Abstract
Macrophage activation syndrome (MAS) is a form of hemophagocytic lymphohistocytosis that occurs in patients with a variety of inflammatory rheumatologic conditions. Traditionally, it is noted in pediatric patients with systemic juvenile idiopathic arthritis and systemic lupus erythematous. It is a rapidly progressive and life-threatening syndrome of excess immune activation with an estimated mortality rate of 40% in children. It has become clear recently that MAS occurs in adult patients with underlying rheumatic inflammatory diseases. In this article, we describe 6 adult patients with likely underlying MAS. This case series will outline factors related to diagnosis, pathophysiology, and review present therapeutic strategies.
Keywords: macrophage activation syndrome, adults
Introduction
Macrophage activation syndrome (MAS) is a subgroup of hemophagocytic lymphohistocytosis (HLH) syndrome occurring in patients with rheumatologic inflammatory disease. Other subgroups include familial HLH (fHLH), infection-associated HLH, and malignancy associated HLH. All share a phenotype of cytokine storm. fHLH is considered a primary disorder associated with a genetic mutation although it is now recognized it may overlap with acquired conditions. MAS is caused by immune dysregulation that causes a defect in cytotoxic pathways natural killer cells and cytotoxic T lymphocytes. Consequently, this immune dysregulation will lead to cytokine storm and accumulation of activated lymphocytes and macrophages in different organs and tissues.1-5
Clinical Presentation
Present understanding of the clinical presentations of MAS is primarily based on observations in systemic juvenile idiopathic arthritis (sJIA) patients and/or pediatric HLH patients. Features of MAS presentation can vary from a “subclinical” to a severe and potentially life-threatening episode. The difficulty in making the diagnosis of MAS lies in the fact that clinical presentations, even severe cases, can vary drastically as summarized in Table 1.
Table 1.
Clinical Presenting Features Suggestive of MAS.
| Clinical presentation | Correlating laboratory findings |
|---|---|
| Fever of unknown origin | Negative blood cultures, elevated ferritin |
| Liver failure | Elevated AST |
| Thrombocytopenia/DIC/coagulopathy | Prolonged PT, elevated fibrin split products |
| Encephalitis | Elevated CSF protein, EEG evidence of encephalopathy |
Abbreviations: AST, aspartate aminotransferase; DIC, disseminated intravascular coagulation; PT, prothrombin time; CSF, cerebrospinal fluid; EEG, electroencephalography.
Typically, the disease presents with a continuous high fever without an identifiable source. Additional symptoms such as lymphadenopathy, rash, hemorrhagic manifestations, or neurological dysfunction including headache, lethargy, irritability, altered mental status, and even seizures or coma can occur. Hepatosplenomegaly is almost always present. Although some of these findings are present in the patient with HLH, they typically worsen acutely in the onset of MAS. More severe and often fatal cases of MAS lead to multi-organ failure.
Herein, we present a retrospective series of patients with underlying inflammatory rheumatic diseases who likely developed MAS and/or cytokine storm syndrome. Informed consent for the patient information to be published in this article was not obtained because they were either deceased or unavailable to consent. The Texas Tech University Health Science Center Institutional Review Board does not require ethical approval for reporting retrospective case series. Because of the difficulty in establishing the diagnosis, an HScore was developed to assist clinicians in predicting the likelihood of having MAS (Table 2). 6
Table 2.
HScore Variables.
| Variable | Points | |
|---|---|---|
| Known underlying immunosuppression | No | 0 |
| Yes | 18 | |
| Temperature, °F (°C) | <101.1 (<38.4) | 0 |
| 101.1-102.9 (38.4-39.4) | 33 | |
| >102.9 (>39.4) | 49 | |
| Organomegaly | No | 0 |
| Hepatomegaly or splenomegaly | 23 | |
| Hepatomegaly and splenomegaly | 38 | |
| Number of cytopenias | 1 lineage | 0 |
| 2 lineages | 24 | |
| 3 lineages | 34 | |
| Ferritin, ng/mL (µg/L) | <2000 | 0 |
| 2000-6000 | 35 | |
| >6000 | 50 | |
| Triglyceride, mg/dL (mmol/L) | <132.7 (<1.5) | 0 |
| 132.7-354 (1.5-4) | 44 | |
| >354 (>4) | 64 | |
| Fibrinogen, mg/dL (g/L) | >250 (>2.5) | 0 |
| ≤250 (≤2.5) | 30 | |
| AST, U/L | <30 | 0 |
| ≥30 | 19 | |
| Hemophagocytosis features on bone marrow aspirate | No | 0 |
| Yes | 35 |
Abbreviation: AST, aspartate aminotransferase.
