Abstract
The aim of this clinical trial was to control the cytokine storm by administering mesenchymal stem cells (MSCs) to critically-ill COVID-19 patients, to evaluate the healing effect, and to systematically investigate how the treatment works. Patients with moderate and critical COVID-19 clinical manifestations were separated as Group 1 (moderate cases, n = 10, treated conventionally), Group 2 (critical cases, n = 10, treated conventionally), and Group 3 (critical cases, n = 10, treated conventionally plus MSCs transplantation therapy of three consecutive doses on treatment days 0, 3, and 6, (as 3 × 106 cells/kg, intravenously). The treatment mechanism of action was investigated with evaluation markers of the cytokine storm, via biochemical parameters, levels of proinflammatory and anti-inflammatory cytokines, analyses of tissue regeneration via the levels of growth factors, apoptosis markers, chemokines, matrix metalloproteinases, and granzyme-B, and by the assessment of the immunomodulatory effects via total oxidant/antioxidant status markers and the levels of lymphocyte subsets. In the assessment of the overall mortality rates of all the cases, six patients in Group-2 and three patients in Group-3 died, and there was no loss in Group-1. Proinflammatory cytokines IFNγ, IL-6, IL-17A, IL-2, IL-12, anti-inflammatory cytokines IL-10, IL-13, IL-1ra, and growth factors TGF-β, VEGF, KGF, and NGF levels were found to be significant in Group-3. When Group-2 and Group-3 were compared, serum ferritin, fibrinogen and CRP levels in Group-3 had significantly decreased. CD45 +, CD3 +, CD4 +, CD8 +, CD19 +, HLA-DR +, and CD16 + / CD56 + levels were evaluated. In the statistical comparison of the groups, significance was only determined in respect of neutrophils. The results demonstrated the positive systematic and cellular effects of MSCs application on critically ill COVID-19 patients in a versatile way. This effect plays an important role in curing and reducing mortality in critically ill patients.
Keywords: COVID-19, cytokines; growth factors, mesenchymal stem cell
Introduction
Coronaviruses, which are large enveloped non-segmented RNA viruses, cause enteric and respiratory diseases in humans 1 . Generally, the clinical manifestations of the patient include fever, non-productive cough, dyspnea, myalgia, fatigue, and pneumonia. Of the patients with dyspnea, more than half require intensive care, and approximately 46% to 65% of patients in the intensive care unit (ICU) have been reported to deteriorate in a short period of time and die due to respiratory failure 1,2 . This situation is characterized by an acute onset of hypoxic respiratory failure, non-cardiogenic pulmonary oedema, and decreased pulmonary compliance, which can subsequently trigger a cascade of serious complications and progress to multiple organ failure 3 –5 .
Cytokine storms are probably responsible for the diverse, local and remote signs associated with COVID-19 infection. This can lead to acute respiratory distress syndrome (ARDS), acute cardiac injury, secondary infection leading to generalized sepsis, and multisystem failure which may lead to death. Thus, avoiding a cytokine storm may be the key to treating COVID-19 infected patients 4 . Evidence has suggested that mesenchymal stem cells (MSCs) bind to activated immune cells, which may keep them in proximity and thus enhance immunosuppressive effects 6 . MSCs have been investigated in several in vivo models of lung disease 7 . MSC application has been proved therapeutically efficient during influenza infection, resulting in reduced impairment of alveolar fluid clearance and lung injury. This has been attributed to attenuation of pro-inflammatory cytokine secretion, inflammatory cell recruitment, and increased alveolar macrophage content 8 .
The aim of this clinical trial was to treat critically ill COVID-19 patients with MSCs therapy and to systemically explore the mechanism of action of the stem cell therapy in taking the ARDS under control. The safety and effectiveness of the MSC therapy was evaluated in the light of inflammatory, immunomodulatory and regeneration markers. The objectives of this clinical trial were: (1) to evaluate the MSCs transplantation therapy in controlling the cytokine storm in COVID-19 infected critically ill patients, (2) to investigate the mechanisms of the systemic effect of MSC therapy, (3) to evaluate the contribution of MSC application to regeneration indirectly through growth factor levels, (4) to determine the effects of MSCs administration on mortality and hospital stay.
Materials and Methods
This trial was conducted in the Bakirkoy Dr. Sadi Konuk Training and Research Hospital and Istinye University. The study protocol was approved by the Clinical Research Ethics Committee of Bakirkoy Dr. Sadi Konuk Training and Research Hospital (no:2020/139). In addition, permission was obtained from the Ministry of Health (no:56733164/203), and the trial has been registered in the clinical.trial.gov (NCT04392778). All patients or patients’ parents provided written informed consent for the collection, analysis, and publication of their outcome data.
Trial Design
The trial was designed as interventional, prospective, three-parallel armed, with two control arms consisting of moderate and critical clinical cases, as illustrated in Fig. 1. The patients were separated into three treatment arms.
Figure 1.
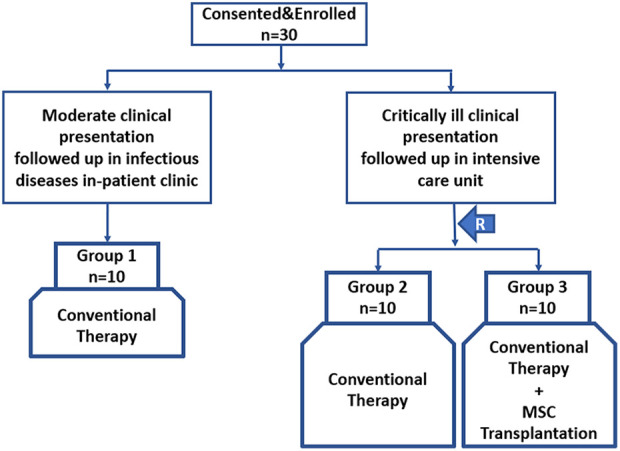
The trial design consisting of three arms. (R: randomization).
Group 1/control ( n = 10): Patients in moderate condition and followed up under the treatment algorithm in the Infectious Diseases Clinic. Patients with clinical and respiratory symptoms with radiological findings of pneumonia but no signs of severe pneumonia and no need for supplemental oxygen were evaluated as moderate disease 9,10 .
Group 2/control ( n = 10): Patients in a critically ill condition, intubated and followed-up under the conventional treatment algorithm in the ICU.
Group 3/experiment ( n = 10): Patients in a critically ill condition, intubated and followed-up under conventional treatment and systemically transplanted MSCs add-on therapy in the ICU.
The critically ill patients were ventilated with invasive mechanical ventilation in ICU. If PaO2/FiO2 was less than 150 with/without RR higher than 30 breaths/min, patients were placed in a prone position. Low tidal volume 6–8 ml/kg PBW with minimal RR to achieve pH above 7.2. PEEP relatively high 10–15 cm H2O; driving pressures below 15 cm H2O; the patients were followed up in the prone position for 16–24 hours. The average number of prone positions of the patients was 6-7. MSC transplantation was applied in three consecutive administrations on day 0, day 3, and day 6.
Patient Selection
The eligibility criteria were defined as 40–60 years old, provision of informed consent, and confirmed COVID-19 related severe ARDS. Patients showing any of the following conditions were excluded: pregnancy, breastfeeding, any current or past malignant tumours, a confirmed co-infection of human immunodeficiency virus (HIV), Hepatitis B or Hepatitis C, any current condition or history of using long-term immunosuppressive agents.
