Highlights
-
•
IFN-γ up-regulated CD47 expression from transcriptional level.
-
•
IFN-γ induced CD47 expression via JAK-STAT1-IRF1 pathway.
-
•
The up-regulation of CD47 expression induced by IFN-γ was widespread among cancer.
Abstract
The anti-phagocytosis signal, CD47, prevents phagocytosis when it interacts with signal-regulatory protein alpha (SIRPα) on macrophages. Given the vital role of CD47 in immune response, further investigation on the regulation of CD47 in tumor microenvironment is needed. Herein, we identified that interferon-gamma (IFN-γ), one of the most important cytokines in the immune and inflammatory response, up-regulated CD47 expression in cancer cells and this effect could be inhibited by the JAK1/2 inhibitor ruxolitinib, as well as siRNA-mediated silencing of JAK1, STAT1, and IRF1. The IFN-γ-induced surface expression of CD47 contributed to a stronger binding affinity to SIRPα and a decrease in phagocytosis of cancer cells by macrophages. Knockdown of JAK1, STAT1, or IRF1 by siRNA reversed the decreased phagocytosis caused by IFN-γ. Besides, analysis from TCGA revealed that IFNG had a positive correlation with CD47 in various types of cancer, which was supported by the increased surface CD47 expression after IFN-γ treatment in different types of cancer cells. The discovery of IFN-γ-induced up-regulation of CD47 in cancer cells unveils another feedback inhibitory mechanism of IFN-γ, thus providing insights into cancer immunotherapy targeting CD47.
Introduction
CD47 acts as a “don't eat me” signal since it exerts its anti-phagocytosis function by interacting with signal-regulatory protein alpha (SIRPα) on myeloid cells including macrophage [1,2]. This transmembrane protein is widely expressed on almost all normal cell but overexpressed on cancer cells [3,4], which assists cancer cells to evade immunosurveillance [5]. Elevated expression of CD47 in mRNA level is associated with a poor probability of patient survival in numerous cancer types [6,4]. Oncoprotein including MYC and hypoxia-inducible factors 1 have been identified to mediate in CD47 expression by directly binding to CD47 promotors [7,8]. Tumor necrosis-factor alpha (TNF-α) regulates CD47 expression via nuclear factor-κB (NF-κB) pathway. Interleukin-6 (IL-6) increases CD47 expression through activating signal transducer and activator of transcription 3 (STAT3), and other interleukins such as IL-4, IL-7, and IL-13 also participate in CD47 regulation [9], [10], [11], [12], [13], [14]. It is believed that the regulation of CD47 in the tumor microenvironment (TME) is complicated and a large part of CD47 regulatory mechanism remains unknown.
Interferon-gamma (IFN-γ) is one of the most essential cytokines in the inflammatory response and immune response. It is mainly produced by natural killer (NK) cells in innate immune system and T cells in adaptive immune system [15]. Like most cytokines, IFN-γ has two sides. On the one side, it exerts anti-tumor effects by directly inhibiting tumor cells proliferation, increasing antigen presentation, modulating macrophage phenotype and augmenting the function of CD8+ cytotoxic T lymphocytes [15]. On the other side, it plays a pro-tumor role mainly through up-regulating inhibitory immune checkpoints such as PD-L1 [16]. Canonically, IFN-γ binds to interferon-gamma receptor, followed by phosphorylation of Janus kinase 1 (JAK1) and JAK2, and then causes signal transducer and activator of transcription 1 (STAT1) phosphorylation. The dimerization of STAT1 translocate into the nuclear to mediate transcription of some IFN-γ-stimulated genes (ISGs) [17]. Interferon regulatory factor 1 (IRF1) acts as an important ISG that is involved in transcription of many secondary response genes [16,18]. IFN-γ could also activate other transcription factors such as STAT3 [19], STAT5 [20] and AP-1 [21].
In early 2000, CD47 had been reported as an ISGs [22]. Slight up-regulation of CD47 expression was found in human melanoma cells after treatment with human IFN-γ [23,24]. However, in murine tumor cell lines Hepa 1.6, MC38, AK-7 and 4T1, CD47 expression was unaltered upon murine IFN-γ stimulation [25]. Besides, there is no other study that focuses on IFN-γ regulation to CD47 on other types of human cancer, much less probe the detailed mechanisms. Given the important role of IFN-γ in the TME, we discovered that CD47 up-regulation induced by IFN-γ was a universal phenomenon in human cancer, and the JAK-STAT1-IRF1 axis was the main pathway mediated in IFN-γ-induced CD47 up-regulation.
Materials and methods
Cell culture
NCI-H1975 (human lung cancer), A549 (human lung cancer), HEY (human ovarian cancer) and MCF-7 (human breast cancer) cells were from American Type Culture Collection (Rockville, MD, USA). HCC827 (human lung cancer) and HCT116 (human colon cancer) were provided by the Shanghai Cell Bank (Shanghai, China). A549, NCI-H1975 and HCC827 cells were cultured in RPMI-1640 medium (Gibco, Carlsbad, CA, USA) with 10% (v/v) Fetal bovine serum (FBS, Gibco, Carlsbad, CA, USA) and 1% (v/v) penicillin (100 units/ml)–streptomycin (100 μg/ml). MCF-7 and HEY were cultured in Dulbecco's Modified Eagle Medium (DMEM, Gibco, Carlsbad, CA, USA) medium with 10% (v/v) FBS and 1% (v/v) penicillin (100 units/ml)–streptomycin (100 μg/ml). HCT116 was cultured in McCoy's 5A Medium (Gibco, Carlsbad, CA, USA) with 10% (v/v) FBS and 1% (v/v) penicillin (100 units/ml)–streptomycin (100 μg/ml). All of the aforementioned cells were maintained with 5% CO2 at 37 °C.
Quantitative real-time PCR
RNA was extracted by TRIzol reagent (Life Technologies, Shanghai, China). The cDNA was synthesized by RevertAid First Strand cDNA Synthesis Kit (Waltham, Massachusetts, USA). SYBR Green reagent (Roche, Penzberg, OBB, Germany) was used as a fluorescent DNA binding dye and the RT-PCR was conducted in ViiATM 7 Real-Time PCR system (Applied Biosystems, Foster City, California, USA). The sequences of the primers of CD47 and β-actin were shown in Table 1. The relative expression of CD47 was normalized to β-actin, and 2−ΔΔCT was used to calculate the relative gene expression.
Table 1.
