Abstract
Background
Cetuximab (chimeric monoclonal antibody to human epidermal growth factor receptor) is used to treat colorectal and head and neck cancers. Due to cross-reactivity with galactose-α-1,3-galactose (alpha-gal), it can induce hypersensitivity even at first administration. We aimed to determine the incidence and clinical manifestation of cetuximab-induced anaphylaxis, and to establish a means of predicting its incidence in patients ahead of treatment.
Methods
Nationwide and single-center pharmacovigilance data from 2010 to 2017 were collected from the Korea Institute of Drug Safety-Korea Adverse Event Reporting System and Severance Regional Pharmacovigilance Center. Patients scheduled for cetuximab administration were enrolled prospectively. A skin prick test was carried out and serum IgE specific to cetuximab and cross-reactive allergens were measured. Reactions were monitored after cetuximab infusion.
Results
Over 8 years, there were 23 reports of anaphylaxis nationwide. In a single-center study, incidence of cetuximab-induced anaphylaxis was 1.1%. Most anaphylaxis occurred at first injection (93.3%), even under pretreatment with anti-allergic drugs. Four of 64 patients (6.3%) experienced severe anaphylaxis. The median cetuximab-specific IgE titer was 6.9 kUA/L in patients experiencing anaphylaxis and 0 kUA/L in those who did not (P < 0.001). The results of alpha-gal, beef sIgE, and cetuximab skin prick testing were similar to those of cetuximab sIgE. Patients who did not experience hypersensitivity were negative in all 4 allergy tests. Its positive and negative predictive values were 100%.
Conclusions
Specific IgE detection of cetuximab or alpha-gal can accurately predict cetuximab-induced anaphylaxis prior to first administration.
Keywords: Anaphylaxis, Cetuximab, Specific IgE
Introduction
Cetuximab is a chimeric anti-epidermal growth factor receptor IgG1 antibody used to treat metastatic colorectal cancer and head and neck cancers, derived from human (70%) and mouse (30%) sources.1 Biological monoclonal antibodies such as cetuximab can induce acute infusion reaction, also known as cytokine release syndrome.2 In addition, the presence of specific IgE (sIgE) to galactose-α-1,3-galactose induced by natural exposure can elicit cetuximab-induced anaphylaxis at first exposure;3 severe hypersensitivity reactions (HSR), which may be life threatening, occur in 3% of cetuximab recipients.4, 5, 6 Due to their similar symptoms and time of onset, it can be difficult to distinguish between cytokine release syndrome and IgE-mediated HSR. Since the two have different mechanisms, prediction of anaphylaxis by identifying sIgE would be useful in developing effective treatment plans.
Previous predictive studies have used laboratory methods such as enzyme-linked immunosorbent assay (ELISA), but this has limitations in real clinical situations. We analyzed the nationwide prevalence of adverse reactions to cetuximab, and investigated means of predicting cetuximab-related anaphylaxis prior to first exposure using clinical methods contrast to laboratory-based methods.
Methods
Nationwide and single-center pharmacovigilance data collection
Pharmacovigilance data on cetuximab were collected from the Korea Institute of Drug Safety-Korea Adverse Event Reporting System (KIDS-KAERS) database of the Korea Institute of Drug Safety and Risk Management. This system consists of 27 regional pharmacovigilance centers in Korea7 including Severance Hospital in Seoul. Adverse drug reaction (ADR) reports from 2010 to 2017 were thoroughly reviewed. Adverse reactions were reported according to World Health Organization-Adverse Reaction Terminology (WHO-ART), including date, age, gender, reporter, clinical manifestations, severity, progress, responses to re-administration, and causality. Causal relationships were assessed using World Health Organization-Uppsala Monitoring Center (WHO-UMC) criteria from unlikely to certain. To overcome limitations of the self-reported anonymized ADR database, ADRs assessed to have stronger relationship (certain and probable/likely) between exposure and outcome was selected. ADRs reporting cetuximab as a concomitant drug only were excluded from the analysis.