Intervention
Patients in Group-1 were treated under the conventional therapy algorithm, and this control arm was used to systematically control disease severity within the study group (Group-3) (Fig. 1). The conventional treatment was as follows: Antibiotics (Piperacillin-tazobactam 3 × 2.5gr IV), Antivirals (Favipiravir, 2 × 1600 mg loading dose, and 2 × 600 mg maintenance), Dexamethasone (1 × 6 mg IV 5–10 days per needed), Hydroxychloroquine (2 × 200 mg 5 days), Enoxaparine (2 × 0.6 ml). The patients in Group 3 were also administered 3 × 106 cell/kg MSC by intravenous infusion as 3 consecutive cures.
Stem Cell Processing, Quality Control, and Preparation
All samples of Wharton Jelly-derived mesenchymal stem cells (WJ-MSCs) as cell therapy medicinal products were isolated, expanded, analysed, and prepared in the cGMP-certified facility at Liv Hospital, as described previously 11 . Cryopreserved vials of MSCs were thawed and mixed in the same tubes before washing. Cell pellets were dissolved in PBS and seeded at a cell density of 4000 cells/cm2. After the harvest at the fourth passage, quality control tests were performed as flow cytometry analysis, endotoxin, rapid microbiological and sterility tests. Cell count and viability tests were perfomed at every harvesting and seeding step. Cell viability was determined using the Vi-CELL XR 2.03 (Beckman Coulter, Miami, FL, USA) using Vi-CELL™ Reagent solutions. The Sensoquest Labcycler and e-Myco Mycoplasma PCR Detection Kit (Intron Biotechnology, US) was used for mycoplasma detection according to the manufacturer’s instructions. Endotoxin levels were analyzed using Pyros Kinetix Flex (Associates of Cape Cod Inc., USA) and Pyrogent 25 Single Test (Lonza Inc., USA) according to the manufacturer’s instructions. Acceptance criteria of the final product were considered to be <0.0625 EU/ml and <50 U/total. MSC sterility was assessed with an automated microbial detection system BacT/Alert for 5 days (BacT/Alert® bioMérieux Corporate–Durham, USA). In addition to automated microbial detection system, sterility of the final product was also confirmed by the rapid microbiological test using the Gram staining method.
The MSCs were slowly drawn into the syringe without pressure to 40 ml volume and suspended in 150 ml of 0.9% NaCl. The MSCs treatment was applied to patients in Group-3 at each stage of the treatment by intravenous infusion for one hour at the same dose on days 0, 3, and 6, respectively.
Laboratory Assessments
Blood sampling was performed for all patients and a complete blood count (CBC), alanine transaminase (ALT), aspartate transaminase (AST), total protein, albumin, total bilirubin, direct bilirubin, ferritin, Triglycerides, D Dimer, Troponin I, myoglobin, procalcitonin (PCT), ammonia, C reactive protein (CRP), pro B-type natriuretic peptide (Pro BNP), creatin kinase (CK), and alkaline phosphatase (ALP) levels were determined.
Flow Cytometry
Anti-CD3-FITC, anti-CD16-PC5, anti-CD56-PE, anti-CD19-ECD, anti-HLA-DR-PC7, anti-CD45-FITC, anti-CD4-PE, anti-CD3-PC5, and anti-CD8-ECD fluorescent conjugated monoclonal antibodies (Beckman Coulter, USA) were used for quantification of major lymphocyte subsets.
ELISA and Luminex Assays
Chemokine (C-C motif) ligand 3 (CCL-3), caspase-3, B-cell lymphoma-2 (BCL2), interleukin (IL)-9, keratinocyte growth factor (KGF), nerve growth factor (NGF), hepatocyte growth factor (HGF), IL12, platelet-derived growth factor (PDGF), transforming growth factor (TGF)-β, interferon (IFN)-γ, IL-6, tumour necrosis factor (TNF)-α, interferon gamma-induced protein (IP)-10, IL-17A, IL-10, IL-2, monocyte chemoattractant protein-1 (MCP-1), granzyme B, IL-1β, total antioxidant status (TAS), total oxidant status (TOS), and vascular endothelial growth factor (VEGF) were measured with the ELISA technique. C-X-C motif chemokine ligand-8 (CXCL8), matrix metalloproteinase (MMP)-9, MMP-13, IL-13, IL-1RA were measured with Luminex Assay Human.
Imaging Assessments
Pulmonary CT and radiography analyses were applied at the beginning and end of the treatment for each patient. The imaging results were evaluated and reported by an experienced specialist physician.
Statistical Analysis
The NCSS (Number Cruncher Statistical System) 2007 (Kaysville, Utah, USA) program was used for statistical analysis. Descriptive statistical methods (mean, standard deviation, median, frequency, ratio, minimum, maximum) were used while evaluating the study data. The conformity of quantitative data to normal distribution was tested with the Kolmogorov-Smirnov, Shapiro-Wilk test, and graphical evaluations. The Mann Whitney U test was used in the comparisons of two groups of data that did not show normal distribution. The Kruskal Wallis test and Bonferroni Dunn test were used for paired comparisons of three and more groups that did not show normal distribution. The Fisher-Freeman-Halton Exact test was used to compare qualitative data. A value of P < .05 was accepted as statistically significant.
Results
Patient Characteristics
Evaluation was made of a total of 30 patients, comprising 11 females (37%) and 19 males (63%), with a mean age of 56 years. There was no statistically significant difference between the groups in respect of demographic data (P > .05).
Laboratory Assessments
When the CRP and PCT levels were evaluated, which are the main markers showing inflammation in COVID-19 infection, the mean pre-treatment value was found to be 73.9 mg / l in Group-1, 165 mg / l in Group-2, and 159.5 in Group-3. PCT levels were 0.15 ng /ml, 1.5 ng /ml, 1.7 ng /ml in Group-1, Group-2 and Group-3, respectively. In Group-3, the CRP values of MSCs were measured as 98.2 mg /l, 108.7 mg /l, 99.2 mg /l on days 0, 3 and 6, respectively, and these values were statistically lower than those of Group 2. The PCT values of MSCs were measured as 1.2 ng /ml, 1.2 ng /ml, 1.1 ng /ml on the days after treatment, respectively, and these values were statistically lower than those of Group-2.The minimum, maximum and average values of the other infection markers of the groups are given in Table 1. When the serum ferritin, fibrinogen, and CRP levels were compared between the groups, the levels in Group-1 were found to be lower (P < .05) than in Group-2 and Group-3. When Group-3 and Group-2 were compared, the serum ferritin, fibrinogen, and CRP levels in Group-3 were significantly more decreased (P < .05) than those in Group-2 after the 4th day. When serum troponin, procalcitonin, and D-dimer levels were compared between the groups, the Group-1 values were found to be lower (P < .05) than those of Group-2 and Group-3. (Table 1). No statistically significant difference was found between the groups in respect of the biochemical parameters.
Table 1.
