortho and Abbott S/CO ratios a
| Predictor | Univariate analysis | Multivariable analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| Ortho S/CO (n = 1080) | Abbott S/CO (n = 389) | Ortho S/CO (n = 349) | Abbott S/CO (n = 88) | |||||
| β (SE) | p‐value | β (SE) | p‐value | β (SE) | p‐value | β (SE) | p‐value | |
| Age | 0.58 (0.38) | NS | 0.06 (0.02) | <0.001 | −0.02 (0.70) | NS | 0.09 (0.03) | 0.001 |
| Gender (ref group: female) | 23.43 (9.93) | 0.019 | 0.91 (0.31) | 0.003 | 12.47 (18.72) | NS | 1.49 (0.69) | 0.034 |
| Ethnicity | ||||||||
| Black | 61.85 (44.90) | NS | 2.41 (1.55) | NS | −21.79 (50.30) | NS | 3.24 (1.78) | NS |
| Hispanic | 85.75 (21.77) | <0.001 | 0.92 (0.69) | NS | 91.76 (26.78) | <0.001 | 2.12 (0.91) | 0.023 |
| Multiracial | 3.33 (46.54) | NS | 0.51 (1.39) | NS | −64.51 (62.46) | NS | 1.05 (2.16) | NS |
| White | Ref group | ‐ | Ref group | ‐ | Ref group | ‐ | Ref group | ‐ |
| Asian | 3.49 (36.16) | NS | 0.31 | NS | 1.53 (43.99) | NS | 1.34 (1.37) | NS |
| BMI categories | ||||||||
| Underweight | 53.48 (93.10) | NS | 4.24 (2.96) | NS | −25.41 (116.12) | NS | n/a | |
| Normal | Ref group | ‐ | Ref group | ‐ | ‐ | ‐ | Ref group | ‐ |
| Overweight | 26.05 (11.48) | NS | 1.43 (0.35) | <0.001 | −43.01 (21.64) | 0.048 | −0.77 (0.78) | NS |
| Obese | 80.95 (12.55) | <0.001 | 1.85 (0.40) | <0.001 | 41.44 (22.9) | NS | −0.78 (0.84) | NS |
| Unknown | 62.35 (54.16) | NS | 2.09 (2.10) | NS | 71.07 (96.65) | NS | n/a | |
| ABO | ||||||||
| AB | −22.48 | NS | −0.85 (0.72) | NS | ||||
| B | −0.84 | NS | 0.27 (0.48) | NS | Not included | Not included | ||
| O | 15.95 | NS | −0.40 (0.34) | NS | ||||
| A | Ref group | ‐ | Ref group | ‐ | ||||
| Symptom duration | 2.34 (1.09) | 0.032 | 0.02 (0.05) | NS | 3.07 (1.10) | 0.006 | 0.052 (0.05) | NS |
| Days between symptom onset and first donation | −0.36 (0.29) | NS | −0.05 (0.01) | <0.001 | −0.71 (0.39) | NS | −0.04 (0.02) | 0.049 |
Abbreviation: NS, not significant.
Significant correlates are shown in bold.
Table 1 also shows the multivariable analysis performed on the subset of donors for whom data on symptom duration and days between symptom onset and first donation was available. All variables except ABO type (because of lack of significance on univariate analysis) were carried into the multivariable analysis. Anti‐spike total Ig and anti‐nucleocapsid IgG S/CO had different correlates: for anti‐spike total Ig, the three correlates were Hispanic ethnicity, overweight BMI, and symptom duration. Being Hispanic predicted a mean S/CO increase of 92 units; being overweight predicted a mean decrease of 43 units compared to normal weight, and each day increase in symptom duration predicted a mean increase of 3 units. For anti‐nucleocapsid IgG S/CO, the four correlates were age, gender, Hispanic ethnicity, and days between symptom onset and first donation. Each year increase in age predicted an S/CO mean increase of 0.1 units; being male predicted a mean increase of 1.5 units compared to females; Hispanic ethnicity predicted a mean increase of 2 units; and each day increase in days between symptom onset and first donation predicted an S/CO decrease of 0.04 units.
