Highlights
-
•
Verbal fluency associated with superior longitudinal fasciculus (SLF/AF) in children.
-
•
Semantic fluency was positively related to right SLF/AF fractional anisotropy (FA).
-
•
Better phonemic fluency was related to higher right and lower left SLF/AF FA.
-
•
Verbal fluency performance in children may rely on right hemisphere structure.
Keywords: Category fluency, Letter fluency, superior longitudinal fasciculus, Diffusion tensor imaging, Surface area, MRI
Abstract
Verbal fluency is the ability to retrieve lexical knowledge quickly and efficiently and develops during childhood and adolescence. Few studies have investigated associations between verbal fluency performance and brain structural variation in children. Here we examined associations of verbal fluency performance with structural measures of frontal and temporal language-related brain regions and their connections in 73 typically-developing children aged 7–13 years. Tract-based spatial statistics was used to extract fractional anisotropy (FA) from the superior longitudinal fasciculus/arcuate fasciculus (SLF/AF), and the white matter underlying frontal and temporal language-related regions. FreeSurfer was used to extract cortical thickness and surface area. Better semantic and phonemic fluency performance was associated with higher right SLF/AF FA, and phonemic fluency was also modestly associated with lower left SLF/AF FA. Explorative voxelwise analyses for semantic fluency suggested associations with FA in other fiber tracts, including corpus callosum and right inferior fronto-occipital fasciculus. Overall, our results suggest that verbal fluency performance in children may rely on right hemisphere structures, possibly involving both language and executive function networks, and less on solely left hemisphere structures as often is observed in adults. Longitudinal studies are needed to clarify whether these associations are mediated by maturational processes, stable characteristics and/or experience.
1. Introduction
Verbal fluency is an important language skill that develops during childhood, with performance reaching maturity in late adolescence (Kavé, 2006; Kavé and Knafo-Noam, 2015; Korkman et al., 2001). Verbal fluency tasks commonly require participants to generate as many words as possible in one minute, either given a specific category (e.g., animals) for semantic fluency, or a specific letter (e.g., letter ‘s’) for phonemic fluency. Phonemic and semantic fluency relies on both common and distinct cognitive processes. Successful performance on verbal fluency tasks require access to language functions, such as lexical knowledge and reading (Nation and Snowling, 2004; Shao et al., 2014), as well as executive functions, such as cognitive flexibility, inhibition, and working memory (Aita et al., 2019; Amunts et al., 2020; Koren et al., 2005; Prigatano et al., 2008; Stolwyk et al., 2015; Welsh et al., 1991). In terms of cognitive strategies, phonemic fluency is thought to rely more on strategic search and switching, to produce words that are not semantically associated, while semantic fluency is thought to depend more on being able to engage underlying semantic networks, and to some extent switching (Sauzéon et al., 2004; Troyer, 2000). Studies suggest phonemic fluency develops more slowly than semantic fluency during childhood because phonemic fluency relies on search strategies that require development of frontal lobe executive functions, while semantic fluency may require lexico-semantic skills dependent on temporal regions (Hurks et al., 2006; Korkman et al., 2001; Riva et al., 2000). Deficits in verbal fluency performance have been reported for children in different clinical populations, including children born pre-term or with low birth weight (Aarnoudse-Moens et al., 2009), or diagnosed with Down Syndrome (Nash and Snowling, 2008), attention deficit/hyperactivity disorder (Pineda et al., 1999), or specific language impairment (Henry et al., 2012). Thus, assessment of verbal fluency during childhood may provide useful information about both language and executive function development.
While verbal fluency develops throughout childhood and adolescence in conjunction with ongoing continuous brain maturation (Jernigan et al., 2016; Wierenga et al., 2014; Zhou et al., 2015), only a few studies have examined the neural correlates of verbal fluency performance in children, and most of the information about the neural correlates of verbal fluency stems from adult studies. Adult lesion studies have found that phonemic fluency performance was most severely impaired with damage to left frontal lobe regions involved in phonetic processing, while impairment in semantic fluency has been associated with damage to left temporal brain regions thought to support mapping of meaning (Baldo et al., 2006, 2001; Bullock and Toribio, 2009; Ghanavati et al., 2019; Jurado et al., 2000). However, another adult lesion study detected anatomical overlap in the left inferior frontal gyrus (IFG) and left insula for semantic and phonemic fluency performance (Biesbroek et al., 2016).
Functional magnetic resonance imaging (fMRI) studies on semantic and phonemic fluency in healthy populations have also reported associations with both shared and distinct brain regions, most consistently in fronto-temporal areas important for executive function and language skills (Costafreda et al., 2006; Wagner et al., 2014). In healthy adults, activation has been reported in the left IFG for both semantic and phonological word retrieval tasks (Birn et al., 2010; Costafreda et al., 2006; Gold and Buckner, 2002; Heim et al., 2008; Katzev et al., 2013; Li et al., 2017; Wagner et al., 2014), although activation in the right hemisphere for semantic fluency has also been reported (Donnelly et al., 2011). FMRI studies using semantic and phonemic fluency tasks report that children show activation in similar frontal brain regions as adults, specifically the left IFG (Gaillard et al., 2000, 2003), although in children more widespread activation across the cortex, including right hemisphere regions such as the IFG, has also been reported for phonemic fluency (Gaillard et al., 2003).
Similarly, MRI studies investigating the structural correlates of verbal fluency also report of both shared and district brain regions in association with semantic and phonemic performance. In elderly adults, phonemic fluency and semantic fluency were both associated with increased cortical thickness in bilateral inferior frontal and superior temporal regions, with semantic fluency also showing more widespread associations within bilateral frontal, temporal and parietal regions (Vonk et al., 2019). In another study in adults, better phonemic fluency performance was associated with increased cortical thickness in the left superior temporal gyrus (STG) and pre- and postcentral gyrus (Phillips et al., 2011). In children and adolescents, better phonemic fluency performance has been associated with decreased cortical thickness in language-related frontal and temporal regions, i.e. left IFG and STG, as well as in regions related to performance monitoring, sustained attention and working memory (Porter et al., 2011). As recent evidence indicates that the cortex undergoes apparent thinning during childhood and adolescence (Amlien et al., 2016; Jernigan et al., 2016; Walhovd et al., 2017; Zhou et al., 2015), decreased cortical thickness within this age range may reflect increased maturation of the cortex. In contrast, surface area exhibits regional-specific curvilinear maturational trajectories, with patterns of increasing surface area during childhood that peak around the age of 10–12 years, after which the surface area undergoes minor decreases during adolescence (Jernigan et al., 2016; Raznahan et al., 2011; Wierenga et al., 2014).
Several studies have examined verbal fluency in relation to white matter microstructure using diffusion tensor imaging (DTI). Common DTI measures are fractional anisotropy (FA), axial diffusivity (AD), and radial diffusivity (RD). Particularly FA and RD have been shown to be sensitive to maturational processes, with FA increasing and RD decreasing during childhood and adolescence in various white matter tracts (Beaulieu, 2009; Lebel et al., 2008; Lebel and Beaulieu, 2011). There are only a few studies that have reported associations of verbal fluency with white matter microstructure of language-related fiber tracts in healthy populations. In adults, better phonemic fluency performance has been associated with higher left superior longitudinal fasciculus/arcuate fasciculus (SLF/AF) AD (Phillips et al., 2011). The SLF and AF are overlapping anatomical structures on MRI, connecting the IFG with parietal and temporal regions (Dick and Tremblay, 2012). In children and adolescents, better phonemic fluency was associated with higher bilateral SLF/AF FA (Peters et al., 2012). However, it is unknown if this is also true for semantic fluency in children since white matter associations with semantic fluency in children have not been previously examined.
In the current study, we investigated to what extent individual differences in phonemic and semantic fluency performance were associated with variability in language-related brain regions in typically-developing children. First, we tested our main hypotheses that better phonemic and semantic fluency performance would be associated with higher left and right SLF/AF FA. Second, we examined if better performance would also be associated with higher FA in the white matter underlying the left frontal and temporal language-related cortices, i.e., IFG, STG and middle temporal gyrus, as well as with larger surface area and thinner apparent cortical thickness of these cortices. Lastly, we explored whether age moderated any potential relationships between brain regions and phonemic and semantic fluency to determine whether these associations differed across the age range of 7–13 years.
2. Material and methods
2.1. Participants
The present study included 73 typically-developing native Danish-speaking children (43 girls, 30 boys) aged 7–13 years (mean (SD) = 10.2 (1.6)). All children were enrolled in the longitudinal HUBU project designed to trace brain and behavioral developmental changes. The HUBU cohort includes 95 typically-developing children (55 girls, 40 boys) recruited from three elementary schools in the Copenhagen suburban area in 2007, when they were between seven and 13 years of age. All children, who volunteered for the HUBU project were included, except those with a known history of neurological or psychiatric disorders or significant brain injury. Prior to participation, informed written consent was obtained from the parents of all participants, and all children assented to partake in the study. The study was approved by the Ethical Committees of the Capital Region of Denmark (H-KF-01–131/03) and conducted in accordance with the Declaration of Helsinki.
Participants in the HUBU cohort have been assessed up to 13 times, with six months intervals for the first 10 visits. The present study included data from the baseline of the HUBU project. After the MR images were evaluated for incidental clinical findings and image quality, 22 participants (12 girls) were excluded due to poor MR-image quality (n = 12), incomplete scanning sessions (n = 4), incidental clinical findings on the MRI (n = 1), the verbal fluency task not being administered due to time constraints (n = 4), or technical problems (n = 1). The sample included two sibling pairs (female siblings aged 8.7 and 12.5 years; male-female siblings aged 10.3 and 11.8 years). According to the Edinburgh Handedness Inventory (EHI), 62 participants were right-handed (EHI score ≥ 40, range: 50–100), 10 participants were left-handed (EHI score ≤ -40, range: -40 - -100), and one participant was ambidextrous (EHI score between -40 and 40). Highest level of maternal and paternal educational degree was acquired for all participants and translated into years of education using national norms. In Denmark, the number of educational years for all primary and secondary education, and all occupations, including professional and trade careers have a standardized length of time to completion. The years of education were then calculated based on the Danish government standards for years of education for compulsory primary and lower secondary education (9 years), plus the number of standard years to complete their highest degree (e.g., plus 3 years for high school, plus 3 years for a Bachelor’s, plus 2 years for a Masters). Standard years for degree completion by occupation can be found in “The Education Guide” by the Ministry of Children and Education in Denmark (www.ug.dk). Average years of parental education, or years of education from one parent if unavailable for both parents, (mean (SD) = 13.5 (2.1), range = 9–18.5) were used in the statistical analyses. Data from the HUBU cohort has previously been used in other cross-sectional (Angstmann et al., 2016; Klarborg et al., 2013; Madsen et al., 2010, 2011, 2018; Vestergaard et al., 2011) and longitudinal (Madsen et al., 2020) studies examining the relationship between behavioral functions and brain microstructural characteristics.
