Abstract
Ehlers–Danlos syndrome (EDS) is a group of inherited connective tissue disorders that may present with a wide range of multisystemic symptoms. Hypermobile EDS, one of 13 identified subtypes of EDS, is the only variant without a known associated genetic mutation. A review of the literature suggests the five primary dermatological changes associated with hypermobile EDS are soft skin, atrophic cutaneous scars, piezogenic papules, hyperextensive stretchability, and hematomas. Our paper will address these cutaneous manifestations and delve into how they affect patients (primarily women). Possible consequences and treatment options for these different dermatological changes, as well as other skin manifestations such as livedo reticularis and elastosis perforans serpiginosa, will also be further explored.
Keywords: Ehlers–Danlos, Ehlers–Danlos syndrome, Skin manifestations, Piezogenic pedal papule, Livedo reticularis, Elastosis perforans serpiginosa
Introduction
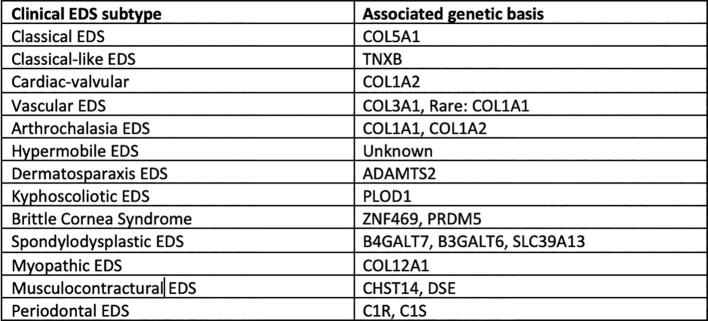
Ehlers–Danlos syndrome (EDS) is a rare heritable connective tissue disorder that is classified into 13 subtypes (Fig. 1). Each subtype has unique clinical diagnostic criteria, with a degree of variability and overlap in symptoms. Most of the subtypes are extremely rare; however, the most common type is hypermobile EDS (hEDS). Out of the 1 in 5000 people worldwide who have EDS, 80% to 90% of cases are classified as hEDS (Tinkle et al., 2017), the majority of which are women (Hugon-Rodin et al., 2016). hEDS follows an autosomal dominant inheritance pattern and is the only subtype that does not have a known genetic mutation. Thus, hEDS is difficult to diagnose because of the lack of genetic markers in confirmatory laboratory and molecular tests (Ritelli and Colombi, 2020). Moreover, hEDS may be misdiagnosed as fibromyalgia, Marfan syndrome, and other related connective tissue disorders because of its clinical overlap in skin hyperextensibility, joint hypermobility, and tissue fragility (Ritelli and Colombi, 2020).
Fig. 1.
Clinical classification of 13 subtypes of Ehlers–Danlos syndrome and associated genetic basis (Malfait et al., 2017).
The aim of the present review is to examine and create awareness of the added impact hEDS has on women and to address the most common detectable skin findings and their role in diagnosis (Fig. 2).
Fig. 2.
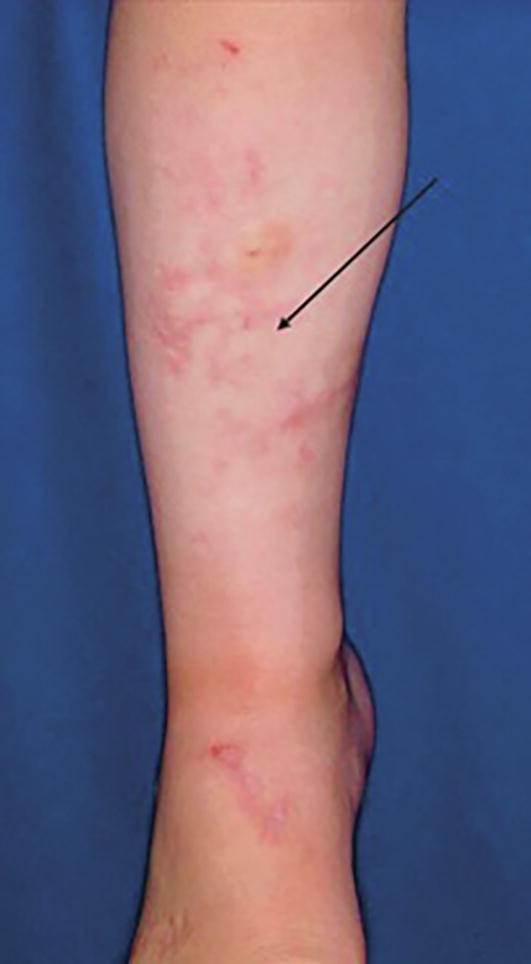
Healed wounds result in atrophic, cutaneous scars (arrow on anterior shin; Kang, et al., 2018; copyright McGraw-Hill Education. All rights reserved).
