Abstract
The impact of SARS‐CoV‐2 infection in pregnant women and their neonates is an area of research interest nowadays. To date, there is limited knowledge about SARS‐CoV‐2 prevalence, maternal and perinatal outcomes of pregnant women at term in middle‐ and low‐income countries. In the present retro‐prospective study, medical records of pregnant women admitted for delivery were reviewed from the largest Covid‐19 dedicated Shri Maharaja Gulab Singh (SMGS) maternity hospital. The SARS‐CoV‐2 screening was carried out for all pregnant women admitted for delivery using RT‐PCR. All neonates born from SARS‐CoV‐2‐positive mothers were isolated and tested for SARS‐CoV‐2 infection. Most of the pregnant women (90.6%) were asymptomatic at the time of admission with a low prevalence (3.4%) of SARS‐CoV‐2. A higher rate of asymptomatic prevalence (86.1%) was found among SARS‐CoV‐2‐positive pregnant women. On the basis of the RT‐PCR result (negative vs. positive), statistically significant differences were found for maternal characteristics, such as mean gestational age (37.5 ± 2.2 vs. 36.6 ± 3.3), medical comorbidity (2.9% vs. 7.4%), and maternal outcomes like the C‐section rate (29.8% vs. 58.3%), preterm delivery (14.6% vs. 28.3), and neonatal outcomes like mean birth weight (2840 ± 450 vs. 2600 ± 600), low Apgar score (2.7% vs. 6.48%), and fetal distress (10.9% vs. 22.2%) among SARS‐CoV‐2 negative and positive cases, respectively. No neonate from SARS‐CoV‐2‐positive pregnant women was found to be positive for SARS‐CoV‐2 infection.
Keywords: COVID‐19, maternal and perinatal outcome, RT‐PCR, SARS‐CoV‐2, seroprevalence
Highlights
SARS‐CoV‐2 infection has deleterious effects on maternal characteristics
obstetric complications
maternal and perinatal outcomes among pregnant women admitted for delivery
1. INTRODUCTION
The novel coronavirus is responsible for the coronavirus disease 2019 (COVID‐19) pandemic and thereby moving an unprecedented public health emergency all around the world. 1 COVID‐19 pandemic, is also termed as a “Systematic Human Development Crisis” by the United Nations Development Programme. 2 COVID‐19 pandemic can have more devastating effects on low‐ and middle‐income countries, which are already under threat to meet the health needs of their population.3, 4 Nowadays, the effect of SARS‐CoV‐2 infection in pregnant women admitted for delivery and their neonates an area of research interest. Reports from the beginning of the COVID‐19 pandemic have characterized pregnant women as a vulnerable group in comparison to the general population for developing severe SARS‐CoV‐2 infection with adverse maternal‐perinatal outcomes as well. 5 More than 100 million pregnant women across the globe are at high risk of SARS‐Cov 2 infection due to various physiological conditions, such as elevation of the diaphragm, decrease residual lung functional capacity, increased oxygen consumption, edema of mucosal membrane of the respiratory tract, and immune‐modulation during pregnancy. Moreover, reports suggest that with viral respiratory infections pregnant women are at a higher risk of obstetric and perinatal complications due to changes in their immune response. 6
The majority of pregnant women have been found to be asymptomatic at the time of admission.7, 8 Asymptomatic pregnant women admitted for delivery can easily transmit disease among mothers, infants, obstetric care providers, and people in general. Adverse effects of SARS‐CoV‐2 infection on mothers and neonates emphasized the critical need for universal screening of admitted pregnant women using RT‐PCR.8, 9, 10 Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) positivity rate has been reported up to 13.5% in asymptomatic pregnant women admitted for delivery.8, 11 SARS‐CoV‐2 infection during pregnancy may lead to mother‐to‐child vertical transmission and unfavorable maternal and perinatal outcomes as well. SARS‐CoV‐2‐positive pregnant women have a high risk of cesarean section and premature delivery, decreased Apgar Score, and low birth weight of the newborn.12, 13, 14 Most of the studies of SARS‐CoV‐2 infected pregnant women admitted for delivery and their maternal‐perinatal outcomes are confined to only high‐income countries. Little is known about the prevalence of SARS‐CoV‐2, and maternal‐perinatal outcomes of admitted pregnant women in middle‐ and low‐income countries.
India, having the second largest population in the world, comes under the category of a middle‐income country with the paucity of data about the rate of SARS‐CoV‐2 positivity and maternal‐perinatal outcomes in pregnant women admitted for delivery. More research work should be focused on maternal and neonatal health issues in India due to the COVID‐19 pandemic. 15 To date there are no reports on the rate of SARS‐CoV‐2 positivity and maternal and perinatal outcomes in pregnant women admitted for delivery in any northern state of India.
The aim of the present study is to investigate the rate of SARS‐CoV‐2 positivity, maternal‐ perinatal outcomes among pregnant women admitted for delivery in COVID‐19 dedicated, Shri Maharaja Gulab Singh (SMGS) maternity hospital located in Jammu region of UT of Jammu and Kashmir (India).
2. MATERIALS AND METHODS
The present retrospective cohort study was conducted in COVID‐19 dedicated Shri Maharaja Gulab Singh (SMGS) maternity hospital located in Jammu, Jammu and Kashmir (India) between September 1, 2020 and November 30, 2020. Shri Maharaja Gulab Singh (SMGS) maternity hospital is one of the largest maternity hospitals in the Jammu region that provides obstetrical care to nearly 15 000 pregnant women per year.
2.1. Study population
All pregnant women admitted to labor and delivery with no prior history of SARS‐CoV‐2 positivity were included in the study. At the time of admission, all pregnant women were screened for COVID‐19‐related symptoms like fever, cough, dyspnea, and anosmia. If one or more symptoms were present, the patient was considered to be symptomatic. Apart from that admitted women patients were also inquired about maternal characteristics like age, gestational age at delivery, gravidity, parity, and presence of medical comorbidities like hypertension, diabetes, thyroidism, and so on. If one or more medical comorbidity were present, the patient was considered to have medical comorbidity. Admitted women patients were inquired about pregnancy outcomes such as mode of delivery (C‐section or vaginal), type of delivery (term or preterm), and obstetric complications, such as hemoglobin (HB < 10), gestational diabetes mellitus (GDM), pregnancy‐induced hypertension (PIH), intrahepatic cholestasis (ICP), antepartum hemorrhage (APH), postpartum hemorrhage (PPH) along with perinatal outcomes (birth weight, Apgar score, fetal distress, neonatal intensive care unit (ICU) Admission, and neonatal deaths). Information regarding the severity of COVID‐19 based on the requirement of oxygen support and treatment regimen received (antibiotics, antivirals, and corticosteroids) were also collected from SARS‐CoV‐2 positive pregnant women admitted for delivery. All neonates born from SARS‐CoV‐2 positive mothers were isolated and tested for SARS‐CoV‐2 infection at 6 h and 48–72 h after delivery.