Primers and siRNA sequences.
| Genes | Analysis | Sequences |
|---|---|---|
| CD47 | qRT-PCR | Forward: 5’-AGAAGGTGAAACGATCATCGAGC-3’ Reverse: 5’-CTCATCCATACCACCGGATCT-3’ |
| β-actin | qRT-PCR | Forward: 5’- AGCGAGCATCCCCCAAAGTT-3’ Reverse: 5’- GGGCACGAAGGCTCATCATT-3’ |
| JAK1 | siRNA | Sense: 5’-GCCUGAGAGUGGAGGUAAC-3’ Antisense: 5’-GUUACCUCCACUCUCAGGC-3’ |
| JAK2 | siRNA | Sense: 5’-CCAUCAUACGAGAUCUUAA-3’ Antisense: 5’-UUAAGAUCUCGUAUGAUGG-3’ |
| STAT1 | siRNA | Sense: 5’-GUUCGGCAGCAGCUUAAAA-3’ Antisense: 5’-UUUUAAGCUGCUGCCGAAC-3’ |
| IRF1 | siRNA | Sense: 5’-GUAAGGAGGAGCCAGAAAUTT-3’ Antisense: 5’-AAAUUUCUGGCUCCUCCUUAC-3’ |
| Negative Control | siRNA | Sense:5’-UUCUCCGAACGUGUCACGUTT-3′ Antisense: 5’-ACGUGACACGUUCGGAGAATT-3 |
Western blot
The process of western blot was described before [26]. The primary antibodies used to detect the targeting proteins expression were as follows: CD47 (Cell Signaling Technology (CST), Beverly, USA, #63000), JAK1 (CST, #3332S), JAK2 (CST, #3230S), STAT1 (CST, #14994), p-STAT1 (Tyr701) (CST, #9167), IRF1 (CST, #8478S) and GAPDH (CST. # 5174S).
Cell surface CD47 detection by flow cytometry
After IFN-γ treatment or other combined treatment with IFN-γ, cells were washed with phosphate buffer saline (PBS) and trypsinized (all steps were conducted at 37 °C). The cells collected by centrifugation were blocked with 0.5% BSA (incubation buffer) for 15 min. Then cells were washed with PBS for one time. The specific antibody against CD47 (Biolegend, San Diego, USA, #CC2C6) was added into the cells for 1 h in dark. After being washed with PBS, cell surface CD47 was detected by flow cytometry and data were analyzed by using FlowJo VX software (Tree Star, Inc, San Carlos, CA, USA).
SiRNA transfection
The siRNAs of JAK1, JAK2, STAT1 and IRF1 were purchased from Shanghai GenePharma (Shanghai, China). Lipofectamine® 3000 Reagent (Invitrogen, Carlsbad, USA) was used to transfect with SiRNA according to the manufacturer's protocol. The siRNA sequences of all target genes were listed in Table 1.
Immunofluorescence
Cells were pretreated with or without 1μM ruxolitinib followed by IFN-γ treatment in the confocal dishes. The cell samples were washed with PBS and fixed with 4% PFA for 0.5 h at 37 °C. After fixation, 0.5% Triton X-100 was used to permeabilize the cells. Subsequently the cells were blocked with 5% BSA for 1 h. The cell samples were incubated with the primary antibody against CD47 (Santa Cruz Biotechnology (SCBT), Dallas, USA, #sc-12730) at 4 °C overnight. Next, cells were incubated with the anti-rabbit IgG (H+L), F(ab')2 Fragment (Alexa Fluor® 488 Conjugate/Alexa Fluor® 594 Conjugate, Thermo Fisher Scientific, Waltham, USA, #A-11008/#A-11012) for 1 h at 37 °C. After incubation, Hoechst 33342 solution was used to stain the cell nucleuses for 5 min. Immunofluorescence images were captured by the confocal laser scanning microscope (Leica TCS SP8, Solms, Germany).
SIRPα binding assay
3 × 105 cancer cells were incubated with 50 μl PE Labeled Human SIRPα/CD172a Protein, Fc tag (0.2 μg/ml, ACRO Biosystems, Beijing, China #SIA-HP252) for 1 h at 4 °C in dark. The cells were washed three times and analyzed via flow cytometry.
Phagocytosis assay
Mouse bone marrow derived macrophages (BMDMs) were derived from 4-6 weeks old C57BL/6 wild-type mice. All experiment operations were strictly complied with the animal ethics application approved by the Animal Ethics Committee of the University of Macau. Mice femora and tibiae were flushed with ice-cold PBS, erythrocytes were lysed with red blood cell lysis buffer, and then bone marrow cells were cultured in the DMEM medium with 10% FBS and 1% (v/v) penicillin (100 units/ml)–streptomycin (100 μg/ml) and 25% L929 supernatant for 7 days. After 7 days, BMDMs were plated in a 24-well plate (1 × 105 per well) in the culture medium overnight. 2 × 105 A549 cells per well were stained with 2.5 μM CFSE at 37 °C for 10 min, and washed with the full culture medium and PBS. Each phagocytosis reaction reported in this work was performed by co-culture of 3 × 105 target cells and 1 × 105 macrophages for 2 h at 37 °C in the incubator. Macrophages were identified with APC-labeled anti-F4/80 (Biolegend, San Diego, USA #123116), and flow cytometry (BD FACS verse) was performed. Phagocytosis was calculated as the percentage of CFSE+F4/80+ cells (Q2) among F4/80+ cells (Q1 + Q2): phagocytosis (%) = [Q2 / (Q1 + Q2)] *100%. The relative phagocytosis index of each group was calculated by the percent of control.
Gene expression profiling
All cancer data were from The Cancer Genome Atlas (TCGA), and downloaded from the cBioPortal website (https://www.cbioportal.org/) [27,28] and UCSC Xena (https://xena.ucsc.edu/) website [29]. The correlation between CD47 and IFNG mRNA expression was analyzed by calculating Pearson correlation coefficients in GraphPad Prism 6 (Graph-Pad Software, Inc, California, USA).
Statistical analysis
The statistical significance was analyzed by using Student's unpaired t-test and one-way ANOVA in GraphPad Prism 6. P <0.05 was regarded as statistical significance.
Results
IFN-γ up-regulates CD47 expression in cancer cells
To find whether IFN-γ impacts CD47 expression in mRNA level, real-time PCR was conducted. CD47 mRNA expression was up-regulated after 24 h treatment with IFN-γ doses ranging from 5 to 100 ng/ml in two cancer cell lines (Fig. 1A). The same concentration of IFN-γ increased CD47 expression in the protein level (Fig. 1B-C). In addition, 24 h treatment with IFN-γ was adequate for CD47 protein accumulation on the cell surface (Fig. 1D). Taken together, IFN-γ up-regulates CD47 expression from transcriptional level and increases cell surface expression of CD47 after 24 h of stimulation.