Soft skin
Patients with hEDS likely present with a velvety, silky, soft skin texture and semitransparent skin that causes veins and tendons to be more visible on the forearms (Tinkle et al., 2017), which predispose to skin lesions and lacerations. Such skin fragility is due to a decreased expression in FLG, a gene that encodes profilaggrin, which is then hydrolyzed to filaggrin protein and plays an important role in cell envelope formation and creating an effective skin barrier. Because FLG is downregulated, this causes a disruption in keratinocyte differentiation, resulting in decreased skin integrity (Chiarelli et al., 2016).
Hematomas
The presentation of bleeding in patients with EDS, including the hEDS subtype, varies from mild bruising to possibly severe or fatal bleeding (Artoni et al., 2018). The most common bleeding symptoms experienced by patients include bruising, hematomas, and menorrhagia (Artoni et al., 2018). A significant factor for bleeding is the delicate subcutaneous and perivascular tissues that are common in collagen disorders (Artoni et al., 2018). A study of data from 112 papers with a total of 467 patients noted that of all bleeding symptoms, the formation of hematomas, especially after minor trauma, was the most frequently reported vascular-related complication (D’hondt et al., 2018). The majority of the hematomas were reported to be subcutaneous. The management of hematomas was described only for patients with musculocontractural EDS in this study. Specific management included transfusions that were required for a number of the hematomas due to minor trauma, intensive care unit admittance, and, in one instance, emergency surgical drainage accompanied by transfusion (D’hondt et al., 2018).
Management is most likely applicable to multiple EDS subtypes despite this study reporting hematoma management of only patients with musculocontractural EDS. Guidelines exist to help prevent the formation of hematomas. Protective bandages and pads, in addition to the avoidance of contact sports and heavy exercise, are recommended to patients with marked bruising (Paepe and Malfait, 2004). Additionally, ascorbic acid supplementation can help decrease the tendency toward bruise development. Because ascorbic acid is a cofactor of prolyl and lysyl hydroxylases (enzymes that aid in the biogenesis of collagen; Castori, 2012), ascorbic acid may facilitate increased dermal stiffness and integrity.
Additionally, conjugated estrogens may activate coagulation pathways, shorten prolonged bleeding times, and stop bleeding in patients with uremia (Mannucci, 1998). As mentioned in a case report, a 23-year-old male patient diagnosed with EDS and a hematoma had been using daily deamino‐delta‐d‐arginine vasopressin (DDAVP) to reduce the bleeding at a hematoma site but had minimal improvement in bleeding time (Boulanger et al., 2003). However, after adding conjugated estrogen intramuscularly with DDAVP, the bleeding time returned to normal and the patient no longer had ongoing hemorrhagic episodes (Boulanger et al., 2003). Thus, both estrogen and DDAVP are helpful agents in reducing bleeding; however, estrogen has a longer duration of effects on bleeding time (10–15 days compared with DDAVP’s average duration of 6 to 8 hours; Mannucci, 1998).
EDS is most prevalent in female patients; thus, this basis of improving bleeding time may be applicable, perhaps with lower amounts of conjugated estrogen required. Of note, however, the use of conjugated estrogen has been shown to increase the risk of thrombotic events. A study composed of both perimenopausal and postmenopausal women found that there was an increased risk of venous thrombosis in women taking conjugated estrogen in comparison with women not using hormones and in women using esterified estrogen (Smith, et al., 2004). Consequently, precaution should be taken when considering conjugated estrogen as a modality of therapy for bleeding prevention.