2.2. Samples
Combined nasopharyngeal and oropharyngeal swabs were collected from all pregnant women unless a prior RT‐PCR report with no more than 48 h was reported at the time of admission whereas only nasopharyngeal swabs were collected from the infants of SARS‐CoV‐2 positive mothers. Samples were collected in Hi Viral Transport Medium (3 ml), labeled, and transported to Viral research diagnostic laboratory (VRDL), Department of Microbiology, Govt. Medical College, Jammu in triple‐layered packed containers ensuring cold chain and processed further.
2.3. Extraction of viral RNA
All the kits used in the present study were approved by Indian Council for Medical Research (ICMR), Govt. of India. Viral RNA was extracted from the collected samples using QIAamp Viral RNA Mini Kit (Qiagen) as per the manufacturer's protocol. The extracted RNA was dissolved in 60 μl of AVE buffer. 5 μl of extracted RNA was used for RT‐PCR and the rest was stored at −20°C.
2.4. RT‐PCR amplification for SARS‐CoV‐2 detection
The RT‐PCR amplification from isolated RNA was performed using Meril COVID‐19 One‐Step RT‐PCR Kit (Meril Diagnostics) in CFX96 Touch Real‐Time PCR 121 Detection System RT‐PCR platform (Bio‐Rad Laboratories, Inc.). Meril COVID‐19 One‐Step RT‐PCR Kit uses Taqman based multicolor probes for ORF1ab (FAM), N gene (HEX), and RNase P (ROX) in a single tube. Meril COVID‐19 One‐Step RT‐PCR Kit has been approved by the Drug Controller General of India (DCGI), Food and Drug Administration (FDA), and Foundation for Innovative New Diagnostics (FIND), Govt. of India. Only Meril COVID‐19 One‐Step RT‐PCR Kit was used in the present study to avoid kit‐to‐kit variations and to resolve the issue of efficiency. The reaction mixture preparation and amplification program was used as per the manufacturer's instruction. The result interpretation was made according to manufacturer recommendation considering threshold cycle value (Ct ≤ 40) for both ORF1ab & N genes and Internal Control (IC).
2.5. Analysis of maternal characteristics, maternal outcomes, obstetric complications, and neonatal outcomes
Maternal characteristics like maternal age, gestational age at delivery, gravidity, parity, medical comorbidities, and maternal outcomes, such as cesarian delivery, vaginal delivery, preterm delivery, and perinatal outcomes, such as birth weight, Apgar scores, fetal distress, neonatal ICU admission, and neonatal deaths were compared between SARS‐CoV‐2‐negative and SARS‐CoV‐2‐positive pregnant women admitted for delivery. Apart from that obstetric complications, such as HB, GDM, PIH, ICP, APH, and PPH were also evaluated among SARS‐CoV‐2‐negative and SARS‐CoV‐2‐positive pregnant women admitted for delivery.
2.6. Treatment of SARS‐CoV‐2 positive pregnant women
SARS‐CoV‐2 positive pregnant women at term were admitted in COVID‐19 specific quarantine ward. Standard medical treatment was given to both SARS‐CoV‐2 positive and SARS‐CoV‐2 negative pregnant women admitted for delivery. Pregnant women at term were given adequate nutritional support along with monitoring of symptoms related to respiratory and heart failure, FiO2 and complete blood count (CBC), liver and renal function, C‐reactive protein (CRP). Oxygen therapy through nasal catheter or mask was done mandatory for SARS‐CoV‐2 positive pregnant women showing severe illness. In both SARS‐CoV‐2 positive and SARS‐CoV‐2 negative pregnant women having gestation ages between 28 and 34 weeks, Antenatal corticosteroids were administered as standard drugs for the maturation of a baby's lungs before being born. However, in the case of the gestational age than 34 termination of pregnancy was considered safe due to the high possibility of newborn survival. However, timely termination of pregnancy was done in both SARS‐CoV‐2 negative and SARS‐CoV‐2 positive pregnant women showing signs of obstetric complications for well being of both pregnant women and infants.
2.7. Medications of SARS‐CoV‐2 positive pregnant women
SARS‐CoV‐2 positive pregnant women medication depends upon the severity of SARS‐CoV‐2 infection. SARS‐CoV‐2 negative pregnant women and asymptomatic SARS‐CoV‐2 positive pregnant women were treated using antibiotics only. Corticosteroids were used as a treatment regime in SARS‐CoV‐2 positive pregnant women showing mild to moderate symptoms with the requirement of oxygen. No SARS‐CoV‐2 positive pregnant women were found to be severely ill and critically ill in the present study.
2.8. Statistical analysis
The statistical analysis was performed using the MedCalc statistical software package for the biomedical sciences. Differences in maternal characteristics, maternal outcomes, obstetric complications, and perinatal outcomes between SARS‐CoV‐2 negative cases and SARS‐CoV‐2 positive cases were compared using the independent sample t‐test for the continuous variables using 95% confidence intervals. Two‐sided p values of less than 0.05 were considered to be statistically significant.
3. RESULTS
In the present cohort, a total of 3165 pregnant women admitted for delivery in Shri Maharaja Gulab Singh (SMGS) maternity hospital have been analyzed during the study period. Out of which, 90.6% (2868/3165) of pregnant women were asymptomatic and 9.4% (297/3165) of pregnant patients were symptomatic at the time of admission. All pregnant women admitted for delivery were universally screened for SARS‐CoV‐2 positivity using RT‐PCR.
Out of 2868 asymptomatic pregnant women, 93 (3.2%) of asymptomatic pregnant women were tested positive and 2772 (96.7%) were tested negative for SARS‐CoV‐2 infection. Out of 297 symptomatic pregnant women 15 (5.1%), symptomatic pregnant women were tested positive and 282 (94.9%) symptomatic pregnant women were tested negative for SARS‐CoV‐2 infection.
Out of 3165 pregnant women admitted for delivery, 108 test positive for SARS‐CoV‐2 infection resulting in 3.4% (108/3165) prevalence of SARS‐CoV‐2 positivity in the present study. Out of 108 SARS‐CoV‐2 positive pregnant women, 93 (86.1%) were asymptomatic and 15 (13.9%) were symptomatic. Among fifteen (15) SARS‐CoV‐2 positive symptomatic pregnant women, the most common presenting symptoms were fever (66.6%), cough (60%), dyspnea (46.6%), and anosmia (33.3%; Figure 1).
Figure 1.