Level of Inflammation Markers in Venous Blood Samples.
| Group 1 | Group 2 | Group 3 | P | |
|---|---|---|---|---|
| CRP (mg/L) | Min-Max Mean ± SD |
|||
| Before procedure | 15.1–104 | 62.3–257 | 9.4–254.7 | <.05* |
| 73.9 ± 40.9 | 165 ± 63 | 159.5 ± 30.1 | ||
| Day 1 | 2.3–243.2 | 38.5–364.6 | 4–276 | <.05** |
| 64.3 ± 84.8 | 173 ± 88.7 | 98.2 ± 87.9 | ||
| Day 4 | 1.3–104 | 33–433 | 4–466 | <.05** |
| 37 ± 36.5 | 157 ± 123.9 | 108.7 ± 101.2 | ||
| Day 7 | 1–71.2 | 62.3–257 | 9.4–254.7 | <.05** |
| 22.5 ± 23.8 | 139.6 ± 72.05 | 99.2 ± 90.8 | ||
| PCT (ng/mL) | ||||
| Before procedure | 0.1–0.2 | 0.1–6.6 | 0.1–7.4 | <.05* |
| 0.15 ± 0.01 | 1.5 ± 1.9 | 1.7 ± 2 | ||
| Day 1 | 0.1–0.2 | 0.1–19.2 | 0.1–4.7 | <.05** |
| 0.18 ± 0.06 | 5.2 ± 1.8 | 1.2 ± 1.1 | ||
| Day 4 | 0.1 -1 | 0.1–10.3 | 0.1–6 | <.05** |
| 0.9 ± 0.06 | 1.9 ± 2.1 | 1.1 ± 2.4 | ||
| Day 7 | 0.1–2 | 0.1–6.5 | 0.1–5.2 | <.05** |
| 0.4 ± 0.04 | 1.6 ± 1.5 | 1.1 ± 1.7 | ||
| D-Dimer (μg/ml) | ||||
| Before procedure | 0.1–0.5 | 0.4–3.6 | 0.4–6.4 | <.05* |
| 0.33 ± 0.3 | 1.7 ± 1 | 2.2 ± 1.7 | ||
| Day 1 | 0.1–1.7 | 0.4–3 | 1–5.7 | <.05** |
| 0.7 ± 0.6 | 1.6 ± 0.8 | 2.8 ± 1.8 | ||
| Day 4 | 0–2.3 | 0.5–3.3 | 0.7–5.5 | <.05* |
| 0.6 ± 0.6 | 1.8 ± 1.1 | 2.3 ± 1.3 | ||
| Day 7 | 0.1–0.9 | 0.3–4.1 | 0.7–8.2 | <.01* |
| 0.5 ± 0.3 | 1.9 ± 1.1 | 3.2 ± 2.7 | ||
| Fibrinogen (mg/dL) | ||||
| Before procedure | 376–643 | 393–850 | 340–759 | NS |
| 509.5 ± 188.8 | 618.8 ± 153.4 | 499.3 ± 134.1 | ||
| Day 1 | 260–651 | 320–850 | 258–590 | <.05** |
| 474.1 ± 147.3 | 622.1 ± 176.3 | 448.6 ± 104.6 | ||
| Day 4 | 258–598 | 412–745 | 250–745 | <.05** |
| 423 ± 107.2 | 612 ± 92.2 | 454.2 ± 138.2 | ||
| Day 7 | 280–467 | 390–850 | 308–773 | NS |
| 400.9 ± 70.6 | 561.3 ± 144.1 | 498.4 ± 146.1 | ||
| Ferritin (μg/L) | ||||
| Before procedure | 27–81.7 | 88–4113 | 60–2022 | <.05* |
| 54.4 ± 38.6 | 822.3 ± 1018.4 | 747.5 ± 701.9 | ||
| Day 1 | 52.9–789 | 229–3387 | 52.4–3585 | <.05* |
| 264.8 ± 282.3 | 954.4 ± 948,8 | 864.8 ± 1002.6 | ||
| Day 4 | 41–576 | 294–1600 | 213.2–966.8 | <.05** |
| 342 ± 296.4 | 670.8 ± 423.8 | 431.4 ± 229.4 | ||
| Day 7 | 35.4–587 | 221–4467 | 135–702 | <.05** |
| 199.3 ± 131.2 | 1088.5 ± 1082.4 | 379.1 ± 175.3 | ||
P-values following comparison between control groups (Group-1 and Group-2) and the study group (Group-3). All values are given as minimum, maximum and mean, NS: not significant, SD: standart deviation. * significant in favor of Group-1, ** significant in favor of Group-3 compared to Group-2 and Group-3.
The levels of proinflammatory cytokines were examined, including TNF, IFNγ, IL-6, IL-17A, IL-2, IL-12, and anti-inflammatory cytokines IL-10, IL-13, and IL-1ra. All the results are given in Table 2. There was no statistical significance between the groups in respect of TNFα, IL-1β, and IL-9 levels (P > .05).
Table 2.
Level of Proinflammatory and Anti-Inflammatory Cytokines in Venous Blood Samples.
| Cytokines | Group 1 | Group 2 | Group 3 | P |
|---|---|---|---|---|
| Proinflammatory cytokines | ||||
| IFN-γ (pg/ml) | Min-Max Mean ± SD |
|||
| Before procedure | 59.5–73.6 | 67–89.8 | 57.3–92.4 | NS |
| 68.9 ± 4.1 | 74.2 ± 8.1 | 80.2 ± 14.7 | ||
| Day 1 | 67.8–80.6 | 65.1–88.3 | 38.1–89.5 | NS |
| 73.3 ± 3.9 | 73.4 ± 8.4 | 67.5 ± 12.7 | ||
| Day 4 | 65.6–79.5 | 66.5–85.4 | 35.1–76.1 | <.05** |
| 70.2 ± 4.2 | 73.5 ± 6.7 | 64.6 ± 11.7 | ||
| Day 7 | 65.5–73.5 | 65.6–87 | 33.2–73.6 | <.005** |
| 68.3 ± 2.9 | 73.3 ± 6.6 | 60 ± 14.5 | ||
| IL-6 (pg/ml) | ||||
| Before procedure | 16.5–72 | 40.2–156 | 38.4–185.6 | NS |
| 51 ± 18.7 | 97 ± 38.3 | 101 ± 28.2 | ||
| Day 1 | 32.4–87 | 56.6–172.4 | 26.5–168.2 | <.05** |
| 55.2 ± 18.2 | 106.1 ± 42.3 | 93.1 ± 91.4 | ||
| Day 4 | 28–87.5 | 55.6–185.2 | 22.2–191 | <.05** |
| 50.1 ± 18.4 | 113.5 ± 44.5 | 90.9 ± 89.2 | ||
| Day 7 | 30.4–82.1 | 60.2–192.5 | 27–195.3 | <.05** |
| 51.7 ± 18.3 | 117.3 ± 44.2 | 91.3 ± 82.1 | ||
| IL-17A (pg/ml) | ||||
| Before procedure | 47.7–128.7 | 58.4–109.5 | 67.4–118.7 | NS |
| 84.6 ± 24.2 | 86 ± 18 | 82.2 ± 15.2 | ||
| Day 1 | 58.4–127 | 62.5–111 | 47.7–93.8 | NS |