Atrophic scars
Compared with patients with other variants of EDS who have cigarette-paper and crumpled scars, patients with hEDS tend to have atrophic, nonpapyraceous cutaneous scars at sites exposed to repeated traumas, such as the knees, elbows, and shins (Castori, 2012). In hEDS, collagen and elastic fibers are altered in the dermis, ligaments, vessel walls, and internal organs, and the size of the flower-like collagen fibers is much smaller than normal (Hermanns-Lê et al., 2016). The scars exhibit abnormal elastic fibers with granulo-filamentous deposits with the presence of large stellate hyaluronic acid-like globules (Hermanns-Lê et al., 2016). Emphasized in the reticular dermis layer, flower-like collagen scaffolding shows uneven fibril sizes and irregular interfibrillar spacing, and elastic fibers demonstrate irregular contours (Hermanns-Lê et al., 2016). Thus, the appearance of these atrophic scars is due to the defective collagen fibrils that precipitate decreased stiffness of the dermis (Hermanns-Lê et al., 2016).
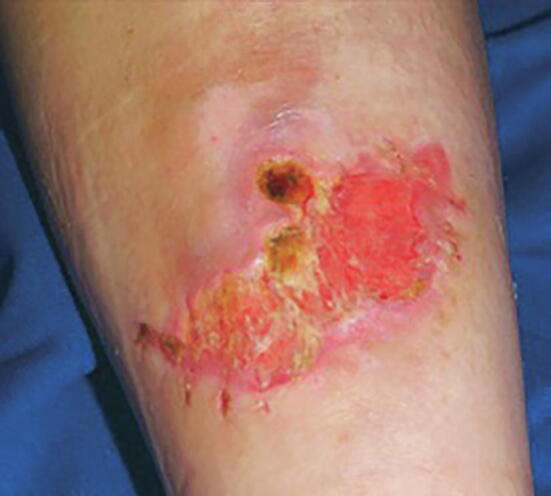
The greatest surgery-related issue of hEDS is the possibility of increased bleeding risk and delay in wound closure and tissue repair (Tinkle et al., 2017). For patients with hEDS, soft-tissue repair takes longer than normal, and surgeries carry a higher risk of unsatisfactory results, such as postsurgical complications and scarring (Tinkle et al., 2017). In some cases, the appearance of scars may be wider than the original wound and/or sunken below the surface of the surrounding skin (Fig. 3; Tinkle et al., 2017). Hence, patients with hEDS should always consider nonsurgical approaches before undergoing invasive procedures. However, if surgical procedures are necessary, they should be carried out with gentle dissection and the use of mild lateral force during incisions, retraction, and suturing (Tinkle et al., 2017). To minimize tension, skin closure should have a sufficient number of deep sutures, and support tape should be used to reinforce the sutures (Burcharth and Rosenberg, 2012). If superficial sutures are used, they should be left in twice as long compared with patients without EDS to avoid wound reopening (Burcharth and Rosenberg, 2012). Careful postoperative planning is critical for patients with EDS and should include pressure bandages and wraps for high-tension sites.
Fig. 3.
Wound dehiscence: Note evidence of former sutures at lower border, now 3 weeks after injury and treatment with antibiotics. Scars tend to stretch further in the 6 months after closure (Kang, et al., 2018; copyright McGraw-Hill Education. All rights reserved).
In addition to using protective bandages and pads, patients may practice low-resistance exercise and physiotherapy that pose minimal injury risk (Zhang et al., 2019). As mentioned, daily consumption of ascorbic acid can ameliorate the fragility in blood vessels and may reduce bruising (Zhang et al., 2019). Contributing to wound healing, estrogen and estrogen-like compounds have positive effects on the homeostasis of connective tissue and promote processes, such as regranulation and inflammation (Horng et al., 2017). For postmenopausal women with an estrogen deficiency, exogenous estrogen treatments could potentially maintain these processes (Horng et al., 2017), especially for patients with hEDS who are constantly at risk for wound problems.
