Abstract
Background:
To evaluate the prognostic role of circulating fibrinogen-to-pre-albumin (FPR) in colorectal cancer (CRC) with different tumor locations, and its involvement in chemosensitivity and chemoresistance.
Patients and methods:
A total of 2917 eligible CRC patients from multiple centers were enrolled in this prospective study, and 3 years follow-up was carried out to obtain the outcome of these patients. Circulating fibrinogen (Fib), pre-albumin (pAlb), carcinoembryonic antigen (CEA), and carbohydrate antigen 19-9 (CA19-9) were detected, and we calculated FPR according to the detected results. Kaplan–Meier curves, Cox proportional regression, time-dependent receiver operating characteristic curves, Harrell’s concordance index, calibration, and decision curves were used to investigate the role of FPR in predicting chemotherapy efficacy and prognosis of CRC patients.
Results:
Our results showed that cancer bulk, its infiltrating depth, and the distal metastasis status of CRC determined circulating FPR levels. A high FPR was associated with a significantly inferior prognosis, while the outcomes of right-sided patients with stage III and IV CRC were worse than left-sided cases. Only FPR was found to be a reliable and independent prognostic factor for each stage of CRC. In addition, the prognostic FPR-contained nomograms were superior to the non-FPR nomograms and FPR in predicting the outcomes in both localized and metastatic CRC patients. The circulating FPR was significantly associated with chemotherapeutic efficacy in stage III and IV CRC patients. In particular, low-grade (FPR < 15) and medium-grade (15 ⩽ FPR < 20) FPR patients exhibited a complete response to chemotherapy and attenuated chemosensitivity, respectively; in contrast, high-grade inflammation (FPR ⩾ 20) conferred resistance to the treatment.
Conclusion:
Circulating FPR is a robust and independent prognostic factor, a simple and economically-friendly predictor of chemotherapy efficacy within cases of localized and metastatic CRC. FPR-contained nomograms are more effective in predicting the prognosis of these patients. FPR and the nomogram can be recommended for the evaluation of chemotherapy efficacy and to aid decision-making associated with the management of these patients.
Keywords: biomarkers, cancer-related inflammation, chemotherapy, colorectal cancer, fibrinogen to pre-albumin ratio
Introduction
Colorectal cancer (CRC) is one of the deadliest malignancies affecting both men and women worldwide; it is the third most commonly diagnosed cancer and the second most common cause of cancer-related deaths. 1 Previous studies have implicated chromosome and microsatellite instability, CpG island methylator phenotypes, and epigenetic alterations in CRC tumorigenesis and metastasis.2–4 Studies have also reported distinct molecular features and varying gut microbiota in CRC patients,5,6 with analysis of clinical therapeutic responses and outcomes between the right- and left-sided disease yielding contrasting results.7–9 In addition, the role of inflammation in the prognosis of CRC patients with different primary tumor locations and possible causes of the heterogeneous outcomes are poorly understood.
In general, chronic inflammation has emerged as a hallmark of different types of cancer, including CRC. 10 In particular, clinically silent systemic inflammation is manifested during disease onset, 11 whereas overt inflammation occurs throughout its progression. 12 Strong epidemiological evidence suggests that low-dose aspirin and other non-steroidal anti-inflammatory drugs are powerful chemo-preventive agents for reducing rates and cancer-related deaths.13–15 In contrast, a dynamic alteration of circulating host inflammatory response biomarkers, such as lactate dehydrogenase (LDH), differential count of peripheral leukocyte, circulating albumin (Alb), pre-albumin (pAlb) and fibrinogen (Fib), and neutrophil-to-lymphocyte ratio (NLR), have been implicated in reflecting a circulating level of the cancer-related inflammation. Our previous studies have shown that the circulating Alb-to-Fib ratio (AFR) and Fib-to-pAlb ratio (FPR) are superior to these single factors and that these ratios independently predict clinical outcomes in many kinds of solid malignancies, including non-small cell lung cancer, 16 esophageal, hepatocellular, and gastric cancers.17–19 We have also demonstrated that preoperative FPR is closely-associated with chemoresistance in metastatic CRC patients. 20 However, the role of quantitative FPR in impairment and resistance to chemotherapy remains unclear. In addition, the relationship between circulating FPR and tumor laterality is unknown. Furthermore, the dynamics of FPR and survival outcomes across stages (I–IV) of CRC needs to be further investigated.
The current prospective study sought to evaluate FPR across all CRC stages. In particular, we determined survival outcomes of the stage I–IV patients based on different FPR and tumor location, as well as the involvement of circulating FPR in chemosensitivity and chemoresistance. We revealed the cut-off values for predicting chemotherapy efficacy. Finally, we evaluated the use of a prognostic nomogram for effective determination of clinical outcomes in patients with localized and metastatic CRC.
Materials and methods
Eligible population
We performed this study on CRC patients enrolled across five surgery centers in Chinese University hospitals between January 2008 and December 2016. The study was approved by the ethics committee of the Second Affiliated Hospital of Nanchang University (No: 2007033). We obtained appropriate written informed consent from each patient, or their legal surrogates, prior to the start of the study. Patients who were hospitalized for at least 1 day with CRC were initially screened and enrolled in the study. Patient diagnosis and pathological staging were performed according to the criteria described in the six and seventh American Joint Committee on Cancer (AJCC) tumor classification.21,22 The pathological examination was carried out by one senior pathologist and the reports were checked by another senior pathologist. More than 12 lymph nodes were detected to ensure the accuracy of pathological staging in each surgical specimen sample. Patients were ultimately enrolled in the study if they met the following criteria: (i) they were newly diagnosed with localized CRC (stage I–III) and the stage IV disease was (primary and metachronous metastasis) based on clinical characteristics, clinical imaging, and histopathological detections; (ii) they did not suffer from ulcerative colitis or hereditary polyposis as well as hereditary nonpolyposis CRC; in addition, they had not received emergency surgery before pre-admission; (iii) they had clinical characteristics data indicating that they did not suffer from other malignancies, recent bacterial or viral infections, autoimmune, hematologic, cardiovascular, or cerebrovascular diseases; (iv) and they had normal liver and kidney function, no diarrhea, and they had not been taking non-steroidal anti-inflammatory, antiplatelet, or anticoagulant drugs, antibacterial agents, or intravenous albumin supplements in the last 3 months. Right-sided CRC was defined as the disease originating from ileocecal to transverse colon; in contrast, left-sided CRC was defined as the disease occurring in the splenic flexure, descending and sigmoid colon, as well as the rectum.
Follow-up
Patient follow-up was performed 3 years after enrollment. The majority of the patients were counterchecked with a frequency of every 3 months in the first 2 years, and every 6 months in the third year. The patients received follow-up investigations, including a physical examination, serum carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) detection, and computed tomography (CT) or magnetic resonance imaging (MRI), as well as colonoscopy. A contrast-enhanced chest CT and bone scanning were selected to detect lung or bone metastasis, respectively. The disease progression was clinically confirmed by one of the following criteria: (a) colonoscopy examination; (b) typical appearances in imaging scan; (c) or more than twice the increase CEA or CA19-9 compared to the last detection. Telephone, email, and network questionnaires were selected to follow-up those who were not regularly checked in the period. The last follow-up was conducted in December 2019. The primary endpoints are recurrence-free survival (RFS) within the stage I–III patients, and progression-free survival (PFS) within the stage IV patients. The secondary endpoint was overall survival (OS). The time from surgical operation to radiologic recurrence (local or distal recurrence of CRC) was considered as RFS. PFS was defined as the time from the disease diagnosis to progression or censored at the deadline in stage IV patients. OS was described as the time taken from operation/diagnosis to death in any cause or censored at the last follow-up.