Case Presentations
Case 1
A 52-year-old female with a 2-year history of dermatomyositis was referred to our facility for refractory disease. Over the prior 2 years, she experienced multiple disease exacerbations. Treatment included methotrexate, mycophenolate mofetil, rituximab, intravenous immunoglobulin, and high-dose corticosteroids with no significant improvement. Within several months of her diagnosis, her ability to complete basic activities of daily living declined to the point that she required gastrostomy tube placement due to dysphagia and became wheelchair bound. One month prior to this hospitalization, she was diagnosed with splenic vein thrombosis. Physical examination revealed a diffuse macular rash on her torso and extremities. She was profoundly weak on muscle testing in all 4 extremities. Anasarca was also evident. She underwent colonoscopy to evaluate abdominal pain and fecal occult blood. Colonoscopy revealed ischemic colitis. Subsequent biopsies detected cytomegalovirus. Laboratory studies are presented in Table 3. Of note, ferritin and soluble interleukin-2 receptor levels were significantly elevated. She also had profound pancytopenia. Workup for malignancy was negative. Peripheral smear showed pancytopenia as well as schistocytes suggestive of microangiopathy. Given the severity of her illness and poor prognosis, her family asked for palliative care only. She passed away several minutes after being compassionately extubated.
Table 3.
Laboratory Values in 6 Presented Cases.
| Case | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Age (years) | 52 | 26 | 29 | 27 | 35 | 24 |
| Gender | Female | Female | Male | Female | Male | Female |
| Inclusion criteria | ||||||
| Underlying rheumatic diagnosis | Dermatomyositis | Systemic lupus erythematosus | Systemic lupus erythematosus | Systemic lupus erythematosus | Systemic lupus erythematosus | Systemic lupus erythematosus |
| Elevated ferritin (ng/mL) | 3126 | 5415 | 6606 | 24 608 | 2937 | 1844 |
| Refractory thrombocytopenia or any other cytopenia (platelet = 163 000-337 000/µL) | 18 | 7 | 3 | 18 | 13 | 6 |
| D-Dimer (<500 ng/mL) | 61 | 3846 | NT | 3816 | NT | 2438 |
| Fibrinogen (200-393 mg/dL) | 6374 | 239 | 114 | 121 | NT | 221 |
| Prothrombin time (9.4-12.5 seconds) | 18.1 | 19.8 | 14.9 | 36.6 | 21.1 | 11.5 |
| Hepatic transaminases | ||||||
| AST (5-37 IU/L) | 115 | 87 | 388 | 2370 | 35 | 25 |
| ALT (5-41 IU/L) | 81 | 91 | 368 | 2575 | 30 | 39 |
| sIL-2R (532-1891 pg/mL) | 10 642 | 7264 | 34 298 | 4246 | 14 360 | 3499 |
| Autoantibodies | NT | Anti-smith, chromatin and dsDNA | ANA, anti-centromere, anti-dsDNA antibodies | Anti-centromere and anti-dsDNA antibodies | Anti-cardiolipin and anti-centromere antibodies | dsDNA Smith and anti-chromatin antibodies |
| Complement determinations | ||||||
| C3 (88-201 mg/dL) | NT | 7 | 28 | 30 | 33 | 80 |
| C4 (15-45 mg/dL) | NT | 3 | 5 | 2 | 3 | 11 |
| IVIG | Yes | No | No | No | Yes | Yes |
| HScore (>169 indicates great likelihood of hemophagocytic syndrome) | 235 (98% to 99%) | 306 (>99%) | 186 (70% to 80%) | 195 (80% to 88%) | 106 (1% to 3%) | 145 (16% to 25%) |
| Modified HScore (using soluble IL-2R >2× normal instead of organomegaly) | 258 (>99%) | 306 (>99%) | 209 (88% to 93%) | 218 (93% to 96%) | 129 (5% to 9%) | 168 (40% to 54%) |
| DIC score >5 indicates overt DIC | 9 | 10 | 9 | 9 | 6 | 9 |
Abbreviations: AST, aspartate aminotransferase; ALT, alanine aminotransferase; IVIG, intravenous immunoglobulin; DIC, disseminated intravascular coagulation.