4. DISCUSSION
Previous studies suggest that people of male gender, 20 older age, 21 higher BMI, 22 and Hispanic and black ethnicity 23 may have higher susceptibility to SARS CoV‐2 infection. Indeed, in NYS, those more likely to be diagnosed with SARS‐CoV‐2 infection were males, those 55 years of older, Hispanics, and blacks. 24 Comparing CCP to WB donors, however, females, older age, and whites were significantly over‐represented. During the study time period, however, NYBC was the beneficiary of CCP donation efforts within the Orthodox Jewish community and also was not collecting blood through mobile drives, where we historically recruit the majority of our non‐white donors.
Previous studies also suggest that the O blood group may be associated with slightly lower and the A blood group with slightly higher susceptibility to SARS‐CoV‐2 infection. 25 , 26 , 27 Comparison to our WB donor ABO type distribution is consistent with the reported risks, with the caveat that NYBC specifically targets O blood type donors for WB donations. We therefore calculated, based on our CCP donor ethnicity distribution, 28 our expected ABO type distribution, which is as follows: 44% O, 36% A, 11% B, and 4% AB. Our actual distribution is significantly different from expected (p < 0.0001 for all ABO types), and consistent with the previously reported risks. Also observed is a relative increase in AB blood type among CCP donors, which is beneficial given that AB donors are universal plasma donors.
Another study 12 also evaluated donation‐related adverse events and reasons for post‐donation deferral. Their group found increased adverse events (significance not provided) in CCP compared to apheresis blood donors. In our study with a similar percentage of first‐time blood donors (64% versus 56% in their study), CCP donors had an increased rate for only vasovagal reactions; their hematoma rate was slightly lower and their citrate reaction rate was similar. Similarly, their study had a higher percentage of CCP products rejected due to insufficient quantity (6.4%) compared to ours (1.4%). Study differences are likely related to our comparison to plasmapheresis only donors, whereas their comparison group was both platelet‐ and plasma‐pheresis donors. Our study is unique in evaluating reasons for on‐site deferral, with Hb/Hct the most common reason for deferral in both CCP and WB donors. The second and third most common reasons for deferrals in CCP donors were vital signs (blood pressure most common) followed by travel/residence, whereas in WB donors these were reversed (travel/residence then vital signs). For post‐donation deferrals, our study also found HLA Ab reactivity to the most common reason for deferral, followed by positive TDT markers; as with the previous study, 12 positive hepatitis B screening was the most common TDT‐related cause for deferral.
Our analysis of the variation in Ab reactivity with the NYS DOH anti‐nucleocapsid total Ig compared to Ortho anti‐spike total Ig highlights the importance of comparing Ab platforms and adjusting as needed. Since 95% and 40% of indeterminate and non‐reactive NYS DOH results were reactive by Ortho anti‐spike Ig testing, reliance on the NYS DOH test alone would have resulted in our ceasing CCP collection on 318 indeterminate and 132 non‐reactive donors. NYBC has continued to adjust its testing algorithm given current data that neutralizing Ab levels correlate best with anti‐S1 IgG and anti‐S1 total Ig levels 29 , 30 , 31 ; we now use Ortho platform anti‐spike IgG and anti‐spike total Ig testing. Ab testing platform is also relevant for distinguishing between vaccine‐induced anti‐spike versus infection‐induced COVID‐19 immunity; Abbott anti‐nucleocapsid IgG testing has good correlation with neutralizing Ab titer 16 , 31 and identifies infection‐induced COVID‐19 immunity.