| 88.2 ± 22.5 | 86.5 ± 17 | 87.4 ± 15.7 | ||
| Day 4 | 58.6–110.2 | 65.9–107.8 | 59.2–97.1 | NS |
| 81.2 ± 16.4 | 88 ± 13.4 | 78 ± 14.3 | ||
| Day 7 | 56.6–104.9 | 67–109.6 | 54–96.3 | <.05** |
| 79.7 ± 15.5 | 90.6 ± 13.5 | 73.2 ± 12.8 | ||
| IL-2 (pg/ml) | ||||
| Before procedure | 82.1–149.4 | 60.4–153.8 | 64.7–162.5 | NS |
| 122.3 ± 20.3 | 127.7 ± 27 | 131.2 ± 29.1 | ||
| Day 1 | 110.3–145.1 | 72.5–151.3 | 83–147.2 | NS |
| 127.8 ± 10.1 | 129.8 ± 24.3 | 124.8 ± 21.4 | ||
| Day 4 | 119.5–132.3 | 81.5–150.5 | 86.4–140.7 | NS |
| 125.6 ± 4.9 | 132.6 ± 22.8 | 121.5 ± 18.6 | ||
| Day 7 | 115.4–131.5 | 82.6–153 | 81.7–142.5 | <.05** |
| 122.4 ± 5.6 | 133.8 ± 22 | 120.4 ± 8.1 | ||
| IL-12 (pg/ml) | ||||
| Before procedure | 5–19.7 | 6–18.9 | 6–18.3 | NS |
| 11.7 ± 4.7 | 10.3 ± 3.8 | 11.4 ± 4.2 | ||
| Day 1 | 6.4–16.2 | 6–18.5 | 6.2–22.9 | NS |
| 10.3 ± 3.1 | 10.7 ± 3.8 | 13.1 ± 5.2 | ||
| Day 4 | 7.5–16.5 | 6.3–19.5 | 6.7–24.8 | NS |
| 11.8 ± 3.4 | 11.1 ± 4 | 14.6 ± 5.8 | ||
| Day 7 | 5.5–15.9 | 6.3–19.5 | 5.6–26.9 | <.05* |
| 9.4 ± 2.6 | 11.6 ± 4 | 16.4 ± 6.8 | ||
| Anti-inflammatory cytokines | ||||
| IL-10 (pg/ml) | ||||
| Before procedure | 9.5–50.3 | 2.7–55.1 | 11–43.1 | <.05* |
| 42.6 ± 12 | 20.5 ± 15.8 | 26.5 ± 21.4 | ||
| Day 1 | 10–47.6 | 3.2–54.9 | 4.8–76 | NS |
| 31.7 ± 18.5 | 20.4 ± 15.6 | 30 ± 24.6 | ||
| Day 4 | 9.5–50.3 | 3.9–55.8 | 2.1–60 | <.05** |
| 35.4 ± 14.1 | 22.4 ± 15.9 | 32.1 ± 15.5 | ||
| Day 7 | 19–47.5 | 4.5–54.8 | 4.2–65.3 | <.05** |
| 35.6 ± 10.5 | 20.3 ± 15.2 | 37.1 ± 17.4 | ||
| IL-13 (pg/ml) | ||||
| Before procedure | 225.2–524.9 | 375–1127.4 | 165.5–1245.6 | <.005*** |
| 404.2 ± 99.7 | 745 ± 231.5 | 630 ± 150.2 | ||
| Day 1 | 341.2–926.5 | 405.6–1122.6 | 468–1240.8 | <.05*** |
| 511 ± 200 | 768 ± 205.3 | 770 ± 260.1 | ||
| Day 4 | 353–831.5 | 406.9–1105.3 | 468–1148.6 | <.05** |
| 555.3 ± 171.5 | 769.7 ± 207.2 | 800.9 ± 281.3 | ||
| Day 7 | 355.7–822.5 | 574.4–1063.3 | 500.2–1111.5 | <.05** |
| 551.6 ± 167.4 | 734.6 ± 141.9 | 808.4 ± 207.8 | ||
| IL-1ra (pg/ml) | ||||
| Before procedure | 338–2781 | 900–8257 | 1078–6684 | <.005*** |
| 1279.7 ± 870.9 | 3363.4 ± 2139.3 | 3134.2 ± 1522.6 | ||
| Day 1 | 715–3758 | 1412–7459 | 1644–6569 | NS |
| 1694.2 ± 1069.5 | 3104.3 ± 2187.6 | 2882.1 ± 1529 | ||
| Day 4 | 276–2653 | 1320–6597 | 1762–5488 | <.05** |
| 1282 ± 720.8 | 2921.8 ± 1780.2 | 3236.8 ± 1256.2 | ||
| Day 7 | 283–2344 | 1237–5987 | 1459–5478 | <0.05** |
| 1257.1 ± | 2843.9 ± 1520.3 | 3149.5 ± 1058.6 | ||
P-values following comparison between control and study groups. All values are provided as minimum, maximum, and mean. NS: not significant, SD: standart deviation. * significant in favor of Group-1, ** significant in favor of Group-3 in comparison with Group-2 and Group-3, ***Significant in favor of critical patient groups in comparison with moderate patients.
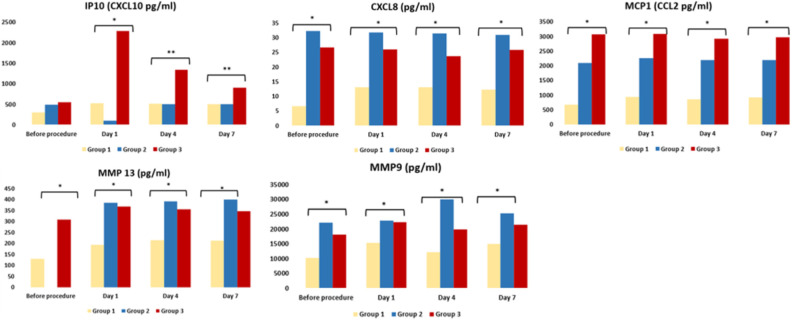
When TAS and TOS levels of the groups were compared, no significant difference was found. CXCL10 (IP10), CXCL8, MCP1, MMP13, MMP9 were analyzed as inflammatory pathway indicators and are illustrated in Fig. 2.
Figure 2.
The changes in the inflammatory pathway indicators. *p < 0.05; **p < 0.005.
Granzyme-B levels (pg/ml) were determined as 40.3, 39.4, 39, 38.2, in Group-1, 35.7, 36, 33.9, 35.9 in Group-2, and 24.2, 22.1, 29.3, 28.5 in Group-3. The effect of MSCs on apoptosis was evaluated by measuring the Caspase-3 and BCL-2 enzyme levels. Caspase-3 enzyme levels (pmol/l) were measured four times, respectively, in Group-1, 60.3, 60.7, 61.5, 60.5, in Group-2, 50.5, 55.1, 55.7, 59.4, and in Group-3 70.3, 58.3, 61.8, 61.9. BCL-2 enzyme levels (µg/l) were determined to be 170.1, 172.4, 173.3, 175.5 in Group-1, 166.4, 157.7, 193.3, 184 in Group-2, and 169, 170.3, 168.9 and 167 in Group-3. There was no significant difference between the groups in espect of the levels of caspase-3, BCL-2, and granzyme B.
In the evaluation of the HGF, PDGF, TGF-β, VEGF, KGF, and NGF serum levels, statistical significance was only determined in respect of the HGF and PDGF levels (Table 3).
Table 3.
Levels of Growth Factors.