2.2. Verbal fluency assessment
Children were asked to name as many different words as possible in the category of animals (semantic fluency) and words starting with the letter S, each given 1 min. They were instructed not to list names of people or places. Words starting with the letter S occur often in the Danish language. Repetitions and responses that did not meet the criteria were excluded from the total score for both semantic and phonemic fluency.
2.3. Image acquisition
On the same day as the verbal fluency assessment, children underwent structural MRI on a 3 T Siemens Magnetom Trio MR scanner (Siemens, Erlangen, Germany) using an eight-channel head coil (Invivo, FL, USA). Two T1-weighted images were acquired using a 3D MPRAGE sequence (TR =1550 ms, TE =3.04 ms, matrix = 256 × 256, 192 sagittal slices, 1 × 1 × 1 mm3 voxels, acquisition time = 6:38). A T2-weighted image was acquired using a 3D turbo spin echo sequence (TR =3000 ms, TE =354 ms, FOV = 282 × 216, matrix = 256 × 196, 192 sagittal slices, 1 × 1 × 1 mm3 voxels, acquisition time = 8:29). Whole brain diffusion-weighted images were acquired using a twice-refocused balanced spin echo sequence that minimized eddy current distortion (Reese et al., 2003) including ten non-diffusion-weighted images (b = 0) and 61 diffusion-weighted images (b = 1200s/mm2) encoded along independent collinear diffusion gradient orientations (TR =8200 ms, TE =100 ms, FOV = 220 × 220, matrix = 96 × 96, GRAPPA: factor = 2, 48 lines, 61 transverse slices with no gap, 2.3 × 2.3 × 2.3 mm3 voxels, acquisition time = 9:50). To correct for B0 field distortions, a gradient echo field map was acquired (TR =530 ms, TE[1] =5.19 ms and TE[2] =7.65 ms, FOV = 256 × 256, matrix = 128 × 128, 47 transverse slices with no gap, voxel size = 2 × 2 × 3 mm3, acquisition time = 2:18).
2.4. Image evaluation
All baseline MRI scans were evaluated by an experienced neuroradiologist for incidental findings and all, but one, were deemed without significant clinical pathology. Prior to analysis and blind to the behavioral data, all raw MR-images were visually inspected to assure sufficient image quality. Based on this inspection, 12 datasets were excluded from further analyses due to poor image quality (see Section 2.1. “Participants”).
2.5. Diffusion-weighted image processing
Diffusion-weighted images were preprocessed as described in Klarborg et al., (2012) and Vestergaard et al. (2011). Preprocessing pipelines were implemented in Matlab using mainly SPM5 coregistration and realignment routines and the batch functionality. The diffusion-weighted images were oriented to the MNI coordinate system by rigidly coregistering the mean b0 image to the T2-weighted image, after which all diffusion-weighted images were coregistered (no reslicing) to the mean b0 image. All co-registered DWI images were then corrected for geometric distortions using a voxel displacement map based on both the acquired B0 field map (Andersson et al., 2001) and a scanner specific map of gradient non-linearities (Jovicich et al., 2006). Subsequently, all transformations and voxel displacement maps were concatenated, and all images were resliced using trilinear interpolation in one interpolation step. The diffusion gradient orientations were adjusted to account for any applied rotation during the registration. The diffusion tensor was fitted with the RESTORE algorithm using a noise standard deviation of 30 (Chang et al., 2005), implemented in Camino (Cook et al., 2006) to calculate FA, AD and RD. Finally, brain masks based on the T2-weighted images were created using segmentation routines and morphological operations implemented in the VBM5 toolbox (http://dbm.neuro.uni-jena.de/vbm/vbm5-for spm5/) in SPM5. Brain masks were visually inspected, and manually edited if necessary, before being applied to the FA and diffusivity images.
Tract-based spatial statistics (Smith et al., 2006), part of FSL 4.1.0, was used to spatially normalize and align fiber tracts across all subjects. The FA images of all subjects were aligned into a common space using the non-linear registration tool FNIRT. A study-specific target, i.e., the group’s most representative FA image, was identified by non-linearly registering all subjects’ FA images to the FA images of every other subject. Using affine registration, the target was aligned to MNI space and subsequently the entire dataset was aligned to this image and transformed into 1 mm3 MNI space. A mean FA image of the entire cohort was created and thinned to generate the mean FA skeleton, representing the centers of all tracts common to the group. All warped FA images were visually inspected to ensure sufficient quality and correct alignment to the mean FA skeleton. The mean FA skeleton was thresholded at FA > 0.25 and contained 103,588 1 mm3 interpolated voxels. All subjects’ aligned FA data were then projected onto the skeleton by locating the voxels with the highest local FA value in the direction perpendicular to the skeleton tracts and assigning this value to the skeleton. In addition, the nonlinear warps, and skeleton projections were applied to the AD and RD data.
2.6. White matter regions-of-interest (ROIs)
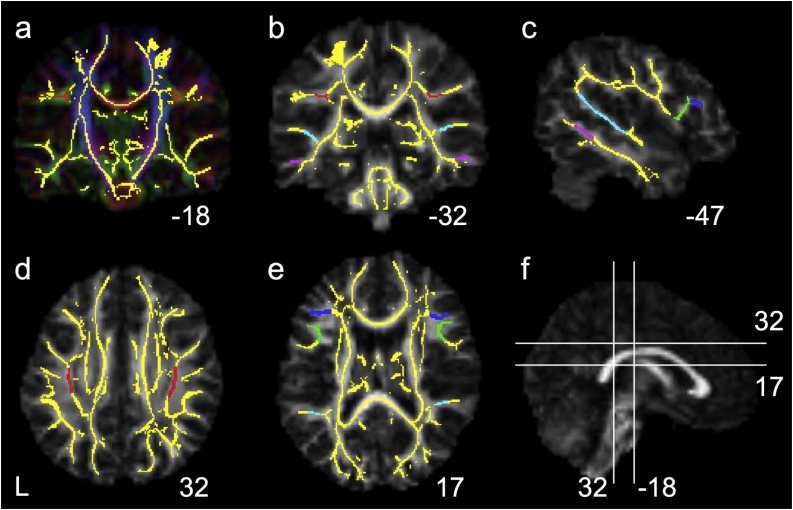
To test our hypotheses, five ROIs were delineated on the mean FA skeleton overlaid on the target FA map using FSLview (Fig. 1). Specifically, ROIs were delineated in the SLF/AF and in the white matter underlying the left pars triangularis, left pars opercularis, left planum temporale (PT), and left middle temporal gyrus (MTG). Similar ROIs were delineated in the right hemisphere. Descriptions of the ROI delineations can be found in the Supplementary methods. Mean FA, AD and RD were extracted from each of the ROIs.
Fig. 1.
Regions-of-interest in the superior longitudinal fasciculus/arcuate fasciculus (SLF/AF, red), as well as in the white matter underlying the pars opercularis (green), pars triangularis (blue), planum temporale (PT, light-blue) and middle temporal gyrus (MTG, magenta) are displayed on the mean skeleton (yellow), overlaid on the target’s fractional anisotropy (FA) map. The FA map are displayed in grey scale in b-f and color-coded in a. MNI coordinates are presented below each image. Images are shown according to neurological convention, where L = left hemisphere is depicted in the left side (for interpretation of the references to color in the Figure Legend, the reader is referred to the web version of this article).
2.7. FreeSurfer analysis and cortical ROIs
All T1-weighted and T2-weighted images were processed using tools available in the FreeSurfer (version 6.0) software suite (Dale et al., 1999; Dale and Sereno, 1993; Desikan et al., 2006). Cortical surface reconstruction was done by applying the following procedures: skull stripping, non-uniformity correction, white matter segmentation, creation of initial mesh, correction of topological defects, and creation of optimal white and pial surfaces (Dale et al., 1999; Dale and Sereno, 1993; Desikan et al., 2006). Cortical grey matter parcellations were based on surface-based nonlinear registration to the Desikan-Killiany atlas based on gyral and sulcal patterns and Bayesian classification rules (Desikan et al., 2006). Apparent cortical thickness was calculated as a measure of the shortest distance between the white and pial surfaces. For each cortical parcel, average thickness and white surface area were calculated. To quality check the FreeSurfer outputs, we used a quality control approach similar to that used in Enigma (http://enigma.ini.usc.edu/protocols/imaging-protocols/), i.e., statistical detection of outliers for the average thickness and surface area of each cortical parcel, which were then visually inspected. The statistical detection on outliers was performed on the age and sex adjusted residuals, to account for age and sex difference in the brain measures. Furthermore, to conform Enigma protocol, we did not perform manual editing on the FreeSurfer 6 outputs, and discarded data of questionable quality. To test our hypothesis, we selected the cortical ROIs in the pars opercularis, pars triangularis, superior temporal gyrus (STG), and middle temporal gyrus (MTG).
