Abstract
Background
Recent studies indicate that, in addition to antibody production, lymphocyte responses to SARS-CoV-2 may play an important role in protective immunity to COVID-19 and a percentage of the general population may exhibit lymphocyte memory due to unknown/asymptomatic exposure to SARS-CoV-2 or cross-reactivity to other more common coronaviruses pre-vaccination. Total joint replacement (TJR) candidates returning to elective surgeries (median age 68 years) may exhibit similar lymphocyte and/or antibody protection to COVID-19 prior to vaccination
Methods
In this retrospective study, we analyzed antibody titters, lymphocyte memory, and inflammatory biomarkers specific for the Spike and Nucleocapsid proteins of the SARS-CoV-2 virus in a cohort of n=73 returning TJR candidates (knees and/or hips) pre-operatively.
Results
Peripheral blood serum of TJR candidate patients exhibited a positivity rate of 18.4% and 4% for IgG antibodies specific for SARS-CoV-2 nucleocapsid and spike proteins, respectively. 13.5% of TJR candidates exhibited positive lymphocyte reactivity (SI > 2) to the SARS-CoV-2 nucleocapsid protein and 38% to the spike protein. SARS-CoV-2 reactive lymphocytes exhibited a higher production of inflammatory biomarkers (i.e., IL-1β, IL-6, TNFα, and IL-1RA) compared to non-reactive lymphocytes.
Conclusions
A percentage of TJR candidates returning for elective surgeries exhibit pre-vaccination positive SARS-CoV-2 antibodies and T cell memory responses with associated pro-inflammatory biomarkers. This is an important parameter for understanding immunity, risk profiles, and may aid pre-operative planning.
Trial registration
Retrospectively registered.
Keyword: Total joint arthroplasty, COVID-19, Antibodies, Lymphocytes, LTT
Background
From the beginning of the COVID-19 pandemic, 83.5% of all elective orthopedic procedures were delayed, postponed, or canceled due to lockdowns and mitigation measures across the USA and the world [1–6]. With the number of infections and fatalities more severely affecting the age group of the general total joint replacement (TJR) population, there continues to be a lack of information on rates of asymptomatic exposure, possible immunity, and overall infection risk in patients returning to elective TJR surgeries. Current epidemiological studies demonstrate that in addition to symptomatic COVID-19 patients, a percentage of the general population exhibit COVID-19 antibody titers due to unknown/asymptomatic exposure to SARS-CoV-2 or cross-reactivity to other more common beta-coronaviruses [7, 8]. Additionally, the literature points to evidence that T lymphocytes specific to SARS-CoV-2 structural proteins play a more important role than previously anticipated in conferring immunity against COVID-19 infection [9–11]. Specifically, in cases of COVID-19 convalescent individuals, not only do they exhibit detectable titers of neutralizing antibodies, but also virus-specific T lymphocytes [9, 12–14]. There is a dearth of information on how a worldwide viral pandemic affected orthopedic patients in general, and if there may be any lasting impact on biological orthopedic implant performance, i.e., immune reactivity to implants/debris. Given the evidence of possible protective immunity to SARS-CoV-2 in a subset of the general population prior to vaccination, it remains unknown whether a subset of TJR candidates returning for primary or revision surgeries (median age 66 years) would exhibit similar lymphocyte and/or antibody protection to the SARS-CoV-2 virus. We hypothesized that prior to available vaccines, a subset of TJR candidates returning from initial months of “shelter in place” orders would exhibit either cellular immunity (lymphocyte responses) and/or detectable humoral immunity (antibody titers) to SARS-CoV-2 due to unknown asymptomatic exposure to COVID-19 or cross-reactivity to other beta-coronaviruses and that these responses would also manifest as detectable increases in inflammatory biomarkers. In a retrospective cohort of primary and revision TJR candidate patients (n=73), we analyzed specific SARS-CoV-2 nucleocapsid or spike protein lymphocyte activation/proliferation and serum IgG antibody titers specific for SARS-CoV-2 at the time of pre-operative blood work.