To obtain more specific clinical information (number of infusion, primary cancer, underlying diseases, and progress of ADR), we also collected data from the Severance Hospital Regional Pharmacovigilance Center operated by Yonsei University in Seoul, Korea. This is a well-established pharmacovigilance center with the assistance of the government and tertiary teaching hospital, which was established in 1990. In 2019, there were 10,322 ADR reports. ADR reports from the period 2010 to 2017 were analyzed in conjunction with reports from KIDS-KAERS and the single pharmacovigilance center. Anaphylaxis was defined as systemic severe allergy during hypersensitivity according to World Allergy Organization (WAO) diagnostic criteria.8
Predictive model subjects
A prospective observational study was designed to predict cetuximab-induced anaphylaxis, based on an allergy prediction model to detect sIgE prior to first administration in patients with colorectal and head and neck cancers. Patients over 18 years of age who were scheduled for cetuximab treatment according to standard guidelines were enrolled based on the judgment of the oncologist participating in the study. This study was approved by the Institutional Review Board of the Yonsei University Health System (No. 4-2016-0811) and registered at the clinical trial database (clinicaltrials.gov NCT03485638). Patients who did not voluntarily consent to the study or who did not complete the written consent form were excluded.
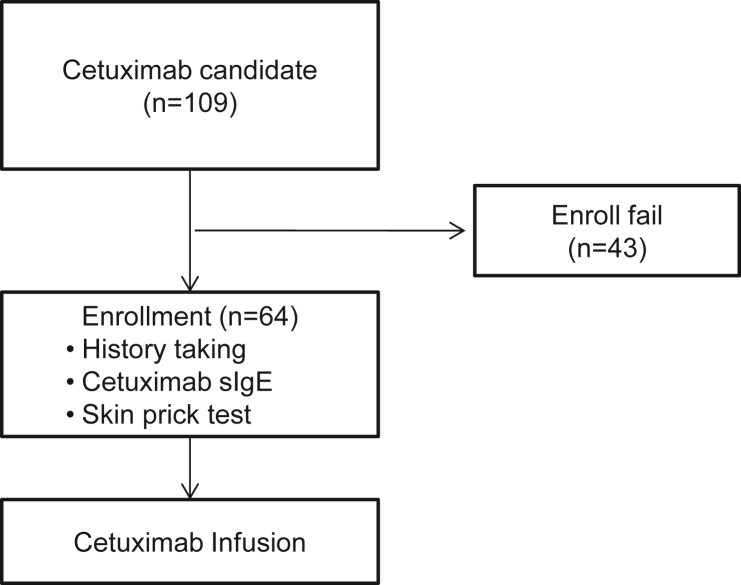
Cetuximab sIgE reactivity was checked using in vivo and in vitro methods, including a skin prick test (SPT), enzyme-linked immunosorbent (ELISA) assay, and ImmunoCAP (Phadia 250, ThermoFisher Scientific, Waltham, USA) assay. Past history, including allergy, was reviewed prior to SPT to cetuximab. After SPT, a 5 mL blood sample was obtained for serum IgE testing, and patients were given antihistamine and systemic corticosteroid as premedication. Cetuximab was infused a 1–2 h period according to body surface area, while patients were admitted or during outpatient chemotherapy injection. After confirming safe administration of cetuximab, subsequent anticancer drugs were administered. Adverse reactions were closely monitored during the chemotherapy by expert nurses. The study scheme is shown in Fig. 1.
Fig. 1.
Study scheme
Detection of specific immunoglobulin E against cetuximab
For SPT, 5 mg/mL cetuximab (Erbitux, Merck, Darmstadt, Germany) was used. Normal saline with 0.3% phenol and 50% glycerol was used as a negative control, and 0.1% histamine (Allergy Therapeutics, Worthing, UK) was used as a positive control. Prior to SPT, all participants were asked to discontinue medications that might influence the results. If the drugs could not be stopped or changed for SPT, the subjects conducted the in vitro test except SPT. SPT results were recorded and interpreted by an allergy specialist clinical research nurse. Wheals and erythema were measured 15 min after pricking; wheal sizes of ≥3 mm were considered positive reactions. No intradermal test was performed.