Immunoassays
Peripheral serum and sodium citrate anticoagulated plasma samples (every 2 ml) were collected when the patients were first admitted to the hospitals; this occurred earlier than when the patients were clinically diagnosed as localized or metastatic CRC. These samples were used for laboratory detection, and all the detections were completed within 2 h after the collection. Plasma Fib was measured by Clauss assay using a SYSMEX CA-7000 machine (Sysmex, Tokyo, Japan), whereas serum pAlb, CEA and CA 19-9 were measured using the immunoturbidimetric and chemiluminescence assays on OLYMPUS AU5400 (Beckman Coulter, Tokyo, Japan) and SIEMENS ADVIA Centaur XP (Siemens, Erlangen, Germany) machines. Inter- and intra-batch variation coefficients for Fib, pAlb, CEA, and CA19-9 kits were less than 5%. We calculated FPR according to the formula: FPR = (circulating Fib/pAlb) × 1000.
Statistical analyses
Continuous variables, with normal and skewed distribution, were expressed as means ± standard deviations (SD) of the mean, medium, and inter-quartile ranger (IQR), respectively. Comparative differences between categorical variables were carried out using the Chi–square or Fisher’s exact tests; in contrast, those across continuous variables with skewed distribution were done using the Mann–Whitney U test. Optimal FPR cut-off points across 3 years OS, at each disease stage, were determined using the X-tile 3.6.1 software (Yale University, New Haven, CT, USA) as described in our previous studies,20,23 and their optimal cut-off values for stage I, II, III, and IV patients were 14.0, 16.5, 19.5, and 22.8, respectively. According to the cut-off values, we divided the patients into high- and low-FPR groups across each stage. We also stratified low- (FPR < 15), medium- (15 ⩽ FPR < 20) and high-grade FPR (FPR ⩾ 20) groups in the overall population.
Differences in survival rates were calculated using Kaplan–Meier curves, with the log-rank test, whereas the prognostic role of clinical baseline characteristics and indicators detected in the laboratory were analyzed using the Cox proportional regression with a hazard ratio (HR) at a 95% confidence interval (CI). Comparisons of baseline and pathological characteristics, treatment, and other confounders were performed using multivariate analysis by backward stepwise Cox regression modeling. Furthermore, the prognostic predictive efficacy of FPR was assessed and compared using time-dependent receiver operating characteristic (ROC) curves. The significant characteristics and FPR were selected to establish the prognostic nomograms for the localized and metastatic CRC patients. In addition, predicted efficacies of Harrell’s concordance index (c-index), time-dependent ROC, and calibration and decision curves were analyzed using ‘rms’, ‘survival’, ‘survivalROC’, ‘tdROC’, and ‘rmda’ packages implemented in R software. All analyses were performed with SPSS version 22.0 (IBM Corp, Armonk, NY, USA), R version 3.6.3 (Institute for Statistics and Mathematics, Vienna, Austria), and GraphPad Prism version 8.2.1 (GraphPad Software Inc., San Diego, USA). All analyses were two-sided, with values followed by p < 0.05 considered statistically significant.
Results
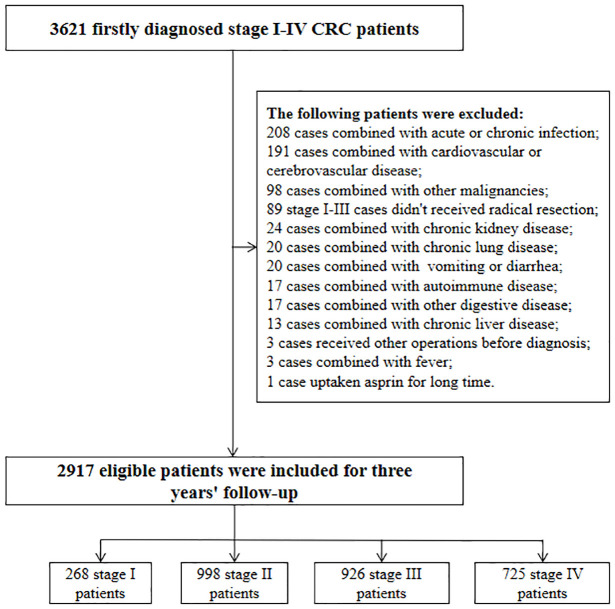
A summary of the study overview and an outline of the eligibility criteria are described in Figure 1. As shown from Figure 1, 268, 998, 926, and 725 first-diagnosed patients with stage I, II, III, and IV CRC were recruited in this study, respectively. The baseline characteristics, pathology, and survival data are outlined in Table 1. All the stage I–III cases received radical resection, while 73.45% and 86.29% of stage II and III patients underwent postoperative adjuvant chemotherapy. Due to unwillingness and poverty, 14.76% of the stage IV patients gave up any treatment after the diagnosis, 97 and 216 cases received the single palliative resection and the resection plus the other treatments. Of these cases, only 68.00% and 6.48% of the patients underwent chemotherapy and targeted therapy, respectively. The median follow-up time was 36 and 13 months for stages I–III and IV patients, respectively.
Figure 1.
Selection flowchart of 2917 eligible colorectal cancer patients.
Table 1.
The baseline characteristics of 2917 stage I–IV colorectal cancer patients in the study.
| Variants | Stage I cases (n = 268) | Stage II cases (n = 998) | Stage III cases (n = 926) | Stage IV cases (n = 725) |
|---|---|---|---|---|
| Age, years | 61 (51–68) | 62 (51–71) | 61 (52–69) | 60 (49–69) |
| Sex | ||||
| Female (%) | 111 (41) | 382 (38) | 355 (38) | 333 (46) |
| Male (%) | 157 (59) | 616 (62) | 571 (62) | 392 (54) |
| Smoking | ||||
| Yes (%) | 35 (13) | 166 (17) | 113 (12) | 125 (17) |
| No (%) | 233 (87) | 832 (83) | 813 (88) | 600 (83) |
| Drinking | ||||
| Yes (%) | 29 (11) | 122 (12) | 89 (10) | 104 (14) |
| No (%) | 239 (89) | 876 (88) | 837 (90) | 621 (86) |
| Diabetes | ||||
| Yes (%) | 24 (9) | 63 (6) | 68 (7) | 53 (7) |
| No (%) | 244 (91) | 935 (94) | 858 (93) | 672 (93) |
| Hypertension | ||||
| Yes (%) | 41 (15) | 147 (15) | 122 (13) | 97 (13) |
| No (%) | 227 (85) | 851 (85) | 804 (87) | 628 (87) |
| pT stage | ||||
| T1 (%) | 58 (22) | 0 | 7 (1) | 1 (0.1) |
| T2 (%) | 210 (78) | 0 | 77 (8) | 10 (1) |
| T3 (%) | 0 | 396 (40) | 269 (29) | 17 (2) |
| T4 (%) | 0 | 602 (60) | 573 (62) | 283 (39) |
| NA (%) | 0 | 0 | 0 | 414 (57) |
| pN stage | ||||
| N0 (%) | 268 (100) | 998 (100) | 0 | 99 (14) |
| N1 (%) | 0 | 0 | 837 (90) | 100 (14) |
| N2 (%) | 0 | 0 | 89 (10) | 104 (14) |
| NA (%) | 0 | 0 | 0 | 422 (58) |
| Distal metastasis | ||||
| No (%) | 268 (100) | 998 (100) | 926 (100) | 0 |
| Yes (%) | 0 | 0 | 0 | 725 (100) |
| Differentiation grade | ||||
| Good (G1) (%) | 32 (12) | 43 (43) | 62 (7) | 9 (1) |
| Median (G2) (%) | 223 (83) | 896 (90) | 747 (81) | 271 (38) |
| Poor (G3) (%) | 13 (5) | 59 (59) | 117 (13) | 49 (7) |
| NA (%) | 0 | 0 | 0 | 396 (55) |
| Location | ||||
| Proximal colon (%) | 30 (11) | 288 (29) | 205 (22) | 192 (27) |
| Distal colon (%) | 47 (18) | 273 (27) | 216 (23) | 210 (29) |
| Rectum (%) | 191 (71) | 437 (44) | 505 (54) | 323 (45) |
| Treatment | ||||
| Operation* (%) | 268 (100) | 998 (100) | 926 (100) | 353 (49) |
| Single chemotherapy (%) | 0 | 683 (68) | 721 (78) | 484 (67) |
| Single radiotherapy (%) | 0 | 3 (0.3) | 3 (0.3) | 1 (0.1) |
| Chemotherapy and radiotherapy (%) | 0 | 50 (5) | 78 (8) | 52 (7) |
| Targeted therapy (%) | 0 | 0 | 0 | 47 (7) |
| Follow-up time, months | 36 | 36 | 36 (25–36) | 13 (7–26) |
| FPR | 12.57 (9.79–16.28) | 15.50 (11.45–21.18) | 14.79 (10.82–20.80) | 23.34 (15.11–34.75) |
| CEA (ng/ml) | 1.74 (1.15–3.20) | 2.87 (1.67–5.84) | 3.45 (1.72–9.22) | 13.14 (3.71–87.76) |
| CA19-9 (U/ml) | 11.45 (7.19–17.43) | 13.71 (7.30–24.77) | 17.40 (8.61–33.77) | 43.49 (1.52–375.18) |
Data are median (IQR) or n (%), Operation*: Radical resection for stage I–III CRC patients, palliative operation for stage IV cases.