Fig. 1.

IFN-γ up-regulated CD47 expression.
In A549 and NCI-H1975 cells, RT-PCR was to detect mRNA expression of CD47 (a), western blot was applied to detect the CD47 protein expression (b), flow cytometry was used to detect cell surface expression of CD47 (c). (d) After treatment with IFN-γ (10 ng/ml) for 3, 6, 12, 24 h in two cancer cell lines, the surface protein expression of CD47 was detected by flow cytometry, respectively. *, P < 0.05; **, P < 0.01; ns, nonsignificant.
Ruxolitinib inhibits CD47 up-regulation induced by IFN-γ
The JAK-STAT pathway is a canonical pathway in IFN-γ-induced response [30]. To reveal the mechanism of how IFN-γ induces CD47 expression, cells were pretreated with 1 μM ruxolitinib, a JAK1/2 inhibitor, for 1 h and subsequently combined with or without 10 ng/ml IFN-γ for another 24 h. The combination of ruxolitinib and IFN-γ significantly inhibited CD47 up-regulation in mRNA level (Fig. 2A). Further, after successfully inhibited the IFN-γ-JAK signal, the up-regulated protein level of CD47 by IFN-γ in the two cancer cell lines was totally disappeared as detected by western blot analysis (Fig. 2C). Immunofluorescence staining also exhibited that CD47 had a higher protein expression no matter on the cell surface or intracellularly when the cells were treated with IFN-γ. But after combined with ruxolitinib, the expression of CD47 almost decreased to the same level as the control group (Fig. 2B). The flow cytometry analysis showed that the up-regulation of CD47 by IFN-γ was abrogated by ruxolitinib (Fig. 2D), implying the irreplaceable role of JAK1 and/or JAK2 in the pathway.
Fig. 2.

Ruxolitinib inhibited CD47 up-regulation induced by IFN-γ.
A549 and NCI-H1975 cells were pretreated with ruxolitinib (1 μM) for 1 hours followed by treatment with IFN-γ (10 ng/ml) for 24 h. The mRNA level of CD47 was detected by RT-PCR (a), Immunofluorescence was used to detected CD47 expression in different treatment groups. The Hoechst 33342 was to stain the cell nuclei and anti-CD47 antibody was to stain CD47 protein. Scale bar: 250 μm (b), Western blot analysis showed p-STAT1 expression and CD47 expression (c), The surface protein expression of CD47 was determined by flow cytometry (d). (e) After A549 cells were treated with or without ruxolitinib, co-culture experiment was conducted by flow cytometry and then the relatively phagocytosis index was calculated. *, P < 0.05; **, P < 0.01.
BMDMs from C57BL/6 and Balb/c mice are two widely-used mouse strains in the phagocytosis assay [31], [32], [33], [34], [35]. From our previous result, no significant difference was observed in the phagocytosis between BMDMs from these two different mouse stains (data not show). We conducted the in vitro phagocytosis experiment by co-culturing BMDMs from C57BL/6 mice with cancer cells and detected a significant decline in the phagocytosis index between IFN-γ treatment group and the control group. However, the decline of phagocytosis was reversed when co-treated A549 cells with ruxolitinib and IFN-γ. Meanwhile, treatment with ruxolitinib alone did not affect phagocytosis of A549 cells (Fig. 2E). All these data indicate that ruxolitinib-mediated inhibition of JAK1/2 counteracts CD47 up-regulation induced by IFN-γ.
Knockdown of JAK1 significantly inhibits IFN-γ-induced CD47 up-regulation
To further confirm the role of JAK1 or JAK2 in IFN-γ-induced CD47 up-regulation, siRNAs to knockdown JAK1 or JAK2 were used. Transfected siRNA of JAK1 or JAK2 for 24 h followed by another 24 h treatment with 10 ng/ml IFN-γ, we observed that silenced JAK1 significantly suppressed IFN-γ-induced CD47 up-regulation while silencing of JAK2 only had slight inhibition effect in two cancer cell lines (Fig. 3A-C). An increase in cell surface expression of CD47 correlates a stronger binding affinity of CD47 to SIRPα. Therefore, SIRPα binding assay was conducted to test whether IFN-γ treatment, as well as knockdown of JAK1, affected the binding of CD47 to SIRPα. IFN-γ-induced CD47 up-regulation led to an enhancement of CD47 binding affinity to SIRPα. Knockdown of JAK1 followed by IFN-γ exposure significantly reduced the binding of SIRPα and CD47 (Fig. 3D). More importantly, the result of BMDMs mediated phagocytosis experiment demonstrated that siRNA of JAK1 totally abolished the decreased phagocytosis index caused by IFN-γ (Fig. 3E). Taken together, both JAK1 and JAK2 are involved in IFN-γ-induced CD47 up-regulation, while JAK1 plays a more critical role in the IFN-γ-CD47 pathway.
Fig. 3.
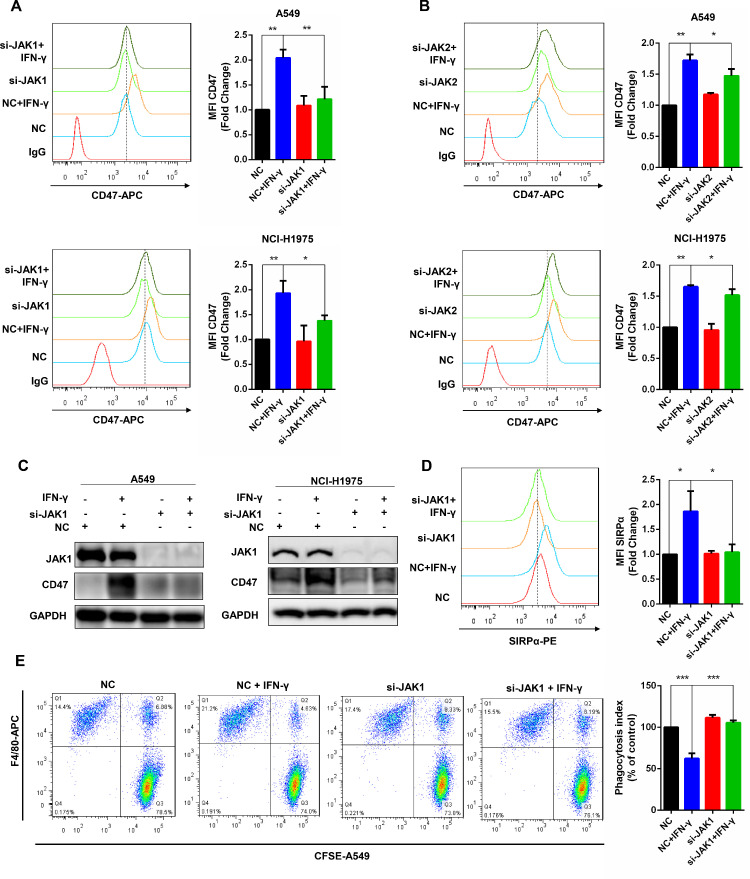
Knockdown of JAK1 significantly inhibits CD47 up-regulation induced by IFN-γ.