The patient had an HScore of 235 suggesting a 98% to 99% likelihood of hemophagocytic syndrome and a disseminated intravascular coagulation (DIC) score of 10 indicating overt DIC. She was thus diagnosed with MAS in the setting of both rheumatic disease and severe infection.
Case 2
A 26-year-old female with 8-year history of systemic lupus erythematosus (SLE) was hospitalized with refractory (>1 year) autoimmune hemolytic anemia and subsequent pancytopenia. Treatment with prednisone and azathioprine, rituximab, and cyclophosphamide failed to improve her hematologic status. Her hospital course included neutropenic fever, sepsis, and encephalopathy. Elevation in ferritin and interleukin 2 receptor and HScore suggested MAS (see Table 3). She received a short course of tocilizumab followed by etoposide. There was temporary improvement from the etoposide but she succumbed from sepsis.
The patient had an HScore of 306 indicating a >99% likelihood of a hemophagocytic syndrome and a DIC score of 10 indicating overt DIC.
Case 3
A 29-year-old male with 10-year history of ESRD (end-stage renal disease) secondary to systemic lupus on peritoneal dialysis presented with aplasia, severe mucositis, rash, and fever. Laboratory studies revealed pancytopenia, positive ANA, and dropping complements (see Table 3). Physical examination showed fever above 102 °F, cervical lymphadenopathy, and diffuse bullous rash. Peritoneal fluid grew Enterobacter cloacae and Enterococcus and he was started on appropriate antibiotics. Due to his serologic findings and cytopenias, he was treated with methylprednisolone, mycophenolate, and hydroxychloroquine. Ferritin was elevated at 6606 and platelets were decreased drastically at 3. Fibrinogen was low at 114 and aspartate aminotransferase was elevated at 388. He was found to have elevated soluble interleukin 2 receptor at 34 298 pg/mL (Table 3). Bone marrow evaluation demonstrated marrow aplasia. The patient’s condition continued to decline despite treatment, and he became unresponsive, eventually went into cardiac arrest and passed away.
His HScore was 186, indicating a 70% to 80% likelihood of hemophagocytic syndrome such as MAS. He was found to have a DIC score of 9 suggesting overt DIC.
Case 4
The patient was a 27-year-old female with 5-year medical history of SLE who presented with deep infection to the leg. The patient was treated with mycophenolate and prednisone for her lupus. She developed renal insufficiency, respiratory failure, hypofibrinogenemia, blood loss anemia, pancytopenia (with profound thrombocytopenia), deep vein thrombosis lower extremity, lactic acidosis, and septic pulmonary embolism during her hospitalization. Bronchoscopy revealed diffuse hemorrhage. Despite various treatments, such as platelets, plasma, and packed red blood cell transfusions, the patient remained pancytopenic with diffuse bleeding. Treatment with high-dose steroids, plaquenil, and mycophenolate were to no avail. Soluble interleukin 2 receptor was elevated at 4246 (pg/mL) and aspartate aminotransferase was dramatically increased at 2370. D-dimer was elevated at 3816 and fibrinogen was decreased at 121. The patient had increased ferritin at 24 608. She had severe coagulopathy and went into cardiac arrest passing away soon after.
The patient had an HScore of 195 indicating an 80% to 88% likelihood of hemophagocytic syndrome and her DIC score was 9 suggesting overt DIC.
Case 5
A 35-year-old male with a long history of antiphospholipid syndrome was admitted with infarcted fingers and severe pulmonary hypertension. Laboratory evaluation suggested an overlap syndrome with the presence of anti-centromere, anti-dsDNA, and low complements (see Table 3). Treatment included high-dose steroids, hydroxychloroquine, and mycophenolate with no significant effect. The patient’s health deteriorated with multi-organ failure including refractory thrombocytopenia.
Intravenous immunoglobulin was not effective despite the presence of megakaryocytes in the bone marrow. MAS was considered based on elevations in ferritin, IL-2 receptor (see Table 3), and refractory thrombocytopenia.