CRC, colorectal cancer; N1*, positive of node metastasis; NA, not available.
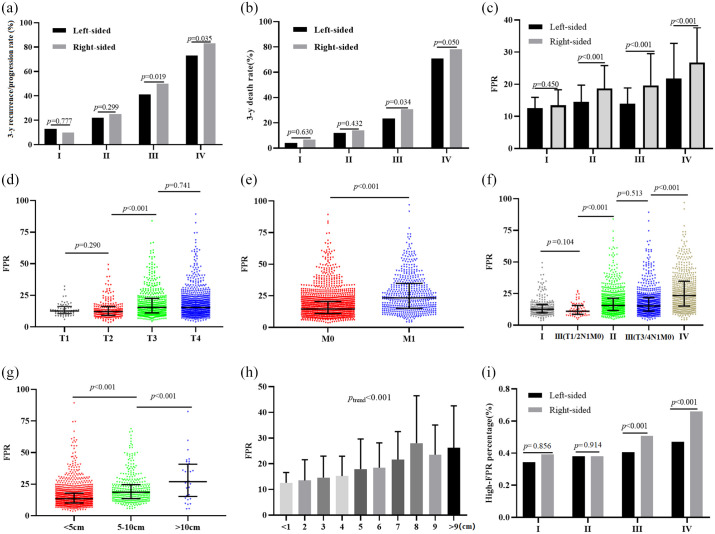
Stratification according to tumor laterality resulted in a significantly higher recurrence rate being observed in patients with stage III right-sided CRC compared to it with the left-sided disease (p = 0.019 for 50% versus 41%), meanwhile, the progression rate with the right-sided stage IV patients was significantly higher than the left-sided patients (p = 0.035 for 83% versus 74%) [Figure 2(a)].
Figure 2.
Primary tumor location, , and survival in colorectal cancer patients. (a) 3-years’ recurrence rate in the right- and left-sided patients; (b) 3-years’ death rate in the right- and left-sided patients; (c) circulating FPR in the right- and left-sided patients; (d) circulating FPR in the T1~4 patients; (e) circulating FPR in the patients with different distal metastatic status; (f) circulating FPR in different TNM stage; (g-i) circulating FPR in the patients with different cancer bulk.
FRP, fibrinogen to pre-albumin ratio; TNM, tumor, nodes and metastases staging.
In addition, right-sided patients exhibited significantly higher mortalities than left-sided tumour, across stages III (p = 0.034 for 30.70% versus 23.40%) and IV (p = 0.050 for 78.10 versus 70.90%) [Figure 2(b)]. Despite this, no difference of recurrence or death rate was observed in the left- or right-sided stage I or II patients. Comparison between left- versus right-sided patients revealed similar survival outcome at the stage I (plog–rank = 0.615 for RFS; plog–rank = 0.582 for OS) and II (plog–rank = 0.224 for RFS; plog–rank = 0.371 for OS) diseases (Table 2). Kaplan–Meier curves revealed significantly worse prognosis in right-sided patients with stage III (plog–rank = 0.006 for RFS; plog–rank = 0.014 for OS) [Supplemental Figure 1(a-b)] and IV (plog–rank = 0.033 for RFS; plog–rank = 0.011 for OS) disease than their left-sided tumour counterparts [Supplemental Figure 1(c-d)]. Adjusting for the baseline, pathological characteristics and FPR, our analysis revealed that primary tumor location was not associated with clinical outcome of patients regardless of TNM stage (Table 2).
Table 2.
Cox analysis of clinical baseline characteristics, tumor laterality and FPR in 2917 stage I–IV colorectal cancer patients.
| Stage | Comparison | RFS (HR and 95% CI) | OS (HR and 95% CI) | ||||
|---|---|---|---|---|---|---|---|
| p-value* | Crude | Adjusted | p-value* | Crude | Adjusted | ||
| Stage I | Male versus female | 0.109 | 1.823 (0.875–3.795) | 2.017 (0.514–7.908) | 0.235 | 2.165 (0.586–7.996) | 3.023 (0.379–24.114) |
| Age (⩾60 versus <60) | 0.131 | 1.970 (0.818–4.745) | 2.750 (0.71910.524) | 0.080 | 3.035 (0.822–11.211) | 8.096 (0.625–40.337) | |
| Smoking (yes versus no) | 0.404 | 2.364 (0.313–17.824) | 1.592 (0.162–15.610) | 0.894 | 1.155 (0.139–9.596) | 1.561 (0.042–58.342) | |
| Drinking (yes versus no) | 0.939 | 1.060 (0.242–4.634) | 1.971 (0.039–100.734) | 0.798 | 1.317 (0.158–10.936) | 1.526 (0.117–19.874) | |
| Diabetes (yes versus no) | 0.716 | 1.314 (0.301–5.748) | 2.321 (0.458–11.768) | 0.079 | 3.904 (0.757–20.126) | 4.418 (0.759–25.718) | |
| Hypertension (yes versus no) | 0.774 | 1.200 (0.345–4.176) | 1.292 (0.290–5.754) | 0.299 | 2.323 (0.451–11.973) | 1.420 (0.208–9.701) | |
| T2 versus T1 | 0.278 | 1.688 (0.655–4.351) | 2.327 (0.285–18.960) | 0.247 | 3.142 (0.406–24.338) | 1.532 (0.063–37.192) | |
| Size (⩾5 versus <5) | 0.527 | 1.386 (0.504–3.814) | 1.398 (0.155–12.609) | 0.104 | 2.733 (0.771–9.687) | 6.579 (0.581–7.692) | |
| Differentiation (G1-2 versus G3-4) | 0.555 | 1.629 (0.352–7.519) | 4.150 (0.383–44.950) | 0.529 | 1.629 (0.352–7.519) | 8.932 (0.576–13.861) | |
| CEA (⩾5 versus <5) | 0.004 | 4.244 (1.569–11.479) | 5.668 (1.628–19.729) | 0.009 | 5.814 (1.301–25.995) | 6.988 (1.402–34.841) | |
| CA199 (⩾37 versus <37)) | 0.592 | 2.126 (0.100–15.326) | 2.238 (0.124–4.339) | 0.605 | 2.128 (0.352–18.653) | 2.345 (0.259–16.554) | |
| Tumor location (Right versus left) | 0.622 | 1.347 (0.413–4.399) | 1.999 (0.229–17.436) | 0.579 | 1.532 (0.336–6.992) | 5.444 (0.251–11.784) | |
| High-FPR versus low-FPR | <0.001 | 6.245 (2.287–17.051) | 6.833 (1.438–32.466) | 0.007 | 6.535 (1.357–31.459) | 6.548 (1.229–30.447) | |
| Stage II | Male versus Female | 0.890 | 1.019 (0.782–1.327) | 1.047 (0.620–1.768) | 0.476 | 1.138 (0.797–1.625) | 1.303 (0.653–2.598) |
| Age (⩾60 versus <60) | 0.002 | 1.673 (1.208–2.317) | 1.075 (0.523–2.212) | <0.001 | 2.211 (1.490–3.280) | 1.147 (0.473–2.779) | |
| Smoking (yes versus no) | 0.749 | 1.074 (0.694–1.661) | 1.109 (0.625–1.968) | 0.753 | 1.095 (0.619–1.937) | 1.351 (0.659–2.769) | |
| Drinking (yes versus no) | 0.602 | 1.143 (0.690–1.893) | 1.281 (0.529–3.106) | 0.301 | 1.462 (0.707–3.021) | 2.197 (0.684–7.058) | |
| Diabetes (yes versus no) | 0.583 | 1.187 (0.642–2.193) | 1.274 (0.548–2.964) | 0.129 | 1.692 (0.850–3.368) | 1.287 (0.451–3.668) | |
| Hypertension (yes versus no) | 0.499 | 1.181 (0.730–1.910) | 1.093 (0.586–2.038) | 0.965 | 1.013 (0.563–1.824) | 1.138 (0.514–2.518) | |