Piezogenic pedal papules
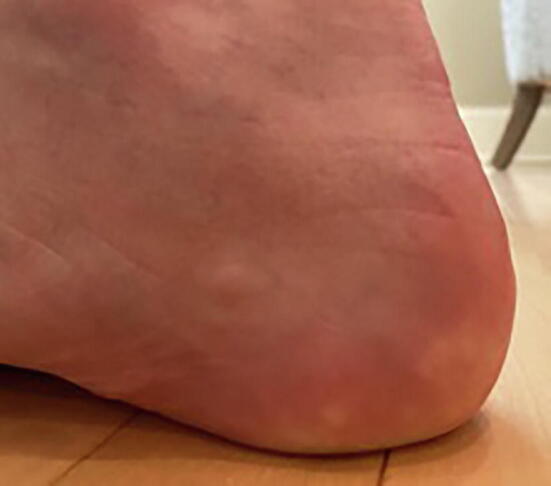
Piezogenic pedal papules are among the multiple dermatologic symptoms associated with hEDS. The papules are described as benign herniations of elastic tissue and subcutaneous fat that break through the reticular dermis (Altin et al., 2016). The condition is exacerbated by pressure and compressive forces (de Oliveira and Dumêt Fernandes, 2015, Karadag et al., 2013), which cause tears in the plantar fascia to make room for subcutaneous fat to seep through (Doukas et al., 2004). Compressive forces, such as prolonged standing, put excessive pressure on the heel, thus exacerbating the papules (Poppe and Hamm, 2013), which are compressible yellowish or skin-colored skin protrusions (Fig. 4; Brown and Cook, 2020, de Oliveira and Dumêt Fernandes, 2015). Biopsy of a piezogenic papule reveals hyperkeratosis with a dense and thick dermis, (Brown and Cook, 2020) with thin subcutaneous fibrous trabeculae, thus allowing infiltration of subcutaneous fat into the dermis (de Oliveira and Dumêt Fernandes, 2015, Kahana et al., 1987).
Fig. 4.
Clinical picture of piezogenic pedal papules (Photo courtesy of Zachary P. Nahmias, MD).
The papules are caused by increased pressure; thus, they are commonly found on the plantar aspect of the foot, primarily along the medial surface of the heel where the body bears most of its weight (Brown and Cook, 2020, Kahana et al., 1987). In most patients, papules occur at random and without pain and as such are often undiagnosed. Painful papules are more commonly seen in patients with connective tissue diseases, such as EDS. (Brown and Cook, 2020, Kahana et al., 1987). In extreme cases, continuous force bearing down on the papules can cut off the blood supply and cause ischemia, which can be responsible for nerve entrapment causing sharp and localized pain (Altin et al., 2016). Less commonly, papules may be found on the wrist (Brown and Cook, 2020, Laing and Fleischer, 1991). Piezogenic papules often recede when the weight-bearing force is removed (Altin et al., 2016).
Although there is no known genetic cause for piezogenic pedal papules, an association with EDS has been demonstrated (van Straaten et al., 1991), likely due to the inherent weakness of the connective tissue (Poppe and Hamm, 2013). Patients with EDS also have a higher likelihood of experiencing pain from their papules secondary to their propensity for slow and impaired wound healing. Although no set treatment is available for pain from piezogenic papules, patients are advised to reduce athletic activities and avoid prolonged periods of standing (Kahana et al., 1987). Additionally, providers should counsel patients on weight loss and avoidance of foot trauma. Patients can also try orthotics and compression stockings to alleviate associated discomfort (Brown and Cook, 2020). Success has been shown with localized injections consisting of either betamethasone and bupivacaine (Doukas et al., 2004) or deoxycholic acid (Turkmani, 2018).
Cutaneous stretchability
Another common symptom of hEDS is the ability of the skin to stretch excessively beyond normal limits and return to its original state upon release (Burrows, 2016). The excessive cutaneous stretchability is due to collagen defects (National Institutes of Health, 2018), a hallmark of patients with EDS. Hyperextensible skin is diagnosed with the rubber-glove skin test (Tinkle et al., 2017) in which a skin fold from the dorsum of the hand is stretched. If the skin stretches >1.5 cm (Burrows, 2016) or stretches beyond the wrist (Tinkle et al., 2017), it can be classified as hyperextensible.
Areas of further research
Additional symptomatic manifestations of hEDS are certainly an area of research that should be further explored, including livedo reticularis and elastosis perforans serpiginosa (EPS). EPS is typically noted in vascular EDS (vEDS) but has been documented in hEDS as well. Further research in these areas may reveal a difference in manifestations and treatment options for patients with hEDS.