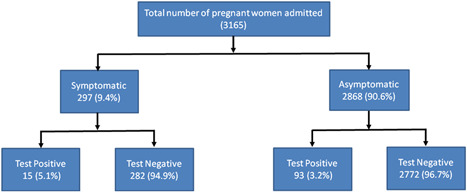
Flow chart indicating the SARS‐CoV‐2 symptoms and RT‐PCR results among 3165 pregnant women admitted for delivery
3.1. Maternal characteristics
Maternal characteristics of all pregnant women admitted for delivery have been summarized in Table 1. The mean age of all the 3165 pregnant women during the universal screening was 24.9 ± 2.2 years and ranged between 17 and 42 years. The mean gestational age at delivery (in weeks) was 37.2 ± 2.8 and ranged between 27 and 42 weeks. Among them 1587 (50.15%) were nullipara, 1578 (49.85%) were multipara, 1587 (50.14%) were with Gravia 1, 947 (29.9%) were with Gravia 2, 631 (19.9%) were with Gravia ≥ 3 and 97 (3.1%) of pregnant women had medical comorbidities.
Table 1.
Maternal characteristics of pregnant women at the time of admission
| Maternal characteristic | Total number of pregnant women (3165) | SARS‐CoV‐2 positive pregnant women (108) | SARS‐CoV‐2 negative pregnant women (3057) | Estimated effect (95% CI) | p |
|---|---|---|---|---|---|
| Maternal age (years) | 24.9 ± 2.2 | 24.7 ± 2.4 | 25.1 ± 2.6 | 0.40 (−0.09 to 0.89) | p = 0.1153 |
| Gestational age at delivery (weeks) | 37.2 ± 2.8 | 36.6 ± 3.3 | 37.5 ± 2.2 | 0.90 (0.46– 1.33) | p < 0.0001 |
| Gravidity | |||||
| 1 | 1587 (50.14%) | 45 (41.6%) | 1512 (49.4%) | 7.8 (−1.79 to 16.82) | p = .1111 |
| 2 | 947 (29.9%) | 48 (44.4%) | 1247 (40.7%) | 3.7 (−5.48 to 13.26) | p = .4421 |
| ≥3 | 631 (19.93) | 15 (13.8%) | 298 (9.7%) | 4.1 (−1.28 to 11.91) | p = .1598 |
| Medical Comorbidity | 97 (3.1%) | 8 (7.4%) | 89 (2.9%) | 4.5 (0.83–11.05) | p = 0.0076 |
| Parity | |||||
| Nullipara | 1587 (50.15%) | 1542 (50.44%) | 45 (41.6%) | 8.8 (−0.60 to 18.25) | p = .067 |
| Multipara | 1578 (49.85) | 1515 (49.56) | 63 (58.3%) | 8.8 (−0.64 to 18.21) | p = .068 |
In the case of 108 SARS‐CoV‐2 positive pregnant women mean age was 24.7 ± 2.4 years and ranged between 24 and 37 years. The mean gestational age was 36.6 ± 3.3 and ranged between 27 and 42 weeks. Among which 1542 (50.44%) were nullipara, 1515 (49.56) were multipara, 45(41.6%) were with Gravia 1, 48 (44.4%) were with Gravia 2, 15 (13.8%) were with Gravia ≥ 3, and 8 (7.4%) had medical comorbidities.
SARS‐CoV‐2 positive and SARS‐CoV‐2 negative pregnant women have been characterized on the basis of maternal age (years), Gestational age at delivery (weeks), gravidity, parity, and medical comorbidity. Out of various maternal characteristics evaluated in the present study, gestational age at delivery (in weeks) and medical comorbidity showed statistically significant difference between SARS‐CoV‐2 negative and SARS‐CoV‐2 positive pregnant women.
3.2. Maternal outcomes and obstetric complications
SARS‐CoV‐2 negative and SARS‐CoV‐2 positive pregnant women have also been characterized on the basis of maternal outcomes such as type of delivery (term, preterm), mode of delivery (vaginal, cesarean), maternal ICU admission maternal deaths (Table 2), and obstetric complications, such as HB < 10, GDM, PIH, ICP, APH, and PPH (Table 3).
Table 2.
Maternal outcomes of SARS‐CoV‐2 positive and SARS‐CoV‐2 negative pregnant women
| Maternal outcome | SARS‐CoV‐2 positive pregnant women (108) | SARS‐CoV‐2 negative pregnant women (3057) | Estimated effect (95% CI) | p |
|---|---|---|---|---|
| Pregnancy outcomes | ||||
| Term | 71.29 (77) | 84.3 (2578) | 13.01 (5.2–22.2) | p = 0.0003 |
| Preterm | 28.3 (31) | 14.6 (14.6) | 13.7 (5.9–22.9) | p = 0.0001 |
| Mode of delivery | ||||
| LSCS | 63 (58.3%) | 914 (29.8%) | 28.5 (18.9–37.4) | p < 0.0001 |
| Vaginal delivery | 45 (41.6%) | 2143 (70.1%) | 28.5 (18.9–37.4) | p < 0.0001 |
| Maternal ICU admission | 2 (1.85%) | 22 (0.71%) | 1.14 (−0.25 to 5.7) | p = 0.1769 |
| Maternal death | 1 (0.9%) | 7 (0.22%) | 0.68 (−0.1 to 4.7) | p = 0.1586 |
Abbreviations: ICU, intensive care unit; LSCS, lower segment cesarian section.
Table 3.
Obstetric complications among SARS‐CoV‐2 positive and SARS‐CoV‐2 negative pregnant women
| Obstetric complications | SARS‐CoV‐2 positive pregnant women (108) | SARS‐CoV‐2 negative pregnant women (3057) | Estimated effect (95% CI) | p |
|---|---|---|---|---|
| Hemoglobin (HB < 10) | 24 (22.2%) | 504 (16.4%) | 5.8 (−1.13 to 14.60) | p = 0.1114 |
| Gestational diabetes mellitus | 16 (14.8%) | 334 (10.9%) | 3.9 (−1.70 to 11.87) | p = 0.2037 |
| Pregnancy‐induced hypertension | 12 (11.1%) | 264 (7.39%) | 3.71 (−1.02 to 11.07) | p = 0.1507 |
| Intrahepatic cholestasis | 10 (9.2%) | 212 (6.93%) | 2.27 (1.97–9.25) | p = 0.3638 |
| Antepartum hemorrhage | 6 (5.55%) | 132 (4.3%) | 1.25 (−1.83 to 7.32) | p = 0.5311 |
| Postpartum hemorrhage | 5 (4.6%) | 110 (3.59%) | 1.01 (−1.71 to 6.78) | p = 0.5811 |
Among 108 SARS‐CoV‐2 positive cases, 31 (28.3%) had a preterm delivery, 77 (71.29%) had term delivery. Vaginal delivery was performed in 45 (41.6%) pregnant women whereas cesarean delivery was performed in 63 (58.3%) pregnant women. Elective cesarean delivery was performed in 21 (19.4%) and 42 (38.9%) underwent emergency cesarean delivery for various maternal‐fetal indications, the most common being fetal distress in 24 (22.2%) pregnant women, 2 (1.85%) SARS‐CoV‐2 positive pregnant women had admitted to ICU and 1 (0.9%) SARS‐CoV‐2 positive pregnant women had maternal death which was not related to SARS‐CoV‐2 infection. Out of various maternal outcomes analyzed in our study, Rates of C‐section and preterm delivery showed statistically significant differences between SARS‐CoV‐2 negative and SARS‐CoV‐2 positive pregnant women.