| Chemotherapy (yes versus no) | 0.077 | 0.775 (0.583–1.030) | 0.559 (0.44–0.909) | 0.001 | 0.545 (0.381–0.779) | 0.506 (0.273–0.936) | |
| Radiotherapy (yes versus no) | 0.017 | 0.537 (0.319–0.904) | 0.305 (0.152–0.610) | 0.486 | 0.760 (0.350–1.650) | 0.275 (0.113–0.672) | |
| T4 versus T3 | 0.002 | 1.505 (1.163–1.948) | 1.137 (0.504–2.567) | 0.124 | 1.306 (0.928–1.838) | 1.411 (0.514–3.874) | |
| Size (⩾5 versus <5) | 0.360 | 1.159 (0.844–1.594) | 1.177 (0.714–1.942) | 0.344 | 1.211 (0.813–1.805) | 1.394 (0.732–2.653) | |
| Differentiation (G1-2 versus G3-4) | 0.185 | 1.531 (0.810–2.894) | 2.853 (0.102–9.075) | 0.001 | 2.870 (1.496–5.505) | 3.139 (0.945–6.544) | |
| CEA (⩾5 versus <5) | 0.005 | 1.686 (1.169–2.433) | 1.032 (0.616–1.729) | 0.013 | 1.756 (1.121–2.753) | 2.513 (1.198–5.272) | |
| CA199 (⩾37 versus <37)) | 0.006 | 1.800 (1.177–2.753) | 2.140 (1.247–3.672) | 0.001 | 2.240 (1366–3.674) | 2.078 (1.040–4.154) | |
| Tumor location (right versus left) | 0.230 | 1.184 (0.897–1.52) | 1.068 (0.608–1.874) | 0.371 | 1.181 (0.819–1.704) | 1.394 (0.695–2.797) | |
| High-FPR versus low-FPR | <0.001 | 3.144 (2.267–4.361) | 3.431 (2.102–5.601) | <0.001 | 4.756 (2.985–7.579) | 7.194 (3.329–15.547) | |
| Stage III | Male versus female | 0.444 | 1.082 (0.882–1.328) | 1.024 (0.746–1.404) | 0.713 | 1.050 (0.808–1.366) | 1.250 (0.834–1.875) |
| Age (⩾60 versus <60) | 0.999 | 1.000 (0.820–1.220) | 1.089 (0.794–1.492) | 0.013 | 1.383 (1.069–1.790) | 1.805 (1.192–2.734) | |
| Smoking (yes versus no) | 0.949 | 1.010 (0.736–1.386) | 1.039 (0.670–1.612) | 0.381 | 1.214 (0.784–1.878) | 1.081 (0.584–2.001) | |
| Drinking (yes versus no) | 0.876 | 1.028 (0.720–1.469) | 1.049 (0.663–1.660) | 0.629 | 1.124 (0.699–1.806) | 1.023 (0.474–2.211) | |
| Diabetes (yes versus no) | 0.058 | 1.539 (0.978–2.422) | 1.467 (0.832–2.586) | 0.051 | 1.862 (0.985–3.521) | 2.339 (0.946–5.782) | |
| Hypertension (yes versus no) | 0.061 | 1.364 (0.982–1.896) | 1.481 (0.969–2.262) | 0.271 | 1.255 (0.835–1.888) | 1.257 (0.713–2.216) | |
| Chemotherapy (yes versus no) | 0.040 | 0.755 (0.575–0.991) | 0.743 (0.493–1.118) | <0.001 | 0.548 (0.395–0.760) | 0.607 (0.360–1.024) | |
| Radiotherapy (yes versus no) | 0.077 | 0.741 (0.529–1.038) | 1.216 (0.764–1.936) | 0.244 | 0.725 (0.420–1.251) | 0.704 (0.298–1.660) | |
| T3-4 versus T1-2 | <0.001 | 3.440 (2.018–5.865) | 2.662 (1.244–5.694) | <0.001 | 4.601 (2.045–10.351) | 4.308 (1.054–17.600) | |
| Size (⩾5 versus <5) | 0.532 | 1.074 (0.856–1.348) | 1.103 (0.813–1.496) | 0.016 | 1.414 (1.062–1.882) | 1.292 (0.862–1.937) | |
| Differentiation (G1-2 versus G3-4) | 0.014 | 1.463 (1.077–1.989) | 1.295 (0.884–1.899) | 0.031 | 1.564 (1.036–2.361) | 1.198 (0.695–2.067) | |
| CEA (⩾5 versus <5) | <0.001 | 1.553 (1.224–1.970) | 1.341 (0.999–1.801) | <0.001 | 1.785 (1.312–2.428) | 1.362 (0.910–2.038) | |
| CA199 (⩾37 versus <37)) | 0.001 | 1.787 (1.372–2.327) | 1.455 (1.053–2.010) | <0.001 | 2.436 (1.767–3.360) | 1.889 (1.243–2.869) | |
| Tumor location (right versus left) | 0.006 | 1.370 (1.093–1.716) | 1.173 (0.831–1.655) | 0.014 | 1.430 (1.071–1.910) | 1.283 (0.817–2.013) | |
| High-FPR versus low-FPR | <0.001 | 2.439 (1.947–3.056) | 2.182 (1.616–2.947) | <0.001 | 2.761 (2.079–3.666) | 2.808 (1.858–4.241) | |
| Stage IV | Male versus female | 0.747 | 1.032 (0.845–1.261) | 1.244 (0.723–2.139) | 0.269 | 1.099 (0.926–1.305) | 1.204 (0.749–1.934) |
| Age (⩾60 versus <60) | 0.097 | 1.324 (0.939–1.866) | 1.148 (0.483–2.729) | <0.001 | 1.713 (1.333–2.201) | 1.548 (0.785–3.054) | |
| Smoking (yes versus no) | 0.200 | 1.210 (0.895–1.636) | 1.111 (0.263–4.692) | 0.673 | 1.049 (0.837–1.313) | 1.261 (0.554–2.868) | |
| Drinking (yes versus no) | 0.023 | 1.439 (1.039–1.992) | 2.851 (0.860–9.446) | 0.195 | 1.175 (0.915–1.508) | 1.096 (0.399–3.010) | |
| Diabetes (yes versus no) | 0.824 | 1.040 (0.727–1.488) | 2.652 (0.793–8.865) | 0.437 | 1.142 (0.810–1.610) | 1.368 (0.152–9.846) | |
| Hypertension (yes versus no) | 0.514 | 1.099 (0.821–1.471) | 1.603 (0.792–3.247) | 0.639 | 1.062 (0.823–1.371) | 1.499 (0.744–3.021) | |
| Palliative operation (yes versus no) | 0.016 | 0.788 (0.644–0.963) | 0.779 (0.315–1.923) | <0.001 | 0.588 (0.495–0.699) | 0.818 (0.315–2.122) | |
| Chemotherapy (yes versus no) | 0.225 | 0.848 (0.644–1.117) | 0.787 (0.318–1.950) | <0.001 | 0.434 (0.361–0.522) | 0.435 (0.249–0.760) | |
| Radiotherapy (yes versus no) | 0.002 | 0.543 (0.362–0.814) | 0.876 (0.198–3.876) | <0.001 | 0.509 (0.348–0.745) | 0.642 (0.145–2.852) | |
| Targeted therapy (yes versus no) | 0.027 | 0.688 (0.488–0.971) | 0.126 (0.031–0.504) | 0.515 | 0.897 (0.642–1.253) | 0.870 (0.176–4.312) | |
| T3-4 versus T1-2 | 0.136 | 1.811 (0.802–4.087) | 1.422 (0.175–11.569) | 0.039 | 3.074 (0.982–9.620) | 1.217 (0.112–8.629) | |
| N1 versus N0 | 0.001 | 1.657 (1.214–2.262) | 2.394 (1.328–4.315) | <0.001 | 1.786 (1.305–2.445) | 2.428 (1.386–4.253) | |
| Size (⩾5 versus <5) | 0.277 | 1.200 (0.856–1.682) | 2.112 (1.236–3.608) | 0.958 | 1.008 (0.752–1.351) | 1.030 (0.607–1.747) | |
| Differentiation (G1-2 versus G3-4) | 0.109 | 1.410 (0.912–2.181) | 2.353 (1.015–5.456) | 0.908 | 1.022 (0.702–1.488) | 1.099 (0.531–2.276) | |