Livedo reticularis
Livedo reticularis often affects young women and is exacerbated by cold temperatures (Sajjan et al., 2015). The rash can be described as a bluish-to-lavender pigmented rash that appears in a net-like distribution (Fig. 5; Wolff et al., 2017) and is commonly seen on the legs (Sajjan et al., 2015). Patches can be temporary or permanent and range from the benign, physiological form (cutis marmorata) to the serious, pathological form associated with systemic illness (lupus vasculitis or rheumatoid vasculitis). The benign cutis marmorata form is likely associated with hEDS. This can be inferred due to the defective collagen found in hEDS, leading to structural defects being the basis of cutis marmorata in hEDS. In both forms of livedo reticularis, the dusky colored rash is seen with any process that decreases oxygen flow to the skin, including cold exposure, which causes increased levels of deoxyhemoglobin. In healthy individuals, the vasospastic response to cold is only temporary, and the vessels return to normal upon rewarming of the skin (Rose et al., 2013). Treatment options involve avoiding the cold and vasodilator therapy. The rash of livedo reticularis may improve with age, and in most cases of EDS, no treatment is necessary unless other comorbid conditions are present (Sajjan et al., 2015).
Fig. 5.
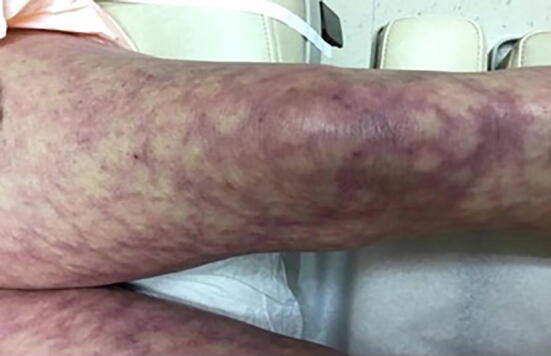
Clinical picture of livedo reticularis (Photo courtesy of Zachary P. Nahmias, MD).
Elastosis perforans serpiginosa
EPS is a rare condition that involves the transepidermal elimination of connective tissue fibers from the dermis through the epidermis (Uldall Pallesen et al., 2019). The typical clinical presentation is hyperkeratotic papules that are arranged in a serpiginous-like manner. These lesions can be seen on the face, neck, arms, and flexural surfaces (Uldall Pallesen et al., 2019). Across numerous articles, EPS has been noted to occur in patients with vEDS. A particular case study discussed an incidence of EPS in a 26-year-old woman with vEDS. She was noted to bruise easily and have prolonged wound healing during childhood, which was followed by atrophic scar formation. The patient developed EPS lesions on her upper arms, thighs, and hips. Biopsy was taken of a lesion and testing demonstrated numerous coarse elastic fibers in the reticular and papillary dermis extruding into the epidermis. EPS has been described as either asymptomatic or pruritic (Uldall Pallesen et al., 2019). Overall, there is poor response to treatment of EPS. Treatment modalities include but are not limited to topical corticosteroids, tretinoin, calcipotriol, tazarotene, narrowband ultraviolet B light radiation, laser therapy, and cryotherapy (Uldall Pallesen et al., 2019).
Conclusion
The dermatological manifestations of hEDS delineated in this paper are the most common, and include soft skin, hematomas, atrophic scars, piezogenic papules, and cutaneous stretchability. Management techniques are especially important to note in practice because these patients may require different modalities. Preventative measures, such as protective wear and ascorbic acid supplementation, are some of the most important points on which practitioners should counsel patients with hEDS. The implementation of these measures may prevent severe cutaneous complications, such as hematomas that require emergent surgical drainage.
Future research in livedo reticularis and EPS, as well as their roles in hEDS, would be helpful in the management of patients who present with such findings. Furthermore, because there has been no genetic cause linked to hEDS, an exploration of how these patients can present clinically is important in developing patient management protocols.
Conflicts of interest
None.
Funding
None.
Study approval
The author(s) confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies.