However, his HScore was low due to missing data. Similarly, his DIC score was only 6 due to absence of D-dimer and fibrinogen determinations. The patient’s condition continued to deteriorate. He was unresponsive and given his poor prognosis no further interventions were attempted and he succumbed shortly thereafter.
Case 6
The patient is a 24-year-old female with medical history of SLE and lupus nephritis who developed refractory thrombocytopenia and pancytopenia. Treatment included cyclophosphamide, mycophenolate mofetil, hydroxychloroquine, and prednisone.
Physical examination was negative for any bruising, rash, or fever, and the patient denied joint pain or bleeding at this time although she had excessive menstrual bleeding. Serologic status is outlined in Table 1. Peripheral smear showed schistocytes suggesting microangiopathy. Prior marrow examination confirmed adequate platelet production. In addition, standard treatment for immune thrombocytopenia was ineffective. She remained refractory with rising D-dimer, low fibrinogen, and elevated IL-2 receptor.
In view of the patient’s refractory thrombocytopenia and evidence for coagulopathy, she was placed on ruxolitinib (a Janus kinase 1 and 2 inhibitor) with resultant improvement in platelet counts and dropping serum ferritin levels. The patient has HScore of 145 indicating 16% to 25% chance of hemophagocytic syndrome and DIC score of 9 suggesting overt DIC.
As presented in Table 3, Patients 1 to 4 likely expired from ongoing cytokine storm or MAS. Patient 5 had an inadequate database but likely died of MAS with features consistent with catastrophic anti-phospholipid syndrome. Patient 6’s HScore is not diagnostic but we felt it was likely MAS as typical treatment for immune thrombocytopenia was unsuccessful over a year’s time with evidence of coagulopathy (elevated D-dimer), hyperferritinemia, and elevated sIL-2 receptor at >2× normal in the context of a normal marrow examination. In view of the above and failure to respond to vigorous immunosuppression including cyclophosphamide, we instituted therapy with ruloxitinib, a Janus 1 and 2 kinase inhibitor reported to be helpful in treating MAS. 5 Since beginning ruloxitinib, she has responded in all clinical parameters. Our observations with this heterogeneous group of patients point to consumptive coagulopathy as the underlying cause of each patients underlying refractory thrombocytopenia (except perhaps Patient 3 who was aplastic).
Discussion
Macrophage activation syndrome is a hemophagocytic syndrome similar to HLH that is found in conjunction with rheumatic disease. It is most commonly associated with sJIA, although it may also occur in patients diagnosed with adult-onset Still’s disease, SLE, Kawasaki disease, 1 and auto-inflammatory diseases. 6 Its incidence in adult patients is increasingly recognized.7-16
Pathophysiology
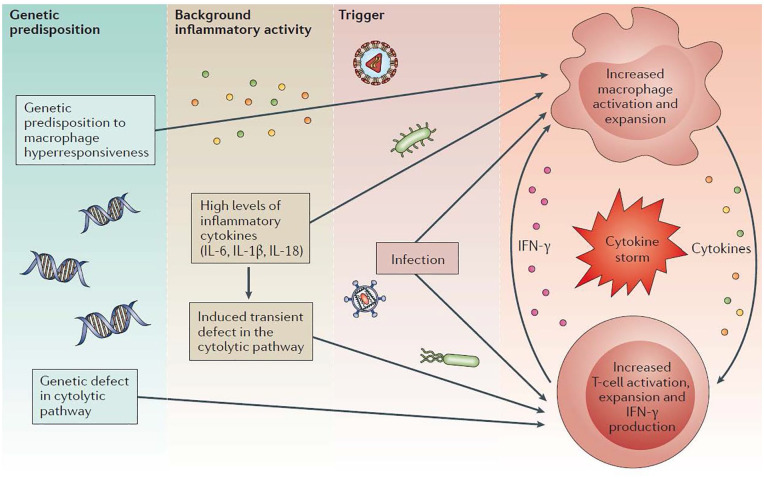
The pathophysiology of MAS is unclear. Presently, it is thought that MAS is due to a combination of genetic mutations affecting the functionality of cytolysis (the destruction of the cell membrane, typically by osmotic forces) and overwhelming IL-18 signaling leading to pro-inflammatory cytokine storm1,17,18 (see Figure 1). Recent studies have found it likely that MAS and fHLH do have genetic overlap, with nearly 40% of patients diagnosed with MAS carrying the same mono-allelic mutation associated with cytotoxicity regulating genes as those with HLH.17,18 The absence of physiologic cytolysis prevents natural killer cell and cytotoxic T-cell mediated suppression of activated lymphocytes and results in cytokine storm most notably mediated by interferon-γ and interleukin-18.