| CEA (⩾5 versus <5) | <0.001 | 1.590 (1.249–2.023) | 1.258 (0.647–2.445) | <0.001 | 1.722 (1.393–2.129) | 1.496 (0.808–2.772) | |
| CA199 (⩾37 versus <37)) | <0.001 | 1.824 (1.454–2.289) | 1.472 (0.879–2.465) | <0.001 | 1.799 (1.489–2.173) | 2.406 (1.468–3.943) | |
| Tumor location (right versus left) | 0.036 | 1.260 (1.007–1.577) | 1.056 (0.600–1.859) | 0.011 | 1.270 (1.051–1.535) | 1.021 (0.586–1.776) | |
| High-FPR versus low-FPR | <0.001 | 2.642 (2.084–3.351) | 1.994 (1.156–3.438) | <0.001 | 2.691 (2.186–3.312) | 2.786 (1.667–4.656) | |
Right-sided CRC was defined as the disease originating from ileocecal to transverse colon, whereas left-sided CRC comprised disease occurring in the splenic flexure, descending and sigmoid colon, as well as the rectum. p-value, p-value of univariable Cox regression; [1]: was adjusted by gender, age, tobacco, alcohol, diabetes, hypertension, chemotherapy, radiotherapy, T, N, differentiation, cancer size, CEA, CA 19-9 for tumor laterality or FPR; [2]: was adjusted by gender, age, tobacco, alcohol, diabetes, hypertension, chemotherapy, radiotherapy, T, N, differentiation, cancer size, CEA, CA199, and tumor laterality for H-FPR versus L-FPR; was adjusted by gender, age, tobacco, alcohol, diabetes, hypertension, chemotherapy, radiotherapy, T, N, differentiation, cancer size, CEA, CA 19-9, and FPR for right- versus left-tumor location; H- and L-FPR within stage I patient: FPR ⩾ 14.6 and FPR < 14.6 within stage I CRC patient; H- and L-FPR within stage I patient: FPR ⩾ 14.6 and FPR < 14.6 within stage I CRC patient; H- and L-FPR within stage II patient: FPR ⩾ 16.5 and FPR < 16.5 within stage II CRC patient; H- and L-FPR within stage I patient: FPR ⩾ 19.5 and FPR < 19.5 within stage III CRC patient; H- and L-FPR within stage IV patient: FPR ⩾ 22.8 and FPR < 22.8 within stage I CRC patient.
CA 19-9; carbohydrate antigen 19-9; CEA, carcinoembryonic antigen; CI, confidence interval; CRC, colorectal cancer; (H/L-) FPR, (high/low-) fibrinogen to prealbumin ratio; HR, hazard ratio; OS, overall survival; RFS, recurrence-free survival.
Our results also observed significantly higher circulating FPR in right-sided than left-sided patients with stage II (p < 0.001), III (p < 0.001), and IV (p < 0.001) CRC [Figure 2(c)]. No differential circulating FPR was observed in patients with stage I–III CRC, based on comparisons between T1 versus T2 (p = 0.290), T3 versus T4 (p = 0.741), and negative-metastasis of lymph nodes (LN−) versus positive-metastasis of lymph nodes (LN+) (p = 0.856) [Figure 2(d)]. However, significantly higher FPR was observed in the T3 subgroup compared to T2 (p < 0.001) patients [Figure 2(d)], and the similar significant result was also observed between the G3-4 and G1-2 differentiated subgroups (p = 0.01), distal metastasis and non-metastasis subgroups (p < 0.001) [Figure 2(e)], respectively. Comparisons revealed significantly higher preoperative FPR in patients with stage IV relative to those with stages I–III or IIIB (T3/4N1M0) (p < 0.001) [Figure 2(f)]. A similar trend was also observed with regard to circulating FPR between patients with stage I and IIIA (T1/2N1M0) CRC (p = 0.104) or those with stage II and IIIB CRC (T3/4N1M0) (p = 0.513) [Figure 2(f)]. In addition, patients with stage II CRC exhibited significantly higher preoperative FPR than those with IIIA (T1/2N1M0) (p < 0.001) [Figure 2(f)]. Finally, a significantly high FPR was observed in patients with stage I–III CRC, with a median of 5~10 cm (p < 0.001) and large cancer bulk (⩾10 cm) (p < 0.001) relative to those with small (<5 cm) bulk [Figure 2(g)], while the median FPR gradually increased from the smallest (<1 cm) to the largest cancer bulk (>9 cm) (p-trend < 0.001) [Figure 2(h)].
In comparing the high- and low-FPR distribution in right- and left-sided CRC patients, we observed significantly higher high-FPR proportion within right-sided than left-sided patients with stage III (p < 0.001) and IV (p < 0.001) CRC [Figure 2(i) and Supplemental Table 1]. H-FPR was found to significantly associated with both high recurrence and death rates compared to the L-FPR patients within each TNM stage CRC (Supplemental Table 1). In addition, Kaplan–Meier curves and univariable Cox regression analyzes showed that patients with high-FPR were significantly associated with worse survival outcome than those with low-FPR at stage I (plog–rank < 0.001 and 0.007 for RFS and OS, respectively), II (all plog–rank < 0.001 for both RFS and OS), III (all plog–rank < 0.001 for both RFS and OS), and IV (all plog–rank < 0.001 for both RFS and OS) CRC [Table 2 and Supplemental Figure 1(e-l)]. Adjusting for the baseline and pathological characteristics as well as tumor laterality, it indicated that high-FPR was still significantly associated with unsatisfactory clinical outcomes for patients with stage I (adjusted HR = 6.658, 95% CI = 1.229–30.447 for OS), II (adjusted HR = 7.194, 95% CI = 3.329–15.547 for OS), III (adjusted HR = 2.808, 95% CI = 1.858–4.241 for OS), and IV (adjusted HR = 2.786, 95% CI = 1.667–4.656 for OS) CRC (Table 2).