ELISA and ImmunoCAP assay were used to detect serum sIgE to cetuximab. For ELISA, a microplate was coated with cetuximab (2 μ/L; Erbitux, Merck, Darmstadt, Germany) in 0.05 M carbonate buffer (pH 9.6) overnight at 4 °C. After blocking with 1% bovine serum albumin in phosphate buffered saline with Tween 20, serum samples (diluted 1:4) were added and the plate was incubated for 1 h. Biotinylated goat anti-human IgE (1:1000; Vector, Burlingame, CA, USA) was added to each well and incubated with streptavidin (1:1000; Sigma-Aldrich) for 30 min. The colorimetric reaction was developed using 3,3′,5,5′-tetramethylbenzidine (Kirkegaard & Perry Laboratories, Gaithersburg, MD, USA). After stopping the enzyme reaction by adding 0.5 M H2SO4, absorbance was measured at 450 nm. The cutoff value was determined as the mean absorbance plus twice the standard deviation obtained from the normal controls.
At present, there are no commercial kits for direct measurement of cetuximab sIgE by ImmunoCAP. Alpha-gal (ImmunoCAP code o215, from bovine thyroglobulin source material) and beef (ImmunoCAP code f27, from raw meat source material) IgE, which cross-react with cetuximab3 were measured first, on the same or subsequent day as blood samples were collected.
To detect cetuximab sIgE using ImmunoCAP, cetuximab was biotinylated using EZ-Link® Sulfo-NHS-LC-Biotin (ThermoFisher Scientific, Waltham, USA). Biotinylated cetuximab diluted 1:9 in PBS was immobilized on a streptavidin CAP microplate. We then used the ImmunoCAP system with a sensitivity range of 0.1 kUA/L to 100 kUA/L; sIgE concentrations ≥0.35 kUA/L were considered positive.
Statistical analysis
We used IBM SPSS Statistics for Windows version 23.0 (IBM Corp Armonk, NY) for data analysis. Nonparametric continuous data were analyzed using a Mann-Whitney U test, and categorical data were analyzed using Fisher's exact or Pearson's chi-squared tests. A P value of <0.05 was considered significant.
Results
Nationwide pharmacovigilance reports on cetuximab (n = 369)
Nationwide pharmacovigilance data for cetuximab across the 8 years surveyed indicated that the most common ADRs were cutaneous manifestations, including rash (53.7%), pruritus (33.9%), and urticaria (18.7%). Median age was 60 years old. Male patients were twice as many as female patients. Fifty patients experienced serious reactions, including life-threatening ADRs, necessity or extension of hospitalization, and important medical issues (specific information could not be checked due to database characteristics). There was no death or significant disability caused by cetuximab ADR. Among the 369 patients, 23 patients (6.2%) suffered from anaphylaxis induced by cetuximab (Table 1) judged by WAO diagnostic criteria.
Table 1.
Nationwide pharmacovigilance data on cetuximab over 8 years in Korea.
| Self-reported ADRs to cetuximab in Korea (N = 369 patients) | |
|---|---|
| Age in year, median (range) | 60.0 (22–89) |
| Gender, N (%) | |
| Male | 250 (68.7) |
| Female | 114 (31.3) |
| WHO-UMC causality assessment | |
| Certain, N (%) | 93 (23.2) |
| Probable/likely, N (%) | 276 (74.8) |
| Serious adverse reactions, N (%) | |