2.8. Statistical analyses
All statistical modeling was completed using R (version 3.4.4) and R Studio (version 1.1.463). All verbal fluency and ROI measures were examined for associations with age, age2, sex, age-by-sex, handedness, and parental education using multiple linear regression analyses. The Shapiro-Wilk test was used to determine that the residuals from the multiple linear regression models did not significantly deviate from the normal distribution. All assumptions for linear regressions were met. To test our primary hypotheses, we used hierarchical regression models. The first step was a reduced model that included age and sex as the only predictor variables of semantic or phonemic fluency. In the second step, we extended the model by entering both left and right SLF/AF FA as additional predictors of semantic or phonemic fluency. A likelihood-ratio chi-square test was used to determine whether the full model explained significantly more of the variance in semantic or phonemic fluency as compared to the reduced model (i.e., R2 change). We applied a Bonferroni correction for our primary hypotheses for two tests, i.e., semantic and phonemic fluency, i.e., all p-values (ps) less than 0.025 were considered significant. Finally, exploratory analyses were conducted to investigate age by SLF/AF FA interaction effects on semantic or phonemic fluency, to investigate if the relationship between verbal fluency and SLF/AF FA differed with age.
Contingent on a significant R2 change for the extended models with left and right SLF/AF FA, several planned follow-up models were conducted. First, we tested the individual contribution of left and right SLF/AF FA in separate follow-up models. Next, we tested for anatomical specificity by including whole skeleton FA as an additional predictor, while simultaneously also correcting for handedness and parental education. Finally, to obtain further information about the nature of observed FA findings, we investigated AD and RD, as higher FA can be due to decreased RD and/or increased AD. This was tested by replacing SLF/AF FA with either AD or RD in the models where SLF/AF FA was significant. For follow-up analyses we considered a p-value of 0.05 as significant.
Our secondary hypotheses regarding associations between verbal fluency performance and FA, apparent cortical thickness or surface area of the left pars opercularis, pars triangularis, PT and MTG ROIs were also tested using hierarchical regression models. The first step included age and sex as the only predictor variables of semantic or phonemic fluency. The second step included the brain structural measures (FA, cortical thickness or surface area) for the four left secondary ROIs as simultaneous predictors of semantic or phonemic fluency. The significance of the R2 change was determined as described above. For the hierarchical models with surface area, to investigate the regionalized effect of the ROIs, the first step also included total surface area, since total surface area is correlated with regional surface area. We applied a Bonferroni correction for eight tests to our secondary hypotheses, i.e., associations of phonemic or semantic fluency with three types of brain measures (FA, thickness and surface area), plus the two primary hypotheses tests, i.e., R2 changes between full and reduced models with p-values less than 0.0065 were considered significant. Finally, in exploratory analyses, we examined associations between semantic or phonemic fluency and structural measures in the right ROIs using similar hierarchical models as for the left ROIs.
2.8.1. Effect size maps
The present study was designed to test specific anatomical hypotheses about the relationship between fronto-temporal language regions and variability in verbal fluency performance in children. However, since TBSS can produce estimates of the effect sizes at each skeleton voxel, we generated voxelwise maps to provide further information about semantic or phonemic fluency associations with FA values across the white matter skeleton, controlling for age and sex. The maps were generated using the Monte Carlo permutation test with 10,000 permutations implemented in the randomise program within FSL (Winkler et al., 2014). We applied the Threshold-Free Cluster Enhancement (TFCE) method using the --T2 option with default parameters, which is optimized for the mainly 2D structure of the TBSS skeleton. To correct for multiple comparisons, FWE correction was applied to the TFCE map. The unthresholded t-maps as well as corrected p-map (for semantic fluency only, see Section 3.5.) have been uploaded to NeuroVault.org (Gorgolewski et al., 2015) and are available at https://neurovault.org/collections/8632/.
Finally, exploratory vertex-wise maps were created to visualize the distribution of associations of surface area across the brain with semantic or phonemic fluency. Surface area was mapped into standardized spherical atlas space (Fischl et al., 1999), with a Jacobian correction to account for the stretching or compression of the registration to atlas at each point on the cortical surface. Iterative smoothing was performed on the cortical surface, after resampling to atlas-space, with a full-width half max filter size of 20 mm (Hagler et al., 2006). Vertex-wise surface-area maps were produced using a general linear model with each vertex as a dependent measure, and either semantic or phonemic fluency as a predictor variable, adjusted for age and sex.
3. Results
3.1. Effects of age, sex, handedness and parental education on verbal fluency performance
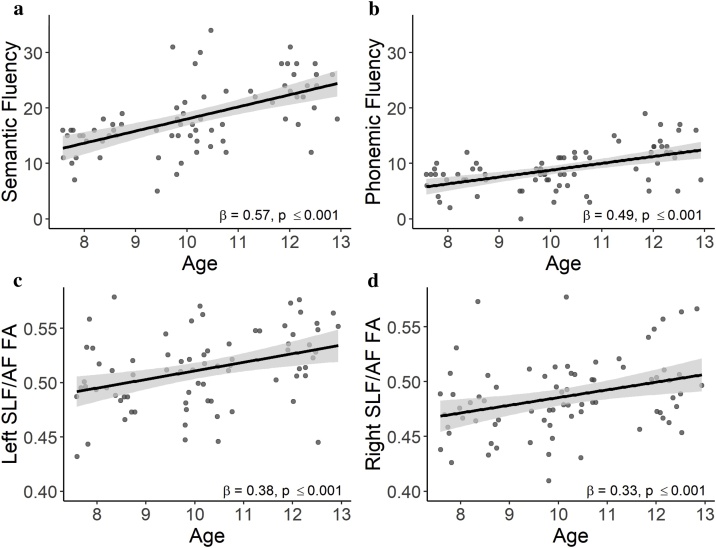
Phonemic fluency performance averaged to 9.01 (SD = 3.79; range: 0–19) words, while semantic fluency performance averaged to 18.41 (SD = 6.16; range: 5–34) words. As expected, performance on both verbal fluency measures increased significantly with age (ps ≤ 0.001, Fig. 2), while there were no significant age2 (ps ≥ 0.08), sex (p ≥ 0.25), or age-by-sex interaction effects (ps > 0.11). Higher parental education showed a modest positive association with verbal fluency (phonemic: p = 0.04; semantic: p = 0.11). No significant associations were observed between verbal fluency and handedness (ps ≥ 0.50).
Fig. 2.
Scatter plots showing significant age-related increases for (a) semantic fluency, (b) phonemic fluency, (c) left superior longitudinal fasciculus/arcuate fasciculus (SLF/AF) fractional anisotropy (FA), and (d) right SLF/AF FA with shaded 95 % confidence intervals.
3.2. Effects of age, sex, handedness and parental education on white matter ROI FA
FA increased significantly with age in the left and right SLF/AF, as well as in the left pars opercularis, right pars triangularis, and right PT white matter ROIs (ps ≤ 0.007), but not in the remaining ROIs (ps ≥ 0.06). Females had higher FA in the left SLF/AF relative to males (p = 0.016), while males had higher FA in the left pars triangularis relative to females (p = 0.009). There were no significant sex effects on FA in the remaining ROIs (p ≥ 0.93). There were no significant age-by-sex interactions (ps ≥ 0.19) or age2 effects (ps ≥ 0.34) on any of the ROI measures. There was an effect of handedness on left SLF/AF FA, such that left-handed participants had higher left SLF/AF FA compared to right-handed participants (p = 0.036). We did not observe any other significant associations between ROI FA and handedness (ps > 0.06) or parental education (ps ≥ 0.10).
3.3. Associations between verbal fluency and SLF/AF microstructure
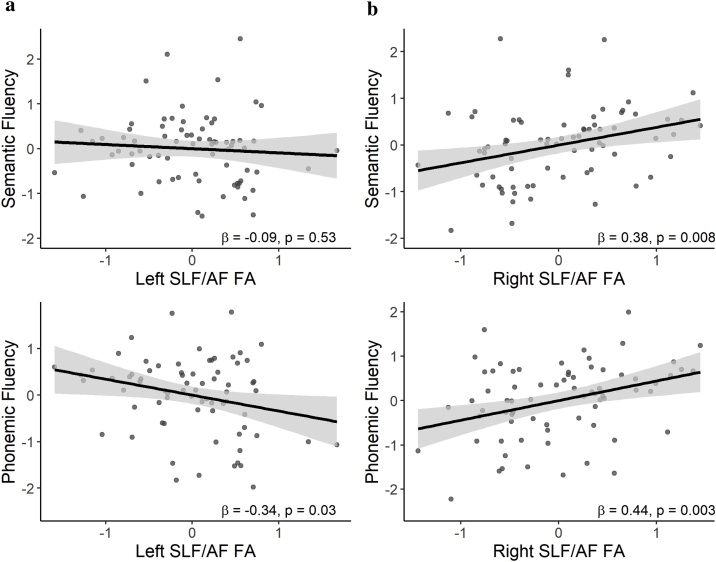
The hierarchical regression models showed that both semantic and phonemic fluency performance were significantly associated with SLF/AF FA, after controlling for age and sex (Table 1; Fig. 3). Inspecting the pattern of the regression coefficients of the left and right SLF/AF ROIs revealed that better semantic fluency performance appeared to be associated with higher right SLF/AF FA, but not with left SLF/AF FA (Table 1; Fig. 3a), while better phonemic fluency performance appeared to be associated with higher right and lower left SLF/AF FA (Table 1; Fig. 3b). No significant interactions of age by FA in left or right SLF/AF on semantic or phonemic fluency (ps > 0.30) were observed, suggesting that the relationship between verbal fluency and SLF/AF FA did not differ with age.
Table 1.