References
- Altin C., Askin U., Gezmis E., Muderrisoglu H. Piezogenic pedal papules with mitral valve prolapse. Indian J Dermatol. 2016;61(2):234. doi: 10.4103/0019-5154.177803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artoni A., Bassotti A., Abbattista M., Marinelli B., Lecchi A., Gianniello F. Hemostatic abnormalities in patients with Ehlers-Danlos syndrome. J Thromb Haemost. 2018;16(12):2425–2431. doi: 10.1111/jth.14310. [DOI] [PubMed] [Google Scholar]
- Boulanger E., Briere J., Gaulard P., Droz D., Oksenhendler E. HHV8-related non-Hodgkin’s lymphoma of the spermatic cord in a patient with HIV-associated multicentric Castleman disease. Am J Hematol. 2003;72(1):70–71. doi: 10.1002/ajh.10246. [DOI] [PubMed] [Google Scholar]
- Brown F., Cook C. StatPearls Publishing; Treasure Island, FL: 2020. Piezogenic pedal papule. [PubMed] [Google Scholar]
- Burrows N. The skin in hypermobile Ehlers-Danlos syndrome [Internet] 2016 [cited 20 August 20]. Available from: https://www.ehlers-danlos.org/information/the-skin-in-hypermobile-ehlers-danlos-syndrome/.
- Burcharth J., Rosenberg J. Gastrointestinal surgery and related complications in patients with Ehlers-Danlos syndrome: A systematic review. Dig Surg. 2012;29(4):349–357. doi: 10.1159/000343738. [DOI] [PubMed] [Google Scholar]
- Castori M. Ehlers-Danlos syndrome, hypermobility type: An underdiagnosed hereditary connective tissue disorder with mucocutaneous, articular, and systemic manifestations. ISRN Dermatol. 2012;2012 doi: 10.5402/2012/751768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiarelli N., Carini G., Zoppi N., Dordoni C., Ritelli M., Venturini M. Transcriptome-wide expression profiling in skin fibroblasts of patients with joint hypermobility syndrome/Ehlers–Danlos syndrome hypermobility type. PLoS One. 2016;11(8) doi: 10.1371/journal.pone.0161347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’hondt S., Van Damme T., Malfait F. Vascular phenotypes in nonvascular subtypes of the Ehlers-Danlos syndrome: A systematic review. Genet Med. 2018;20(6):562–573. doi: 10.1038/gim.2017.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira Rocha B., Dumêt Fernandes J., Ventin de Oliveira Prates F. Piezogenic pedal papules. An Bras Dermatol. 2015;90(6):928–929. doi: 10.1590/abd1806-4841.20154884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doukas D.J., Holmes J., Leonard J.A. A nonsurgical approach to painful piezogenic pedal papules. Cutis. 2004;73(5):339–340. 346. [PubMed] [Google Scholar]
- Hermanns-Lê T., Piérard G.E., Manicourt D., Piérard-Franchimont C. Clinical and ultrastructural skin alterations in the Ehlers-Danlos syndrome, hypermobility type. Open Dermatol J. 2016;1(1):22–26. [Google Scholar]
- Horng H.C., Chang W.H., Yeh C.C., Huang B.S., Chang C.P., Chen Y.J. Estrogen effects on wound healing. Int J Mol Sci. 2017;18(11):2325. doi: 10.3390/ijms18112325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugon-Rodin J., Lebègue G., Becourt S., Hamonet C., Gompel A. Gynecologic symptoms and the influence on reproductive life in 386 women with hypermobility type Ehlers-Danlos syndrome: A cohort study. Orphanet J Rare Dis. 2016;11(1):124. doi: 10.1186/s13023-016-0511-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana M., Feinstein A., Tabachnic E., Schewach-Millet M., Engelberg S. Painful piezogenic pedal papules in patients with Ehlers-Danlos syndrome. J Am Acad Dermatol. 1987;17(2 Pt 1):205–209. doi: 10.1016/s0190-9622(87)70192-3. [DOI] [PubMed] [Google Scholar]
- Kang S., Amagai M., Bruckner A.L., Enk A.H., Margolis D.J., McMichael A.J. 9th edition. McGraw-Hill Education; New York, NY: 2018. Fitzpatrick’s dermatology. [Google Scholar]
- Karadag A.S., Bilgili S.G., Guner S., Yilmaz D. A cases series of piezogenic pedal papules. Indian Dermatol Online J. 2013;4(4):369–371. doi: 10.4103/2229-5178.120647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laing V.B., Fleischer A.B. Piezogenic wrist papules: A common and asymptomatic finding. J Am Acad Dermatol. 1991;24(3):415–417. doi: 10.1016/0190-9622(91)70062-7. [DOI] [PubMed] [Google Scholar]
- Malfait F., Francomano C., Byers P., Belmont J., Berglund B., Black J. The 2017 international classification of the Ehlers-Danlos syndromes. Am J Med Genet Part C Semin Med Genet. 2017;175C:8–26. doi: 10.1002/ajmg.c.31552. [DOI] [PubMed] [Google Scholar]
- Mannucci P.M. Hemostatic drugs. N Engl J Med. 1998;339(4):245–253. doi: 10.1056/NEJM199807233390407. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. Hypermobile Ehlers–Danlos syndrome [Internet]. 2018 [cited 2020 August 20]. Available from: https://rarediseases.info.nih.gov/diseases/2081/hypermobile-ehlers-danlos-syndrome.