Among 108 SARS‐CoV‐2 positive pregnant women, 24 (22.2%) had HB < 10, 16 (14.8%) had GDM, 12 (11.1%) had PIH, 10 (9.2%) had ICP, 6 (5.5%) had APH, and 5 (4.6%) had PPH. In the present study, obstetric complications were found to comparatively higher in SARS‐CoV‐2 positive pregnant women in comparison with SARS‐CoV‐2 negative pregnant women. However, none of the obstetric complications showed a statistically significant difference between SARS‐CoV‐2 positive and SARS‐CoV‐2 pregnant women admitted for delivery.
3.3. Perinatal outcomes
Neonates from SARS‐CoV‐2 negative and SARS‐CoV‐2 positive pregnant women were also evaluated on the basis of perinatal characteristics such as birth weight (g), 5th‐min Apgar score ≤ 7, fetal distress, neonatal ICU admission, neonatal death, and SARS‐CoV‐2 RT‐PCR positivity (Table 3).
Neonates from SARS‐CoV‐2 positive mothers showed the mean birth weight of 2600 ± 600 g, 7 (6.48%) neonates had Apgar score ≤ 7, 24 (22.2%) neonates had fetal distress, 14 (12.9%) neonates had admitted to NICU, and 2 (1.9%) neonates had IUD death which was not related to SARS‐CoV‐2 infection. In the present study, perinatal characteristics as birth weight (g), 5th‐min Apgar score ≤ 7 and fetal distress showed a statistically significant difference between SARS‐CoV‐2 negative and SARS‐CoV‐2 positive pregnant women (Table 4).
Table 4.
Perinatal outcomes of SARS‐CoV‐2 positive and SARS‐CoV‐2 negative pregnant women
| Perinatal outcome | SARS‐CoV‐2 positive pregnant women (108) | SARS‐CoV‐2 negative pregnant women (3057) | Estimated effect (95% CI) | p |
|---|---|---|---|---|
| Birth weight (g) | 2600 ± 600 | 2840 ± 450 | 240 (152.4–327.5) | p < 0.0001 |
| 5th‐min Apgar score ≤ 7 | 7 (6.48%) | 83 (2.7%) | 3.78 (0.41–10.0) | p = 0.0199 |
| Fetal distress | 24 (22.2%) | 334 (10.9) | 11.3 (4.3–20.0) | p = 0.0003 |
| Neonatal ICU admission | 14 (12.9%) | 254 (8.3%) | 4.6 (−0.5 to 12.2) | p = 0.0914 |
| Neonatal deaths | 2 (1.9%) | 42 (1.3%) | 0.6 (0.8–5.2) | p = 0.5914 |
| SARS‐CoV‐2 RT‐PCR positivity | Nil | Nil | NA | NA |
Abbreviation: ICU, intensive care unit.
Apart from that, no neonate from SARS‐CoV‐2 positive showed positive test for SARS‐CoV‐2 infection.
4. DISCUSSION
Our study represents one of the few published studies from low‐income countries having a large sample size, employing RT‐PCR‐based universal testing, illustrating maternal and perinatal outcomes in pregnant women admitted for delivery in COVID 19 dedicated maternity hospital.
In our study, 90.6% of total pregnant women were asymptomatic at the time of admission. Maru et al. 16 find out that 72% of pregnant women were asymptomatic at the time of admission in the largest maternity hospital in New York. As per reports of Vintzileos et al. 10 77% of admitted pregnant women for delivery were asymptomatic. In another study, 84% of pregnant women were found to asymptomatic at the time of admission. 8 Our observed rate of asymptomatic pregnant women is in correlation with other similar studies where more than 90% of pregnant women admitted for delivery were asymptomatic.17, 18, 19
In the present large cohort, 3.4% prevalence of SARS‐CoV‐2 positivity was found during the study period. However, the prevalence of SARS‐CoV‐2 infection in a large diverse cohort of pregnant women at term was found to be 0.43% from Southern California. 20 Most of the previous similar studies are confined to high‐income countries having a small sample size and case studies only.21, 22, 23 Abeysuriya et al. 21 reported SARS‐CoV‐2 prevalence of 3.9% at Newham university hospital, in east London whereas 2.7% prevalence of SARS‐CoV‐2 was found in Seattle, Washington using universal testing. 22 3.6% of pregnant women were tested positive for SARS‐CoV‐2 by Miller et al. 23 at Northwestern Memorial, Chicago. Hospital. Dıaz‐Corvillon et al. 24 revealed a 6.35% prevalence of SARS‐CoV‐2 infection among pregnant women at term in South America. As per reports of Campbell et al., 25 5.5% of admitted pregnant women were diagnosed with SARS‐CoV‐2 infections in Southern Connecticut. Sutton et al. 8 asserted SARS‐CoV‐2 positivity in 13.5% of admitted pregnant patients in New York. In another study, SARS‐CoV‐2 prevalence of 12.3% was found in Maharashtra, India from 15 COVID‐19 dedicated maternity hospitals. 26
In the present study, a higher proportion (86.1%) of SARS‐CoV‐2 positive pregnant women cases were asymptomatic at the time of admission. 43.5% of SARS‐CoV‐2 positive cases were reported asymptomatic in Northwestern Memorial, Chicago 23 and 73.3% of asymptomatic women patients were tested positive for SARS‐CoV‐2 in Southern Connecticut. 25 Our results are in comparison with findings of Abeysuriya et al. 21 who reported a high proportion (87.9%) of asymptomatic SARS‐CoV‐2 positive women. Sutton et al. 8 also identified a higher proportion (88%) of asymptomatic SARS‐CoV‐2 positive cases in the high prevalence region of New York. In a similar study, 88.5% of SARS‐CoV‐2 positive pregnant women were found to be asymptomatic in a high prevalence region of Maharashtra, India. 26 As per reports of Doria et al., 27 91.6% of SARS‐CoV‐2 positive pregnant cases were found to be asymptomatic. Although there is variation in rates of asymptomatic prevalence in both high‐ and low‐prevalence regions of COVID‐19. Our study favors universal testing of SARS‐CoV‐2 infection in pregnant women admitted for delivery to control the spread of the virus among women themselves, their newborns, and health workers. Our findings suggest a low prevalence of SARS‐CoV‐2 and a higher representation of asymptomatic pregnant women in the present cohort. Our study showed similarities as well as differences in comparison to the current literature on rates of SARS‐CoV‐2 positivity and asymptomatic prevalence among SARS‐CoV‐2 positive pregnant women.