Results for the main hierarchical linear regression models and follow-up models of the associations between left and right superior longitudinal fasciculus/arcuate fasciculus (SLF/AF) fractional anisotropy (FA) and semantic or phonemic fluency performance.
| Age | Sex | Left SLF/AF FA | Right SLF/AF FA | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Main Hypothesesa | R2 (df) | ß | p | ß | p | ß | p | ß | p |
| Semantic Fluency | |||||||||
| Reduced Model | 0.32 (70) | 0.57 | <0.001 | −0.16 | 0.43 | -- | -- | ||
| Full Model | 0.40 (68) | 0.48 | <0.001 | −0.18 | 0.35 | −0.09 | 0.53 | 0.38 | 0.008 |
| Model Comparison | ΔR2 = 0.08; χ2 (2, N = 73) = 5.7, p = 0.005* | ||||||||
| Phonemic Fluency | |||||||||
| Reduced Model | 0.27 (70) | 0.49 | <0.001 | 0.23 | 0.25 | -- | -- | -- | -- |
| Full Model | 0.34 (68) | 0.51 | <0.001 | 0.33 | 0.11 | −0.34 | 0.03 | 0.44 | 0.003 |
| Model Comparison | ΔR2 = 0.07; χ2 (2, N = 73) = 4.6, p = 0.01* | ||||||||
| Follow-up Modelsb | |||||||||
| Semantic Fluency | |||||||||
| Right SLF/AF FA | 0.40 (69) | 0.46 | <0.001 | −0.22 | 0.235 | -- | -- | 0.32 | 0.001 |
| Left SLF/AF FA | 0.34 (69) | 0.49 | <0.001 | −0.26 | 0.194 | 0.201 | 0.063 | -- | -- |
| Phonemic Fluency | |||||||||
| Right SLF/AF FA | 0.30 (69) | 0.46 | <0.001 | 0.19 | 0.343 | -- | -- | 0.21 | 0.046 |
| Left SLF/AF FA | 0.26 (69) | 0.53 | <0.001 | 0.23 | 0.275 | −0.001 | 0.993 | -- | -- |
The results of the likelihood-ratio chi-square tests of the R2 change between the full and reduced models are shown below each model coefficients. * Below Bonferroni corrected α (p < 0.025) for two main hypotheses.
Left and right SLF/AF FA are in the same model.
Left and right SLF/AF FA are in separate models.
Fig. 3.
Partial regression plots of the associations between (a) semantic fluency or (b) phonemic fluency and left and right superior longitudinal fasciculus/arcuate fasciculus (SLF/AF) fractional anisotropy (FA) modelled simultaneously, controlling for age, sex, with shaded 95 % confidence intervals.
Follow-up analyses of the contributions of left and right SLF/AF FA individually confirmed that better semantic fluency performance was associated with higher right SLF/AF FA but not with left SLF/AF FA (Table 1). Thus, follow-up models for semantic fluency were only conducted with the right SLF/AF. The follow-up model with right SLF/AF FA still significantly explained more variance in semantic fluency than the reduced model when additionally controlling for whole skeleton FA, parental education and handedness (R2 change = 0.07, p = 0.038).
For phonemic fluency, analyses of the contributions of left and right SLF/AF FA individually, showed that right SLF/AF FA was modestly associated with performance while left SLF/AF FA was no longer significant (Table 1). Since having both left and right SLF/AF FA in the model explained more variance in phonemic fluency, follow-up models included both the left and right SLF/AF. These remained significant in explaining more variance in phonemic fluency than the reduced model when additionally controlling for whole skeleton FA, parental education and handedness (R2 change = 0.08, p = 0.035).
Finally, in follow-up analyses examining the contribution of RD and AD for the apparent positive association for right SLF/AF FA with semantic fluency, and for the apparent association of higher relative to left SLF/AF FA with phonemic fluency. Better semantic fluency performance was associated with lower RD (p = 0.0036) but not with higher AD (p = 0.083) in the right SLF/AF. Better phonemic fluency performance was associated with higher RD in left (p = 0.004) and lower RD in right (p = 0.006) SLF/AF, and to a lesser extent with higher right SLF/AF AD (p = 0.021), but not with left SLF/AF AD (p = 0.858). Together, these findings suggest that the observed FA associations for semantic and phonemic fluency were mainly driven by variability in RD.
3.4. Associations between verbal fluency and FA in frontal and temporal white matter ROIs
No significant associations were observed between left pars opercularis, pars triangularis, PT, or MTG white matter FA with either semantic fluency or phonemic fluency performance (Table 2). Further, there were no significant interactions of age by FA in any of the left frontal and temporal ROIs for semantic or phonemic fluency (ps > 0.27). Exploratory analyses of right frontal and temporal ROI FA did not reveal any significant models with semantic fluency or phonemic fluency (see Supplementary Table 1).
Table 2.
Results for the hierarchical linear regression models for fractional anisotropy (FA) and cortical grey matter measures in the left frontal and temporal ROIs in association with semantic or phonemic fluency performance.
|
Age |
Sex |
Left Pars Opercularis |
Left Pars Triangularis |
Left PT |
Left MTG |
Whole brain |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | ß | p | ß | p | ß | p | ß | p | ß | p | ß | p | ß | p | |
| White Matter FA | |||||||||||||||
| Semantic Fluency | |||||||||||||||
| Reduced Model | 0.32 | 0.57 | <0.001 | −0.16 | 0.43 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Full Model | 0.34 | 0.55 | <0.001 | 0.01 | 0.97 | 0.04 | 0.70 | 0.27 | 0.01 | −0.12 | 0.28 | 0.06 | 0.55 | -- | -- |
| Model comparison | ΔR2 = 0.03; χ2 (4, N = 70) = 4.5, p = 0.143 | ||||||||||||||
| Phonemic Fluency | -- | -- | |||||||||||||
| Reduced Model | 0.27 | 0.53 | <0.001 | 0.23 | 0.25 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Full Model | 0.28 | 0.59 | <0.001 | 0.37 | 0.09 | −0.17 | 0.13 | 0.13 | 0.25 | −0.08 | 0.51 | 0.11 | 0.34 | -- | -- |
| Model comparison | ΔR2 = 01; χ2 (4, N = 70) = 3.5, p = 0.300 | ||||||||||||||
| Surface Area | |||||||||||||||
| Semantic Fluency | |||||||||||||||
| Reduced Model | 0.37 | 0.57 | <0.001 | 0.16 | 0.50 | -- | -- | -- | -- | -- | -- | -- | -- | 0.32 | 0.01 |
| Full Model | 0.35 | 0.56 | <0.001 | 0.15 | 0.59 | 0.001 | 0.99 | −0.05 | 0.67 | −0.16 | 0.30 | −0.02 | 0.89 | 0.48 | 0.04 |
| Model comparison | ΔR2 = -0.02; χ2 (4, N = 69) = 0.92, p = 0.85 | ||||||||||||||
| Phonemic Fluency | |||||||||||||||
| Reduced Model | 0.35 | 0.55 | <0.001 | 0.57 | 0.02 | -- | -- | -- | -- | -- | -- | -- | -- | 0.28 | 0.02 |
| Full Model | 0.34 | 0.55 | <0.001 | 0.53 | 0.03 | −0.21 | 0.123 | 0.17 | 0.20 | 0.04 | 0.75 | 0.10 | 0.52 | 0.17 | 0.44 |
| Model comparison | ΔR2 = -0.01; χ2 (4, N = 69) = 2.5, p = 0.85 | ||||||||||||||
| Mean Cortical Thickness | |||||||||||||||
| Semantic Fluency | |||||||||||||||
| Reduced Model | 0.31 | 0.59 | <0.001 | −0.20 | 0.35 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| Full Model | 0.32 | 0.60 | <0.001 | −0.19 | 0.38 | −0.10 | 0.42 | −0.01 | 0.92 | 0.11 | 0.63 | −0.18 | 0.15 | -- | -- |
| Model comparison | ΔR2 = 0.01; χ2 (4, N = 69) = 3.1, p = 0.360 | ||||||||||||||
| Phonemic Fluency | |||||||||||||||
| Reduced Model | 0.30 | 0.57 | <0.001 | 0.25 | 0.23 | ||||||||||
| Full Model | 0.34 | 0.56 | <0.001 | 0.18 | 0.38 | 0.06 | 0.631 | −0.08 | 0.57 | 0.13 | 0.265 | −0.29 | 0.02 | -- | -- |
| Model comparison | ΔR2 = 0.04; χ2 (4, N = 69) = 5.6, p = 0.07 | ||||||||||||||
For each brain measure, we show model comparisons of a full model (ROIs + covariates) relative to the reduced model (covariates only) for both semantic fluency and phonemic fluency. The results of the likelihood-ratio chi-square tests of the R2 change between the full and reduced models are shown below each model coefficients. Abbreviations: MTG = middle temporal gyrus, PT = planum temporale.
3.5. Voxelwise maps of the association between verbal fluency and white matter FA
Voxelwise analyses were conducted to further explore the distribution of semantic or phonemic fluency performance associations with FA across the white matter skeleton, adjusted for age and sex. The full uncorrected t-maps as well as the FWE corrected TFCE p-map for semantic fluency can be downloaded from https://neurovault.org/collections/8632/.
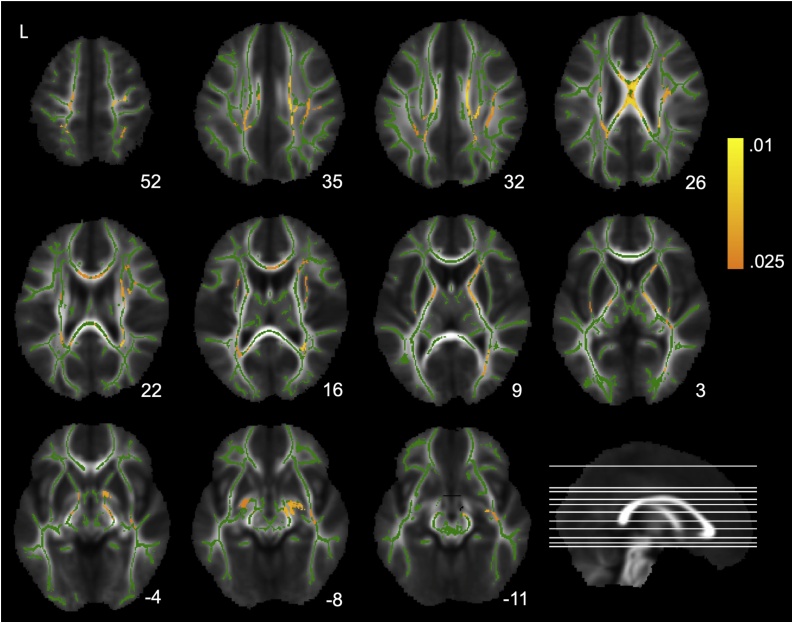
Better semantic fluency performance was associated with higher FA (FWE corrected p ≤ 0.025, equivalent to a two-tailed p ≤ 0.05) in multiple locations across the white matter skeleton (Fig. 4). These clusters included the white matter underlying the bilateral superior parietal cortex (52), bilateral corticospinal tract (52), right SLF/AF (35, 32, 26, 22), left SLF/AF (32), body of corpus callosum (35, 32, 26, 22, 16), bilateral superior corona radiata (35, 32, 22), bilateral posterior corona radiata (32, 26, 22), bilateral external/extreme capsule (16, 3), right anterior (9, 3) and bilateral posterior (9, 3, -4) limb of the internal capsule, right posterior thalamic radiation (9), right inferior fronto-occipital fasciculus (9, 3, -4, -8, -11), and the anterior commissure (-4, -8).