(a-b) After successfully knockdown of JAK1 or JAK2, the cell surface CD47 expression with or without IFN-γ exposure was detected by flow cytometry in A549 and NCI-H1975 cells. (c) The JAK1/2 and CD47 expression was showed by western blot after knockdown of JAK1/2 with or without IFN-γ treatment. (d) SIRPα binding assay was conducted to exhibit the binding ability of CD47 to SIRPα in A549 cells after silenced JAK1. (e) In A549 cells and BMDMs co-culture system, the phagocytosis index changes after knockdown JAK1 combined with or without IFN-γ were analyzed by flow cytometry. *, P < 0.05; **, P < 0.01.
IFN-γ up-regulates CD47 expression through JAK-STAT1 pathway
Upon IFN-γ stimulation, JAKs mediate tyrosine-phosphorylation (p-Tyr) of STATs. The type II interferon elicits a stronger STAT1 response [17]. We showed that the different concentrations of IFN-γ activated STAT1 in two cancer cell lines (Fig. 1B). In order to address the position of STAT1 in the signaling pathway, western blot was conducted and the result indicated that silencing of STAT1 reduced CD47 up-regulation stimulated by IFN-γ in cancer cells, which was confirmed by flow cytometry results (Fig. 4A-B). Moreover, pretreated cancer cells with 1 μM fludarabine, an STAT1 specific inhibitor, for 24 h then stimulated by IFN-γ for another 24 h, the up-regulation effect of IFN-γ to CD47 expression was partially abolished (Fig. 4C). The binding affinity of SIRPα and CD47 in the silencing STAT1 with IFN-γ group had a significant reduction when compared with the only IFN-γ treatment group (Fig. 4D). The phagocytosis index of the combination group was similar to the control one (Fig. 4E). SiRNA-mediated silencing of STAT1 partially inhibits CD47 up-regulation induced by IFN-γ, indicating that IFN-γ regulates CD47 expression through the JAK-STAT1 pathway.
Fig. 4.
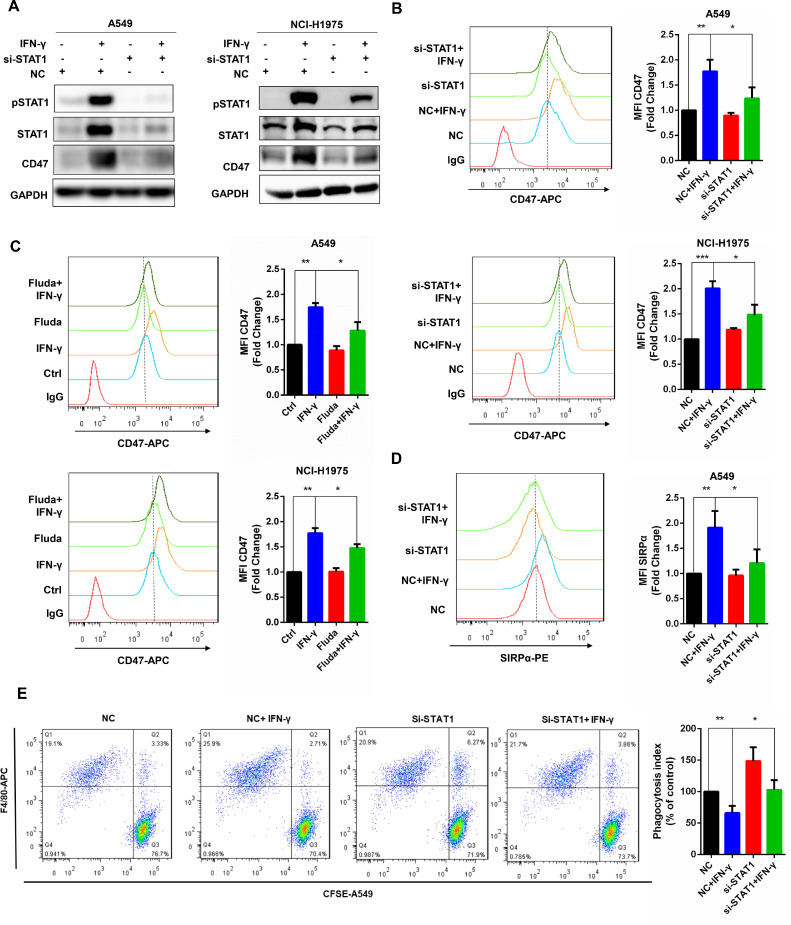
Knockdown of STAT1 inhibited CD47 up-regulation induced by IFN-γ.
(a) The protein expression of STAT1 and CD47 was analyzed by western blot after knockdown of STAT1 by siRNA in A549 and NCI-H1975 cells. (b) The cell surface CD47 expression was revealed by flow cytometry after knockdown of STAT1 with or without IFN-γ treatment. (c) Flow cytometry was used to detect the surface expression of CD47 after the STAT1 inhibitor fludarabine treatment. (d) After knockdown of STAT1, the binding of CD47 to SIRPα was determined by flow cytometry in A549 cells. (e) The relative phagocytosis index of BMDMs to A549 cells was calculated based on flow cytometry result. *, P < 0.05; **, P < 0.01.
IRF1 is the key factor mediating CD47 up-regulation upon IFN-γ exposure
It had been reported that IRF1 was the downstream protein of the JAK-STAT pathway which bound to PD-L1 promoter to increase PD-L1 expression [16]. In our study, after integrating and analyzing the data from the GEO database (GSE5542, the cancer cell line is same as ours), IRF1 was ranked top transcriptional factor with the highest fold change upon IFN-γ exposure for 6 hours and 24 h (data not show). Furthermore, flow cytometry and western blot results showed that CD47 up-regulation was partly inhibited by knockdown of IRF1 in two cancer cell lines (Fig. 5A-B). Compared with the IFN-γ treatment group, reduced binding of SIRPα and CD47 was shown in the co-treatment group (Fig. 5C). More importantly, knockdown of IRF1 reversed the decline of phagocytosis index caused by IFN-γ and treatment with siRNA of IRF1 alone had no obvious impact on phagocytosis index (Fig. 5D). These results support that IRF1 is the key factor in CD47 up-regulation after IFN-γ stimulation.