Materials and methods
Subject groups and parameters
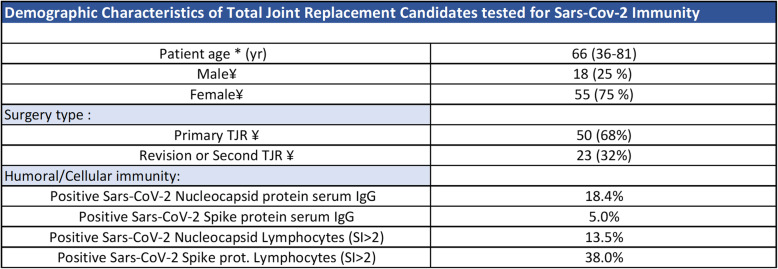
Retrospective blinded de-identified data from a cohort of n=73 returning TJR candidates (knees and/or hips) tested for lymphocyte function with an in vitro lymphocyte transformation test (LTT) was studied (approved under Rush University IRB). A group of male (n=18) and female (n=55) returning TJR candidates referred for lymphocyte transformation testing were screened for COVID-19 antibody titers and lymphocyte reactivity to SARS-CoV-2 nucleocapsid and spike proteins during pre-op testing (Table 1); none reported having a confirmed COVID-19 positive test or being exposed COVID-19 at time of sample collection.
Table 1.
Demographics of TJR candidates tested for SARS-CoV-2 humoral and cellular immunity. *The values are given as the mean, with the range in parentheses for patient age. ¥The values are given as the number, with the percentage in parentheses
Sample collection and lymphocyte transformation test for SARS-CoV-2
Whole blood was collected by venipuncture from TJR patients referred from healthcare facilities representative of 18 states in the nation using a specialized blood collection kit to ensure the quality of blood draw supplies and sample temperature stability during transport. All samples were transported to the testing facility priority overnight and processed within 24 h of initial collection. Peripheral blood mononuclear cells (PBMCs) were collected from 30 ml of peripheral blood by Ficoll gradient separation. Collected PBMCs (white buffy coat) were washed in PBS and re-suspended in RPMI-1640 with 10% autologous serum and cultured with 5μg/ml of SARS-CoV-2 nucleocapsid or spike protein (Miltenyi Biotec, CA) at 5% CO2 and 37 °C for 6 days. 3H thymidine was added at day 5 of culture. 3H thymidine incorporation in unchallenged (control) and metal-treated PBMCs was analyzed using a beta scintillation counter at day 6. A stimulation index (SI) of reactivity was calculated by dividing scintillation counts per minute of SARS-CoV-2-challenged cells over untreated controls. Results were designated as non-reactive for SI < 2, or reactive for SI > 2.
Serum SARS-CoV-2 nucleocapsid IgG detection
Enzyme-linked immunoassay
Serum samples were collected from whole blood of TJR candidates. Sandwich enzyme-linked immunoassays for SARS-Cov-2 nucleocapsid (Epitope Diagnostics, CA) and spike proteins (Bethyl Labs, TX) were performed with the manufacturer’s provided buffers and protocols.
Multiplex Luminex assays
Luminex MultiAnalyte Assay (EMD Millipore) analysis was used to detect human IL-1β, TNF-α, IL-6, IL-1RA, and IL-10 in SARS-CoV-2 peripheral blood mononuclear cell culture supernatants. Samples were collected at day 5 of culture, aliquoted, and stored at − 80 °C until time of analysis.
Positivity rates and statistical analysis
Rate of SARS-Cov-2 nucleocapsid and spike protein IgG positivity was calculated by the percentage of TJR positive serum samples (OD > 0.3 for nucleocapsid protein and OD > 0.15 for spike protein). Rate of lymphocyte reactivity to SARS-CoV-2 nucleocapsid and spike proteins was calculated by the percent of reactive (SI > 2.0) subjects in each group. Statistical differences in pro-inflammatory cytokine production were analyzed with parametric t test reaching significance at p < 0.05.