| Growth Factors | Group 1 | Group 2 | Group 3 | P |
|---|---|---|---|---|
| TGF-β (µg/ml) | Min-Max Mean ± SD |
|||
| Before procedure | 26.8–152.4 | 22.7–81.4 | 16.4–108.3 | NS |
| 55.7 ± 43.6 | 38.4 ± 18.3 | 42.5 ± 22.9 | ||
| Day 1 | 20.7–35.6 | 22.1–80.5 | 24.5–403.2 | <.05 |
| 26.9 ± 4.4 | 38.4 ± 18.4 | 84.9 ± 48.9 | ||
| Day 4 | 24.5–33.5 | 21.5–79.7 | 25.9–272.1 | <.05 |
| 28.5 ± 3.2 | 37.2 ± 17.9 | 86 ± 81.5 | ||
| Day 7 | 24.8–32.6 | 21.5–80.1 | 15.7–281.4 | <.05 |
| 29 ± 2.6 | 38.2 ± 11.9 | 89.2 ± 88.9 | ||
| VEGF (pg/ml) | ||||
| Before procedure | 1306–3813 | 1150–3796 | 1496–3528 | NS |
| 2797 ± 716.1 | 2263 ± 999.4 | 2842 ± 660.2 | ||
| Day 1 | 1576–3748 | 1470–3788 | 2024–4679 | P < .05 |
| 2703 ± 685.9 | 2270 ± 1006.9 | 3222 ± 1048 | ||
| Day 4 | 1254–3625 | 1204–3958 | 2170–5124 | P < .05 |
| 2524 ± 787.3 | 2373 ± 938.5 | 3138 ± 963.5 | ||
| Day 7 | 1514–3152 | 1100–3854 | 1506.4–5241.6 | P < .05 |
| 2300 ± 560.2 | 2314 ± 892.9 | 3333 ± 1060 | ||
| KGF (ng/ml) | ||||
| Before procedure | 138.3–172.3 | 101.1–591.2 | 130.4–473.9 | NS |
| 155.1 ± 11 | 194.8 ± 140.6 | 218.4 ± 111.7 | ||
| Day 1 | 142.8–182.4 | 111.5–595.3 | 106.5–584.6 | NS |
| 161.8 ± 11.8 | 196.8 ± 141.7 | 216 ± 147.2 | ||
| Day 4 | 147.4–180.5 | 107.5–497 | 132.6–397.6 | NS |
| 165.7 ± 9.9 | 197.2 ± 141.6 | 206.1 ± 81.5 | ||
| Day 7 | 149–178.5 | 111.4–597.9 | 157.7–502.2 | P < .05 |
| 164.8 ± 9 | 196.2 ± 142 | 228.2 ± 73.4 | ||
| NGF (ng/ml) | ||||
| Before procedure | 1.3–65 | 0.2–20.4 | 1.2–15.4 | NS |
| 12.2 ± 19.1 | 8.5 ± 12.4 | 5.8 ± 5.4 | ||
| Day 1 | 0.6–15.8 | 0.1–22.5 | 0.1–21.1 | NS |
| 7.5 ± 5.2 | 8.4 ± 12 | 6.7 ± 6.6 | ||
| Day 4 | 2–16.3 | 1–20.5 | 2.1–16.8 | NS |
| 7.7 ± 5.3 | 8.3 ± 9.6 | 12.1 ± 5.5 | ||
| Day 7 | 1.5–15.5 | 0.9–17.1 | 2.1–24.6 | P < .05 |
| 7.8 ± 4.8 | 8.4 ± 12.3 | 13.7 ± 6.7 | ||
P-values following comparison between control groups (Group1 and Group-2) and the study group (Group-3). All values are given as minimum, maximum and mean, NS: not significant, SD: standart deviation.
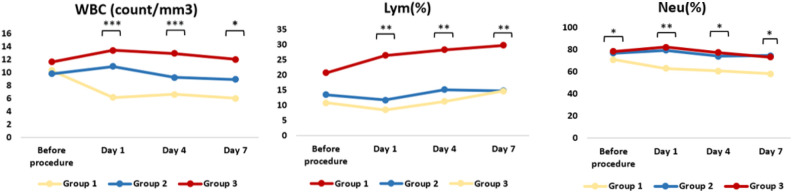
The main lymphocyte subgroups (CD45+, CD3+, CD4+, CD8+, CD19+, HLA-DR+ and CD16+CD56+) were evaluated in all groups. The statistical comparisons of the groups are shown in Table 4. The WBC, lymphocytes, and neutrophil leukocytes were also counted and the results are presented in Fig. 3.
Table 4.
Levels of the Main Lymphocyte Subgroups.
| Group 1 | Group 2 | Group 3 | P | |
|---|---|---|---|---|
| CD45+ | Min-Max Mean ± SD |
|||
| Before procedure | 10.5–38.4 | 3–22.1 | 2.1–10.4 | <.05* |
| 20.1 ± 10.6 | 8.3 ± 5.4 | 5.4 ± 2.6 | ||
| Day 7 | 13.3–45.4 | 3.3–21.5 | 3.1–13 | <.005* |
| 25.8 ± 10.1 | 8.7 ± 5.1 | 6.4 ± 4.3 | ||
| HLADR+ | ||||
| Before procedure | 0.1–0.8 | 0.4–3.1 | 0.5–4.1 | NS |
| 0.5 ± 0.1 | 1.1 ± 0.5 | 0.7 ± 0.7 | ||
| Day 7 | 0.9–2.3 | 0.3–2.1 | 0.6–4 | <.05** |
| 1.7 ± 0.5 | 0.8 ± 0.6 | 1.8 ± 1.1 | ||
| CD16+ | ||||
| Before procedure | 41.2–75.3 | 50.4–61.8 | 50.3–62.5 | NS |
| 58.1 ± 10.1 | 52.8 ± 3.9 | 52.2 ± 4.1 | ||
| Day 7 | 43.9–77.2 | 57.2–82.5 | 38.8–83.3 | <.05* |
| 59 ± 10.6 | 74 ± 7.9 | 63 ± 14.2 | ||
| CD16+CD56+ | ||||
| Before procedure | 0.4–6.3 | 0.2–1.3 | 0.1–1.5 | <.05* |
| 2.4 ± 2.1 | 0.5 ± 0.2 | 0.2 ± 0.3 | ||
| Day 7 | 0.4–6.2 | 0.5–2.4 | 0.1–1.4 | <.05* |
| 2.5 ± 2 | 1.1 ± 0.6 | 0.6 ± 0.5 | ||
| Granulocyte | ||||
| Before procedure | 28.6–76 | 50–74 | 33–81 | <.05* |
| 52 ± 13.4 | 68 ± 8 | 60 ± 6.8 | ||
| Day 7 | 28.9–77 | 52.3–81.2 | 41.9–82 | <.05** |
| 52.2 ± 13.6 | 72.1 ± 10 | 64.5 ± 13.2 | ||
| CD3+CD4+ | ||||
| Before procedure | 28–52.4 | 15.7–65.2 | 15.7–65.1 | NS |
| 41.3 ± 7.7 | 38.3 ± 8.9 | 42.1 ± 9.9 | ||
| Day 7 | 30–54.4 | 17.7–67.2 | 29.7–66 | NS |
| 42.3 ± 7.2 | 39.3 ± 14.2 | 44.6 ± 10.7 | ||
| CD3+CD8+ | ||||
| Before procedure | 11.7–42.6 | 7.8–43.4 | 15.7–43.8 | NS |
| 26 ± 9.4 | 26.1 ± 8.6 | 24.6 ± 12.7 | ||
| Day 7 | 16.4–42.6 | 17.5–43.4 | 14–75.8 | NS |
| 26.4 ± 9.5 | 28.2 ± 8.6 | 28.4 ± 10.8 | ||
P-values following comparison between control groups (Group1 and Group-2) and the study group (Group-3) All values are given as minimum, maximum and mean, NS: not significant. * significant in favor of Group-1, ** significant in favor of Group-3 in comparison with Group-2 and Group-3.
Figure 3.
The changes in the white blood cells (WBC), lymphocytes (Lym), and neutrophils (Neu). *p < 0.05; **p < 0.005; ***p < 0.001.
Patient Safety and Mortality
No adverse or serious adverse events occurred related to the MSCs therapy. No complications or mortality was observed in Group-1. In the evaluation of the mortality rates in the intensive care groups, 6 patients (60%) in Group-2 and 3 patients in Group-3 (30%) died. When Group-2 and Group-3 were compared, the mortality rate in Group 3 was found to be statistically lower (P < .001) than in Group-2. In the examination of the causes of death of the patients, secondary infections due to bacteria were the most common, followed by myocardial infarction and thromboembolism. The rates of comorbidity in patients were not statistically significant between the two groups (P > .05). When comorbidity was examined in patients who died, hypertension, DM and chronic obstructive pulmonary disease (COPD) were common. During the follow-up period in the hospital, all 21 patients recovered clinically, laboratory (RT-PCR -) and were discharged. The length of stay in hospital and ICU of Group-2 and Group-3 patients who were followed up in the ICU were examined in detail in the study. In Group-2, the duration of hospitalization was minimum 31—maximum 62 days and the average length of stay was 45 days. In Group-3, these values were minimum 27—maximum 108 days and the average length of stay was 47 days. When the groups were compared, no statistical difference (P > .05) was found. When the length of stay in the ICU was examined, in Group-2, it was minimum 26 - maximum 62 days and the average length of stay was 41 days; Group-3 was minimum 20 - maximum 101 days, and the average was 31 days. When the groups were compared statistically, this was found to be significant for Group-3 (P < .05).