Fig. 4.
Associations between semantic fluency and fractional anisotropy (FA) across the white matter skeleton, after correction for age and sex, overlaid on the mean FA map and the mean FA skeleton (green). Warm colored voxels represent clusters (FWE corrected p ≤ 0.025) where better semantic fluency performance was significantly associated with higher FA. The MNI Z coordinates for the axial slices are displayed below each image. Images are shown according to neurological convention, where L = left hemisphere is depicted in the left side. The depicted axial slices are displayed on a midsagittal image in the bottom right corner (for interpretation of the references to color in the Figure Legend, the reader is referred to the web version of this article).
No significant associations were observed between phonemic fluency and FA across the white matter skeleton after FWE correction of the TFCE map. However, to inform future studies an effect size map of the uncorrected t-values has been provided in the Supplementary material (Supplementary Fig. 1).
3.6. Surface area
3.6.1. Effects of age, sex, handedness and parental education on surface area
There were no significant associations of age (ps ≥ 0.20), age2 (ps ≥ 0.37), nor age-by-sex (ps ≥ 0.05) with total or left ROI surface area. Males showed significantly larger total surface area, and larger surface area in the left PT and MTG compared to females (ps ≤ 0.001), but not in the remaining left ROIs (ps ≥ 0.05). Parental education was significantly positively associated with larger total surface area and in the left MTG surface area (ps < 0.01), but not with any other ROIs (ps ≥ 0.17). There were no significant associations with handedness in any of the ROIs (ps ≥ 0.23).
3.6.2. Associations between verbal fluency and surface area
The results of the hierarchical regression models are shown in Table 2. For semantic and phonemic fluency, we did not observe any significant associations with surface area of the left frontal and temporal ROIs. While total surface area was positively associated with semantic fluency and to a lesser extent with phonemic fluency, these associations would not survive correction for multiple comparisons at the individual ROI level. Moreover, there were no significant interactions of age by surface area in any of the left frontal and temporal ROIs for semantic or phonemic fluency (ps > 0.08). Likewise, exploratory models investigating the right frontal and temporal ROIs were not statistically significant for semantic nor phonemic fluency (see Supplementary Table 1).
3.6.3. Effect size maps of the association between verbal fluency and surface area
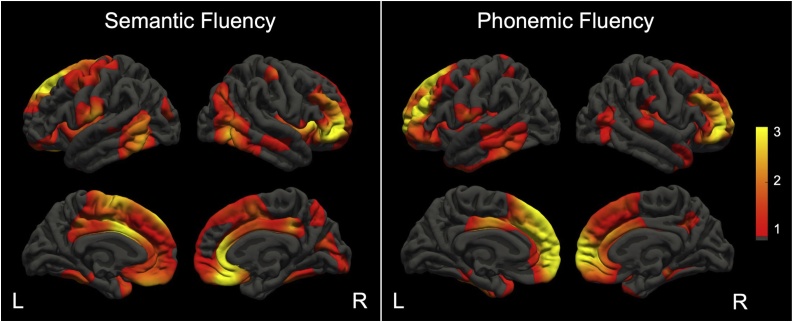
Exploratory vertex-wise effect size maps, displaying the uncorrected -log10(p)-values for surface area associations with semantic fluency and phonemic fluency, controlling for age and sex, are shown in respectively Fig. 5a and b. Apparent positive associations with semantic fluency were seen in the left superior frontal cortex, left mid-cingulate cortex, right middle frontal gyrus and pars orbitalis, right ventromedial prefrontal cortex, and right anterior cingulate cortex (Fig. 5a). For phonemic fluency, apparent positive associations were seen in the left superior frontal gyrus, left middle frontal gyrus, left dorsomedial prefrontal cortex, right middle frontal gyrus and pars orbitalis, and the frontal pole bilaterally (Fig. 5b).
Fig. 5.
Vertex-wise effect size maps for surface area displaying the range of uncorrected -log10(p)-values for associations with semantic fluency and phonemic fluency performance adjusted for age and sex. Warm colors (red-yellow) display regions in which better verbal fluency performance was associated with larger surface area. The -log10(p)-values in the color bar correpond to uncorrected p-values of 1: p = 0.1, 2: p = 0.01, 3: p = 0.001 (for interpretation of the references to color in the Figure Legend, the reader is referred to the web version of this article).
3.7. Apparent cortical thickness
3.7.1. Effects of age, sex, handedness and parental education on apparent cortical thickness
There were no significant age (ps ≥ 0.20), age2 (ps ≥ 0.42), sex (ps ≥ 0.21), or age-by-sex interaction (ps ≥ 0.50) effects on apparent cortical thickness in left pars opercularis, pars triangularis, PT, or MTG, nor with parental education or handedness (ps ≥ 0.21).
3.7.2. Associations between verbal fluency and apparent cortical thickness
Cortical thickness in the left frontal and temporal ROIs was not significantly associated with semantic or phonemic fluency, over and beyond age and sex (Table 2). Furthermore, there were no significant interactions of age by cortical thickness in any of the left frontal and temporal ROIs for semantic or phonemic fluency (ps > 0.05). The exploratory model investigating the right frontal and temporal ROIs revealed an association between phonemic fluency and cortical thickness of the right ROIs (p = 0.01), but this association did not survive correction for multiple comparisons (see Supplementary Table 1). Furthermore, no significant associations were observed between semantic fluency and the right ROIs.
4. Discussion
We examined associations of both phonemic and semantic fluency with measures of white and grey matter structure in language-related brain regions in typically-developing children. Better semantic and phonemic fluency performance were associated with higher right SLF/AF FA, while better phonemic fluency was also modestly associated with lower left SLF/AF FA. Importantly, these associations remained after controlling for whole skeleton FA, suggesting that the associations of verbal fluency with SLF/AF FA were not due to global differences in white matter FA. Moreover, we found no evidence that the relationship between verbal fluency and brain structural measures differed with age. Associations between verbal fluency measures and language-related frontal and temporal ROI cortical thickness, surface area or white matter FA were not significant.
Our study is the first, to the best of our knowledge, to report an association between SLF/AF and semantic fluency in children. Furthermore, our results corroborate previous findings on the importance of the SLF/AF for phonemic fluency (Peters et al., 2012; Phillips et al., 2011). For phonemic fluency, including both right and left SLF/AF FA explained significantly more variance in performance, than age and sex alone, even after additionally correcting for several confounders. However, it was unexpected to find that better phonemic fluency performance was associated with higher right SLF/AF FA, but with lower left SLF/AF FA. Nevertheless, follow-up analyses suggested that phonemic fluency, like semantic fluency, was more strongly related to right SLF/AF FA. Our findings differ in laterality from the associations reported by the few other DTI studies on phonemic fluency. Better phonemic fluency performance has been associated with higher bilateral SLF/AF FA in a cohort of 8−21-year-olds and with higher left SLF/AF AD in adults (Phillips et al., 2011). It is unclear if these apparent discrepancies are related to differences in the specific age ranges investigated, i.e., 7–13-years in the present study. In support of such notion, a recent fMRI study reported that while children, similar to adults, showed left-lateralized language activation, a large proportion of the youngest children also exhibited significant right-lateralized language activation in notably the IFG and STG (Olulade et al., 2020). Interestingly, this right-lateralized language activation decreased with age. Though we did not find evidence that relationship between verbal fluency and SLF/AF FA differed with age in the present study of children aged 7–13 years, it may be that a wider age range and/or longitudinal designs are needed to detect possible differences in the verbal fluency-SLF/AF relationship across age. Observed discrepancies could also be related to methodological differences, e.g., we used TBSS to extract DTI measures from SLF/AF ROIs, while the previous studies used tractography or voxel-based TBSS (Peters et al., 2012; Phillips et al., 2011). Future studies, preferably longitudinal in design, should investigate if and how the associations between SLF/AF and phonemic fluency, as well as semantic fluency, differ or change across childhood, adolescence and adulthood.
Despite the discrepancies discussed above, there are some lesion and fMRI studies supporting right hemisphere involvement in verbal fluency. Lesion studies have found that semantic fluency, but not phonemic fluency, was related to the IFG specifically in the right hemisphere (Biesbroek et al., 2016; Loring et al., 1994; Martin et al., 1990; Perret, 1974), which projects to the right SLF/AF. Early studies in children using fMRI reported associations of phonemic and semantic fluency with bilateral frontal language regions, i.e., the IFG (Gaillard et al., 2000, 2003). Moreover, a recent fMRI study in children and adolescents found that higher right hemisphere activation in regions homologous to left hemisphere language-related regions was associated with better semantic fluency performance (Bartha-Doering et al., 2018), as well as better performance on other language tasks such as verb generation tasks (Holland et al., 2001; Lidzba et al., 2011; Yeatman et al., 2010). Together, these findings suggest that in children, verbal fluency performance may not only rely on left, but also on right hemisphere language networks, for which homologous language-related regions such as the right SLF/AF have been associated with reading comprehension and semantic processing in children (Horowitz-Kraus et al., 2015). Furthermore, these findings are consistent with behavioral studies that find deficits in verbal fluency performance among children with specific language impairment and dyslexia, who also show difficulties in reading skills (Cohen et al., 1999; Henry et al., 2012; Weckerly et al., 2001). Moreover, in lesion studies, damage to the right hemisphere has been linked to deficits in language skills such as discourse, inferential processes and non-literal language, i.e., in applications that would support meaning or comprehension of language (Johns et al., 2008). While the left hemisphere may play a more dominant role in speech processing, the right hemisphere is important in activation and processing of broader semantic information (Johns 2008, Beeman and Chiarello). Thus, not all language skills may be lateralized to the left hemisphere, and may rely on the right hemisphere, including in children.