Results
Rate of positive SARS-CoV-2 nucleocapsid and spike protein IgG antibodies in TJR candidates
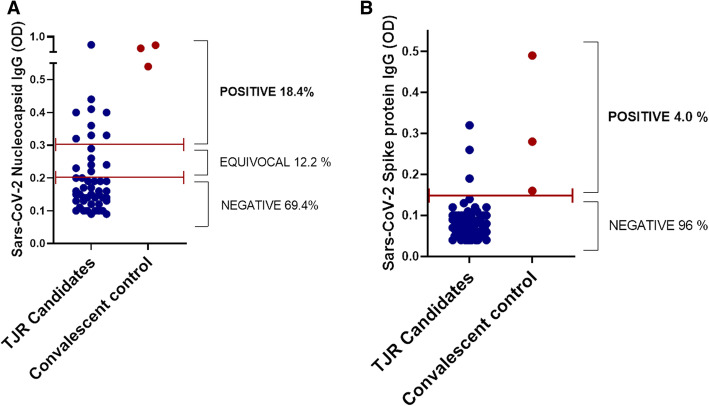
To measure the level of serum SARS-CoV-2 nucleocapsid and spike protein antibodies (IgG) in TJR candidates, peripheral blood serum collected pre-operatively was tested via enzyme-linked immunoassay for SARS-CoV-2 nucleocapsid (n=49) and spike protein (n=65) IgG antibodies (Fig. 1). Nine (18.4%) of 49 TJR candidates exhibited elevated serum levels of SARS-CoV-2 nucleocapsid IgG while 6 (12.2%) had borderline/equivocal positive results and 69.4% of TJR candidates tested negative for SARS-CoV-2 nucleocapsid protein IgG (Fig. 1A, Table 1). Contrary to nucleocapsid IgG titers, only 3 (5%) of 65 TJR candidates exhibited positive antibody titers for spike protein IgG with the majority of patients testing negative (95%) (Fig. 1B, Table 1). Convalescent serum from polymerase chain reaction (PCR) confirmed cases of COVID-19 exhibited elevated titers of both nucleocapsid and spike SARS-CoV-2 proteins (Fig. 1).
Fig. 1.
SARS-CoV-2 Antibody titers in TJR candidates. A Nucleocapsid protein serum IgG was measured in TJR candidates (n=49). B Spike protein serum IgG was measured in TJR candidates (n=64). Each dot represents each subject tested. Convalescent serum of COVID-19 confirmed cases were used as positive controls for nucleocapsid and spike protein IgG. Dotted bars indicate the threshold of antibody levels based on the manufacturer’s instructions. OD, optical density
Rate of lymphocyte memory to SARS-CoV-2 in TJR candidates
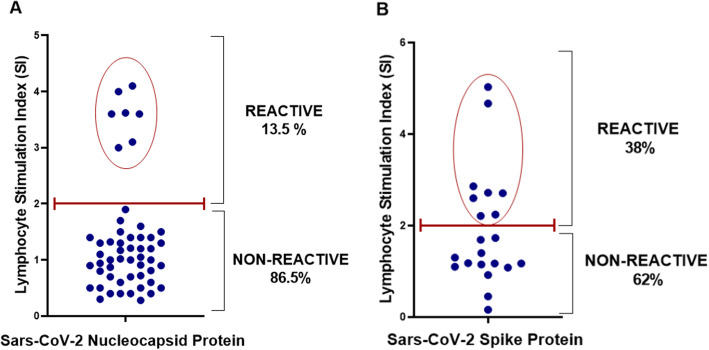
In the incidence of adaptive cellular immunity to SARS-CoV-2, TJR was compared (n=73) when tested for lymphocyte reactivity using a lymphocyte proliferation test in response to SARS-CoV-2 nucleocapsid (n=52) or spike proteins (n=21) (Fig. 2). Eight (15%) of the 52 subjects tested for lymphocyte activation/proliferation to the SARS-CoV-2 nucleocapsid protein showed reactivity with a stimulation index higher than 2-fold (SI > 2) and 44 (85%) did not exhibit any significant lymphocyte proliferation (SI < 2) to this specific protein (Fig. 2A). Additionally, eight (38%) of the 21 subjects tested for the SARS-CoV-2 spike protein exhibited lymphocyte activation/proliferation (SI > 2) while 13 (62%) showed no significant lymphocyte proliferation (SI < 2) to this specific protein (Fig. 2B). The combined overall rate of adaptive lymphocyte memory to either the SARS-CoV-2 nucleocapsid or spike proteins in TJR candidates returning to elective surgery was 21% (Fig. 2).