Pregnant women patients with COVID‐19 generally experience similar symptoms to what has been reported in the general population but the difference in symptoms frequency is still unknown. There are reports revealing cough, fever, dyspnea, and anosmia as the most frequently reported symptoms in SARS‐CoV‐2 infected pregnant women. In the present study, fever (66.6%), cough (60%), dyspnea (46.6%), and anosmia (33.3%) were common symptoms in symptomatic SARS‐CoV‐2 positivity pregnant cases. Pereira et al. 28 also reported fever (75.5%), cough (75.5%), dyspnea (37.8%) as most frequent symptoms in their study. Symptoms such as fever, cough, and fatigue were reported in 83%, 60%, and 38% of patients, respectively, in a meta‐analysis of 43 studies. 29
The most common presenting symptom in the current study was fever (66.6%) corroborating to other similar studies.30, 31 In another study, fever (58%) has been reported as the most presenting symptom followed by cough (50.6%) in symptomatic SARS‐CoV‐2 positive pregnant women. 32 Another systematic review also revealed fever and cough as most dominant initial symptoms in pregnant women infected with SARS‐CoV‐2. 33 However, the majority of the previous studies also asserted fever and cough as the most common symptoms in SARS‐CoV‐2 infected pregnant women.34, 35, 36
According to reports of Trahan et al., 37 symptoms such as cough (53%), fever (37%), dyspnea (30%), and anosmia (20%) were more frequent in symptomatic SARS‐CoV‐2 pregnant patients. The most common symptoms cough (72%), runny nose (57%), myalgia (52%), and fever (42%) were reported in another study. 38 In the present study, cough and fever were reported less as well as high in comparison to other studies. Our study demonstrated similarities as well as differences in comparison to past studies on COVID‐19 symptoms in pregnant women admitted for delivery.
In the present study, the mean maternal age of all pregnant women admitted for delivery was 25 years. In a systematic review, Smith et al. 39 reported mean maternal age of 36 years in pregnant women at term. Ayed et al. 32 demonstrated maternal age of 31 years from a nationwide study of pregnant women admitted for delivery in Kuwait. In another report, Breslin et al. 34 affirmed a mean maternal age of 29.7 years from pair of New York City hospitals. In the current study, mean maternal age has been found comparatively less in comparison to other similar research studies due to the large youth population in India.
In our study, maternal characteristics, such as gestational age at delivery (in weeks) and medical comorbidity, showed a statistically significant difference between and SARS‐CoV‐2 negative and SARS‐CoV‐2 positive pregnant women. There are few reports comparing the maternal characteristics in SARS‐CoV‐2 negative and SARS‐CoV‐2 positive women admitted for delivery.19, 20, 24 However only Tanacana et al., 19 observed a statistically significant difference between the high‐ and low‐risk pregnancy groups on the basis of maternal characteristics, such as gestational age at delivery and medical comorbidity. Our findings suggest that pregnant women with medical comorbidities are more susceptible to SARS‐CoV‐2 infection. However, less gestational age at delivery in SARS‐CoV‐2 positive pregnant can be also attributed to SARS‐CoV‐2 infection also.
In our study, 28.3% of neonates were born preterm and 58.3% of neonates were delivered by cesarean section with fetal distress in 22.2% of SARS‐CoV‐2 positive pregnant women. Cesarean delivery rates (58.3%) were found to be higher in comparison to WHO's recommended cesarean delivery rates of 1%–5% to avoid mortality and morbidity in both mother and infants.
Antoun et al. 35 reported preterm birth in 36.8% and cesarean delivery in 84% of SARS‐CoV‐2 positive pregnant women from high SARS‐CoV‐2 prevalence region of United Kingdom. Ayed et al. 32 revealed preterm births and cesarean delivery in 26.6% and 47.8% of the neonates from SARS‐CoV‐2 positive pregnant women respectively. Another similar study elucidates that among SARS‐CoV‐2 positive pregnant women 27% of the neonates had preterm births and 59% had cesarean births. 36 A systematic review comprising of 33 studies described a preterm birth rate of 15.2% in SARS‐CoV‐2 infected pregnant women. 37
In the present study, elective cesarean delivery in 21 (19.4%) and fetal distress in 24 (22.2%) pregnant women have abruptly increased the rate of cesarean delivery in SARS‐CoV‐2 positive pregnant women. There are reports demonstrating that majority of cesarean deliveries were performed due to fetal distress.12, 30, 38 Clinical characteristics such as placental insufficiency and hypoxia may be responsible for fetal distress in SARS‐CoV‐2 infected pregnant women. Juan et al. 5 reported that cesarean delivery was performed in SARS‐CoV‐2 positive pregnant women to prevent neonatal transmission of the virus. A systematic review also affirmed cesarean delivery as most the frequent mode of delivery in SARS‐CoV‐2 positive pregnant women.