- Paepe A.D., Malfait F. Bleeding and bruising in patients with Ehlers-Danlos syndrome and other collagen vascular disorders. Br J Haematol. 2004;127(5):491–500. doi: 10.1111/j.1365-2141.2004.05220.x. [DOI] [PubMed] [Google Scholar]
- Poppe H., Hamm H. Piezogenic papules in Ehlers-Danlos syndrome. J Pediatr. 2013;163(6):1788. doi: 10.1016/j.jpeds.2013.07.035. [DOI] [PubMed] [Google Scholar]
- Ritelli M., Colombi M. Molecular genetics and pathogenesis of Ehlers-Danlos syndrome and related connective tissue disorders. Genes (Basel) 2020;11(5):547. doi: 10.3390/genes11050547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose A.E., Saggar V., Boyd K.P., Patel R.R., McLellan B. Livedo reticularis. Online Dermatol J. 2013;19:12. [PubMed] [Google Scholar]
- Sajjan V.V., Lunge S., Swamy M.B., Pandit A.M. Livedo reticularis: A review of the literature. Indian Dermatol Online J. 2015;6(5):315. doi: 10.4103/2229-5178.164493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith N.L., Heckbert S.R., Lemaitre R.N., Reiner A.P., Lumley T., Weiss N.S. Esterified estrogens and conjugated equine estrogens and the risk of venous thrombosis. JAMA. 2004;292(13):1581–1587. doi: 10.1001/jama.292.13.1581. [DOI] [PubMed] [Google Scholar]
- Tinkle B., Castori M., Berglund B., Cohen H., Grahame R., Kazkaz H. Hypermobile Ehlers-Danlos syndrome (a.k.a. Ehlers-Danlos syndrome Type III and Ehlers-Danlos syndrome hypermobility type): Clinical description and natural history. Am J Med Genet Part C Semin Med Genet. 2017;175(1):48–69. doi: 10.1002/ajmg.c.31538. [DOI] [PubMed] [Google Scholar]
- Turkmani M.G. Piezogenic pedal papules treated successfully with deoxycholic acid injection. JAAD Case Rep. 2018;4(6):582–583. doi: 10.1016/j.jdcr.2018.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uldall Pallesen K.A., Lindahl K.H., Bygum A. Elastosis perforans serpiginosa related to vascular Ehlers-Danlos syndrome. Online Dermatol J. 2019;25(3) [PubMed] [Google Scholar]
- van Straaten E.A., van Langen I.M., Oorthuys J.W., Oosting J. Piezogenic papules of the feet in healthy children and their possible relation with connective tissue disorders. Pediatr Dermatol. 1991;8(4):277–279. doi: 10.1111/j.1525-1470.1991.tb00932.x. [DOI] [PubMed] [Google Scholar]
- Wolff K., Johnson R.A., Saavedra A.P., Roh E. 8th edition. McGraw-Hill Medical; New York, NY: 2017. Fitzpatrick’s color atlas and synopsis of clinical dermatology. [Google Scholar]
- Zhang W., Windsor K., Jones R., Taunton D.O. Hypermobile type Ehlers-Danlos syndrome associated with hypogammaglobulinemia and fibromyalgia: A case-based review on new classification, diagnosis, and multidisciplinary management. Clin Case Rep. 2019;7(4):680–685. doi: 10.1002/ccr3.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]