| Life-threatening | 5 (1.4) |
| Hospitalization | 18 (4.9) |
| Important medical event | 27 (7.3) |
| Re-challenge information available | |
| Challenge results reproduced | 42 (11.3% of the total patients) |
| Clinical manifestations, N (%) | |
| Cutaneous | |
| Rash | 198 (53.7) |
| Pruritis | 125 (33.9) |
| Urticaria | 69 (18.7) |
| Cardiovascular | |
| Shock | 37 (10.0) |
| Chest discomfort | 32 (8.7) |
| Tachycardia | 8 (2.2) |
| Systemic | |
| Chills | 28 (7.6) |
| Anaphylaxis | 23 (6.2) |
| Fever | 6 (5.4) |
| Gastrointestinal | |
| Nausea | 60 (16.3) |
| Vomiting | 37 (10.0) |
| Diarrhea | 28 (7.6) |
| Neurologic | |
| Dizziness | 19 (5.1) |
| Headache | 9 (2.4) |
| Mental change | 6 (1.6) |
| Others | |
| Cytopenia | 15 (4.1) |
| Hepatitis | 7 (1.9) |
| Hypomagnesemia | 3 (0.8) |
ADR, adverse drug reaction; WHO-UMC, World Health Organization-Uppsala Monitoring Center
Single-center pharmacovigilance reports
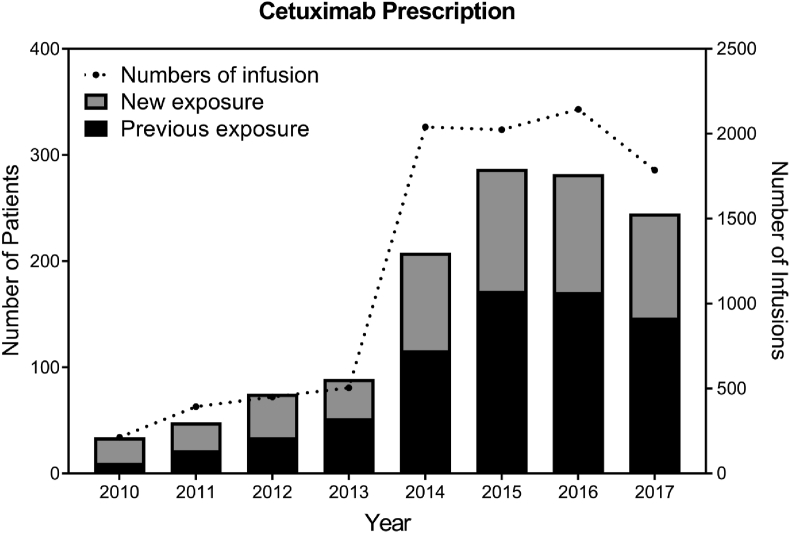
There were 1268 patients treated with cetuximab over 9551 infusions during the period of study (Fig. 2). During this time, the number of patients treated with cetuximab increased, but the incidence of anaphylaxis did not increase (data not shown). A total of 15 patients suffered from anaphylaxis, at a rate of 1.2%, in spite of preventive antihistamine and systemic steroid drugs. In these cases, anaphylaxis usually developed at first dose of cetuximab (13/15, 86.7%). One patient underwent cardiopulmonary resuscitation but recovered without complication. Because of these unexpected ADRs, 9 out of 15 (60%) anaphylactic patients changed or gave up on the first line treatment. Two patients were able to continue the use of cetuximab after desensitization.
Fig. 2.
Number of cetuximab prescription over time
Predictive model (n = 64)
Among patients who visited the oncology department, 109 who were scheduled for first administration of cetuximab were screened. Since the screening subjects were first diagnosed with stage IV cancer and planned for their first chemotherapy, 39.4% of the participation refused to participated to the prospective observational allergy prediction study. After exclusions were applied, 64 adult patients with stage IV colorectal cancers were included (Table 2).
Table 2.
Baseline characteristics of patients included in predictive modeling analysis.
| No Anaphylaxis (N = 60) | Anaphylaxis (N = 4) | P value | |
|---|---|---|---|
| Age in years, median (range) | 55.5 (30–80) | 73.5 (57–76) | 0.012 |
| Gender, N (%) | >0.999 | ||
| Male | 38 (63.3) | 3 (75) | |
| Female | 22 (36.7) | 1 (25) | |
| Allergy History, N (%) | |||
| Asthma | 1 (1.7) | 0 (0) | >0.999 |
| Food allergy | 0 (0) | 0 (0) | |
| Drug allergy (any) | 1 (1.7) | 0 (0) | >0.999 |
| Laboratory findings in median (range) | |||
| Eosinophil (numbers/μL) | 145 (0–900) | 205 (90–2,088) | 0.227 |
| AST (IU/L) | 20.5 (7–166) | 22 (21–32) | 0.428 |
| ALT (IU/L) | 20 (6–138) | 16.5 (6–22) | 0.285 |
| BUN (mg/dL) | 12.5 (4.5–31.0) | 14.8 (13.6–22.1) | 0.367 |
| Creatinine (mg/dL) | 0.8 (0.3–1.4) | 0.9 (0.7–0.9) | 0.718 |
| EGFR (ml/min/1.73 m2) | >90 (52->90) | 87 (61->90) | 0.353 |