Most evidence for the neural correlates of phonemic and semantic fluency, mainly derived from studies investigating adults, involve anatomical structures corresponding to the dorsal stream of language processing, i.e., the IFG, PT, and the SLF, thought to support mapping of sound to speech production (Hickok and Poeppel, 2004). Our findings suggest that in the age range of 7–13 years, phonemic and semantic fluency are also both associated with white matter connections in the dorsal stream (i.e., SLF/AF). Recent studies have reported associations between semantic fluency in association with anatomical structures of the ventral stream of language processing (i.e., inferior fronto-occipital fasciculus, IFOF), thought to map sound to meaning (Almairac et al., 2015; Houston et al., 2019). While we did not a priori investigate white matter fiber tracts in the ventral stream, visual inspection of our corrected voxelwise maps suggested that semantic fluency performance may be correlated with other white matter tracts, including the IFOF, supporting the possible involvement of the ventral stream in semantic fluency. Furthermore, clusters were observed in the corpus callosum, suggesting that interhemispheric integration may also be important for semantic fluency performance in children. This appears to be supported by findings from a recent study in children aged 6–12 years, in which better semantic fluency performance was associated with larger posterior corpus callosum volume (Bartha-Doering et al., 2021). Future studies, preferably longitudinal in design, should investigate both dorsal and ventral pathways, as well as the corpus callosum, in relation to verbal fluency during childhood and whether these relationships differ or change across childhood, adolescence and adulthood.
Alternatively, as verbal fluency performance is also closely linked with executive function performance, it is also plausible that the involvement of right hemisphere regions, such as the right SLF/AF, may reflect recruitment of executive function processes, such as inhibition, monitoring, and working memory (Aita et al., 2019; Stolwyk et al., 2015; Welsh et al., 1991), to sustain better verbal fluency performance in children. Notably, in the same cohort included in the present study, we previously found that higher right SLF/AF FA was associated with better sustained attention performance, an executive function skill that requires selective attention and working memory (Klarborg et al., 2013). Thus, it is plausible that in children verbal fluency performance is supported by either right-sided language networks or executive networks, or by both. Overall, our findings suggest the SLF/AF in the right hemisphere may be important for both phonemic and semantic fluency in children, indicating there may be shared neural networks and possibly also cognitive processes supporting phonemic and semantic fluency in children. However, we cannot determine whether they correspond specifically to executive function or language skills, or both. Future studies should investigate to which extent verbal fluency and other language and executive functions show associations in overlapping brain regions, including the right SLF, during childhood.
Contrary to our expectations, we did not observe any significant associations between phonemic or semantic fluency and apparent cortical thickness nor surface area in the fronto-temporal language regions in our cohort of 7–13-year-old children. Studies investigating associations between structural grey matter measures and verbal fluency in children are very sparse. One study found that better phonemic fluency performance was associated with decreased cortical thickness in the left fronto-temporal language regions, the bilateral precuneus and left cingulate cortex in children and adolescents aged 9–23 years (Porter et al., 2011). While we did not observe significant associations in our hypothesized regions, the provided exploratory voxel- and vertex-wise maps show that associations of semantic and phonemic fluency may be distributed across the brain.
It is important to note that while we report associations that are age-adjusted, it is unclear what these age-independent associations reflect. The associations may reflect phase differences in structural maturation, i.e., children of similar age may have less or more mature brain structure that supports relatively poorer or better verbal fluency performance. Alternatively, the associations may reflect stable individual differences in the underlying neural architecture that arise earlier in childhood and remain stable, despite superimposed ongoing maturation. Finally, these age-independent verbal fluency associations may reflect differences in experience-related brain plasticity, with frequent practice supporting better verbal fluency performance.
4.1. Limitations and strengths
We tested specific a priori hypotheses about associations of semantic and phonemic fluency with structural measures of frontal and temporal language-related brain regions and their connections. We found significant effects confirming our main hypothesis about the relationship between individual variability in semantic and phonemic fluency performance and white matter microstructural properties in language-related white matter fiber tracts (SLF/AF). Although, our sample size is comparable to other studies on white matter and verbal fluency (Peters et al., 2012; Phillips et al., 2011), given the possible limitations of small sample size studies in susceptibility to low replicability and inflated effect sizes (Button et al., 2013), our hypothesis driven findings should be replicated in larger samples. Moreover, novel methodologies for whole-brain analyses suggest that variability in behavioral phenotypes, such as verbal fluency, may be associated with distributed patterns of effects across the brain (Zhao et al., 2020), beyond specific associations between a phenotype and an ROI. While we cannot infer statistical significance from our white matter and surface area uncorrected effect size maps (Jernigan et al., 2003), they can inform future studies that aim to investigate such associations of global brain patterns and individual variability in verbal fluency performance. Finally, while we have controlled for factors that might influence the observed associations, we cannot exclude that there might be other latent variables influencing the observed associations.
5. Conclusion
In typically-developing children, better semantic and phonemic fluency performance was associated with higher right SLF/AF FA, while better phonemic fluency performance was also modestly associated with lower left SLF/AF FA. Overall, our results suggest that right hemisphere structures may be particularly important for verbal fluency performance during childhood, with contribution of brain regions related to language and/or executive functions. Given verbal fluency is related to both language and executive function skills, understanding the underlying neural pathways in relation to verbal fluency may further our understanding of the shared neural architecture that may support both language and executive function skills during childhood. Future studies examining the neural and developmental correlates of verbal fluency during childhood and adolescence should assess if and how the associations between SLF/AF and verbal fluency change across childhood, adolescence and adulthood.
Author contributions
Marybel Robledo Gonzalez: Conceptualization, methodology, formal analysis, visualization, writing - original draft. William F.C. Baaré: Software, resources, writing - review & editing, project administration, funding acquisition. Donald J. Hagler Jr.: Software, formal analysis, visualization, writing - review & editing. Sarah Archibald: Writing - review & editing, supervision. Martin Vestergaard: Investigation, writing - review & editing. Kathrine Skak Madsen: Conceptualization, methodology, software, formal analysis, investigation, resources, writing - original draft, visualization, supervision, project administration, funding acquisition.
Data statement
Individual level data for the HUBU Study is confidential. However, the unthresholded t-maps have been uploaded to NeuroVault.org (Gorgolewski et al., 2015) and are available at https://neurovault.org/collections/FSNZIXKW/.
Funding
This work was supported by the Danish Council for Independent Research | Medical Sciences [grant numbers 09–060166, 0602–02099B], the Lundbeck Foundation [grant number R32-A3161] and the Lundbeck Foundation Center of Excellence grant to The Center for Integrated Molecular Brain Imaging.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank the children and their parents for their participation in the HUBU study.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2021.100982.
Contributor Information
Marybel Robledo Gonzalez, Email: marybel.robledo@gmail.com.
William F.C. Baaré, Email: wimb@drcmr.dk.
Donald J. Hagler, Jr., Email: dhagler@mail.ucsd.edu.
Sarah Archibald, Email: sarchibald@ucsd.edu.
Martin Vestergaard, Email: martinvh@drcmr.dk.
Kathrine Skak Madsen, Email: kathrine@drcmr.dk.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Aarnoudse-Moens C.S.H., Smidts D.P., Oosterlaan J., Duivenvoorden H.J., Weisglas-Kuperus N. Executive function in very preterm children at early school age. J. Abnorm. Child Psychol. 2009;37(7):981–993. doi: 10.1007/s10802-009-9327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aita S.L., Beach J.D., Taylor S.E., Borgogna N.C., Harrell M.N., Hill B.D. Executive, language, or both? An examination of the construct validity of verbal fluency measures. Applied Neuropsychology:Adult. 2019;26(5):441–451. doi: 10.1080/23279095.2018.1439830. [DOI] [PubMed] [Google Scholar]
- Almairac F., Herbet G., Moritz-Gasser S., de Champfleur N.M., Duffau H. The left inferior fronto-occipital fasciculus subserves language semantics: a multilevel lesion study. Brain Struct. Funct. 2015;220(4):1983–1995. doi: 10.1007/s00429-014-0773-1. [DOI] [PubMed] [Google Scholar]
- Amlien I.K., Fjell A.M., Tamnes C.K., Grydeland H., Krogsrud S.K., Chaplin T.A., Rosa M.G.P., Walhovd K.B. Organizing principles of human cortical development - thickness and area from 4 to 30 years: insights from comparative primate neuroanatomy. Cereb. Cortex. 2016;26(1):257–267. doi: 10.1093/cercor/bhu214. [DOI] [PubMed] [Google Scholar]