Fig. 5.

Knockdown of IRF1 inhibited CD47 up-regulation induced by IFN-γ.
(a) The protein expression of IRF1 and CD47 was analyzed by western blot analysis after knockdown of IRF1 by siRNA in A549 and NCI-H1975 cells. (b) Surface expression of CD47 was detected by flow cytometry after silenced IRF1 in two lung cancer cell lines. (c) SIRPα binding assay was presented to showed CD47 binding ability to SIRPα after knockdown of IRF1 in A549 cells. (d) Co-culture A549 cells with BMDMs, the phagocytosis index change was measured based on flow cytometry result. *, P < 0.05; **, P < 0.01.
The up-regulation of CD47 expression induced by IFN-γ is widespread in cancer
To further unveil the role of IFN-γ to CD47, we analyzed the correlation between CD47 and IFN-γ from TCGA database. All cancer types in the database were collected and analyzed, and we found that CD47 had positive correlation with IFNG in 15 cancer types including bladder carcinoma, skin cutaneous melanoma, uveal melanoma, breast invasive carcinoma, head and neck squamous cell carcinoma, kidney renal clear cell carcinoma, stomach adenocarcinoma, cervical squamous cell carcinoma, colorectal adenocarcinoma, liver hepatocellular carcinoma, ovarian serous cystadenocarcinoma, esophageal adenocarcinoma, pancreatic adenocarcinoma, pan-lung cancer and prostate adenocarcinoma (Fig. 6A). Considering the positive correlation between these two genes might also due to an increased T cells or natural killer (NK) cells infiltrate expressing IFNG, since higher expression of CD47 on T cells and NK cells assists them to keep homeostasis [36,37], further experiments was conducted. After 24 h treatment, 10 ng/ml IFN-γ increased CD47 surface expression in lung cancer cells (A549, NCI-H1975, and HCC827), colon cancer cells (HCT116), ovarian cancer cells (HEY) and breast cancer cells (MCF-7) (Fig. 6B). Combined the TCGA data analysis and the experiment results, CD47 expression is up-regulated by IFN-γ in various cancer types.
Fig. 6.

IFN-γ induced CD47 up-regulation was a widespread phenomenon in cancer cells.
(a) The correlations between CD47 and IFNG in different cancer cell types were analyzed by calculating the Pearson correlation coefficients. (b) In different cancer cell lines, the up-regulation of CD47 induced by IFN-γ was detected by flow cytometry. *, P < 0.05; **, P < 0.01.
Discussion
Discovering the regulation of CD47 by IFN-γ uncovers a new inhibitory mechanism of type II interferon, further determined its pro-tumor role mediated in immunosurveillance. Combined our study with previous findings in melanoma cells [23,24], the increased expression of CD47 induced by IFN-γ might be a widespread phenomenon within cancer. Other cytokines such as TNF-α also increased CD47 expression in various cancer types including breast cancer, hepatocellular carcinoma and lung cancer [9,10,14]. The up-regulation effect caused by these cytokines commonly existed in the TME is a favorable explanation for the higher expression of CD47 in cancer [5].
Both JAK1 and JAK2 could be activated by IFN-γ. However, IFN-γ-induced CD47 expression was mainly through the JAK1-STAT1-IRF1 axis based on the results from our research, while JAK2 participated less in this process. The uncanonical finding was supported by previous study, which reported that, in JAK2 deficient cells, the single JAK1 had capacity to compensate the loss of JAK2 by activating the subsequently signal protein STAT1 thus to complete the transcription of ISGs [20]. Utilized siRNA to silence STAT1 partially, but not totally inhibited CD47 up-regulation, indicating that other proteins might replace the role of STAT1 in the pathway once STAT1 was silenced. Upon IFN-γ stimulation, a strong STAT1 response and a weak STAT3 response exist simultaneously [19]. However, whether STAT3 is involved in the IFN-γ-induced pathway needed further investigation. Another speculation might be attribute to the moderate knockdown effect of siRNA to STAT1 (Fig. 4A), i.e., the imperfectly abolished protein expression of STAT1 may result in a mild up-regulation of CD47. The participation of STAT1 in the signaling pathway is consolidated since the specific STAT1 inhibitor fludarabine also partially reversed IFN-γ-induced CD47 up-regulation in cancer cells (Fig. 4C). Besides, knockdown of STAT1 alone significantly increased phagocytosis ability (Fig. 4E). One supposition is the loss of STAT1 in cancer cells decreases the expression of major histocompatibility complex class I (MHC class I), which is a newly found anti-phagocytosis checkpoint in cancer [38,39].
IFN-γ participates macrophage polarization [40]. Researchers polarize BMDMs with IFN-γ to transmit them into M1 type of macrophages since it has a stronger phagocytic ability [41]. However, it is speculated that a contrary effect on macrophage phagocytosis may occur simultaneously when treated cancer cells with IFN-γ. Its amplification effect to the anti-phagocytosis signal led to a reduced phagocytosis rate as well as stronger binding affinity to SIRPα. We noticed that IFN-γ caused nearly 2-fold increase of CD47 expression in mRNA and protein level, and from others findings, 2-3 folds increase of CD47 expression by TNF-α or IL-6 in mRNA level also led to a significant decline in macrophage phagocytosis [10,12]. Although we did not test the expression of other phagocytosis-related signals upon IFN-γ exposure, from the decrease phagocytosis index caused by IFN-γ we can conclude that the anti-phagocytosis signal dominates in the process. Moreover, the regulation of IFN-γ to the anti-phagocytosis signal on macrophage, SIRPα, is worth to detect in our future study for its equivalent exposure under IFN-γ treatment in the TME. Previously IFN-γ has been reported to increase SIRPα expression in monocyte, one type of cells which is closely related with macrophage in the mononuclear phagocyte system [42,43].
More importantly, our study might provide some new clues for future immunotherapy targeting immune checkpoints. Since PD-L1 was also reported to be up-regulated by IFN-γ [16] and recently one clinical research found a positive correlation between CD47/PD-L1 co-expression and CD8+ T cell density [44], it is convincible that IFN-γ may exert “linkage effect” between CD47 and PD-L1. The two immune checkpoints shared some common up-regulators such as MYC and JUN [8,45], meanwhile, CD47 and PD-L1 also co-expressed on cancer cells [46]. These studies combined with our finding augments the feasibility of developing bi-specific antibody which co-targeting CD47 and PD-L1. Recently, one of the bi-specific antibodies is going through phase I clinical trials for hematologic malignancy (NCT04795128). The bi-specific antibodies also exhibited improved phagocytosis and better tumor control [47], [48], [49], [50].