Fig. 2.
SARS-CoV-2 lymphocyte reactivity in TJR candidates. SARS-CoV-2 adaptive immunity was determined by lymphocyte transformation testing against SARS-CoV-2 nucleocapsid (A) and spike (B) proteins in TJR candidates (n=73). Lymphocyte stimulation index (SI) was achieved by normalizing nucleocapsid- and spike protein-treated lymphocyte counts per minute (cpms) to unchallenged lymphocyte controls. Each dot represents each subject tested and an SI > 2 was considered a positive reactive result
Pro-inflammatory cytokine production in response to SARS-CoV-2 proteins in TJR candidates
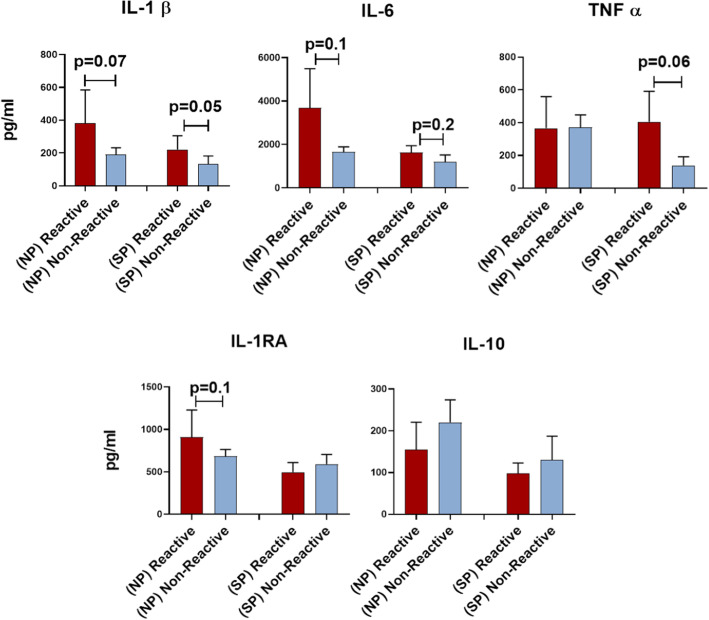
SARS-CoV-2 lymphocyte culture supernatants from TJR candidates were collected and analyzed for pro-inflammatory cytokine production at day 5 of culture. Not surprisingly, orthopedic patients with SARS-CoV-2 lymphocyte reactivity exhibited higher production of pro-inflammatory cytokines compared to those non-LTT reactive. SARS-CoV-2 nucleocapsid protein reactive lymphocytes exhibited a higher production of IL-1β (382.92 pg/ml) compared to non-reactive subjects (190.5 pg/ml) (p=0.07). Similarly, IL-6 and IL-1RA were found in greater quantities (p ≤ 0.1) in nucleocapsid reactive subjects at 3685 pg/ml and 909.82 pg/ml compared to non-reactive subjects at 1659.5 pg/ml and 684.6 pg/ml, respectively (Fig. 3A). TNFα was not higher in nucleocapsid reactive subjects (365.38 pg/ml) compared to non-reactive subjects (370.6 pg/ml). Anti-inflammatory IL-10 differences did not significantly differ, but lower concentrations in nucleocapsid reactive subjects (154.44 pg/ml) compared to non-reactive subjects were detected (219.9 pg/ml) (Fig. 3A). A similar association of pro-inflammatory cytokine production was observed for spike protein reactive subjects compared to non-reactive subjects. Spike protein reactive subjects exhibited significantly higher production of IL-1β (221.1 pg/ml, p=0.05), TNFα (405.15 pg/ml, p=0.06), and IL-6 (1632 pg/ml, p=0.2) compared to non-reactive subjects which only exhibited 132.1 pg/ml, 138.5 pg/ml, and 1194.6 pg/ml of IL-1β, TNFα, and IL-6, respectively (Fig. 3B). Non-significant differences in anti-inflammatory cytokines IL-10 (97.6 pg/ml) and ILRA (495.8 pg/ml) were observed between the reactive group compared to the non-reactive spike protein group with 130.1 pg/ml of IL-10 and 590.4 pg/ml of IL1RA (Fig. 3B).