Discussion
To date, there is no definitive therapy proven to control the cytokine storm completely and to fully restore the organ damage caused by COVID-19 infection. The main objective of this trial was to systemically explore the mechanism of action of the stem cell therapy to take the ARDS under control by means of controlling the cytokine storm caused by COVID-19 infection. The main reason for using a double control group was to understand the immuno-pathogenetic difference in moderate and critical patients, while also assessing the effects of MSC add-on treatment in critically ill patients.
Cytokines and chemokines have long been known to play important roles in immunity and immunopathology during viral infections. A rapid and well-coordinated innate immune response is the first line of defence against viral infections, but dysregulated and excessive immune responses may cause immunopathology 12 –14 . Previous studies have shown that MSC transplantation could act as an immune modulator in cytokine storm development due to inflammation. Exogenous MSCs have been used as an immune regulator in cytokine storm due to acute pancreatitis and acute and chronic pulmonary disease 15,16 . Clinical studies have revealed that COVID-19 patients admitted to ICU have increased expression of inflammatory cytokines. Cytokine release syndrome (CRS) is caused by vast numbers of immune cells producing pro-inflammatory cytokines in a positive feedback loop and is one of the key elements contributing to the mortality and morbidity of COVID-19. The hallmarks of CRS are elevated serum concentrations of pro-inflammatory cytokines and CRP 17 . A meta-analysis of nine studies showed significantly higher IL-6 levels in patients with severe COVID-19 18 . Moreover, IL-2, IL-10, IFNγ, IL-7, IP10 (CXCL10), MIP-1A, and TNFα were seen to be the main factors causing the cytokine storm 19 . In this study, a wide range of pro-inflammatory and anti-inflammatory cytokines were evaluated at four different times. The aim of this was to evaluate the differences between the three groups and to show on which day the MSC transplant became effective on the cytokine storm. When the IL-6 and CRP levels were compared between the groups, the levels of critically ill Group-2 and Group-3 patients were seen to be significantly higher than those of Group-1. When Group 3 was compared with Group-2 after MSC transplantation, the IL-6 and CRP levels were found to be significantly lower (P < .05) than in Group-2. Levels of other pro-inflammatory cytokines, IFNγ, IL-2, IL-12, and IL-17A appeared to have steadily decreased in the MSC transplanted Group-3. This level of reduction was statistically significant in Group 3, especially after the third dose of MSC therapy. There was no statistically significant decrease in IL-β and TNF-α levels in MSC transplant Group-3. Anti-inflammatory cytokines played an important role in the regulation and control of the inflammatory response. In this study, the IL-10, IL-13, and IL-1ra levels appeared to increase steadily from baseline. This increase was found to be statistically significant in favor of Group-3, who received MSC transplants, especially after the 4th day. The IL-13 and IL-1ra values in Group-2 and Group-3 were found to be higher than in Group-1 from the outset. The increase in these values was thought to be a natural response to COVID-19, as the two groups were seriously ill patients.
CXCL-10 is a pro-inflammatory chemokine with a well-established role in the COVID-19-related cytokine storm, and which is also crucially involved in the development of severe lung impairment observed in these patients. The role of one specific chemokine, CXCL-10, has been highlighted in ARDS in both experimental models and patients 20,21 . CXCL-10 has been reported as the only chemokine whose levels showed a positive and significant correlation with the viral load. In contrast, CXCL-10 levels were reported as significantly lower in patients with moderate disease 21 . In the current study, the CXCL-10 level in Group-1 was found to be significantly lower than in the severely ill Group-2 and Group-3. This indicated that the level of CXCL-10, as a proinflammatory pathway indicator, increased in a correlation with the severity of the disease. In addition, the high level of CXCL-10 in the MSC transplanted Group-3 showed that add on-MSCs had no additional action on this pathway.
MMPs play essential roles in infection and in host defense against infection 22 . In acute lung injury, MMP-9 released from neutrophils promotes inflammation and degradation of the alveolar-capillary barrier, further stimulating migration of inflammatory cells and destruction of lung tissue 23 . In this study, the MMP-9 and MMP-13 levels of patients who were taken into ICU were found to be higher than those of patients followed in the infection clinic. The MMP-9 and MMP-3 levels were decreased in add-on MSC transplanted critical cases, especially when compared to only conventionally treated critical cases. This decrease in Group-3 was found to be statistically significant (P < .05), especially after the 4th day. This result suggested that the immunomodulatory effect of MSCs was involved in the regulation of the MMP levels.
Previously, transplanted MSCs have been shown to have a high expression of anti-inflammatory and trophic factors such as TGF-β, HGF, FGF, VEGF, EGF, and NGF 23,24 . Signaling by TGF-β family ligands is of high importance in cell differentiation and proliferation, as well as in the maintenance of pluripotency in stem cells 23 . VEGF can act like a pneumotrophic factor and thus help in tissue repair during the recovery process from lung injury. HGF also supports growth in epithelial and endothelial cells by inducing the mitogenic process, and by enhancing the survival of pulmonary endothelial and alveolar type II epithelial cells by blocking the apoptosis process 23,25 . MSCs can release VEGF and HGF, which work together to stabilize the endothelial barrier function by restoring pulmonary capillary permeability. KGF secreted by MSCs can reduce injury and promote the proliferation and repair of alveolar epithelial cells by increasing surface-active substances 26 . KGF helps in the repair of the alveolar-capillary barriers seen in ARDS 27 . The results of this study showed that although PDGF and HGF levels of growth factors were found to be slightly higher than for the other groups in the stem cell transplantation Group-3, they were not statistically significant in comparison with the control groups. The TGF-β and VEGF levels were significantly higher in MSC treated critical cases than in the control groups. This increase in TGF-β level in the MSC treated cases was approximately twice that of the other groups. Although there was no statistically significant increase in KGF and NGF levels in the early period, it was found to be significant after the seventh day. In our opinion and experience, an increase in the NGF level occurs later, after transplantation of MSCs. There is also a relationship and correlation between NGF and VEGF. Human neural stem cells (hNSCs) have also been reported to support angiogenesis in vitro and in vivo, the mechanism of action probably governed by VEGF 28 . We suggest that MSC add-on therapy potentiated to increase the level of many growth factors as regeneration indicators. The effect of MSCs on Caspase-3 levels and BCL-2 pathways of apoptosis was also evaluated and no statistically significant increase was found in the MSC transplanted Group 3 compared to the control groups.
The Apache-2 score was used to determine the patient’s current physiological state and overall disease severity. Organ failure evaluations of the patients were conducted with SOFA scores. The Horowitz Index was used to evaluate lung function in ventilators and the degree of damage to the lungs in intensive care patients. When the Apache-2, SOFA scores, and Horowitz Index of the patients in the ICU were compared at the beginning of the treatment, there was no statistically significant difference. In the subsequent follow-ups of the patients in the ICU, the comparison of Apache-2 scores in Group-3 who underwent stem cell transplantation was lower than in Group-2, and was statistically significant. When Group-2 and Group-3 were compared in terms of SOFA scores and the Horowitz index during the treatment, the values were lower in the stem cell transplantation Group-3, but not statistically significant.