In conclusion, for the first time we defined the up-regulation of CD47 expression induced by IFN-γ was widespread among human cancer as well as mapped the JAK-STAT1-IRF1 signaling pathway in the IFN-γ-induced CD47 expression. Our study completes the regulatory mechanism of CD47 in the TME and provides useful information to future immunotherapy targeting CD47.
CRediT authorship contribution statement
Zi-Han Ye: Conceptualization, Methodology, Formal analysis, Writing – original draft, Investigation. Xiao-Ming Jiang: Conceptualization, Methodology, Data curation. Mu-Yang Huang: Methodology, Data curation. Yu-Lian Xu: Methodology, Data curation. Yu-Chi Chen: Visualization, Data curation. Luo-Wei Yuan: Data curation. Can-Yu Huang: Data curation. Wei-Bang Yu: Data curation. Xiuping Chen: Writing – review & editing. Jin-Jian Lu: Supervision, Writing – review & editing, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that no conflict of interest.
Acknowledgments
This work was supported by The Science and Technology Development Fund, Macau SAR (File no. 0129/2019/A3). We sincerely thank Dr. Lu Qi for help with signaling data analysis.
References
- 1.Jiang P., Lagenaur C.F., Narayanan V. Integrin-associated protein is a ligand for the P84 neural adhesion molecule. J. Biol. Chem. 1999;274:559–562. doi: 10.1074/jbc.274.2.559. [DOI] [PubMed] [Google Scholar]
- 2.Brown E.J., Frazier W.A. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 2001;11:130–135. doi: 10.1016/s0962-8924(00)01906-1. [DOI] [PubMed] [Google Scholar]
- 3.Jaiswal S., Jamieson C.H., Pang W.W., Park C.Y., Chao M.P., Majeti R., Traver D., van Rooijen N., Weissman I.L. CD47 is upregulated on circulating hematopoietic stem cells and leukemia cells to avoid phagocytosis. Cell. 2009;138:271–285. doi: 10.1016/j.cell.2009.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willingham S.B., Volkmer J.P., Gentles A.J., Sahoo D., Dalerba P., Mitra S.S., Wang J., Contreras-Trujillo H., Martin R., Cohen J.D., Lovelace P., Scheeren F.A., Chao M.P., Weiskopf K., Tang C., Volkmer A.K., Naik T.J., Storm T.A., Mosley A.R., Edris B., Schmid S.M., Sun C.K., Chua M.S., Murillo O., Rajendran P., Cha A.C., Chin R.K., Kim D., Adorno M., Raveh T., Tseng D., Jaiswal S., Enger P.O., Steinberg G.K., Li G., So S.K., Majeti R., Harsh G.R., van de Rijn M., Teng N.N., Sunwoo J.B., Alizadeh A.A., Clarke M.F., Weissman I.L. The CD47-signal regulatory protein alpha (SIRPa) interaction is a therapeutic target for human solid tumors. Proc. Natl. Acad. Sci. USA. 2012;109:6662–6667. doi: 10.1073/pnas.1121623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Logtenberg M.E.W., Scheeren F.A., Schumacher T.N. The CD47-SIRPalpha immune checkpoint. Immunity. 2020;52:742–752. doi: 10.1016/j.immuni.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Majeti R., Chao M.P., Alizadeh A.A., Pang W.W., Jaiswal S., Gibbs K.D., Jr., van Rooijen N., Weissman I.L. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–299. doi: 10.1016/j.cell.2009.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang H., Lu H., Xiang L., Bullen J.W., Zhang C., Samanta D., Gilkes D.M., He J., Semenza G.L. HIF-1 regulates CD47 expression in breast cancer cells to promote evasion of phagocytosis and maintenance of cancer stem cells. Proc. Natl. Acad. Sci. USA. 2015;112:E6215–E6223. doi: 10.1073/pnas.1520032112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casey S.C., Tong L., Li Y., Do R., Walz S., Fitzgerald K.N., Gouw A.M., Baylot V., Gutgemann I., Eilers M., Felsher D.W. MYC regulates the antitumor immune response through CD47 and PD-L1. Science. 2016;352:227–231. doi: 10.1126/science.aac9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lo J., Lau E.Y., Ching R.H., Cheng B.Y., Ma M.K., Ng I.O., Lee T.K. Nuclear factor kappa B-mediated CD47 up-regulation promotes sorafenib resistance and its blockade synergizes the effect of sorafenib in hepatocellular carcinoma in mice. Hepatology. 2015;62:534–545. doi: 10.1002/hep.27859. [DOI] [PubMed] [Google Scholar]
- 10.Betancur P.A., Abraham B.J., Yiu Y.Y., Willingham S.B., Khameneh F., Zarnegar M., Kuo A.H., McKenna K., Kojima Y., Leeper N.J., Ho P., Gip P., Swigut T., Sherwood R.I., Clarke M.F., Somlo G., Young R.A., Weissman I.L. A CD47-associated super-enhancer links pro-inflammatory signalling to CD47 upregulation in breast cancer. Nat. Commun. 2017;8:14802. doi: 10.1038/ncomms14802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu F., Dai M., Xu Q., Zhu X., Zhou Y., Jiang S., Wang Y., Ai Z., Ma L., Zhang Y., Hu L., Yang Q., Li J., Zhao S., Zhang Z., Teng Y. SRSF10-mediated IL1RAP alternative splicing regulates cervical cancer oncogenesis via mIL1RAP-NF-kappaB-CD47 axis. Oncogene. 2018;37:2394–2409. doi: 10.1038/s41388-017-0119-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J., Zheng D.X., Yu X.J., Sun H.W., Xu Y.T., Zhang Y.J., Xu J. Macrophages induce CD47 upregulation via IL-6 and correlate with poor survival in hepatocellular carcinoma patients. Oncoimmunology. 2019;8 doi: 10.1080/2162402X.2019.1652540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson L.D.S., Banerjee S., Kruglov O., Viller N.N., Horwitz S.M., Lesokhin A., Zain J., Querfeld C., Chen R., Okada C., Sawas A., O'Connor O.A., Sievers E.L., Shou Y., Uger R.A., Wong M., Akilov O.E. Targeting CD47 in Sezary syndrome with SIRPalphaFc. Blood Adv. 2019;3:1145–1153. doi: 10.1182/bloodadvances.2018030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X., Wang Y., Fan J., Chen W., Luan J., Mei X., Wang S., Li Y., Ye L., Li S., Tian W., Yin K., Ju D. Blocking CD47 efficiently potentiated therapeutic effects of anti-angiogenic therapy in non-small cell lung cancer. J. Immunother. Cancer. 