The time-dependent area under the curves (AUCs) for FPR were 0.724 and 0.720 in stage I patients, 0.657 and 0.694 in stage II patients, 0.623 and 0.634 in stage III patients to predict RFS and OS, respectively, and were 0.714 and 0.723 for stage IV to predict 3 years’ PFS and OS [Supplemental Figure 2(a-h)]. Measuring FPR resulted in a better prediction efficacy than CEA, CA19-9, and primary tumor location, since it resulted in the highest AUC for predicting prognosis across the disease with each TNM stage of CRC [Supplemental Figure 2(i-p)].
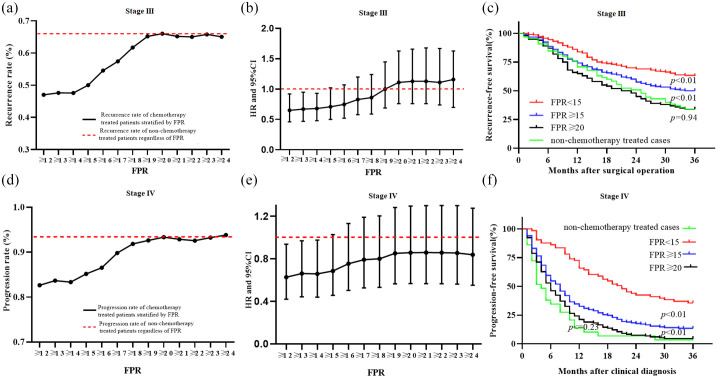
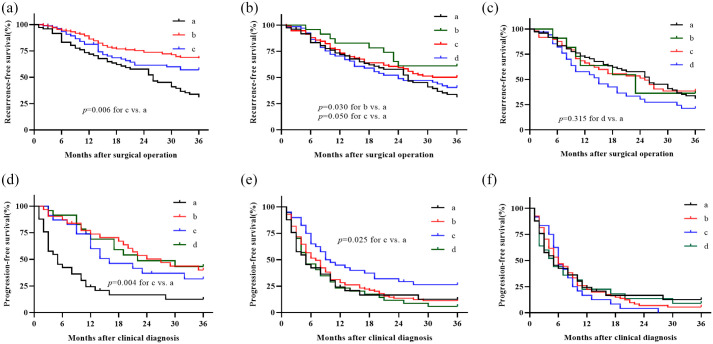
To further understand the effect of chronic inflammation in adjuvant chemotherapy in CRC patients, we analyzed recurrence rates and survival differences in stage III and IV patients under varying FPR concentrations. Summarily, a 3-year follow-up showed recurrence and progression rates of 66.15% and 93.33%, respectively, in the two-stage non-chemotherapy treated patients regardless of FPR [Figure 3(a) and (d)]. On the other hand, recurrence and progression rates in chemotherapy-treated patients with low-grade FPR (<15), with stage III and IV CRC, were nearly 47.50% and 83.50%, respectively, although the rates gradually increased with medium-grade FPR (15⩽FPR < 20). However, higher stable recurrence and progression rates of 66.00% and 93.00% within the chemotherapy-treated stage III and IV cases, respectively, were observed when circulating FPR was larger than 20 [Figure 3(a) and (d)]. It should be noted that the lowest recurrence and progression rates and the best survival outcomes were observed in stage III and IV CRC patients with FPR < 15; significant recurrence and progression rates as well as survival differences were also found in the patients with FPR ⩾ 15 and ⩾20, respectively [Figure 3(a), (c–d) and (f)]. No RFS or PFS was observed in chemotherapy-treated stage III (plog–rank = 0.94) and IV (plog–rank = 0.23) CRC patients with FPR ⩾ 20 compared to the non-chemotherapy treated cases regardless of FPR [Figure 3(c) and (f)]. A comparison with stage III chemotherapy-untreated patients revealed significantly lower HR predicting recurrence in chemotherapy-treated patients with FPR ⩽ 15, although this value gradually increased to nearly 1.0 for the treated cases with FPR ranging from 15 to 19. In contrast, HR remained stable, at 1.0, in patients with FPR20. A similar HR trend was observed in patients with stage IV CRC, who exhibited low- (FPR < 15), medium- (15 ⩽ FPR < 20) and high-FPR (FPR ⩾ 20), relative to those without any treatment [Figure 3(b) and (d)]. Moreover, the RFS and PFS of stage III and IV patients with FPR < 15 subgroups stratified by adjuvant chemotherapy regimen was significantly better than non-chemotherapy treated patients [Figure 4(a) and (d)]. The similar poor survival was observed in the stage III and IV FPR ⩾ 20 subgroup stratified by adjuvant chemotherapy regimen and non-chemotherapy treated patients [Figure 4(c) and (f)]. In contrast, the RFS of 5-FU and oxaliplatin and capecitabine (XELOX) treated patients with 20 > FPR ⩾ 15 was better than non-chemotherapy treated patients in stage III population [Figure 4(b)], and PFS of stage IV 20 > FPR ⩾ 15 patients with XELOX treatment was better than non-chemotherapy treated patients [Figure 4(e)].
Figure 3.
Circulating FPR, recurrence, and progression in stage I–III and IV CRC patients. (a) recurrence rate according to increased FPR in the chemotherapy treated or non-chemotherapy treated stage III patients after curable operation; (b) HR and 95% CI change in recurrence-free survival comparison of stage I–III chemotherapy-treated patients with increased FPR and the cases without chemotherapy; (c) Kaplan–Meier curve in stage I–III patients; (d) progression rate according to increased circulating FPR in the chemotherapy treated or non-chemotherapy treated stage IV patients after clinical diagnosis; (e) HR and 95% CI change in progression-free survival comparison of stage IV chemotherapy-treated patients with increased FPR and the cases without chemotherapy; (f) Kaplan–Meier curve in stage IV patients.
CI, confidence interval; CRC, colorectal cancer; FPR, fibrinogen to pre-albumin ratio; HR, hazard ratio.
Figure 4.
Clinical survival comparison in different chemotherapy regimens treated stage III and IV patients stratified by circulating FPR and non-chemotherapy treated patients. (a) Stage III patients with FPR < 15, a: overall non-chemotherapy patients; b: XELOX regimen; c: FOLFOX regimen; (b) stage III patients with 15⩽FPR < 20, a: overall non-chemotherapy patients; b: capecitabine; c: XELOX regimen; d: FOLFOX regimen; (c) stage III patients with FPR ⩾ 20, a: overall non-chemotherapy patients; b: capecitabine; c: XELOX regimen; d: FOLFOX regimen; (d) stage IV patients with FPR < 15, a: overall non-chemotherapy patients; b: XELOX regimen; c: FOLFOX regimen; d: FOLFIRI regimen; (e) stage IV patients with 15 ⩽ FPR < 20, a: overall non-chemotherapy patients; b: XELOX regimen; c: FOLFOX regimen; d: FOLFIRI regimen; (f) stage IV patients with FPR ⩾ 20, a: overall non-chemotherapy patients; b: XELOX regimen; c: FOLFOX regimen; d: FOLFIRI regimen.