| Allergy serum test (positive ratio) | |||
| Cetuximab sIgE | 0/60 (0%) | 4/4 (100%) | < 0.001 |
| Cetuximab sIgE | 0/60 (0%) | 4/4 (100%) | < 0.001 |
| Alpha-gal sIgE | 0/60 (0%) | 4/4 (100%) | < 0.001 |
| Beef sIgE | 0/60 (0%) | 4/4 (100%) | < 0.001 |
| Allergy skin test (positive ratio) | |||
| Cetuximab SPT | 0/60 (0%) | 2/2 (100%) | < 0.001 |
| Premedication, N (%) | |||
| Anti-histamine | 60 (100) | 4 (100) | |
| Corticosteroid | 60 (100) | 4 (100) | |
| Combination therapy, N (%) | 0.502 | ||
| Irinotecan | 2 (3.3) | 0 (0) | |
| Fluorouracil and Irinotecan | 51 (85.0) | 3 (75) | |
| Fluorouracil and Oxaliplatin | 7 (11.7) | 1 (25) |
EGFR, estimated glomerular filtration rate; sIgE, specific immunoglobulin E; O.D, optical density measured by ELISA; SPT, skin prick test; Gender, allergy history, cetuximab SPT, and combination therapy were analyzed using Fisher's exact test. Age, laboratory findings, and sIgEs were analyzed using a Mann-Whitney U test. Values with a P value less than 0.05 are displayed in bold.
Four patients (6.3%) who received cetuximab experienced anaphylaxis despite the use of anti-allergic premedication. These patients were 18 years older than the control group (P = 0.012), but there was no difference in history of allergic diseases, blood tests, whether to use anti-allergic premedication, or the type of anticancer drugs administered in concert. In both groups, the proportion of male patients was high, and 3 out of 4 who developed anaphylaxis were male.
Anti-cetuximab sIgE and anaphylaxis
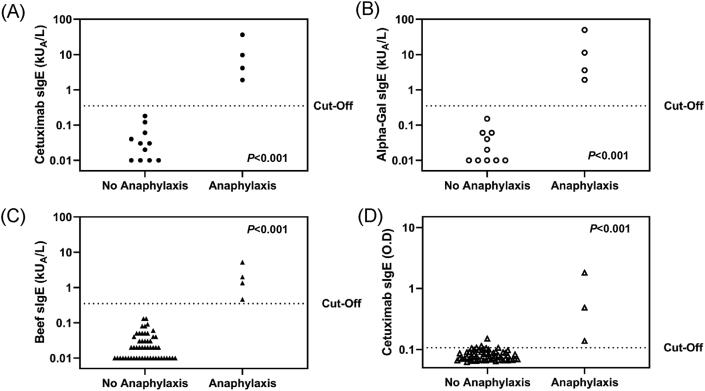
ImmunoCAP assay indicated significantly higher concentration of sIgE to cetuximab (Fig. 3A), alpha-gal (Fig. 3B), and beef (Fig. 3C) in patients experiencing anaphylaxis (P < 0.001). The median and range values were shown in Table 2. Based on the 0.35 kUA/L positive cut-off, all of the sensitivity, specificity, and positive predictive values, and negative predictive values of beef, alpha-gal, and cetuximab were 100%. Although there was no difference in positive and negative predictive values for each sIgE antigen measured, cetuximab and alpha-gal sIgE concentrations were significantly higher than beef sIgE (P = 0.043).
Fig. 3.
Comparison of cetuximab specific IgE responses between groups
Optical density detected using ELISA also showed significant differences between patients who experienced anaphylaxis and those who did not (Fig. 3D, P < 0.001). Of the 62 patients who underwent SPT, 2 who had a positive SPT result showed anaphylactic reaction at cetuximab first administration.
Characteristics of cetuximab-induced anaphylaxis
When anaphylaxis occurred, it did so an average of 17 min after cetuximab administration (Table 3). All affected patients suffered hypotension, respiratory difficulty, and change in mental status. Three patients (75%) were needed intramuscular epinephrine to treat anaphylaxis. Despite these severe reactions, there were no casualties. All of the anaphylactic patients stopped the use of cetuximab and changed the chemotherapy agent. None of the anaphylactic patients had food allergy especially to the red meat.