Fig. 3.
Cytokine production in response to SARS-CoV-2 nucleocapsid (NP) and spike proteins (SP) in TJR candidates. Peripheral blood mononuclear cell (PBMC) pro-inflammatory Il-1β, IL-6, TNFα and anti-inflammatory IL-10, and IL-RA were measured in response to SARS-CoV-2 nucleocapsid protein (NP) and spike protein (SP) using a multiplex analyte assay. Error bars represent the standard error of the mean for each group and p values between groups are listed
Discussion
Results of this study support our hypothesis that a subpopulation of returning TJR candidates have specific immunological memory to SARS-CoV-2 due to unknown asymptomatic exposure to the virus or previous exposure to other common beta coronaviruses and indicate that this immune response is associated with elevated inflammatory biomarkers in vitro. These data show that 18.5% of TJR candidates preparing for elective surgery have a significant level of IgG antibodies specific for the nucleocapsid protein of SARS-CoV-2 and another 12% exhibited borderline levels for the antibody. Surprisingly, only 5% of TJR candidates exhibited elevated levels of spike protein antibodies (IgG). This could have been the result of the more transient levels of spike protein neutralizing antibodies observed in mild or asymptomatic infections compared to the more immunogenic properties of nucleocapsid proteins [15]. While it is still unclear what specific levels of SARS-CoV-2 antibodies may confer any individual immune/protected to subsequent infection, it is remarkable that almost one in five TJR candidates likely possess some level of humoral protection. It is important to note that the higher incidence of antibodies detected was specific for the more immunogenic nucleocapsid protein of SARS-CoV-2, which is a highly conserved protein among the family of coronaviruses. However, the most significant cytokine differences between LTT reactive and non-reactive groups were to the spike protein recall response. It is possible that patients with high levels of nucleocapsid protein and low or no levels of spike protein antibodies may have been infected by SARS-CoV-2 but may experience a milder form of the disease due to immunological memory to the nucleocapsid antigen, which would be detected shortly after infection due to its abundance. Interestingly, in addition to detecting humoral (antibody) immunity to SARS-CoV-2, our data indicates that TJR candidates also exhibited specific adaptive immunity to both the nucleocapsid and spike proteins of SARS-CoV-2. Initial immune recognition of foreign antigens by lymphocytes such as CD4+ helper T cells precedes antibody production by B lymphocytes and is crucial for the development of productive humoral immunity if necessary. Using a lymphocyte transformation test modified for the detection of SARS-CoV-2 nucleocapsid and spike proteins, we found that 13.5% of TJR candidates exhibited lymphocyte reactivity to the nucleocapsid protein while 38% exhibited reactivity to the spike protein. These data are important now, given that it was obtained in the early months of the pandemic prior to vaccination and thus represents a unique data point to compare to current post-vaccine incidence, reactivity, and related implant performance. Additionally, this has important implications in understanding which patients can be considered protected/immune, given that only antibody testing is currently available to assess conferred immunity and may not reflect a complete assessment of true immune status. There are no commercially available tests that examine specific lymphocyte memory (adaptive immunity) to SARS-CoV-2, which likely is a more sensitive measure of immunity even in the absence of circulating antibodies as it is the case in other viral diseases [16]. Not surprisingly, culture supernatants from SARS-CoV-2 reactive lymphocytes exhibited higher production of potent pro-inflammatory biomarkers like IL-1β, TNFα, and IL-6 compared to non-reactive lymphocytes. This supports the notion that patients with adaptive immunological memory may mount a stronger and more productive response to SARS-CoV-2, which may render them more protected during infection [11]. The potential significant increase in inflammatory biomarkers (i.e., IL-1β and IL-6) post COVID-19 infection/resolution indicates there may be a lasting altered pre-operative immune profile/environment capable of inducing musculoskeletal symptoms and affecting orthopedic surgery post-infection and recovery [17]. This may have important ramifications to implant performance. If this association is consistent in a larger scale study, it indicates that people who have had symptomatic or asymptomatic COVID-19 may require increased surveillance/follow-up after orthopedic surgery (i.e., total joint arthroplasty). One caveat in our study is that all antibody-positive subjects, except for one, did not exhibit lymphocyte reactivity (SI < 2) and had lower pro-inflammatory cytokine production compared to antibody-negative subjects. This suggests that SARS-CoV-2 IgG serum antibodies found may be of neutralizing nature, rendering lymphocytes unable to mount a productive response in an in vitro cell culture system where the amount of antigen used is limited to the culture system and not able to overburden neutralizing antibodies. This represents an important limitation of results interpretation that may be generalizable to ex vivo measured lymphocyte responses in other assays as well.