In the present study, a statistically significant difference was found between SARS‐CoV‐2 negative and SARS‐CoV‐2 positive pregnant women on the basis of maternal and perinatal outcomes such as C‐section rate, prematurity, birth weight (g), 5th‐min Apgar score, and fetal distress. There are reports suggesting a risk of low birth weight, low Apgar score, fetal distress, and preterm delivery in SARS‐CoV‐2 infected pregnant women.40, 41 Tanacan et al. 19 also revealed a statistically significant difference between birth weight (g) and Apgar score at birth among high‐ and low‐risk pregnancy groups during COVID‐19 pandemic. Clinical significant differences with no statistically significant differences have been found for C‐section rate, prematurity, NICU admission, and neonatal mortality in small sample size studies.16, 19, 42
The human placenta not only acts as an immunological barrier to the entry of pathogens but is also responsible for maintaining the immune tolerance of the fetal cells. Various RNA viral infections such as Zika virus, cytomegalovirus, and dengue virus have shown remarkable ill effects on growing placenta during pregnancy.43, 44, 45 SARS‐CoV‐2 viral load can be used as an excellent biomarker to predict the severity of the disease in COVID‐19 pregnant women. In case of severe illness of SARS‐CoV‐2 positive pregnant women, Maternal immune response is attributed with the production of a large amount of inflammatory cytokines causing damage to the placenta. Apart from that maternal hypoxia during severe COVID‐19 infection is responsible for overwhelmed production of inflammatory cytokines leading to placental hypoperfusion and subsequent hypoxic–ischemic injury to the placenta. 46 Placental injury accompanied by abnormal uterine perfusion promotes adverse changes such as accelerated villous maturation, increased perivillous and intervillous fibrin deposition, villous infarction, and intervillous thrombosis. 47 Immunomodulatory changes in the placenta are accounted for adverse perinatal outcomes such as preterm birth, fetal growth restriction and even fetal death in case of SARS‐CoV‐2 positive pregnant women admitted for delivery.6, 48, 49 Our results are in accordance with previous reports demonstrating that viral infections during pregnancy lead to higher rates of complications in fetuses including spontaneous abortion, premature birth, and intrauterine growth restriction (IUGR) in pregnant women.6, 50, 51 Before the SARS‐CoV‐2 pandemic, a cohort study comparing pregnant women with and without pneumonia also affirmed that preterm birth, low birth weight, and fetal growth restriction were significantly more prevalent in women with pneumonia. 52
Management and treatment of SARS‐CoV‐2 positive pregnant women varied according to the institution. Various therapies such as antibiotics based therapy (cefoperazone, sulbactam, ceftriaxone, cefazolin, and azithromycin), antiviral therapy (lopinavir, ritonavir, remdesivir, oseltamivir, and ganciclovir), corticosteroid therapy (prednisone, dexamethasone, and hydrocortisone), interferon beta, chloroquine, hydroxychloroquine, and ivermectin may be used for effective treatment of SARS‐CoV‐2 positive pregnant women has been reported by various researchers.53, 54, 55, 56 Due to the nonavailability of data regarding the safety and efficacy of most of the COVID‐19 treatments for pregnant women available in the literature. 57 We have used only antibiotics and corticosteroids such as dexamethasone only in the current study. In the present cohort, most of the SARS‐CoV‐2 positive pregnant women showed mild to moderate illness with no cases of severe and critical illness. None of SARS‐CoV‐2 positive pregnant women needed oxygen support and ventilator support in the present study. Antibiotics play a vital role in preventing secondary bacterial infections and strengthening the immune system of pregnant women with a significant reduction in complications and mortality as well. 58 In our study, both SARS‐CoV‐2 positive and SARS‐CoV‐2 negative pregnant women were treated using antibiotics only due to less severity of SARS‐CoV‐2 infection where dexamethasone was administered in SARS‐CoV‐2 positive pregnant women showing moderate illness needing no oxygen support. Horby et al. 59 reported dexamethasone as an effective drug in the treatment of SARS‐CoV‐2 positive pregnant women with no pregnancy‐associated adverse outcomes as well.
In the present study, obstetric complications were found to comparatively higher in SARS‐CoV‐2 positive pregnant women in comparison to SARS‐CoV‐2 negative pregnant women. However, none of the obstetric complications showed a statistically significant difference between SARS‐CoV‐2 positive and SARS‐CoV‐2 pregnant women admitted for delivery.
Various previous studies also asserted comparatively higher obstetric complications in SARS‐CoV‐2 positive pregnant women in comparison to SARS‐CoV‐2 negative pregnant women with no statistically significant difference. 60 Therefore, obstetrical complications, particularly related to immune‐inflammatory conditions, may have a link to long‐term NCD in affected children. 61 Our findings support the hypothesis that SARS‐CoV‐2 infection is responsible for adverse maternal, obstetric complications, and perinatal outcomes in pregnant women admitted for delivery.
In the present study, no neonates from SARS‐CoV‐2 positive cases showed positive tests for SARS‐CoV‐2 infection similar to other studies. 20 Our study revealed that SARS‐CoV‐2 infection in neonates is independent of the mode of delivery rejecting the hypothesis of vertical transmission of SARS‐CoV‐2 infection.
4.1. Strengths
The main strength of our study is the inclusion of the largest COVID 19 dedicated maternity hospital of the region, large sample size, inclusion of both asymptomatic and symptomatic pregnant women, and universal screening using RT‐PCR. Apart from that majority of the past similar studies have included SARS‐COV‐2 positive pregnant women only. In the present study, we have not only analyzed but also have also compared SARS‐COV‐2 negative and SARS‐COV‐2 positive pregnant women on the basis of maternal characteristics, maternal and perinatal outcomes as well.
4.2. Limitations
The present study has several limitations as well due to its retrospective study design. No maternal serological testing, nonestimation of virus clearance from SARS‐COV‐2 positive pregnant women, and nonavailability of radiological data are key limitations of our study.
5. CONCLUSION
In the present study, our experience at a large COVID‐19 dedicated maternity hospital during the SARS‐CoV‐2 pandemic is quite instructive. The majority of the pregnant women were asymptomatic at the time of admission. Universal testing predicted higher rates of asymptomatic prevalence among SARS‐CoV‐2 positive pregnant women as well. The majority of SARS‐CoV‐2 positive pregnant women had mild symptoms with no severe illness. Our study finds out that SARS‐CoV‐2 infection can lead to unfavorable maternal and perinatal outcomes including higher rates of cesarean delivery, preterm birth, fetal distress, low birth weight, and low Apgar score and higher rates of obstetric complications in pregnant women admitted for delivery. Mother‐to‐child vertical transmission of SARS‐CoV‐2 may be possible. However, vertical transmission of SARS‐CoV‐2 from mother to infant could not be confirmed in the present study. Extensive research work should be carried out to evaluate the long‐term outcomes and the potential of vertical transmission of SARS‐CoV‐2 to infants.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ETHICS STATEMENT
This study has been approved by the Institutional Ethics Committee of Government medical college (GMC) Jammu.
ACKNOWLEDGMENTS
The authors would like to acknowledge the staff of the Department of Microbiology, GMC Jammu, and the staff of the Department of Obstetrics and Gynae, Shri Maharaja Hari Singh (SMGS) Hospital, Jammu, Jammu and Kashmir for delivering their best during the COVID‐19 pandemic and research support as well. The authors are also thankful to Virus Research and Diagnostic Laboratory (VRDL), GMC, Jammu for providing all necessary facilities to carry out research work. One of the authors Puneet Gupta is highly thankful to the National Health Mission (NHM) India for financial support in the form of salary.
Gupta P, Kumar S, Sharma SS. SARS‐CoV‐2 prevalence and maternal‐perinatal outcomes among pregnant women admitted for delivery: Experience from COVID‐19‐dedicated maternity hospital in Jammu, Jammu and Kashmir (India). J Med Virol. 2021;93:5505‐5514. 10.1002/jmv.27074
REFERENCES
- 1. World Health Organization . Naming the coronavirus disease (COVID‐2019) and the virus that causes it. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-andthe-virus-that-causes-it. Accessed December 2020.