- Amunts J., Camilleri J.A., Eickhoff S.B., Heim S., Weis S. Executive functions predict verbal fluency scores in healthy participants. Sci. Rep. 2020;10(1) doi: 10.1038/s41598-020-65525-9. 11141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J.L.R., Hutton C., Ashburner J., Turner R., Friston K. Modeling geometric deformations in EPI time series. NeuroImage. 2001;13(5):903–919. doi: 10.1006/nimg.2001.0746. [DOI] [PubMed] [Google Scholar]
- Angstmann S., Madsen K.S., Skimminge A., Jernigan T.L., Baaré W.F.C., Siebner H.R. Microstructural asymmetry of the corticospinal tracts predicts right–left differences in circle drawing skill in right-handed adolescents. Brain Struct. Funct. 2016;221(9):4475–4489. doi: 10.1007/s00429-015-1178-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo J.V., Shimamura A.P., Delis D.C., Kramer J., Kaplan E. Verbal and design fluency in patients with frontal lobe lesions. J. Int. Neuropsychol. Soc. 2001;7(5):586–596. doi: 10.1017/S1355617701755063. [DOI] [PubMed] [Google Scholar]
- Baldo J.V., SChwartz S., Wilkins D., Dronkers N.F. Role of frontal versus temporal cortex in verbal fluency as revealed by voxel-based lesion symptom mapping. J. Int. Neuropsychol. Soc. 2006;12(6):896–900. doi: 10.1017/S1355617706061078. [DOI] [PubMed] [Google Scholar]
- Bartha-Doering L., Kollndorfer K., Kasprian G., Novak A., Schuler A.L., Fischmeister F.P.S., Alexopoulos J., Gaillard W.D., Prayer D., Seidl R., Berl M.M. Weaker semantic language lateralization associated with better semantic language performance in healthy right-handed children. Brain Behav. 2018;8(11) doi: 10.1002/brb3.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartha-Doering L., Kollndorfer K., Schwartz E., Fischmeister F.P.S., Alexopoulos J., Langs G., Prayer D., Kasprian G., Seidl R. The role of the corpus callosum in language network connectivity in children. Dev. Sci. 2021;24(2) doi: 10.1111/desc.13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C. Diffusion MRI. 2009. The biological basis of diffusion anisotropy; pp. 105–126. [DOI] [Google Scholar]
- Biesbroek J.M., van Zandvoort M.J.E., Kappelle L.J., Velthuis B.K., Biessels G.J., Postma A. Shared and distinct anatomical correlates of semantic and phonemic fluency revealed by lesion-symptom mapping in patients with ischemic stroke. Brain Struct. Funct. 2016;221(4):2123–2134. doi: 10.1007/s00429-015-1033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn R.M., Kenworthy L., Case L., Caravella R., Jones T.B., Bandettini P.A., Martin A. Neural systems supporting lexical search guided by letter and semantic category cues: a self-paced overt response fMRI study of verbal fluency. NeuroImage. 2010;49(1):1099–1107. doi: 10.1016/j.neuroimage.2009.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullock B.E., Toribio A.J., editors. Cambridge Handbook of Linguistic Code-Switching. Cambridge University Press; 2009. http://www.cambridge.org/catalogue/catalogue.asp?isbn=0521875919 [Google Scholar]
- Button K.S., Ioannidis J.P.A., Mokrysz C., Nosek B.A., Flint J., Robinson E.S.J., Munafò M.R. Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 2013;14(5):365–376. doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Chang L.-C., Jones D.K., Pierpaoli C. RESTORE: robust estimation of tensors by outlier rejection. Magn. Reson. Med. 2005;53:1088–1095. doi: 10.1002/mrm.20426. [DOI] [PubMed] [Google Scholar]
- Cook P.A., Bai Y., Nedjati-Gilani S., Seunarine K.K., Hall M.G., Parker G.J., Alexander D.C. Camino: open-source Diffusion-MRI reconstruction and processing. Proc. Int. Soc. Magn. Reson. Med. 2006;14 http://www.cs.ucl.ac.uk/research/medic/camino [Google Scholar]
- Costafreda S.G., Fu C.H.Y., Lee L., Everitt B., Brammer M.J., David A.S. A systematic review and quantitative appraisal of fMRI studies of verbal fluency: role of the left inferior frontal gyrus. Hum. Brain Mapp. 2006;27:799–810. doi: 10.1002/hbm.20221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A.M., Sereno M.I. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J. Cogn. Neurosci. 1993;5(2):162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis: I. Segmentation and surface reconstruction. NeuroImage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Maguire R.P., Hyman B.T., Albert M.S., Killiany R.J. 2006. An Automated Labeling System for Subdividing the Human Cerebral Cortex on MRI Scans Into Gyral Based Regions of Interest. [DOI] [PubMed] [Google Scholar]
- Dick A.S., Tremblay P. Vol. 135. Oxford University Press; 2012. Beyond the arcuate fasciculus: consensus and controversy in the connectional anatomy of language; pp. 3529–3550. (Brain). Issue 12. [DOI] [PubMed] [Google Scholar]
- Donnelly K.M., Allendorfer J.B., Szaflarski J.P. Right hemispheric participation in semantic decision improves performance. Brain Res. 2011;1419:105–116. doi: 10.1016/j.brainres.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Tootell R.B.H., Dale A.M. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum. Brain Mapp. 1999;8(4):272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard W.D., Hertz-Pannier L., Mott S.H., Barnett A.S., LeBihan D., Theodore W.H. Functional anatomy of cognitive development: fMRI of verbal fluency in children and adults. Neurology. 2000;54(1):180–185. doi: 10.1212/WNL.54.1.180. [DOI] [PubMed] [Google Scholar]
- Gaillard W.D., Sachs B.C., Whitnah J.R., Ahmad Z., Balsamo L.M., Petrella J.R., Braniecki S.H., McKinney C.M., Hunter K., Xu B., Grandin C.B. Developmental aspects of language processing: fMRI of verbal fluency in children and adults. Hum. Brain Mapp. 2003;18(3):176–185. doi: 10.1002/hbm.10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanavati E., Salehinejad M.A., Nejati V., Nitsche M.A. Differential role of prefrontal, temporal and parietal cortices in verbal and figural fluency: implications for the supramodal contribution of executive functions. Sci. Rep. 2019;9(1):1–10. doi: 10.1038/s41598-019-40273-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold B.T., Buckner R.L. Common prefrontal regions coactivate with dissociable posterior regions during controlled semantic and phonological tasks. Neuron. 2002;35(4):803–812. doi: 10.1016/S0896-6273(02)00800-0. [DOI] [PubMed] [Google Scholar]
- Gorgolewski K.J., Varoquaux G., Rivera G., Schwarz Y., Ghosh S.S., Maumet C., Sochat V.V., Nichols T.E., Poldrack R.A., Poline J.-B., Yarkoni T., Margulies D.S. NeuroVault.org: a web-based repository for collecting and sharing unthresholded statistical maps of the human brain. Front. Neuroinform. 2015;9(April):8. doi: 10.3389/fninf.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler D.J., Saygin A.P., Sereno M.I. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. NeuroImage. 2006;33(4):1093–1103. doi: 10.1016/j.neuroimage.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim S., Eickhoff S.B., Amunts K. Specialisation in Broca’s region for semantic, phonological, and syntactic fluency? NeuroImage. 2008;40(3):1362–1368. doi: 10.1016/j.neuroimage.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Henry L.A., Messer D.J., Nash G. Executive functioning in children with specific language impairment. J. Child Psychol. Psychiatry. 2012;53(1):37–45. doi: 10.1111/j.1469-7610.2011.02430.x. [DOI] [PubMed] [Google Scholar]
- Hickok G., Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92(1–2):67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Holland S.K., Plante E., Weber Byars A., Strawsburg R.H., Schmithorst V.J., Ball W.S. Normal fMRI brain activation patterns in children performing a verb generation task. NeuroImage. 2001;14(4):837–843. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- Houston J., Allendorfer J., Nenert R., Goodman A.M., Szaflarski J.P. White matter language pathways and language performance in healthy adults across ages. Front. Neurosci. 2019;13 doi: 10.3389/fnins.2019.01185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurks P.P.M., Vles J.S.H., Hendriksen J.G.M., Kalff A.C., Feron F.J.M., Kroes M., Van Zeben T.M.C.B., Steyaert J., Jolles J. Semantic category fluency versus initial letter fluency over 60 seconds as a measure of automatic and controlled processing in healthy school-aged children. J. Clin. Exp. Neuropsychol. 2006;28(5):684–695. doi: 10.1080/13803390590954191. [DOI] [PubMed] [Google Scholar]
- Jernigan T.L., Gamst A.C., Fennema-Notestine C., Ostergaard A.L. More “mapping” in brain mapping: statistical comparison of effects. Hum. Brain Mapp. 2003;19(2):90–95. doi: 10.1002/hbm.10108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan T.L., Brown T.T., Bartsch H., Dale A.M. Toward an integrative science of the developing human mind and brain: focus on the developing cortex. Dev. Cogn. Neurosci. 2016;18:2–11. doi: 10.1016/j.dcn.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovicich J., Czanner S., Greve D., Haley E., Van Der Kouwe A., Gollub R., Kennedy D., Schmitt F., Brown G., MacFall J., Fischl B., Dale A. Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. NeuroImage. 2006;30(2):436–443. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Jurado M.A., Mataro M., Verger K., Bartumeus F., Junque C. Phonemic and semantic fluencies in traumatic brain injury patients with focal frontal lesions. Brain Inj. 2000;14(9):789–795. doi: 10.1080/026990500421903. [DOI] [PubMed] [Google Scholar]
- Katzev M., Tüscher O., Hennig J., Weiller C., Kaller C.P. Revisiting the functional specialization of left inferior frontal gyrus in phonological and semantic fluency: the crucial role of task demands and individual ability. J. Neurosci. 2013;33(18):7837–7845. doi: 10.1523/JNEUROSCI.3147-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavé G. The development of naming and word fluency: evidence from Hebrew-speaking children between ages 8 and 17. Dev. Neuropsychol. 2006;29(3):493–508. doi: 10.1207/s15326942dn2903_7. [DOI] [PubMed] [Google Scholar]
- Kavé G., Knafo-Noam A. Lifespan development of phonemic and semantic fluency: universal increase, differential decrease. J. Clin. Exp. Neuropsychol. 2015;37(7):751–763. doi: 10.1080/13803395.2015.1065958. [DOI] [PubMed] [Google Scholar]