Conclusions
Our combined data suggests that a subgroup of TJR candidates may have a complex background of humoral and/or cellular immunological memory to SARS-CoV-2 associated with pro-inflammatory cytokine production (primarily IL-1β) which may render a certain level of protection to SARS-CoV-2. Additionally, humoral (IgG antibodies) and cellular immunity (lymphocyte memory) may not necessarily occur concomitantly but may provide protective immunity via different immune mechanisms; both of which have the potential to affect immune homeostasis and impact biologic reactivity to orthopedic implants. This is an important parameter for understanding risk profiles and may aid pre-operative planning.
Abbreviations
- TJR
Total joint replacement
- LTT
Lymphocyte transformation test
- ELISA
Enzyme-linked immunoassay
- IgG
Immunoglobulin G
- COVID-19
Coronavirus disease 19
- NP
Nucleocapsid protein
- SP
Spike protein
Authors’ contributions
MC: Conception and design of the work: immunological assay setup and design. VF: Acquisition and analysis of data: lymphocyte transformation testing, ELISAs. AP: Acquisition and analysis of data: data retrieval and analysis. LS: Acquisition and analysis of data: multiplex cytokine luminex assays. JJ: Interpretation of data, revision of work. NH: Interpretation of data, revision of work. The authors read and approved the final manuscript.
Funding
Funding provided by Orthopedic Analysis, LLC, Dept of Orthopedic Surgery and Crown Family endowed Chair.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Declarations
Ethics approval and consent to participate
The study was granted exemption from IRB review in accordance to 45 CFR 46.101(b) Categories of Exempt Human Subjects Research:
Research involving the collection or study of existing data, documents, records, pathological specimens, or diagnostic specimens, if these sources are publicly available or the information is recorded by the investigator in such a manner that the subjects cannot be identified directly or through identifiers linked to the subjects.
Institutional Federwide Assurance number: FWA00000482
Consent for publication
N/A
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bedard NA, Elkins JM, Brown TS. Effect of COVID-19 on hip and knee arthroplasty surgical volume in the United States. J Arthroplasty. 2020;35(7S):S45–S48. doi: 10.1016/j.arth.2020.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vannini F, Mazzotti A, Stefanini N, Faldini C. Coronavirus disease 2019 pandemic: should we delay cartilage regenerative procedures and accept the consequences, or can we find a new normality? Int Orthop. 2020;44(10):2189–2190. doi: 10.1007/s00264-020-04741-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wong JSH, Cheung KMC. Impact of COVID-19 on orthopaedic and trauma service: an epidemiological study. J Bone Joint Surg Am. 2020;102(14):e80. doi: 10.2106/JBJS.20.00775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tijerina JD, Cohen SA, Parham MJ, Debbaut C, Cohen L, Stevanovic M, Lefebvre R. Public interest in elective orthopedic surgery following recommendations during COVID-19: a Google trends analysis. Cureus. 2020;12(12):e12123. doi: 10.7759/cureus.