Although there was no statistically significant difference when Group-2 and Group-3 were compared in terms of time connected to the ventilator, it was found to be significantly lower for Group-3 after the 7th day strongly suggesting that the MSC add-on therapy was associated with shortening the mechanical ventilation period in critical cases.
The highest mortality rate was in the conventionally treated critical cases (67%), whereas this rate was dramatically decreased in the critical cases that received MSC add-on therapy (33%). No mortality was seen in the moderate cases. The hospital stay duration was 45 days and 47 days in critical cases treated conventionally and with add-on MSC therapy, respectively, with no statistical significance. Here, the length of hospital stay in Group-3 appears to be slightly higher. The reason for this is that the mortality rate was twice as high in Group-2 than in Group-3, and the relative duration of hospital stay was lower in Group-2. Length of ICU stay decreased statistically significantly in the MSC add-on treated critical cases compared to the only conventionally treated cases. This result demonstrates that the MSC therapy had an impact on shortening the ICU stay of the critical cases, as supported by the curative effects of anti-inflammation and immunomodulation.
In respect of the effect of cellular adaptive immunity in the relationship between lymphocyte subsets levels and COVID-19 infection, the study results indicated that there was no statistically significant difference in the levels of CD56+, monocyte, CD3+, CD3+CD4+, CD3+CD8+ and CD19+ cells among the study arms. Although the percentage of CD45+ cells slightly increased in the MSC treated cases, the pre-procedure and day-7 CD45+ levels of intensive care patients were still significantly lower than those of the moderate cases group. Similarly, Shi et al. showed that there was no significant difference in the subsets of peripheral lymphocyte counts (CD4+ T cells, CD8+ T cells, B cells, NK cells) 29 . The total expression of CD16 by neutrophils, monocytes, NK cells, and immature granulocytes was assessed and the CD16+ levels of Group-2 were found to be significantly higher than those of Group-1 on day 7. However, critical cases had significantly lower circulating CD16+CD56+ cells, and the reduction was only seen in Group-3 after MSC treatment.
Since COVID-19 is a systemic and complex disease affecting multiple organs, it may take more time for NK cells to reach normal levels, which would explain why NK cells did not increase immediately after MSC treatment. In addition, the pre-procedure and day 7 granulocyte measurements of critical cases were significantly higher than those of moderate cases. Chen et al. noted that SARS-CoV-2 infection can cause a significant reduction in circulating lymphocytes and T cell subsets, reporting that the number of CD4+ and CD8+ T cells was markedly lower in severe cases than moderate cases 30 . In another study, the patients were administered UC-MSC intravenously 3 times (5 × 107 cells/kg each time) on days 13, 16, and 19 and the results of that study showed that after the second UC-MSC administration, the white blood cell count and neutrophil count decreased to normal levels, while the lymphocyte count increased to the normal level. In addition, the counts of CD3+ T cell, CD4+ T cell, and CD8+ T cell also increased remarkably to normal levels 31 . It is highly likely that an early CD4+ and CD8+ T cell response against SARS-CoV-2 is protective, but an early response is difficult to generate because of efficient innate immune evasion mechanisms for SARS-CoV-2 in humans 32 . Immune evasion by SARS-CoV-2 is probably exacerbated by reduced antigen-presenting cell (APC) function. In the current study, day 7 HLA-DR+ cell levels in Group-2 were significantly lower than in Group-3 and Group-1. The course of the patients in Group-1 may have been aggravated as time progressed, and the variability in the MSC treated critical cases group can be attributed to the fact that the stem cells entered into the system and became active. One of the limitations of this study is that the current improvement could not be clearly demonstrated with the 7th day data, which was the last follow-up day for flow cytometric analysis. Laboratory analysis and immunological follow-up of patients until discharge would help clarify the pathogenesis of the disease.
The immunomodulatory characteristics of MSCs indicate that MSCs can be used as a supportive treatment option for better recovery of critically ill COVID-19 patients. In severe cases, immune system dysfunction is the major cause of death in patients as infection stimulates inflammatory cytokines. MSCs are thought to balance the immune system and stop its overactivation 33 . Currently, there are no detailed and definitive data regarding the dose, when to start, or the route of administration of MSCs in critically ill patients with COVID-19 infection. The organ most frequently affected in COVID 19 infection is known to be the lungs. Our opinion is that this favors systemic MSC transplantation, because the vast majority of systemically administered cells are trapped inside the lungs 34 . This ensures that the systemic and local effects of the MSCs given could work faster and more efficiently on COVID-19 infection. In this study, MSCs were administered to those patients whose general condition did not improve after 48 hours of follow-up. As a matter of discussion, it could be asked why this was not started earlier. First of all, when planning this study, we had no experience of the application of MSCs in cases of COVID-19, and initially there were very few studies in the literature. The other issue is the administration of dexamethasone to patients. A limitation of this trial was the inclusion of dexamethasone in the treatment regimens of all the groups, which may have resulted in a certain suppression of the cytokine storm and therefore could have been a confounding variable. Another point was that the critical cases were taken to ICU and the immunopathological responses of the patients to the antiviral and supportive treatments were not fully known.
Ultimately, the results demonstrated that the recovery of the patients was dramatically improved according to the statistical significance of all the indicators of anti-inflammation, antifibrosis signs in the lungs, and immunmodulatory markers in the MSC treated group. The study has also demonstrated in a multi-faceted way the positive systematic and cellular effects of MSC administration to critically ill COVID-19 patients. This effect has been shown to play a statistically significant role in reducing mortality in intensive care patients. The administration of MSCs seems to decrease the pro-inflammatory cytokines and increase the anti-inflammatory cytokines, as well as regulating the cytokine storm in a controlled manner. Moreover, it regulates the chemokine pathway and decreases the levels of matrix metalloprotein involved in infection, thereby contributing to the healing of organ damage, mediated by increasing the level of many growth factors.
In conclusion, conventional treatment with add-on MSC transplantation seems to bring the cytokine storm under control and attenuate disease progression. MSC mediated growth and differentiation decreased the harm to, and accelerated the recovery of damaged organs. The results of this study, suggest that in addition to reduced mortality, decreased ICU stay, and a promising safety profile, MSCs play a specific therapeutic role in the treatment of critically ill COVID-19 patients.
Acknowledgments
This work was supported by the Health Institutes of Turkey in 2020 (project number: 8860/9193).
Contributors: AG, CZ, YKK, IN conceived and designed the study. KP, YR, YZ, KID, KE made substantial contributions in reviewing the design of the study and acquiring the data. IN, KP, YR coordinated sample collection and oversaw data collection. AG, IN, KE conducted and analysed the laboratory results. AG, CZ, YKK, YR, IN, KP, YZ, KID, KE analysed, interpreted the data, conducted the literature review and drafted the manuscript. AG, YKK, CZ, IN and KE contributed by revising the manuscript critically for important intellectual content and critically reviewed the manuscript. All authors contributed to final approval of the version to be submitted.
Ethical Approval: This trial was undertaken in the Bakirkoy Dr. Sadi Konuk Training and Research Hospital and Istinye University. The study protocol was approved by the Clinical Research Ethics Committee of Bakirkoy Dr. Sadi Konuk Training and Research Hospital (no:2020/139). Permission was obtained from the Ministry of Health (no:56733164/203).
Statement of Human and Animal Rights: This study was conducted in accordance with the Helsinki Declaration for human studies.
Statement of Informed Consent: Written informed consent was obtained from the patients or patients’ parents for the collection and publication of clinical data.