2019;7:346. doi: 10.1186/s40425-019-0812-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ivashkiv L.B. IFNgamma: signalling, epigenetics and roles in immunity, metabolism, disease and cancer immunotherapy. Nat. Rev. Immunol. 2018;18:545–558. doi: 10.1038/s41577-018-0029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garcia-Diaz A., Shin D.S., Moreno B.H., Saco J., Escuin-Ordinas H., Rodriguez G.A., Zaretsky J.M., Sun L., Hugo W., Wang X., Parisi G., Saus C.P., Torrejon D.Y., Graeber T.G., Comin-Anduix B., Hu-Lieskovan S., Damoiseaux R., Lo R.S., Ribas A. Interferon Receptor Signaling Pathways Regulating PD-L1 and PD-L2 Expression. Cell Rep. 2017;19:1189–1201. doi: 10.1016/j.celrep.2017.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Villarino A.V., Kanno Y., O'Shea J.J. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat. Immunol. 2017;18:374–384. doi: 10.1038/ni.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castro F., Cardoso A.P., Goncalves R.M., Serre K., Oliveira M.J. Interferon-gamma at the crossroads of tumor immune surveillance or evasion. Front. Immunol. 2018;9:847. doi: 10.3389/fimmu.2018.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qing Y., Stark G.R. Alternative activation of STAT1 and STAT3 in response to interferon-gamma. J. Biol. Chem. 2004;279:41679–41685. doi: 10.1074/jbc.M406413200. [DOI] [PubMed] [Google Scholar]
- 20.Majoros A., Platanitis E., Kernbauer-Holzl E., Rosebrock F., Muller M., Decker T. Canonical and non-canonical aspects of JAK-STAT signaling: lessons from interferons for cytokine responses. Front. Immunol. 2017;8:29. doi: 10.3389/fimmu.2017.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gough D.J., Sabapathy K., Ko E.Y., Arthur H.A., Schreiber R.D., Trapani J.A., Clarke C.J., Johnstone R.W. A novel c-Jun-dependent signal transduction pathway necessary for the transcriptional activation of interferon gamma response genes. J. Biol. Chem. 2007;282:938–946. doi: 10.1074/jbc.M607674200. [DOI] [PubMed] [Google Scholar]
- 22.de Veer M.J., Holko M., Frevel M., Walker E., Der S., Paranjape J.M., Silverman R.H., Williams B.R. Functional classification of interferon-stimulated genes identified using microarrays. J. Leukoc Biol. 2001;69:912–920. [PubMed] [Google Scholar]
- 23.Sockolosky J.T., Dougan M., Ingram J.R., Ho C.C., Kauke M.J., Almo S.C., Ploegh H.L., Garcia K.C. Durable antitumor responses to CD47 blockade require adaptive immune stimulation. Proc. Natl. Acad. Sci. USA. 2016;113:E2646–E2654. doi: 10.1073/pnas.1604268113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basile M.S., Mazzon E., Russo A., Mammana S., Longo A., Bonfiglio V., Fallico M., Caltabiano R., Fagone P., Nicoletti F., Avitabile T., Reibaldi M. Differential modulation and prognostic values of immune-escape genes in uveal melanoma. PLoS One. 2019;14 doi: 10.1371/journal.pone.0210276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gauttier V., Pengam S., Durand J., Biteau K., Mary C., Morello A., Neel M., Porto G., Teppaz G., Thepenier V., Danger R., Vince N., Wilhelm E., Girault I., Abes R., Ruiz C., Trilleaud C., Ralph K., Trombetta E.S., Garcia A., Vignard V., Martinet B., Glemain A., Bruneau S., Haspot F., Dehmani S., Duplouye P., Miyasaka M., Labarriere N., Laplaud D., Bas-Bernardet S.Le, Blanquart C., Catros V., Gouraud P.A., Archambeaud I., Auble H., Metairie S., Mosnier J.F., Costantini D., Blancho G., Conchon S., Vanhove B., Poirier N. Selective SIRPalpha blockade reverses tumor T cell exclusion and overcomes cancer immunotherapy resistance. J. Clin. Investig. 2020;130:6109–6123. doi: 10.1172/JCI135528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang X.M., Xu Y.L., Huang M.Y., Zhang L.L., Su M.X., Chen X., Lu J.J. Osimertinib (AZD9291) decreases programmed death ligand-1 in EGFR-mutated non-small cell lung cancer cells. Acta Pharmacol. Sin. 2017;38:1512–1520. doi: 10.1038/aps.2017.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cerami E., Gao J., Dogrusoz U., Gross B.E., Sumer S.O., Aksoy B.A., Jacobsen A., Byrne C.J., Heuer M.L., Larsson E., Antipin Y., Reva B., Goldberg A.P., Sander C., Schultz N. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2:401–404. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao J., Aksoy B.A., Dogrusoz U., Dresdner G., Gross B., Sumer S.O., Sun Y., Jacobsen A., Sinha R., Larsson E., Cerami E., Sander C., Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldman M.J., Craft B., Hastie M., Repecka K., McDade F., Kamath A., Banerjee A., Luo Y., Rogers D., Brooks A.N., Zhu J., Haussler D. Visualizing and interpreting cancer genomics data via the Xena platform. Nat. Biotechnol. 2020;38:675–678. doi: 10.1038/s41587-020-0546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stark G.R., Darnell J.E., Jr. The JAK-STAT pathway at twenty. Immunity. 2012;36:503–514. doi: 10.1016/j.immuni.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen J., Zhong M.C., Guo H., Davidson D., Mishel S., Lu Y., Rhee I., Perez-Quintero L.A., Zhang S., Cruz-Munoz M.E., Wu N., Vinh D.C., Sinha M., Calderon V., Lowell C.A., Danska J.S., Veillette A. SLAMF7 is critical for phagocytosis of haematopoietic tumour cells via Mac-1 integrin. Nature. 2017;544:493–497. doi: 10.1038/nature22076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang X., Fan J., Wang S., Li Y., Wang Y., Li S., Luan J., Wang Z., Song P., Chen Q., Tian W., Ju D. Targeting CD47 and autophagy elicited enhanced antitumor effects in non-small cell lung cancer. Cancer Immunol. Res. 2017;5:363–375. doi: 10.1158/2326-6066.CIR-16-0398. [DOI] [PubMed] [Google Scholar]