Figure 1.
Multilayer model of pathogenic events leading to the development of macrophage activation syndrome (MAS) in the context of rheumatic diseases. Genetic factors and the inflammatory milieu created by the underlying rheumatic disease act synergistically to reach the threshold for MAS in the presence of an infectious trigger. Absence of perforin-dependent cytotoxicity in natural killer cells and cytotoxic T lymphocytes leads to unbridled macrophage and T cell expansion and cytokine release. 3
Besides genetic predisposition, infectious triggers have been identified. In fact, in nearly half of all hemophagocytic cases, infection has been found to be associated. Epstein-Barr virus, tuberculosis, malaria, salmonella, and typhoid have been identified in rheumatic disease patients with MAS.15,16,19 Cases 1 and 3 were likely triggered by a combination of the patient’s rheumatic disease and infection.
Diagnosis
Understanding of the clinical presentations of MAS is primarily based on observations in sJIA patients. Disease presentation can vary from a “subclinical” presentation to a severe and potentially life-threatening episode which occurs in roughly 10% of those with sJIA. 2 The difficulty in making the diagnosis of MAS lies in the fact that clinical presentations, even severe, can vary as summarized in Table 1. For comparison, the criteria for diagnosis in sJIA are presented in Table 4. 20
Table 4.
Criteria for Diagnosis of MAS in sJIA.
| A febrile patient with known/suspected sJIA can be diagnosed with MAS if the following criteria are met: |
| 1. A ferritin level >684 ng/mL PLUS any 2 of the following: |
| a. Platelet count ≤181 × 109/L |
| b. Aspartate aminotransferase level >48 U/L |
| c. Triglyceride level >156 mg/dL |
| d. Fibrinogen level ≤360 mg/dL |
Abbreviations: MAS, macrophage activation syndrome; sJIA, systemic juvenile idiopathic arthritis.
Our observations note significant differences in clinical presentation of adult patients with rheumatologic disease with progression to MAS. Other reports note considerable similarity with sJIA and criteria for diagnosis in SLE has been suggested.21-23 The HScore (summarized in Table 2) was developed and validated with this in mind. However, rheumatic disease patients represented a minority of patients in that study. HScore differs from the diagnostic criteria for HLH by not including sIL-2 receptor concentrations. Based on the HScore validation study published by Debaugnies et al, a cutoff value of 169 corresponds to a sensitivity of 93% and specificity of 86%. 6
Our patients did not have fever (perhaps because they were already on glucocorticoids) and organomegaly was not seen. MAS in sJIA is commonly seen after years of chronic inflammation likely leading to hepatosplenomegaly. The lowest levels of sIL-2 receptor in our patients were >2× the upper limit of normal. 2 In addition, our patient’s exhibited uniform evidence for ongoing DIC. All patients were thrombocytopenic refractory to standard therapies with evidence of adequate marrow-based platelet production.
Treatment
The treatment for MAS remains problematic. While recommendations include vigorous treatment of the underlying rheumatic disease, we made the diagnosis based on refractoriness to standard therapy including high-dose steroids and in most patients, cyclophosphamide. There exist reports of improvement with IL-1 inhibition, IL-6 inhibition, cyclosporine, and other immunosuppressive agents that may represent instances of subclinical MAS.3,24-27 Unfortunately, standard vigorous immunosuppression therapies were not very successful in our patients.
We did note positive response to etoposide and ruxolitinib. 5 Unfortunately, Patient 3 could not be supported after developing severe cytopenia. Patient 6 clearly responded although her clinical criteria were less severe than the other patients her platelet counts, ferritin level, and D-dimer only responded after ruxolitinib was started.