- 2. United Nations Development Programme (UNDP) . COVID‐19: new UNDP data dashboards reveal huge disparities among countries in ability to cope and recover. 2020. https://www.undp.org/content/undp/en/home/newscentre/news/2020/COVID19_UNDP_data_dashboards_reveal_disparities_among_countries_to_cope_and_recover.html. Accessed December 2020.
- 3. Hopman J, Allegranzi B, Mehtar S. Managing COVID‐19 in low‐ and middle‐income countries. J Am Med Assoc. 2020;323(16):1549‐1550. [DOI] [PubMed] [Google Scholar]
- 4. Shadmi E, Chen Y, Dourado I, et al. Health equity and COVID‐19: global perspectives. Int J Equity Health. 2020;19(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Juan J, Gil MM, Rong Z, et al. Effects of coronavirus disease 2019 (COVID‐19) on maternal, perinatal and neonatal outcomes: a systematic review. Ultrasound Obstet Gynecol. 2020;56(1):15‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wong SF, Chow KM, Leung TN, et al. Pregnancy and perinatal outcomes of women with severe acute respiratory syndrome. Am J Obstet Gynecol. 2004;191(1):292‐297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pardilla MBE, Aguilar AC, Puig BM, et al. Spanish registry of Covid‐19 screening in asymptomatic pregnants. Rev Esp Salud Publica. 2020;94:e202009092. [PMC free article] [PubMed] [Google Scholar]
- 8. Sutton D, Fuchs K, D'alton M, Goffman D. Universal screening for SARS‐CoV‐2 in women admitted for delivery. N Engl J Med. 2020;382(22):2163‐2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bai Y, Yao L, Wei T, et al. Presumed asymptomatic carrier transmission of COVID‐19. J Am Med Assoc. 2020;323(14):1406‐1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vintzileos WS, Muscat J, Hoffmann E, et al. Screening all pregnant women admitted to labor and delivery for the virus responsible for coronavirus disease 2019. Am J Obstet Gynecol. 2020;223(2):284‐286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crovetto F, Crispi F, Llurba E, Figueras F, Gómez‐Roig MD, Gratacós E. Seroprevalence and presentation of SARS‐CoV‐2 in pregnancy. Lancet. 2020;396(10250):530‐531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID‐19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;95(10226):809‐815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rasmussen SA, Smulian JC, Lednicky JA, Wen TS, Jamieson DJ. Coronavirus disease 2019 (COVID‑19) and pregnancy: what obstetricians need to know. Am J Obstet Gynecol. 2020;222(5):415‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhu H, Wang L, Fang C, et al. Clinical analysis of 10 neonates born to mothers with 2019‐nCoV pneumonia. Transl Pediatr. 2020;9(1):51‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kumar C, Sodhi C, Jaleel CP A. Reproductive, maternal and child health services in the wake of COVID−19: insights from India. J Glob Health Sci. 2020;2(2):e28. [Google Scholar]
- 16. Maru S, Patil U, Carroll‐Bennett R, et al. Universal screening for SARS‐CoV‐2 infection among pregnant women at Elmhurst Hospital Center, Queens, New York. PLOS One. 2020;15(12):e0238409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gagliardi L, Danieli R, Suriano G, et al. Universal SARS‐CoV‐2 testing of pregnant women admitted for delivery in two Italian regions. Am J Obstet Gynecol. 2020;223(2):291‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Herraiz I, Folgueira D, Villalaín C, et al. Universal screening for SARS‐CoV‐2 before labor admission during Covid‐19 pandemic in Madrid. J Perinat Med. 2020;48(9):981‐984. [DOI] [PubMed] [Google Scholar]
- 19. Tanacan A, Erol SA, Turgay B, et al. The rate of SARS‐CoV‐2 positivity in asymptomatic pregnant women admitted to hospital for delivery: experience of a pandemic center in Turkey. Eur J Obstet Gynecol Reprod Biol. 2020;253:31‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fassett MJ, Lurvey LD, Yasumura L, et al. Universal SARS‐Cov‐2 screening in women admitted for delivery in a large managed care organization. Am J Perinatol. 2020;37(11):1110‐1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abeysuriya S, Wasif S, Counihan C, et al. Universal screening for SARS‐CoV‐2 in pregnant women at term admitted to an East London maternity unit. Eur J Obstet Gynecol Reprod Biol. 2020;252:444‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. LaCourse SM, Kachikis A, Blain M, et al. Low prevalence of SARS‐CoV‐2 among pregnant and postpartum patients with universal screening in Seattle, Washington. Clin Infect Dis. 2021;72(5):869‐872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miller ES, Grobman WA, Sakowicz A, Rosati J, Peaceman AM. Clinical implications of universal severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) testing in pregnancy. Obstet Gynecol. 2020;136(2):232‐234. [DOI] [PubMed] [Google Scholar]
- 24. Dıaz‐Corvillon P, Monckeberg M, Barros A. Routine screening for SARS CoV‐2 in unselected pregnant women at delivery pregnancy. PLOS One. 2020;15(9):e0239887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Campbell KH, Tornatore JM, Lawrence KE, et al. Prevalence of SARS‐CoV‐2 among patients admitted for childbirth in Southern Connecticut. J Am Med Assoc. 2020;323(24):2520‐2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Waghmare R, Gajbhiye R, Mahajan NN, Modi D, Mukherjee S, Mahale SD. Universal screening identifies asymptomatic carriers of SARS‐CoV‐2 among pregnant women in India. Eur J Obstet Gynecol Reprod Biol. 2020;256:503‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dória M, Peixinho C, Laranjo M, Mesquita Varejão A, Silva PT. Covid‐19 during pregnancy: a case series from an universally tested population from the north of Portugal. Eur J Obstet Gynecol Reprod Biol. 2020;250:261‐262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pereira A, Cruz‐Melguizo S, Adrien M, Fuentes L, Marin E, Perez‐Medina T. Clinical course of coronavirus disease‐2019 in pregnancy. Acta Obstet Gynecol Scand. 2020;99(7):839‐847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fu L, Wang B, Yuan T, et al. Clinical characteristics of coronavirus disease 2019 (COVID‐19) in China: a systematic review and meta‐analysis. J Infect. 2020;80(6):656‐665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang L, Jiang Y, Wei M, et al. Analysis of pregnancy outcomes of pregnant women during the epidemic of new coronavirus pneumonia in Hubei province. Zhonghua Fu Chan Ke Za Zhi. 2020;55(3):166‐167. [DOI] [PubMed] [Google Scholar]
