Funding sources
None.
Conflict of interest
None.
Editor
As vaccination campaign against SARS‐CoV‐2 is ongoing worldwide, dermatologists are witnessing an increasing number of cutaneous adverse events. Delayed hypersensitivity reactions at the site of injections with mRNA‐1273 (Moderna) 1 and BNT162b2 (Pfizer‐BioNtech) vaccines 2 are now known as ‘COVID‐arms’. Besides, cases of Rowell’s syndrome 24 h after vaccination 3 and persistent exanthema have been recently reported. 4 Bostan and Yalici‐Armagan 5 described the case of a 78‐year‐old man with thoracic herpes zoster (HZ) 5 days after COVID‐19 vaccination. We wish to report an additional case from Finland.
A 44‐year‐old healthcare provider received his first injection of BNT162b2 mRNA COVID‐19 vaccine on 2nd February. He had a history of dyslipidemia and active smoking. He presented pain and local redness after vaccination, as widely reported. 2 However, after a week, pain had not subsided and extended to the neck and the left hand. It also changed to a neuropathic pain. He also developed intense tiredness and noticed a rash on the left upper limb. Upon full examination, he displayed an herpetiform vesicular and erythematous rash on the left upper back (Fig. 1a) and lateral side and inner side of the left arm (Fig. 1b) that followed approximately C5–C6 dermatomes. A diagnosis of HZ was made. He still presented the local reaction at the site of injection (Fig. 1c). He received oral valaciclovir thrice a day for 2 weeks. Evolution was favourable, although postzoster neuropathic pain lasted for a month. He had mild varicella during childhood and acknowledged that the very same period was stressful, but denied any other immunosuppressive factor. He received his second injection of BNT162b2 vaccine on 21st April without side effect.
Figure 1.
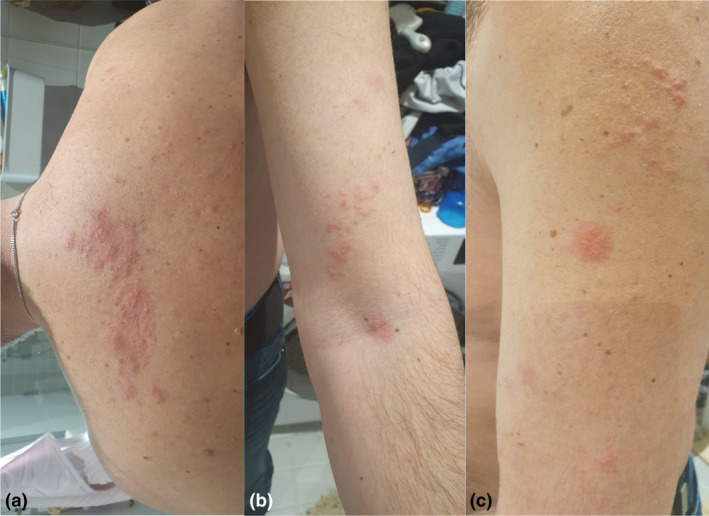
Vesicles and erythematous patches in clusters evocative of a herpes virus infection. The lateralized distribution on the left side of the upper back (a) and arm (b) favours herpes zoster on C5‐C6 dermatomes. Local hypersensitivity reaction after vaccination on the left upper arm (‘COVID‐arm’) is still notable (c).
In the multinational placebo‐controlled study that included 43 548 participants for BNT162b2 vaccination, 6 27%, 21% and 1% of the patients in the vaccination group reported an adverse event, a related event and severe event, respectively. However, HZ was not mentioned. At the time of writing this manuscript, we had found no report of HZ after mRNA‐1273 vaccine.
Arguments that support in our case a link between SARS‐CoV‐2 vaccination and HZ include the following: (i) short delay of onset after vaccination; (ii) eruption on the same side as the vaccinated arm; (iii) a previous case of HZ after SARS‐CoV‐2 vaccination 5 ; (iv) numerous cases of HZ have been described in critically‐ill or seemingly immunocompetent COVID‐19 patients 7 , 8 , 9 ; and (v) cases of herpes viruses reactivations reported after various vaccinations. 10 HZ in COVID‐19 patients may be related to physical and emotional stress associated with acute illness and also to COVID‐19‐induced lymphopenia and functional impairment of T cells. 8 , 9 It is likely that immune dysregulation created by the vaccine played a role in the reactivation of latent Varicella Zoster Virus infection in our case as in the previous on, 5 although we cannot rule out a fortuitous event.
Acknowledgements
The patient in this manuscript has given written informed consent to publication of their case details
References
- 1. Blumenthal KG, Freeman EE, Saff RR et al. Delayed large local reactions to mRNA‐1273 vaccine against SARS‐CoV‐2. N Engl J Med 2021; 384: 1273–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fernandez‐Nieto D, Hammerle J, Fernandez‐Escribano M et al. Skin manifestations of the BNT162b2 mRNA COVID‐19 vaccine in healthcare workers. ‘COVID‐arm’: a clinical and histological characterization. J Eur Acad Dermatol Venereol 2021; 35: e425–e427. 10.1111/jdv.17250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gambichler T, Scholl L, Dickel H, Ocker L, Stranzenbach R. Prompt onset of Rowell’s syndrome following the first BNT162b2 SARS‐CoV‐2 vaccination. J Eur Acad Dermatol Venereol 2021; 35: e415–e416. 10.1111/jdv.17225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ackerman M, Henry D, Finon A, Binois R, Esteve E. Persistent maculopapular rash after the first dose of Pfizer‐BioNTech COVID‐19 vaccine. J Eur Acad Dermatol Venereol 2021; 35: e423–e425. 10.1111/jdv.17248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bostan E, Yalici‐Armagan B. Herpes zoster following inactivated COVID‐19 vaccine: a coexistence or coincidence? J Cosmet Dermatol 2021; 20: 1566–1567. 10.1111/jocd.14035 [DOI] [PubMed] [Google Scholar]
- 6. Polack FP, Thomas SJ, Kitchin N et al. C4591001 Clinical trial group. safety and efficacy of the BNT162b2 mRNA covid‐19 vaccine. N Engl J Med 2020; 383: 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xu R, Zhou Y, Cai L et al. Co‐reactivation of the human herpesvirus alpha subfamily (herpes simplex virus‐1 and varicella zoster virus) in a critically ill patient with COVID‐19. Br J Dermatol 2020; 183: 1145–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tartari F, Spadotto A, Zengarini C et al. Herpes zoster in COVID‐19‐positive patients. Int J Dermatol 2020; 59: 1028–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nofal A, Fawzy MM, Sharaf El Deen SM, El‐Hawary EE. Herpes zoster ophthalmicus in COVID‐19 patients. Int J Dermatol 2020; 59: 1545–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Walter R, Hartmann K, Pool V, Gargiullo P, Kuhn M. Reaktivierung von Herpesviren‐Infektionen durch Impfungen: Evidenz oder Koinzidenz? [Reactivation of herpes virus infections by vaccination: evidence or coincidence?]. Schweiz Med Wochenschr 2000; 130: 1685–1688. [PubMed] [Google Scholar]