Conclusion
Macrophage activation syndrome is increasingly recognized in ill patients with rheumatic diseases. The presence of refractory thrombocytopenia (lack of response to glucocorticoids, intravenous immunoglobulin, and or immunosuppressive agents) should prompt a laboratory evaluation of circulating ferritin levels, ongoing fibrinolysis, and elevation in sIL-2R. Our experience suggests treatment with etoposide or ruxolitinib can at least partially reverse ongoing cytokine storm.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Support provided by the J.T. and Margaret Talkington Endowment (JSP).
Ethics Approval: The Texas Tech University Health Science Center Institutional Review Board does not require ethical approval for reporting retrospective case series.
Informed Consent: Informed consent for the patient information to be published in this article was not obtained because the patients were either deceased or unavailable to consent.
ORCID iD: Kenneth Iwuji  https://orcid.org/0000-0001-5489-233X
https://orcid.org/0000-0001-5489-233X
References
- 1. Ravelli A, Davi S, Minoia F, et al. Macrophage activation syndrome. Hematol Oncol Clin North Am. 2015;29:927-941. doi: 10.1016/j.hoc.2015.06.010 [DOI] [PubMed] [Google Scholar]
- 2. Risma K, Jordan MB. Hemophagocytic lymphohistiocytosis: updates and evolving concepts. Curr Opin Pediatr. 2012;24:9-15. doi: 10.1097/MOP.0b013e32834ec9c1 [DOI] [PubMed] [Google Scholar]
- 3. Grom AA, Horne A, De Benedetti F. Macrophage activation syndrome in the era of biologic therapy. Nat Rev Rheumatol. 2016;12:259-268. doi: 10.1038/nrrheum.2015.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Canna SW, Behrens EM. Making sense of the cytokine storm: a conceptual framework for understanding, diagnosing, and treating hemophagocytic syndromes. Pediatr Clin North Am. 2012;59:329-344. doi: 10.1016/j.pcl.2012.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med. 2020;383:2255-2273. doi: 10.1056/NEJMra2026131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fardet L, Galicier L, Lambotte O, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66: 2613-2620. doi: 10.1002/art.38690 [DOI] [PubMed] [Google Scholar]
- 7. Grom AA, Mellins ED. Macrophage activation syndrome: advances towards understanding pathogenesis. Curr Opin Rheumatol. 2010;22:561-566. doi: 10.1097/01.bor.0000381996.69261.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dall’Ara F, Cavazzana I, Frassi M, et al. Macrophage activation syndrome in adult systemic lupus erythematosus: report of seven adult cases from a single Italian rheumatology center. Reumatismo. 2018;70:100-105. doi: 10.4081/reumatismo.2018.1023 [DOI] [PubMed] [Google Scholar]
- 9. Cho J, Jong SC, Ng SB. A woman with fever and lymphadenopathy. JAMA. 2018;319:2552-2553. doi: 10.1001/jama.2018.6469 [DOI] [PubMed] [Google Scholar]
- 10. Ruscitti P, Rago C, Breda L, et al. Macrophage activation syndrome in Still’s disease: analysis of clinical characteristics and survival in paediatric and adult patients. Clin Rheumatol. 2017;36:2839-2845. doi: 10.1007/s10067-017-3830-3 [DOI] [PubMed] [Google Scholar]
- 11. Watanabe E, Sugawara H, Yamashita T, et al. Successful tocilizumab therapy for macrophage activation syndrome associated with adult-onset Still’s disease: a case-based review. Case Rep Med. 2016;2016:5656320. doi: 10.1155/2016/5656320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parisi F, Paglionico A, Varriano V, et al. Refractory adult-onset Still disease complicated by macrophage activation syndrome and acute myocarditis: a case report treated with high doses (8 mg/kg/d) of anakinra. Medicine (Baltimore). 2017;96:e6656. doi: 10.1097/MD.0000000000006656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Junga Z, Stitt R, Tracy C, Keith M. Novel use of rituximab in macrophage activation syndrome secondary to systemic lupus erythematosus. BMJ Case Rep. 2017;2017:bcr2017221347. doi: 10.1136/bcr-2017-221347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kumar B, Aleem S, Saleh H, et al. A personalized diagnostic and treatment approach for macrophage activation syndrome and secondary hemophagocytic lymphohistiocytosis in adults. J Clin Immunol. 2017;37:638-643. doi: 10.1007/s10875-017-0439-x [DOI] [PubMed] [Google Scholar]