Table 3.
Clinical manifestations of anaphylactic patients
| No | Age | Gender | Onset | Clinical manifestations |
|---|---|---|---|---|
| 1 | 76 | M | 1 min | Shock, Dyspnea, Syncope |
| 2 | 57 | F | 20 min | Shock, Dyspnea, Drowsy mental status, Urticaria, Nausea |
| 3 | 73 | M | 15 min | Shock, Dyspnea, Drowsy mental status, Vomiting, Nausea |
| 4 | 74 | M | 13 min | Shock, Wheezing, Stupor mental status, Urination, Defecation |
Discussion
This study synthesizes comprehensive data on cetuximab-induced ADRs over an 8-year period, and helps to shed light on IgE-mediated HSR to versus cytokine release syndrome. Both in vivo (SPT) and in vitro (serum sIgE: ImmunoCAP, ELISA) methods used in this study showed significant predictive power.
Each test method has its own advantages and disadvantages. SPT results can be interpreted within 15 min, but a commercial cetuximab SPT reagent is not available, and an accurate test requires the interruption of drugs which can affect its outcome, including antihistamine, systemic steroids, and antidepressants. Meanwhile, both ELISA and ImmunoCAP assays were predictive of anaphylaxis. ImmunoCAP in particular can reduce the required incubation time for antigens and antibodies, and is automated to minimize technical errors; it is also the gold standard sIgE quantitation assay recommended by the World Health Organization. Quantitative sIgE titer can be reported in about 7 h using the ImmunoCAP (Phadia 250 version). Alpha-gal (and cross-reactive cetuximab) is component antigens that induce severe allergic reaction among beef antigens. In our study, sIgE concentration was higher in the component antigens compared to crude beef allergen. In a medical setting when the limited allergens can be selected for diagnosis, it may be more helpful to measure the sIgE of the component allergen.
When IgE-mediated HSR is confirmed, safe exposure can be established through either desensitization or graded challenge.9 Successful desensitization of patients who experienced cetuximab-induced anaphylaxis has been reported.10,11 Advance prediction of HSR risk can help enable safe adherence to first-line cancer treatment, and improve treatment outcomes and quality of life in advanced cancer patients. Prediction can also reduce unnecessary medical costs. Although products for cetuximab sIgE measurement are not yet available, alpha-gal sIgE measurement was similarly predictive and ready for immediate clinical application. In addition, though we aimed to predict HSR prior to first exposure, our findings can be also be used to observe sIgE generated during the course of treatment due to repeated exposures. This early diagnosis of HSR can predict which patients may be vulnerable to severe hypersensitivity.
The results of the present study correspond well with those found in the previous studies. Prediction studies were performed using cetuximab sIgE by ELISA method,12, 13, 14, 15 and basophil activation testing.13 An additional study detected alpha-gal sIgE using ImmunoCAP assay with a custom protein not yet approved for diagnostic use by the US Food and Drug Administration, which found that patients who tested negative for alpha-gal sIgE did not develop anaphylaxis (n = 37).16 Retrospective trials to detect cetuximab sIgE using ImmunoCAP using products on development,17 and ELISA have also been carried out.18
Atopy status, age, gender, race, concomitant therapy, and primary cancer site have been proposed as risk factors for cetuximab-induced anaphylaxis, but these hypotheses are unresolved.19 Our findings did not indicate significant differences between compared groups, except for age and sIgE reactivity.
Some additional research will need to be carried out to verify our interpretations, due to key study limitations. First, prevalence of cetuximab-related HSR can vary by geographic distribution4,20 and our entire test subject was Korean. It is known that alpha-gal sensitization is caused by a lone star tick,21 but since there is no such tick in Korea, it is presumed that sensitization was preceded by other mechanisms. Second, cetuximab-related HSR is reported to occur at higher incidence in patients with head and neck cancer compared to those with colorectal cancer,22,23 who made up our study population. Third, only a limited number of patients agreed to SPT. Non-irritating concentration was not defined due to the limitations of testing of anti-cancer drugs in healthy patients. However, 2 patients who were allergic to cetuximab reacted to 5 mg/mL of cetuximab at SPT.