Among patients hospitalized with COVID‐19 and not receiving mechanical ventilation, transfusion of high‐titer CCP reduced COVID‐19 progression and was associated with a lower risk of death. 7 , 8 With the FDA now requiring transfusion of high‐titer CCP only (currently based on an S/CO cutoff with the Ortho VITROS IgG assay of 9.5 or greater), identifying predictive factors for CCP donors likely to have high titers would be useful. Our analysis of correlates for both anti‐spike total Ig and anti‐nucleocapsid IgG S/CO ratios, both of which correlate well with neutralizing Ab titer, 31 is notable for its sample size of up to 1080 donors with univariate and 349 donors with multivariate analysis, compared to previous studies 13 , 14 , 15 with sample sizes of no more than 250 donors.
Previous analyses, in either donor or patient populations, found associations of Ab titer with older age, male gender, AB versus O blood type, fever during acute illness, and hospitalization. 13 , 14 , 15 , 16 , 17 , 18 On univariate analysis, we also found that older age and male gender were correlates of Ab S/CO ratios, but did not find any association with ABO type. We also identified correlates, such as ethnicity, BMI, and symptom duration, that were not previously shown to correlate with Ab S/CO ratios. In fact, previous smaller studies found no correlation of BMI and symptom duration with initial antibody titer. 13 , 17 However, a negative association of BMI is plausible given the greater risk of mortality among overweight patients. 32 Association of longer symptom duration is also plausible given its correlation with disease severity, 17 which is a predictor of Ab titer. 16 , 17 , 33
We also found days between symptom onset and first donation to be a negative predictor of anti‐nucleocapsid IgG S/CO ratio, a plausible association given typically declining anti‐spike and anti‐nucleocapsid IgG levels over time. 17 , 33 , 34 Indeed, recent FDA guidance specifies that CCP donors who also receive SARS‐CoV‐2 vaccination can only donate within 6 months after symptom resolution. Our data, considered with data observing relatively low anti‐spike and anti‐nucleocapsid IgG S/CO ratios prior to 28 days from symptom onset to first donations, 35 suggest that the optimal period of time to recruit donors for CCP donation is between 1 and 6 months post‐symptom onset.
There are some limitations to this study. The period of analysis covers the first pandemic wave, but not the second one, and CCP against the strains present during the first pandemic may be less effective against the current circulating variants. 36 Our uni‐ and multi‐variate analysis for correlates of Ab S/CO titer was retrospective only, without a prospective validation cohort, and did not include some potentially easily collectable variables, such as history of hospitalization.
Collection and usage of CCP is currently waning given increasing vaccination rates and availability of alternative early‐stage treatments. Nevertheless, our data on CCP donor characteristics, on‐site and post‐donation donation deferrals, donation adverse events, utility of evolving Ab testing platforms, and clinical correlates of Ab S/CO ratios will help blood centers to better prepare for any future pandemics or any recurrent waves of COVID‐19 infection due to evolution of more virulent SARS‐CoV‐2 strains.
CONFLICT OF INTEREST
The authors have disclosed no conflicts of interest.
Supporting information
Table S1. Summary of antibody assays of CCP donors, including cutoffs for reactivity.
ACKNOWLEDGMENTS
We thank the CCP donors who were the subjects of this study for their dedication to help others in need.