- 15. D’Errico MM CF, Biancardi C, Torcoletti M, et al. YIM-P58. Macrophage activation syndrome: the role of infectious triggers. Pediatr Rheumotol. 2014;12:Y5. doi: 10.1186/1546-0096-12-S1-Y5 [DOI] [Google Scholar]
- 16. Szolga B, Filipescu I, Damian L, Rednic S, Rednic N. A9.1 Macrophage activation syndrome after mycoplasma pneumoniae infection. Ann Rheum Dis. 2014;73(suppl 1). doi: 10.1136/annrheumdis-2013-205124.214 [DOI] [Google Scholar]
- 17. Vastert SJ, van Wijk R, D’Urbano LE, et al. Mutations in the perforin gene can be linked to macrophage activation syndrome in patients with systemic onset juvenile idiopathic arthritis. Rheumatology (Oxford). 2010;49:441-449. doi: 10.1093/rheumatology/kep418 [DOI] [PubMed] [Google Scholar]
- 18. Girard-Guyonvarc’h C, Palomo J, Martin P, et al. Unopposed IL-18 signalling leads to severe TLR9-induced macrophage activation syndrome in mice. Blood. 2018;131:1430-1441. [DOI] [PubMed] [Google Scholar]
- 19. Karthik R. Infectious causes of macrophage activation syndrome. J Assoc Physicians India. 2007;55:877-878. [PubMed] [Google Scholar]
- 20. Davì S, Minoia F, Pistorio A, et al. Performance of current guidelines for diagnosis of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. Arthritis Rheumatol. 2014;66:2871-2880. doi: 10.1002/art.38769 [DOI] [PubMed] [Google Scholar]
- 21. Parodi A, Davì S, Pringe AB, et al. Macrophage activation syndrome in juvenile systemic lupus erythematosus: a multinational multicenter study of thirty-eight patients. Arthritis Rheum. 2009;60:3388-3399. doi: 10.1002/art.24883 [DOI] [PubMed] [Google Scholar]
- 22. Gavand PE, Serio I, Arnaud L, et al. Clinical spectrum and therapeutic management of systemic lupus erythematosus-associated macrophage activation syndrome: a study of 103 episodes in 89 adult patients. Autoimmun Rev. 2017;16:743-749. doi: 10.1016/j.autrev.2017.05.010 [DOI] [PubMed] [Google Scholar]
- 23. Naveen R, Jain A, Muhammed H, et al. Macrophage activation syndrome in systemic lupus erythematosus and systemic-onset juvenile idiopathic arthritis: a retrospective study of similarities and dissimilarities. Rheumatol Int. 2021;41:625-631. doi: 10.1007/s00296-020-04763-6 [DOI] [PubMed] [Google Scholar]
- 24. Ravelli A, De Benedetti F, Viola S, Martini A. Macrophage activation syndrome in systemic juvenile rheumatoid arthritis successfully treated with cyclosporine. J Pediatr. 1996;128:275-278. doi: 10.1016/s0022-3476(96)70408-0 [DOI] [PubMed] [Google Scholar]
- 25. Miettunen PM, Narendran A, Jayanthan A, Behrens EM, Cron EQ. Successful treatment of severe paediatric rheumatic disease-associated macrophage activation syndrome with interleukin-1 inhibition following conventional immunosuppressive therapy: case series with 12 patients. Rheumatology (Oxford). 2011;50:417-419. doi: 10.1093/rheumatology/keq218 [DOI] [PubMed] [Google Scholar]
- 26. Canna SW, Girard C, Malle L, et al. Life-threatening NLRC4-associated hyperinflammation successfully treated with IL-18 inhibition. J Allergy Clin Immunol. 2017;139:1698-1701. doi: 10.1016/j.jaci.2016.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shakoory B, Carcillo JA, Chatham WW, et al. Interleukin-1 receptor blockade is associated with reduced mortality in sepsis patients with features of macrophage activation syndrome: reanalysis of a prior phase III trial. Crit Care Med. 2016;44:275-281. doi: 10.1097/CCM.0000000000001402 [DOI] [PMC free article] [PubMed] [Google Scholar]