There are fewer studies on alpha-gal syndrome in Asian countries than in western countries. One Korean alpha-gal syndrome patient in an earlier study experienced cetuximab-induced anaphylaxis at first exposure.24 However, none of the cetuximab-allergic patients enrolled in this study experienced an allergic reaction after eating red meat. Further research on alpha-gal syndrome in Asia may shed light on risk factors and mechanisms of cetuximab-induced HSR.
In conclusion, automated quantitative sIgE detection of alpha-gal or cetuximab can be helpful to predict cetuximab-induced anaphylaxis prior to its first administration, allowing for safe clinical planning.
Abbreviations
ADR, adverse drug reaction; EGFR, estimated glomerular filtration rate; ELISA, enzyme-linked immunosorbent assay; HSR, hypersensitivity reaction; KIDS-KAERS, Korea Institute of Drug Safety-Korea Adverse Event Reporting System; O.D., optical density; sIgE, specific Immunoglobulin E; SPT, skin prick test; WHO-UMC, World Health Organization-Uppsala Monitoring Center
Disclosures
Pharmacovigilance data were provided by the Korea Institute of Drug Safety and Risk Management (Ministry of Food and Drug Safety). This study was supported by the Severance Regional Pharmacovigilance Center (Seoul, Korea).
Authorship
KH Park and JW Park designed the study and wrote the manuscript. KH Park, J Lee, SH Beom, SJ Shin, JB Ahn, SR Kim, JH Lee, JW Park contributed to data collection. KH Park, SR Kim, JH Lee and JW Park performed statistical analysis and interpretation of the results. All authors read and approved the submitted manuscript.
Availability of data and materials
Available.
Ethics approval
All study participants provided informed consent, and the study design was sanctioned by appropriate ethics review boards.
Declaration of competing interest
The authors have no conflict of interest to declare. There was no funding source for the ImmunoCAP assay.
Footnotes
Full list of author information is available at the end of the article
References
- 1.Hopps S., Medina P., Pant S., Webb R., Moorman M., Borders E. Cetuximab hypersensitivity infusion reactions: incidence and risk factors. J Oncol Pharm Pract. 2013;19:222–227. doi: 10.1177/1078155212462440. [DOI] [PubMed] [Google Scholar]
- 2.Joshi S.R., Khan D.A. Anaphylaxis induced by biologics. Curr Treat Options Allergy. 2019;6:125–141. [Google Scholar]
- 3.Chung C.H., Mirakhur B., Chan E. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. 2008;358:1109–1117. doi: 10.1056/NEJMoa074943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.George T.J., Jr., Laplant K.D., Walden E.O. Managing cetuximab hypersensitivity-infusion reactions: incidence, risk factors, prevention, and retreatment. J Support Oncol. 2010;8:72–77. [PubMed] [Google Scholar]
- 5.Patel D.D., Goldberg R.M. Cetuximab-associated infusion reactions: pathology and management. Oncology (Williston Park) 2006;20:1373–1382. discussion 82, 92-4, 97. [PubMed] [Google Scholar]
- 6.Zhang D., Ye J., Xu T., Xiong B. Treatment related severe and fatal adverse events with cetuximab in colorectal cancer patients: a meta-analysis. J Chemother. 2013;25:170–175. doi: 10.1179/1973947813Y.0000000070. [DOI] [PubMed] [Google Scholar]
- 7.Park K.H., Pai J., Song D.G. Ranitidine-induced anaphylaxis: clinical features, cross-reactivity, and skin testing. Clin Exp Allergy. 2016;46:631–639. doi: 10.1111/cea.12708. [DOI] [PubMed] [Google Scholar]
- 8.Simons F.E., Ardusso L.R., Bilo M.B. World allergy organization guidelines for the assessment and management of anaphylaxis. World Allergy Organ J. 2011;4:13–37. doi: 10.1097/WOX.0b013e318211496c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Picard M., Galvao V.R. Current knowledge and management of hypersensitivity reactions to monoclonal antibodies. J Allergy Clin Immunol Pract. 2017;5:600–609. doi: 10.1016/j.jaip.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 10.Jerath M.R., Kwan M., Kannarkat M. A desensitization protocol for the mAb cetuximab. J Allergy Clin Immunol. 2009;123:260–262. doi: 10.1016/j.jaci.2008.09.046. [DOI] [PubMed] [Google Scholar]