- Klarborg B., Skak Madsen K., Vestergaard M., Skimminge A., Jernigan T.L., Baaré W.F.C. Sustained attention is associated with right superior longitudinal fasciculus and superior parietal white matter microstructure in children. Hum. Brain Mapp. 2013;34(12):3216–3232. doi: 10.1002/hbm.22139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren R., Kofman O., Berger A. Analysis of word clustering in verbal fluency of school-aged children. Arch. Clin. Neuropsychol. 2005;20(8):1087–1104. doi: 10.1016/j.acn.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Korkman M., Kemp S.L., Kirk U. Effects of age on neurocognitive measures of children ages 5 to 12: a cross-sectional study on 800 children from the United States. Dev. Neuropsychol. 2001;20(1):331–354. doi: 10.1207/S15326942DN2001_2. [DOI] [PubMed] [Google Scholar]
- Lebel C., Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J. Neurosci. 2011;31(30):10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebel C., Walker L., Leemans A., Phillips L., Beaulieu C. Microstructural maturation of the human brain from childhood to adulthood. NeuroImage. 2008;40(3):1044–1055. doi: 10.1016/j.neuroimage.2007.12.053. [DOI] [PubMed] [Google Scholar]
- Li Y., Li P., Yang Q.X., Eslinger P.J., Sica C.T., Karunanayaka P. Lexical-semantic search under different covert verbal fluency tasks: an fMRI study. Front. Behav. Neurosci. 2017;11:131. doi: 10.3389/fnbeh.2017.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidzba K., Schwilling E., Grodd W., Krägeloh-Mann I., Wilke M. Language comprehension vs. Language production: age effects on fMRI activation. Brain Lang. 2011;119(1):6–15. doi: 10.1016/j.bandl.2011.02.003. [DOI] [PubMed] [Google Scholar]
- Loring D.W., Meador K.J., Lee G.P. Effects of temporal lobectomy on generative fluency and other language functions. Arch. Clin. Neuropsychol. 1994;9(3):229–238. doi: 10.1016/0887-6177(94)90028-0. [DOI] [PubMed] [Google Scholar]
- Madsen K.S., Baaré W.F.C., Vestergaard M., Skimminge A., Ejersbo L.R., Ramsøy T.Z., Gerlach C., Åkeson P., Paulson O.B., Jernigan T.L. Response inhibition is associated with white matter microstructure in children. Neuropsychologia. 2010;48:854–862. doi: 10.1016/j.neuropsychologia.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Madsen K.S., Baaré W.F.C., Skimminge A., Vestergaard M., Siebner H.R., Jernigan T.L. Brain microstructural correlates of visuospatial choice reaction time in children. Neuroimage. 2011;58:1090–1100. doi: 10.1016/j.neuroimage.2011.07.032. [DOI] [PubMed] [Google Scholar]
- Madsen K.S., Jernigan T.L., Vestergaard M., Mortensen E.L., Baaré W.F.C. Neuroticism is linked to microstructural left-right asymmetry of fronto-limbic fibre tracts in adolescents with opposite effects in boys and girls. Neuropsychologia. 2018;114:1–10. doi: 10.1016/J.NEUROPSYCHOLOGIA.2018.04.010. [DOI] [PubMed] [Google Scholar]
- Madsen K.S., Johansen L.B., Thompson W.K., Siebner H.R., Jernigan T.L., Baaré W.F.C. Maturational trajectories of white matter microstructure underlying the right presupplementary motor area reflect individual improvements in motor response cancellation in children and adolescents. NeuroImage. 2020;220 doi: 10.1016/j.neuroimage.2020.117105. [DOI] [PubMed] [Google Scholar]
- Martin R.C., Loring D.W., Meador K.J., Lee G.P. The effects of lateralized temporal lobe dysfunction on normal and semantic word fluency. Neuropsychologia. 1990;28(8):823–829. doi: 10.1016/0028-3932(90)90006-A. [DOI] [PubMed] [Google Scholar]
- Nash H.M., Snowling M.J. Semantic and phonological fluency in children with Down syndrome: Atypical organization of language or less efficient retrieval strategies? Cogn. Neuropsychol. 2008;25(5):690–703. doi: 10.1080/02643290802274064. [DOI] [PubMed] [Google Scholar]
- Nation K., Snowling M.J. Beyond phonological skills: broader language skills contribute to the development of reading. J. Res. Read. 2004;27(4):342–356. doi: 10.1111/j.1467-9817.2004.00238.x. [DOI] [Google Scholar]
- Olulade O.A., Seydell-Greenwald A., Chambers C.E., Turkeltaub P.E., Dromerick A.W., Berl M.M., Gaillard W.D., Newport E.L. The neural basis of language development: changes in lateralization over age. Proc. Natl. Acad. Sci. U.S.A. 2020;117(38):23477–23483. doi: 10.1073/pnas.1905590117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perret E. The left frontal lobe of man and the suppression of habitual responses in verbal categorical behaviour. Neuropsychologia. 1974;12(3):323–330. doi: 10.1016/0028-3932(74)90047-5. [DOI] [PubMed] [Google Scholar]
- Peters B.D., Szeszko P.R., Radua J., Ikuta T., Gruner P., Derosse P., Zhang J.P., Giorgio A., Qiu D., Tapert S.F., Brauer J., Asato M.R., Khong P.L., James A.C., Gallego J.A., Malhotra A.K. White matter development in adolescence: diffusion tensor imaging and meta-analytic results. Schizophr. Bull. 2012;38(6):1308–1317. doi: 10.1093/schbul/sbs054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips O.R., Clark K.A., Woods R.P., Subotnik K.L., Asarnow R.F., Nuechterlein K.H., Toga A.W., Narr K.L. Topographical relationships between arcuate fasciculus connectivity and cortical thickness. Hum. Brain Mapp. 2011;32(11):1788–1801. doi: 10.1002/hbm.21147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda D., Ardila A., Rosselli nica. Neuropsychological and behavioral assessment of ADHD in seven-to twelve-year-Old children: a discriminant analysis. J. Learn. Disabil. 1999;32(2):159–173. doi: 10.1177/002221949903200206?casa_token=-b5kzHL9oH4AAAAA:8dudSwc1Zbo0-H9dX5z6MVj7lTiiaHdF0WK9T6Arn0PMpdJdsUVSFtB4EMHgEBY6MtJIfw2wOZyReg. [DOI] [PubMed] [Google Scholar]
- Porter J.N., Collins P.F., Muetzel R.L., Lim K.O., Luciana M. Associations between cortical thickness and verbal fluency in childhood, adolescence, and young adulthood. NeuroImage. 2011;55(4):1865–1877. doi: 10.1016/j.neuroimage.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigatano G.P., Gray J.A., Lomay V.T. Verbal (animal) fluency scores in age/grade appropriate minority children from low socioeconomic backgrounds. J. Int. Neuropsychol. Soc. 2008;14(1):143–147. doi: 10.1017/S1355617708080089. [DOI] [PubMed] [Google Scholar]
- Raznahan A., Shaw P., Lalonde F., Stockman M., Wallace G.L., Greenstein D., Clasen L., Gogtay N., Giedd J.N. 2011. Brief Communications How Does Your Cortex Grow? [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva D., Nichelli F., Devoti M. Developmental aspects of verbal fluency and confrontation naming in children. Brain Lang. 2000;71(2):267–284. doi: 10.1006/brln.1999.2166. [DOI] [PubMed] [Google Scholar]
- Sauzéon H., Lestage P., Raboutet C., N’Kaoua B., Claverie B. Verbal fluency output in children aged 7-16 as a function of the production criterion: qualitative analysis of clustering, switching processes, and semantic network exploitation. Brain Lang. 2004;89(1):192–202. doi: 10.1016/S0093-934X(03)00367-5. [DOI] [PubMed] [Google Scholar]
- Shao Z., Janse E., Visser K., Meyer A.S. What do verbal fluency tasks measure? Predictors of verbal fluency performance in older adults. Front. Psychol. 2014;5(July):772. doi: 10.3389/fpsyg.2014.00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S., Jenkinson M., Johansen-Berg H., Rueckert D. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006 doi: 10.1016/j.neuroimage.2006.02.024. http://www.sciencedirect.com/science/article/pii/S1053811906001388 [DOI] [PubMed] [Google Scholar]
- Stolwyk R., Bannirchelvam B., Kraan C., Simpson K. The cognitive abilities associated with verbal fluency task performance differ across fluency variants and age groups in healthy young and old adults. J. Clin. Exp. Neuropsychol. 2015;37(1):70–83. doi: 10.1080/13803395.2014.988125. [DOI] [PubMed] [Google Scholar]
- Troyer A.K. Normative data for clustering and switching on verbal fluency tasks. J. Clin. Exp. Neuropsychol. 2000;22(3):370–378. doi: 10.1076/1380-3395(200006)22:3;1-V;FT370. [DOI] [PubMed] [Google Scholar]
- Vestergaard M., Madsen K., Baaré W., Skimminge A., Ejersbo L., Ramsøy T., Gerlach C., Åkeson P., Paulson O., Jernigan T. White matter microstructure in superior longitudinal fasciculus associated with spatial working memory performance in children. Cognitive Neuroscience, Journal Of. 2011;23(9):2135–2146. doi: 10.1162/jocn.2010.21592. [DOI] [PubMed] [Google Scholar]
- Vonk J.M.J., Rizvi B., Lao P.J., Budge M., Manly J.J., Mayeux R., Brickman A.M. Letter and category fluency performance correlates with distinct patterns of cortical thickness in older adults. Cereb. Cortex. 2019;29(6):2694–2700. doi: 10.1093/cercor/bhy138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S., Sebastian A., Lieb K., Tüscher O., Tadić A. A coordinate-based ALE functional MRI meta-analysis of brain activation during verbal fluency tasks in healthy control subjects. BMC Neurosci. 2014;15(1):1–13. doi: 10.1186/1471-2202-15-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walhovd K.B., Fjell A.M., Giedd J., Dale A.M., Brown T.T. Through thick and thin: a need to reconcile contradictory results on trajectories in human cortical development. Cereb. Cortex. 2017;27(2):1–10. doi: 10.1093/cercor/bhv301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh M.C., Pennington B.F., Groisser D.B. A normative-developmental study of executive function: a window on prefrontal function in children. Dev. Neuropsychol. 1991;7(2):131–149. doi: 10.1080/87565649109540483. [DOI] [Google Scholar]
- Wierenga L.M., Langen M., Oranje B., Durston S. Unique developmental trajectories of cortical thickness and surface area. NeuroImage. 2014;87:120–126. doi: 10.1016/j.neuroimage.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Winkler A.M., Ridgway G.R., Webster M.A., Smith S.M., Nichols T.E. Permutation inference for the general linear model. NeuroImage. 2014;92:381–397. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman J.D., Ben-Shachar M., Glover G.H., Feldman H.M. Individual differences in auditory sentence comprehension in children: an exploratory event-related functional magnetic resonance imaging investigation. Brain Lang. 2010;114(2):72–79. doi: 10.1016/j.bandl.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W., Palmer C.E., Thompson W.K., Chaarani B., Garavan H.P., Casey B.J., Jernigan T.L., Dale A.M., Fan C.C. Individual differences in cognitive performance are better predicted by global rather than localized BOLD activity patterns across the cortex. Cereb. Cortex. 2020 doi: 10.1093/cercor/bhaa290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D., Lebel C., Treit S., Evans A., Beaulieu C. Accelerated longitudinal cortical thinning in adolescence. NeuroImage. 2015;104:138–145. doi: 10.1016/j.neuroimage.2014.10.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.