FOLFIRI, folinic acid, 5-fluorouracil, and irinotecan; FOLFOX, folinic acid, 5-fluorouracil, and oxaliplatin; FPR, fibrinogen to pre-albumin ratio; XELOX, oxaliplatin and capecitabine
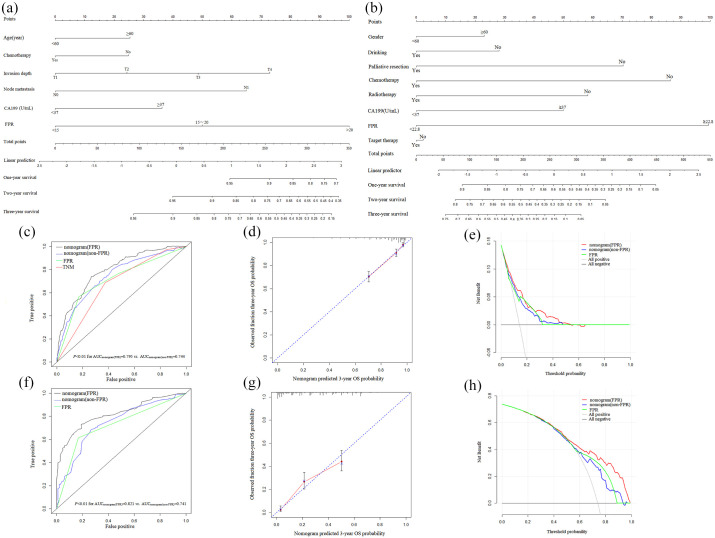
Furthermore, FPR and clinical risk factors were used to successfully establish relevant quantitative prognostic nomograms to predict prognosis in patients with localized and metastatic CRC (Figure 5 and Supplemental Figure 3). To summarize, C-indexes of 0.760 and 0.780 for stage I–III nomograms (FPR) to predict 3 years RFS and OS, respectively, and 0.671 and 0.718 for stage IV nomogram (FPR) to predict PFS and OS (Table 3) were calculated. These were significantly higher than non-FPR nomograms, FPR, and TNM. In addition, time-dependent AUCs of the prognostic nomograms (FPR) (AUCs = 0.791 and 0.795 for 3 years RFS and OS in stage I–III population, respectively; AUCs = 0.777 and 0.821 for 3 years PFS and OS in stage IV population, respectively) were significantly higher than the non-FPR nomograms, FPR, and TNM for predicting 1-, 2- and 3-year survival rates [Table 3, Figure 5(c) and (f), and Supplemental Figure 3(c) and (f)]. Calibration plots showed that the prognostic nomograms (FPR) for localized and metastatic CRC performed well compared with the performance of an ideal model [Figure 5(d) and (g), Supplemental Figure 3(d) and (g)]. In addition, results from decision curve analysis indicated that the prognostic nomogram (FPR) were better than non-FPR nomograms and FPR in predicting survival outcomes of localized and metastatic CRC patients, respectively [Figure 5(e) and (h), Supplemental Figure 3(e) and (h)].
Figure 5.
Building and evaluation of prognostic nomogram. (a) OS predicted nomogram (FPR) in stage I–III operated CRC patients; (b) OS predicted nomogram (FPR) in stage IV CRC patients; (c) time-dependent ROC analysis in stage I–III operated CRC patients; (d) calibration curve of OS predicted nomogram (FPR) in stage I–III operative CRC patients; (e) decision curve of OS predicted nomogram (FPR) in stage I–III operated CRC patients; (f) time-dependent ROC analysis in stage IV CRC patients; (g) calibration curve of OS predicted nomogram (FPR) in stage IV CRC patients; (h) decision curve of OS predicted nomogram (FPR) in stage IV CRC patients.
CRC, colorectal cancer; FPR, fibrinogen to pre-albumin ratio; OS, overall survival; ROC, receiver operating characteristic curve.
Table 3.
Comparison of predicted efficacy between prognostic nomogram and other prognostic biomarkers in stage I–III and IV CRC patients.
| Stage | Outcome | Variants | 12-month survival | 24-month survival | 36-month survival | |
|---|---|---|---|---|---|---|
| AUROC (95% CI) | AUROC (95% CI) | AUROC (95% CI) | C-index (95% CI) | |||
| I–III | RFS | Nomogram (FPR) | 0.790 (0.761–0.823) | 0.784 (0.755–0.830) | 0.791 (0.756–0.839) | 0.760 (0.735–0.785) |
| Nomogram (non-FPR) | 0.762 (0.732–0.804)* | 0.753 (0.714–0.806)* | 0.760 (0.722–0.805)* | 0.726 (0.693–0.747)* | ||
| FPR | 0.664 (0.599–0.709)** | 0.646 (0.601–0.685)** | 0.653 (0.622–0.689)** | 0.634 (0.601–0.667)** | ||
| TNM | 0.689 (0.644–7.35)** | 0.695 (0.633–0.746)** | 0.701 (0.660–0.742)** | 0.673 (0.636–0.700)** | ||
| OS | Nomogram (FPR) | 0.772 (0.722–0.813) | 0.803 (0.755–0.842) | 0.795 (0.751–0.849) | 0.780 (0.750–0.810) | |
| Nomogram (non-FPR) | 0.717 (0.648–0.753)** | 0.745 (0.698–0.801)* | 0.744 (0.699–0.796)* | 0.730 (0.690–0.770)* | ||
| FPR | 0.713 (0.655–0.752)** | 0.714 (0.644–0.766)** | 0.714 (0.668–0.742)** | 0.694 (0.652–0.736)** | ||
| TNM | 0.652 (0.604–0.698)** | 0.676 (0.639–0.736)** | 0.672 (0.624–0.716)** | 0.660 (0.624–0.696)** | ||
| IV | PFS | Nomogram (FPR) | 0.737 (0.688–0.779) | 0.773 (0.724–0.822) | 0.777 (0.739–0.822) | 0.671 (0.641–0.701) |
| Nomogram (non-FPR) | 0.644 (0.594–0.696)** | 0.670 (0.644–0.724)** | 0.693 (0.652–0.752)** | 0.615 (0.581–0.649)** | ||
| FPR | 0.695 (0.658–0.762)* | 0.720 (0.639–0.762)* | 0.714 (0.659–0.748)* | 0.632 (0.605–0.659)* | ||
| OS | Nomogram (FPR) | 0.779 (0.739–0.812) | 0.794 (0.733–0.845) | 0.821 (0.766–0.853) | 0.718 (0.6935–0.743) | |
| Nomogram (non-FPR) | 0.694 (0.638–0.751)** | 0.719 (0.647–0.751)** | 0.741 (0.701–0.749)* | 0.678 (0.650–0.705)* | ||
| FPR | 0.674 (0.630–0.712)** | 0.690 (0.633–0.740)** | 0.723 (0.677–0.765)** | 0.629 (0.605–0.654)** | ||
Discussion
A simple, economically-friendly, practical, and new, powerful CRC biomarker is necessary to guide development of chemotherapeutical approaches, alleviate the risk of recurrence and progression, and enhance prognosis of the disease. In this prospective study, we found that FPR not only indicated disease burden but also predicted CR to chemotherapy, impaired chemosensitivity and chemoresistance. Specifically, high FPR was associated with poor survival of CRC patients, with circulating differential FPR accounting for survival differences between right- and left-sided patients with stage III and IV CRC. In addition, FPR was an independent prognostic factor for each stage of the disease, with nomogram containing FPR effectively predicting the survival of CRC patients.
Previous studies have demonstrated the key roles played by Fib and pAlb in acute and the chronic phases of malignancies such as CRC. 24 In particular, Fib is a driver of chronic low-grade inflammation, owing to its effect on platelets, leukocyte migration, and its role in promoting carcinogenic properties. Moreover, it has been shown to function as a scaffold for cancer growth, migration, and metastasis.25–27 On the other hand, Alb and pAlb represent the main sources of energy and nutrition for tumor growth. Previous studies have demonstrated that inflammatory cytokines produced from CRC microenvironment and kupfer cells, including interleukin-6 (IL-6), can effectively suppress Alb and preAlb synthesis by hepatocytes. 28 As a result, CRC patients, especially those at advanced stages, have been found to commonly manifest malnutrition or hypoalbuminemia.20,29 Although circulating FPR was gradually elevated according to increased tumor invasion depth and cancer bulk, we also found significantly high FPR in the large cancer bulk subgroup and the patients with distal metastasis compared to their counterparts, respectively. These findings indicate that circulating FPR is determined by the cancer and can be attributed to an uncontrolled inflammatory response. As a consequence, it can be used to evaluate cancer burden.