- 31. Zaigham M, Andersson O. Maternal and perinatal outcomes with COVID‐19: a systematic review of 108 pregnancies. Acta Obstet Gynecol Scand. 2020;99(7):823‐829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ayed A, Embaireeg A, Benawadh A, et al. Maternal and perinatal characteristics and outcomes of pregnancies complicated with COVID‐19 in Kuwait. BMC Pregnancy Childbirth. 2020;20(1):754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Muhidin S, Behboodi Moghadam Z, Vizheh M. Analysis of maternal coronavirus infections and neonates born to mothers with 2019‐nCoV; a systematic review. Arch Acad Emerg Med. 2020;8(1):e49. [PMC free article] [PubMed] [Google Scholar]
- 34. Breslin N, Baptiste C, Gyamfi‐Bannerman C, et al. COVID‐19 infection among asymptomatic and symptomatic pregnantwomen: two weeks of confirmed presentations to an affiliated pair of New York City hospitals. Am J Obstet Gynecol MFM. 2020;2(3):100118. 10.1016/j.ajogmf.2020.100118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Antoun L, Taweel NE, Ahmed I, Patni S, Honest H. Maternal COVID‐19 infection, clinical characteristics, pregnancy, and neonatal outcome: a prospective cohort study. Eur J Obstet Gynecol Reprod Biol. 2020;252:559‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Knight M, Bunch K, Vousden N, et al. Characteristics and outcomes of pregnant women admitted to hospital with confirmed SARS‐CoV‐2 infection in UK: national population based cohort study. BMJ. 2020;369:m2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Elshafeey F, Magdi R, Hindi N, et al. A systematic scoping review of COVID‐19 during pregnancy and childbirth. Int J Gynecol Obstet. 2020;150(1):47‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu H, Liu F, Li J, Zhang T, Wang D, Lan W. Clinical and CT imaging features of the COVID‐19 pneumonia: focus on pregnant women and children. J Infect. 2020;80(5):e7‐e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Smith V, Seo D, Warty R, et al. Maternal and neonatal outcomes associated with COVID‐19 infection: A systematic review PLoS ONE. 2020;15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Favre G, Pomar L, Musso D, Baud D. 2019‑nCoV epidemic: what about pregnancies? Lancet. 2020;395(10224):e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hassan N, Muzamil M, Bandey D. COVID‐19 infection during pregnancy – maternal and perinatal outcomes: a tertiary care centre study. Int J Reprod Contracept Obstet Gynecol. 2020;9(9):3764‐3769. [Google Scholar]
- 42. Cuñarro‐López Y, Cano‐Valderrama Ó, Pintado‐Recarte P, et al. Maternal and perinatal outcomes in patients with suspected COVID‐19 and their relationship with a negative RT‐PCR result. J Clin Med. 2020;9(11):3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. De Noronha L, Zanluca C, Burger M, et al. Zika virus infection at different pregnancy stages: anatomopathological findings, target cells and viral persistence in placental tissues. Front Microbiol. 2018;25(90):2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Uenaka M, Morizane M, Tanimura K, et al. Histopathological analysis of placentas with congenital cytomegalovirus infection. Placenta. 2019;75:62‐67. [DOI] [PubMed] [Google Scholar]
- 45. Ribeiro CF, Lopes VGS, Brasil P, Pires A, Rohloff R, Nogueira R. Dengue infection in pregnancy and its impact on the placenta. Int J Infect Dis. 2017;55:109‐112. [DOI] [PubMed] [Google Scholar]
- 46. Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M, Murphy SR, Granger JP. Pathophysiology of hypertension during preeclampsia: linking placental ischemia with endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2008;294:H541‐H550. [DOI] [PubMed] [Google Scholar]
- 47. Ernst LM. Maternal vascular malperfusion of the placental bed. APMIS. 2018;126(7):551‐560. [DOI] [PubMed] [Google Scholar]
- 48. Kim ML, Maloney C, Klimova N, et al. Repeated lipopolysaccharide exposure leads to placental endotoxin tolerance. Am J Reprod Immunol. 2019;81(2):e13080. [DOI] [PubMed] [Google Scholar]
- 49. Kiefer DG, Keeler SM, Rust OA, Wayock CP, Vintzileos AM, Hanna N. Is midtrimester short cervix a sign of intraamniotic inflammation? Am J Obstet Gynecol. 2009;200(374):e371‐e375. [DOI] [PubMed] [Google Scholar]
- 50. Silasi M, Cardenas I, Kwon JY, Racicot K, Aldo P, Mor G. Viral Infections during pregnancy. Am J Reprod Immunol. 2015;73(3):199‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kwon JY, Romero R, Mor G. New insights into the relationship between viral infection and pregnancy complications. Am J Reprod Immunol. 2014;71(5):387‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chen YH, Keller J, Wang IT, Lin CC, Lin HC. Pneumonia and pregnancy outcomes: a nationwide population‐based study. Am J Obstet Gynecol. 2012;207(4):288.e1‐288.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Saito M, Gilder ME, McGready R, Nosten F. Antimalarial drugs for treating and preventing malaria in pregnant and lactating women. Expert Opin Drug Saf. 2018;17(11):1129‐1144. [DOI] [PubMed] [Google Scholar]
- 54. Mulangu S, Dodd LE, Davey RT Jr, et al. A randomized, controlled trial of Ebola virus disease therapeutics. N Engl J Med. 2019;381(24):2293‐2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Thiel S, Langer‐Gould A, Rockhoff M, et al. Interferon‐beta exposure during first trimester is safe in women with multiple sclerosis‐a prospective cohort study from the German multiple sclerosis and pregnancy registry. Mult Scler. 2016;22(6):801‐809. [DOI] [PubMed] [Google Scholar]
- 56. Bandoli G, Palmsten K, Forbess Smith CJ, Chambers CD. A review of systemic corticosteroid use in pregnancy and the risk of select pregnancy and birth outcomes. Rheum Dis Clin N Am. 2017;43(3):489‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Taylor MM, Kobeissi L, KIM C, et al. Inclusion of pregnant women in COVID‐19 treatment trials: a review and global call to action. Lancet Glob Health. 2021;9(3):e366‐e371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Horby P, Lim WS, Emberson JR,et al. Dexamethasone in hospitalized patients with Covid‐19. N Engl J Med. 2021;384(8):693‐704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yadav V, Goel N, Afreen N, Chutani N, Agarwal S. COVID 19 in pregnancy; obstetrical and neonatal outcomes: a retrospective comparative study. Indian J Obstet Gynecol Res. 2020;7(4):584‐589. [Google Scholar]
- 61. Rogers LK, Velten M. Maternal inflammation, growth retardation, and preterm birth: insights into adult cardiovascular disease. Life Sci. 2011;89(13–14):417‐421. [DOI] [PubMed] [Google Scholar]