- 11.Solduzian M., Anvari S., Taghvaye Masoumi H., Shahi F., Jahangard-Rafsanjani Z. Successful desensitization of a patient with cetuximab hypersensitivity: a case report. J Oncol Pharm Pract. 2019;25:1726–1730. doi: 10.1177/1078155218793505. [DOI] [PubMed] [Google Scholar]
- 12.Dupont B., Mariotte D., Dugue A.E. Utility of serum anti-cetuximab immunoglobulin E levels to identify patients at a high risk of severe hypersensitivity reaction to cetuximab. Br J Clin Pharmacol. 2017;83:623–631. doi: 10.1111/bcp.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwamoto T., Okamoto A., Ishinaga H. A novel approach to predict cetuximab-induced hypersensitivity reaction: detection of drug-specific IgE on basophils. Cancer Med. 2016;5:1004–1012. doi: 10.1002/cam4.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mariotte D., Dupont B., Gervais R. Anti-cetuximab IgE ELISA for identification of patients at a high risk of cetuximab-induced anaphylaxis. mAbs. 2011;3:396–401. doi: 10.4161/mabs.3.4.16293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dupont B., Mariotte D., Moldovan C. Case report about fatal or near-fatal hypersensitivity reactions to cetuximab: anticetuximab IgE as a valuable screening test. Clin Med Insights Oncol. 2014;8:91–94. doi: 10.4137/CMO.S13897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiss J., Olson J.G., Deal A.M. Using the galactose-alpha-1,3-galactose enzyme-linked immunosorbent assay to predict anaphylaxis in response to cetuximab. Cancer. 2016;122:1697–1701. doi: 10.1002/cncr.29978. [DOI] [PubMed] [Google Scholar]
- 17.Maier S., Chung C.H., Morse M. A retrospective analysis of cross-reacting cetuximab IgE antibody and its association with severe infusion reactions. Cancer Med. 2015;4:36–42. doi: 10.1002/cam4.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dupont B., Mariotte D., Clarisse B. Risk factors associated with hypersensitivity reactions to cetuximab: anti-cetuximab IgE detection as screening test. Future Oncol. 2014;10:2133–2140. doi: 10.2217/fon.14.153. [DOI] [PubMed] [Google Scholar]
- 19.Pointreau Y., Commins S.P., Calais G., Watier H., Platts-Mills T.A. Fatal infusion reactions to cetuximab: role of immunoglobulin e-mediated anaphylaxis. J Clin Oncol. 2012;30:334. doi: 10.1200/JCO.2011.38.4701. author reply 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Neil B.H., Allen R., Spigel D.R. High incidence of cetuximab-related infusion reactions in Tennessee and North Carolina and the association with atopic history. J Clin Oncol. 2007;25:3644–3648. doi: 10.1200/JCO.2007.11.7812. [DOI] [PubMed] [Google Scholar]
- 21.Platts-Mills T.A.E., Li R.C., Keshavarz B., Smith A.R., Wilson J.M. Diagnosis and management of patients with the alpha-gal syndrome. J Allergy Clin Immunol Pract. 2020;8:15–23 e1. doi: 10.1016/j.jaip.2019.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palomar Coloma V., Bravo P., Lezghed N. High incidence of cetuximab-related infusion reactions in head and neck patients. ESMO Open. 2018;3 doi: 10.1136/esmoopen-2018-000346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grandvuillemin A., Disson-Dautriche A., Miremont-Salame G., Fourrier-Reglat A., Sgro C., Reseau des Centres Regionaux de Pharmacovigilance F. Cetuximab infusion reactions: French pharmacovigilance database analysis. J Oncol Pharm Pract. 2013;19:130–137. doi: 10.1177/1078155212457965. [DOI] [PubMed] [Google Scholar]
- 24.Sim D.W., Lee J.S., Park K.H. Accurate assessment of alpha-gal syndrome using cetuximab and bovine thyroglobulin-specific IgE. Mol Nutr Food Res. 2017;61 doi: 10.1002/mnfr.201601046. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available.