Jain S, Garg K, Tran SM, et al. Characteristics of coronavirus disease 19 convalescent plasma donors and donations in the New York metropolitan area. Transfusion. 2021;61:2374–2383. 10.1111/trf.16421
REFERENCES
- 1. Marano G, Vaglio S, Pupella S, Facco G, Catalano L, Liumbruno GM, et al. Convalescent plasma: new evidence for an old therapeutic tool? Blood Transfus. 2016;14:152–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Selvi V. Convalescent plasma: a challenging tool to treat COVID‐19 patients—a lesson from the past and new perspectives. Biomed Res Int. 2020;2020:2606058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. Effectiveness of convalescent plasma therapy in severe COVID‐19 patients. Proc Natl Acad Sci U S A. 2020;117:9490–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rajendran K, Krishnasamy N, Rangarajan J, Rathinam J, Natarajan M, Ramachandran A. Convalescent plasma transfusion for the treatment of COVID‐19: systematic review. J Med Virol. 2020;92:1475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu STH, Lin HM, Baine I, Wajnberg A, Gumprecht JP, Rahman F, et al. Convalescent plasma treatment of severe COVID‐19: a propensity score‐matched control study. Nat Med. 2020;26:1708–13. [DOI] [PubMed] [Google Scholar]
- 6. Salazar E, Christensen PA, Graviss EA, Nguyen DT, Castillo B, Chen J, et al. Significantly decreased mortality in a large cohort of coronavirus disease 2019 (COVID‐19) patients transfused early with convalescent plasma containing high‐titer anti‐severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) spike protein IgG. Am J Pathol. 2021;191:90–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Libster R, Perez Marc G, Wappner D, Coviello S, Bianchi A, Braem V, et al. Early high‐titer plasma therapy to prevent severe Covid‐19 in older adults. N Engl J Med. 2021;384:610–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Joyner MJ, Carter RE, Senefeld JW, Klassen SA, Mills JR, Johnson PW, et al. Convalescent plasma antibody levels and the risk of death from Covid‐19. N Engl J Med. 2021;384:1015–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fontanet A, Cauchemez S. COVID‐19 herd immunity: where are we? Nat Rev Immunol. 2020;20:583–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Department of Health NYS . NYSDOH COVID‐19 Tracker. https://covid19tracker.health.ny.gov/views/NYS-COVID19-Tracker/NYSDOHCOVID-19Tracker-Map?%3Aembed=yes&%3Atoolbar=no&%3Atabs=n, 2021.
- 11. Budhai A, Wu AA, Hall L, Strauss D, Paradiso S, Alberigo J, et al. How did we rapidly implement a convalescent plasma program? Transfusion. 2020;60:1348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lasky B, Goodhue Meyer E, Steele WR, Crowder LA, Young PP. Covid‐19 convalescent plasma donor characteristics, product disposition and comparison with standard apheresis donors. Transfusion. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Madariaga MLL, Guthmiller JJ, Schrantz S, Jansen MO, Christensen C, Kumar M, et al. Clinical predictors of donor antibody titre and correlation with recipient antibody response in a COVID‐19 convalescent plasma clinical trial. J Intern Med. 2020;289:559–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boonyaratanakornkit J, Morishima C, Selke S, Zamora D, McGuffin SA, Shapiro AE, et al. Clinical, laboratory, and temporal predictors of neutralizing antibodies to SARS‐CoV‐2 among COVID‐19 convalescent plasma donor candidates. J Clin Invest. 2020;131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klein SL, Pekosz A, Park HS, Ursin RL, Shapiro JR, Benner SE, et al. Sex, age, and hospitalization drive antibody responses in a COVID‐19 convalescent plasma donor population. J Clin Invest. 2020;130:6141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang Y, Zhang L, Sang L, Ye F, Ruan S, Zhong B, et al. Kinetics of viral load and antibody response in relation to COVID‐19 severity. J Clin Invest. 2020;130:5235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen Y, Zuiani A, Fischinger S, Mullur J, Atyeo C, Travers M, et al. Quick COVID‐19 healers sustain anti‐SARS‐CoV‐2 antibody production. Cell. 2020;183:1496–507 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li K, Huang B, Wu M, Zhong A, Li L, Cai Y, et al. Dynamic changes in anti‐SARS‐CoV‐2 antibodies during SARS‐CoV‐2 infection and recovery from COVID‐19. Nat Commun. 2020;11:6044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Department of Health NYC . COVID‐19: Data. https://www1.nyc.gov/site/doh/covid/covid-19-data-totals.page#zip, 2021.