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simon MJK, Regan WD. COVID-19 pandemic effects on orthopaedic surgeons in British Columbia. J Orthop Surg Res. 2021;16(1):161. doi: 10.1186/s13018-021-02283-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luceri F, Morelli I, Accetta R, Mangiavini L, Maffulli N, Peretti GM. Italy and COVID-19: the changing patient flow in an orthopedic trauma center emergency department. J Orthop Surg Res. 2020;15(1):323. doi: 10.1186/s13018-020-01816-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Long QX, Tang XJ, Shi QL, Li Q, Deng HJ, Yuan J, Hu JL, Xu W, Zhang Y, Lv FJ, Su K, Zhang F, Gong J, Wu B, Liu XM, Li JJ, Qiu JF, Chen J, Huang AL. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26(8):1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 8.Long QX, Liu BZ, Deng HJ, Wu GC, Deng K, Chen YK, Liao P, Qiu JF, Lin Y, Cai XF, Wang DQ, Hu Y, Ren JH, Tang N, Xu YY, Yu LH, Mo Z, Gong F, Zhang XL, Tian WG, Hu L, Zhang XX, Xiang JL, du HX, Liu HW, Lang CH, Luo XH, Wu SB, Cui XP, Zhou Z, Zhu MM, Wang J, Xue CJ, Li XF, Wang L, Li ZJ, Wang K, Niu CC, Yang QJ, Tang XJ, Zhang Y, Liu XM, Li JJ, Zhang DC, Zhang F, Liu P, Yuan J, Li Q, Hu JL, Chen J, Huang AL. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med. 2020;26(6):845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- 9.Chen Z, John WE. T cell responses in patients with COVID-19. Nat Rev Immunol. 2020;20(9):529–536. doi: 10.1038/s41577-020-0402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathew D, Giles JR, Baxter AE, Oldridge DA, Greenplate AR, Wu JE, et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science. 2020;369(6508). [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 11.Sattler A, Angermair S, Stockmann H, Heim KM, Khadzhynov D, Treskatsch S, Halleck F, Kreis ME, Kotsch K. SARS-CoV-2-specific T cell responses and correlations with COVID-19 patient predisposition. J Clin Invest. 2020;130(12):6477–6489. doi: 10.1172/JCI140965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grifoni A, Weiskopf D, Ramirez SI, Mateus J, Dan JM, Moderbacher CR, Rawlings SA, Sutherland A, Premkumar L, Jadi RS, Marrama D, de Silva AM, Frazier A, Carlin AF, Greenbaum JA, Peters B, Krammer F, Smith DM, Crotty S, Sette A. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181(7):1489–1501. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rydyznski MC, Ramirez SI, Dan JM, Grifoni A, Hastie KM, Weiskopf D, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183(4):996–1012. doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meckiff BJ, Ramirez-Suastegui C, Fajardo V, Chee SJ, Kusnadi A, Simon H, et al. Imbalance of regulatory and cytotoxic SARS-CoV-2-reactive CD4(+) T cells in COVID-19. Cell. 2020;183(5):1340–1353. doi: 10.1016/j.cell.2020.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dutta NK, Mazumdar K, Gordy JT. The nucleocapsid protein of SARS-CoV-2: a target for vaccine development. J Virol. 2020;16, 94(13). [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 16.Schulien I, Kemming J, Oberhardt V, Wild K, Seidel LM, Killmer S, Sagar, Daul F, Salvat Lago M, Decker A, Luxenburger H, Binder B, Bettinger D, Sogukpinar O, Rieg S, Panning M, Huzly D, Schwemmle M, Kochs G, Waller CF, Nieters A, Duerschmied D, Emmerich F, Mei HE, Schulz AR, Llewellyn-Lacey S, Price DA, Boettler T, Bengsch B, Thimme R, Hofmann M, Neumann-Haefelin C. Characterization of pre-existing and induced SARS-CoV-2-specific CD8(+) T cells. Nat Med. 2021;27(1):78–85. doi: 10.1038/s41591-020-01143-2. [DOI] [PubMed] [Google Scholar]
- 17.Cipollaro L, Giordano L, Padulo J, Oliva F, Maffulli N. Musculoskeletal symptoms in SARS-CoV-2 (COVID-19) patients. J Orthop Surg Res. 2020;15(1):178. doi: 10.1186/s13018-020-01702-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.