- 33.Wu Z., Weng L., Zhang T., Tian H., Fang L., Teng H., Zhang W., Gao J., Hao Y., Li Y., Zhou H., Wang P. Identification of Glutaminyl Cyclase isoenzyme isoQC as a regulator of SIRPalpha-CD47 axis. Cell Res. 2019;29:502–505. doi: 10.1038/s41422-019-0177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morrissey M.A., Kern N., Vale R.D. CD47 ligation repositions the inhibitory receptor SIRPA to suppress integrin activation and phagocytosis. Immunity. 2020;53:290–302. doi: 10.1016/j.immuni.2020.07.008. e296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cao X., Li B., Chen J., Dang J., Chen S., Gunes E.G., Xu B., Tian L., Muend S., Raoof M., Querfeld C., Yu J., Rosen S.T., Wang Y., Feng M. Effect of cabazitaxel on macrophages improves CD47-targeted immunotherapy for triple-negative breast cancer. J. Immunother. Cancer. 2021;9 doi: 10.1136/jitc-2020-002022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nath P.R., Gangaplara A., Pal-Nath D., Mandal A., Maric D., Sipes J.M., Cam M., Shevach E.M., Roberts D.D. CD47 expression in natural killer cells regulates homeostasis and modulates immune response to lymphocytic choriomeningitis virus. Front. Immunol. 2018;9:2985. doi: 10.3389/fimmu.2018.02985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strizova Z., Vachtenheim J., Jr., Snajdauf M., Lischke R., Bartunkova J., Smrz D. Tumoral and paratumoral NK cells and CD8(+) T cells of esophageal carcinoma patients express high levels of CD47. Sci. Rep. 2020;10:13936. doi: 10.1038/s41598-020-70771-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee C.K., Gimeno R., Levy D.E. Differential regulation of constitutive major histocompatibility complex class I expression in T and B lymphocytes. J. Exp. Med. 1999;190:1451–1464. doi: 10.1084/jem.190.10.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barkal A.A., Weiskopf K., Kao K.S., Gordon S.R., Rosental B., Yiu Y.Y., George B.M., Markovic M., Ring N.G., Tsai J.M., McKenna K.M., Ho P.Y., Cheng R.Z., Chen J.Y., Barkal L.J., Ring A.M., Weissman I.L., Maute R.L. Engagement of MHC class I by the inhibitory receptor LILRB1 suppresses macrophages and is a target of cancer immunotherapy. Nat. Immunol. 2018;19:76–84. doi: 10.1038/s41590-017-0004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glass C.K., Natoli G. Molecular control of activation and priming in macrophages. Nat. Immunol. 2016;17:26–33. doi: 10.1038/ni.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murray P.J., Allen J.E., Biswas S.K., Fisher E.A., Gilroy D.W., Goerdt S., Gordon S., Hamilton J.A., Ivashkiv L.B., Lawrence T., Locati M., Mantovani A., Martinez F.O., Mege J.L., Mosser D.M., Natoli G., Saeij J.P., Schultze J.L., Shirey K.A., Sica A., Suttles J., Udalova I., van Ginderachter J.A., Vogel S.N., Wynn T.A. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Almeida A.C., Barbosa S.M., de Lourdes Rios Barjas-Castro M., Olalla-Saad S.T., Condino-Neto A. IFN-beta, IFN-gamma, and TNF-alpha decrease erythrophagocytosis by human monocytes independent of SIRP-alpha or SHP-1 expression. Immunopharmacol. Immunotoxicol. 2012;34:1054–1059. doi: 10.3109/08923973.2012.697470. [DOI] [PubMed] [Google Scholar]
- 43.Hume D.A., Irvine K.M., Pridans C. The mononuclear phagocyte system: the relationship between monocytes and macrophages. Trends Immunol. 2019;40:98–112. doi: 10.1016/j.it.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 44.Yang Z., Peng Y., Guo W., Xu J., Li L., Tian H., Li R., Liu L., Tan F., Gao S., He J. PD-L1 and CD47 co-expression predicts survival and enlightens future dual-targeting immunotherapy in non-small cell lung cancer. Thorac Cancer. 2021 doi: 10.1111/1759-7714.13989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cui L., Chen S.Y., Lerbs T., Lee J.W., Domizi P., Gordon S., Kim Y.H., Nolan G., Betancur P., Wernig G. Activation of JUN in fibroblasts promotes pro-fibrotic programme and modulates protective immunity. Nat. Commun. 2020;11:2795. doi: 10.1038/s41467-020-16466-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu B., Guo H., Xu J., Qin T., Guo Q., Gu N., Zhang D., Qian W., Dai J., Hou S., Wang H., Guo Y. Elimination of tumor by CD47/PD-L1 dual-targeting fusion protein that engages innate and adaptive immune responses. MAbs. 2018;10:315–324. doi: 10.1080/19420862.2017.1409319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu X., Liu L., Ren Z., Yang K., Xu H., Luan Y., Fu K., Guo J., Peng H., Zhu M., Fu Y.X. Dual targeting of innate and adaptive checkpoints on tumor cells limits immune evasion. Cell Rep. 2018;24:2101–2111. doi: 10.1016/j.celrep.2018.07.062. [DOI] [PubMed] [Google Scholar]
- 48.Lian S., Xie R., Ye Y., Lu Y., Cheng Y., Xie X., Li S., Jia L. Dual blockage of both PD-L1 and CD47 enhances immunotherapy against circulating tumor cells. Sci. Rep. 2019;9:4532. doi: 10.1038/s41598-019-40241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi R., Chai Y., Duan X., Bi X., Huang Q., Wang Q., Tan S., Gao G.F., Zhu J., Yan J. The identification of a CD47-blocking "hotspot" and design of a CD47/PD-L1 dual-specific antibody with limited hemagglutination. Signal Transduct. Target Ther. 2020;5:16. doi: 10.1038/s41392-020-0121-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Y., Ni H., Zhou S., He K., Gao Y., Wu W., Wu M., Wu Z., Qiu X., Zhou Y., Chen B., Pan D., Huang C., Li M., Bian Y., Yang M., Miao L., Liu J. Tumor-selective blockade of CD47 signaling with a CD47/PD-L1 bispecific antibody for enhanced anti-tumor activity and limited toxicity. Cancer Immunol. Immunother. 2021;70:365–376. doi: 10.1007/s00262-020-02679-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