- 20. Vahidy FS, Pan AP, Ahnstedt H, Munshi Y, Choi HA, Tiruneh Y, et al. Sex differences in susceptibility, severity, and outcomes of coronavirus disease 2019: cross‐sectional analysis from a diverse US metropolitan area. PLoS One. 2021;16:e0245556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Davies NG, Klepac P, Liu Y, Prem K, Jit M, group CC‐w, Eggo RM . Age‐dependent effects in the transmission and control of COVID‐19 epidemics. Nat Med. 2020;26:1205–11. [DOI] [PubMed] [Google Scholar]
- 22. Aung N, Khanji MY, Munroe PB, Petersen SE. Causal inference for genetic obesity, Cardiometabolic profile and COVID‐19 susceptibility: a Mendelian randomization study. Front Genet. 2020;11:586308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mackey K, Ayers CK, Kondo KK, Saha S, Advani SM, Young S, et al. Racial and ethnic disparities in COVID‐19‐related infections, hospitalizations, and deaths: a systematic review. Ann Intern Med. 2020;174:362–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rosenberg ES, Tesoriero JM, Rosenthal EM, Chung R, Barranco MA, Styer LM, et al. Cumulative incidence and diagnosis of SARS‐CoV‐2 infection in New York. Ann Epidemiol. 2020;48:23–9.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ray JG, Schull MJ, Vermeulen MJ, Park AL. Association between ABO and Rh blood groups and SARS‐CoV‐2 infection or severe COVID‐19 illness: a population‐based cohort study. Ann Intern Med. 2020;174:308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zietz M, Zucker J, Tatonetti NP. Associations between blood type and COVID‐19 infection, intubation, and death. Nat Commun. 2020;11:5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wu BB, Gu DZ, Yu JN, Yang J, Shen WQ. Association between ABO blood groups and COVID‐19 infection, severity and demise: a systematic review and meta‐analysis. Infect Genet Evol. 2020;84:104485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Garratty G, Glynn SA, McEntire R, Retrovirus Epidemiology Donor S . ABO and Rh(D) phenotype frequencies of different racial/ethnic groups in the United States. Transfusion. 2004;44:703–6. [DOI] [PubMed] [Google Scholar]
- 29. McAndrews KM, Dowlatshahi DP, Dai J, Becker LM, Hensel J, Snowden LM, et al. Heterogeneous antibodies against SARS‐CoV‐2 spike receptor binding domain and nucleocapsid with implications for COVID‐19 immunity. JCI Insight. 2020;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Legros V, Denolly S, Vogrig M, Boson B, Siret E, Rigaill J, et al. A longitudinal study of SARS‐CoV‐2‐infected patients reveals a high correlation between neutralizing antibodies and COVID‐19 severity. Cell Mol Immunol. 2021;18:318–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Luchsinger LL, Ransegnola BP, Jin DK, Muecksch F, Weisblum Y, Bao W, et al. Serological assays estimate highly variable SARS‐CoV‐2 neutralizing antibody activity in recovered COVID‐19 patients. J Clin Microbiol. 2020;58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hussain A, Mahawar K, Xia Z, Yang W, El‐Hasani S. Obesity and mortality of COVID‐19. Meta‐analysis Obes Res Clin Pract. 2020;14:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33. Ripperger TJ, Uhrlaub JL, Watanabe M, Wong R, Castaneda Y, Pizzato HA, et al. Orthogonal SARS‐CoV‐2 serological assays enable surveillance of low‐prevalence communities and reveal durable humoral immunity. Immunity. 2020;53:925–33 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Patel MM, Thornburg NJ, Stubblefield WB, Talbot HK, Coughlin MM, Feldstein LR, et al. Change in antibodies to SARS‐CoV‐2 over 60 days among health care personnel in Nashville, Tennessee. JAMA. 2020;324:1781–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li L, Tong X, Chen H, He R, Lv Q, Yang R, et al. Characteristics and serological patterns of COVID‐19 convalescent plasma donors: optimal donors and timing of donation. Transfusion. 2020;60:1765–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Peng P, Hu J, Deng HJ, Liu BZ, Fang L, Wang K, et al. Changes in the humoral immunity response in SARS‐CoV‐2 convalescent patients over 8 months. Cell Mol Immunol. 2021;18:490–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary of antibody assays of CCP donors, including cutoffs for reactivity.