Disclaimer: This article includes the results of the small sized-single center clinical trial and COVID-19 conventional treatment information is based on current available literature. The data included herein reflects the evidence as of the date posted in the document. However, recognizing that there are numerous ongoing clinical studies, our research group will update the information in subsequent trials with corresponding recommendations as new evidence becomes available
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: R Yilmaz, MD  https://orcid.org/0000-0003-1707-4607
https://orcid.org/0000-0003-1707-4607
N Isiksacan, PhD  https://orcid.org/0000-0002-0230-6500
https://orcid.org/0000-0002-0230-6500
References
- 1. Li CY, Bai ZW, Hashikawa T. The neuro invasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92(6):552–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, et al. Clinical characteristic of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Han J, Li Y, Li Y. Strategies to enhance Mesenchymal stem cell-based therapies for acute respiratory distress syndrome. Stem Cells Int. 2019;1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Atluri S, Manchikanti L, Hirsch AJ. Expanded umblical cord mesenchymal stem cells (UC-MSCs) as a therapeutic strategy in managing critically ill COVID-19 patients: the case for compassionate use. Pain Physician. 2020;23(2):E71–E83. [PubMed] [Google Scholar]
- 5. Wu Z, Mcgoogan JM. Characteristic of and important lessons from the coronavirus disease 2019 (C0VID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1249. [DOI] [PubMed] [Google Scholar]
- 6. Li H, Shen S, Fu H, Wang Z, Li X, Sui X, Yuan M, Liu S, Wang G, Guo Q. Immunomodulatory functions of mesenchymal stem cells in tissue engineering. Stem Cells Int. 2019;2019:9671206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee JW, Fang X, Krasnodembskaya A, Howard JP, Matthaya M. Concise review: mesenchymal stem cells for acute lung injury: role of paracrine soluble factors. Stem Cells. 2011;29(6);913–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Behnke J, Kremer S, Shahzad T, Chao CM, Böttcher-Friebertshäuser E, Morty ER, Bellusci S, Ehrhardt H. MSC based therapies-new perspectives for the injured lung. J Clin Med. 2020;9(3);682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Peng F, Tu L, Yang Y, Hu P, Wang R, Hu Q, Cao F, Jiang T, Sun J, Xu G, Chang C. Management and treatment of COVID-19: the Chinese experience 2019. Can J Cardiol. 2020;36(6);915–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Poston JT, Patel BK, Davis AM. Management of critically ill adults with COVID-19. JAMA. 2020;323(18):1839–1841. doi:10.1001/jama.2020.4914 [DOI] [PubMed] [Google Scholar]
- 11. Kabatas S, Civelek E, Inci C, Yalcınkaya EY, Gunel G, Kır G, Albayrak E, Ozturk E, Adas G, Karaoz E. Wharton’s jelly-derived mesenchymal stem cell transplantation in a patient with hypoxic-ischemic encephalopathy: a pilot study. Cell Transplant. 2018;27(10):1425–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Channappanavar R, Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin Immunpathol. 2017;39(5):529–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Davidson S, Maini MK, Wack A. Disease-promoting effects of type 1 interferons in viral, bacterial, and coinfections. J Interf Cytokine Res. 2015:35(4):252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koyuncu ID, Darici H, Karaoz E. Stem cell-based therapy option in COVID-19: is it really promising? Aging Dis. 2020;11(4):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xia S, Zhou C, Kalionis B, Shuang X, GE H, Gao W. Combined antioxidant, anti-inflammaging and mesenchymal stem cell treatment: a possible therapeutic direction in elderly patients with chronic obstructive pulmonary disease. Aging Dis. 2020;11(1):129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ahmed MS, Morsi M, Ghoneim IN, Abdel-Daim M, Badri EN. Mesenchymal stromal cell therapy for pancreatitis: a systemic review. Oxidat Med Cellu Longev. 2018:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhand S, Jazi MS, Mohammadi S, Tarighati Rasekhi R, Rostamian G, Kalani MR, Aida Rostamian A, George J, Douglas MW. COVID-19: The immune responses and Clinical therapy candidates. Int J Mol Sci. 2020;21(15):5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aziz M, Fatima R, Assaly R. Elevated interleukin-6 and severe COVID-19: a meta analysis. J Med Virol. 2021;93(1):35–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chenliang G, Yan H. In silico prediction of molecular targets of astragaloside iv for alleviation of COVID-19 hyperinflammation by systems network pharmacology and bioinformatic gene expression analysis. Front Pharmacol. 2020;11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu M, Guo S, Hibbert MJ, Jain V, Singh N, Wilson ON, Stiles KJ. CXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implications. Cytokine Growth Factor Rev. 2011;22(3):121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oliviero A, Castro Coperchini F, Chiovato L, Rotondi M. COVID-19 pulmonary and olfactory dysfunctions: is the chemokine CXCL10 the common denominator? The Neuroscientist. 2021;27(3):214–221. [DOI] [PubMed] [Google Scholar]
- 22. Sekhon SB. Matrix metalloproteinases – an overview. Res Rep Biol. 2010;1:1–20. [Google Scholar]
- 23. Esquivel D, Mishra R, Soni P, Seetharaman R, Mahmood A, Srivastava1 A. Stem cells therapy as a possible therapeutic option in treating COVID-19 patients. Stem Cell Rev Rep. 2020;17(1):144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, Shan G, Meng F, Du D, Wang S, Fan F, et al. Transplantation of ACE2- mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 2020;11(2);216–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Panganiban AM, Day RM. Hepatocyte growth factor in lung repair and pulmonary fibrosis. Acta Pharmacol Sin. 2011;32(1):12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Xiao K, Hou F, Huang X, Li B, Qian RZ, Xiao XL. Mesenchymal stem cells: current clinical progress in ARDS and COVID-19. Stem Cell Res Ther. 2020;11(1);305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kumar A, Ghosh B. Emerging treatment options of regenerative medicine in severe corona virüs/COVID-19 infections. Int J of Stem cells. 2020;13(3):305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thej C, Ramadasse B, Walvekar A, Majumdar SA, Balasubramanian S. Development of a surrogate potency assay to determine the angiogenic activity of Stempeucel, a pooled, ex-vivo expanded, allogeneic human bone marrow mesenchymal stromal cell product. Stem Cell Res Ther. 2017;8(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shi L, Huang H, Lu X, Yan X, Jiang X, Xu R, Wang S, Zhang C, Yuan X, Xu Z, Huang L, et al. Treatment with human umbilical cord-derived mesenchymal stem cells for COVID-19 patients with lung damage: a randomised, double-blind, placebo-controlled phase 2 trial. medRxiv preprint. 2020.10.15.20213553; doi: 10.1101/2020.10.15.20213553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H, Zhang X, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020; 130(5): 2620–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liang B, Chen J, Li T, Wu H, Yang W, Li Y, Li J, Yu C, Nie F, Ma Z, Yang M, et al. Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells: a case report. Medicine. 2020;99(31):e21429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, Chen L, Li M, Liu Y, Wang G, Yuan Z, et al. Reduction and functional exhaustion of t cells in patients with coronavirus disease 2019 (COVID-19). Front Immunol. 2020;11:827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Choudlery SM, Harris TD. Stem cell therapy for COVID-19: possibilities and challenges. Cell Biol Int. 2020;44(11):2182–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fischer MU, Harting TM, Jimenez F, Monzon-Posadas WO, Xue H, Savitz SI, Laine GA, Cox CS, Jr. Pulmonary passage is a major obstacle for intravenous stem cell delivery: the pulmonary first-pass effect. Stem Cells Dev. 2009;18(5):683–692. [DOI] [PMC free article] [PubMed] [Google Scholar]