In our previous studies, we found an association between circulating high FPR and poor prognosis in CRC patients.20,23 In the current study, univariate Cox regression revealed a significant association between high FPR and poor disease outcomes across each stage. Adjusting for common confounders, CEA, CA19-9, and tumor location, high FPR was still robustly associated with poor prognosis of the patients across each stage. Although we found significant differences in survival outcome between left- and right-sided patients with the stage III and IV disease, no association was found between them following adjustment for other confounders, including FPR. These results revealed that preoperative FPR, and not tumor laterality, was an independent prognostic factor for CRC patients at each stage of disease progression. Furthermore, we found extremely higher circulating FPR and high-FPR distribution in the stage III and IV right-sided patients than the left-sided cases. Therefore, the presence of high-grade inflammation in the right-tumor location might be a main cause contributing to the significantly poor survival of those patients. In addition, time-dependent FPR AUCs were superior to CEA, CA19-9, and tumor location in predicting the prognosis of the patients at each TNM stage; in contrast, c-indexes and the AUCs of the prognostic nomograms (FPR) were found to be significantly higher than those from the single factor or non-FPR nomograms. This indicated that FPR was an effective biomarker for predicting survival outcomes of the disease outcomes. As a result, it can be used to improve prediction efficacies of prognostic nomogram in patients with localized and metastatic CRC.
Previous studies have shown that an interaction among cancerous or stem cells, different immune and inflammatory cells as well as various mediators, such as cytokines and gut microbiota, sharpens the inflammatory microenvironment and promotes initiation and progression of CRC.30,31 For example, cancer-associated stromal and inflammatory cells, such as fibroblasts, macrophages and neutrophils, as well as colon cancer cells, were found to activate mitogen-activated protein kinase (MAPK), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB), and the phosphoinositide 3-kinase (PI3K) signaling pathway; as a result, this promotes resistance to various conventional chemotherapy agents.32–34 Moreover, gut microbiota, such as Fusobacterium nucleatum, have been implicated in regulation of a molecular network of the Toll-like receptor, microRNAs, and autophagy, thereby promoting chemoresistance. 35 In the current study, chemotherapy-treated patients with preoperative FPR < 15 exhibited the best outcomes, whereas those with FPR ⩾ 20 had the worst, in both stages III and IV of CRC. Moreover, the clinical outcomes of chemotherapy-treated stage III and IV patients with FPR ⩾ 15 were superior to those without chemotherapy, as well as those who underwent chemotherapy with FPR ⩾ 20. However, no recurrence or death rate difference was observed between the chemotherapy-treated stage III and IV patients with high-grade chronic inflammation (FPR ⩾ 20) and those without treatment of chemotherapy. These results indicated that FPR might be an effective factor for predicting chemotherapy efficacy in CRC patients. In particular, FPR < 15 and FPR ⩾ 20 might indicate complete response and resistance to chemotherapy, respectively; in contrast, 15 ⩽ FPR < 20 could imply impaired sensitivity to the treatment. Overall, these findings show that clinical outcomes of right-sided stage III and IV patients are worse than those of left-sided location, and high FPR effectively predicts poor survival rates in CRC patients.
This is the prospective study reporting a comprehensive analysis of prognostic and predictive significance of preoperative FPR in chemotherapy across stages I–IV in CRC patients, with different tumor locations. Based on results from a large sample size, used herein, it is evident that medium- and high-grade chronic inflammation-attenuated-chemosensitivity or triggered chemoresistance, and conferred poor outcomes within patients with stage III and IV disease. Preoperative FPR was a robust predictor and prognostic factor for CRC patients, following chemotherapy. However, other prospective studies are needed to validate our findings. In particular, functional and mechanistic analysis should be carried out to elucidate the association between FPR, inflammation, and chemoresistance.
In conclusion, CRC-related inflammation affects the response to chemotherapy and clinical outcomes. The circulating FPR represents a simple, economically-friendly and robust independent prognostic factor; it should act as an effective predictor for evaluating the efficacy of chemotherapy at each stage of CRC. In addition, prognostic FPR-contained nomogram is superior to the non-FPR counterpart and FPR in predicting outcomes of CRC patients. Targeting chronic inflammation and its corresponding signaling pathway, coupled with measuring FPR and FPR-contained prognostic nomogram, presents a novel approach to clinical management of CRC.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359211022886 for The value of circulating fibrinogen-to-pre-albumin ratio in predicting survival and benefit from chemotherapy in colorectal cancer by Hou-Qun Ying, Fan Sun, Yu-Cui Liao, Dan Cai, Ying Yang and Xue-Xin Cheng in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-2-tam-10.1177_17588359211022886 for The value of circulating fibrinogen-to-pre-albumin ratio in predicting survival and benefit from chemotherapy in colorectal cancer by Hou-Qun Ying, Fan Sun, Yu-Cui Liao, Dan Cai, Ying Yang and Xue-Xin Cheng in Therapeutic Advances in Medical Oncology
Supplemental material, sj-pdf-3-tam-10.1177_17588359211022886 for The value of circulating fibrinogen-to-pre-albumin ratio in predicting survival and benefit from chemotherapy in colorectal cancer by Hou-Qun Ying, Fan Sun, Yu-Cui Liao, Dan Cai, Ying Yang and Xue-Xin Cheng in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tif-4-tam-10.1177_17588359211022886 for The value of circulating fibrinogen-to-pre-albumin ratio in predicting survival and benefit from chemotherapy in colorectal cancer by Hou-Qun Ying, Fan Sun, Yu-Cui Liao, Dan Cai, Ying Yang and Xue-Xin Cheng in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tif-5-tam-10.1177_17588359211022886 for The value of circulating fibrinogen-to-pre-albumin ratio in predicting survival and benefit from chemotherapy in colorectal cancer by Hou-Qun Ying, Fan Sun, Yu-Cui Liao, Dan Cai, Ying Yang and Xue-Xin Cheng in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tif-6-tam-10.1177_17588359211022886 for The value of circulating fibrinogen-to-pre-albumin ratio in predicting survival and benefit from chemotherapy in colorectal cancer by Hou-Qun Ying, Fan Sun, Yu-Cui Liao, Dan Cai, Ying Yang and Xue-Xin Cheng in Therapeutic Advances in Medical Oncology
Footnotes
Author contributions: Hou-Qun Ying, Fan, Sun and Yu-Cui Liao (co-first authors): conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing. Dan Cai and Ying Yang: statistical analysis of data, collection and assembly of data, interpretation of results. Xue-Xin Cheng: conception and design, collection and assembly of data, data analysis and interpretation, manuscript revision and financial support.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Natural Science Youth Foundation of Jiangxi Province (grant number: 20171BAB215054), the Key Technology Research and Development Program of Jiangxi Province (grant number: 20171BBG70049) and 2020 Scientific Fund of Health and Family Planning Commission of Jiangxi Province (20204251).
Ethics approval and consent to participate: The study was approved by the Ethics committees of the Second Affiliated Hospital of Nanchang University [(No: 2007033)], and appropriate written informed consent obtained from each patient, or their legal surrogates, before enrollment.
ORCID iDs: Hou-Qun Ying  https://orcid.org/0000-0003-3858-4761
https://orcid.org/0000-0003-3858-4761
Xue-Xin Cheng  https://orcid.org/0000-0003-2241-2535
https://orcid.org/0000-0003-2241-2535
Availability of data and materials: The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Hou-Qun Ying, Department of Nuclear Medicine, Jiangxi Province Key Laboratory of Laboratory Medicine, The Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, China.
Fan Sun, Department of Laboratory Medicine, Jiangxi Province Key Laboratory of Laboratory Medicine, The Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, China.
Yu-Cui Liao, Jiangxi Medical College, Nanchang University, Nanchang, Jiangxi, China Biological Resource Center, The Second Affiliated Hospital of Nanchang University, Nanchang, Jiangxi, China.
Dan Cai, Jiangxi Provincial Key Laboratory of Preventive Medicine, School of Public Health, Nanchang University, Nanchang, Jiangxi, China.
Ying Yang, Jiangxi Provincial Key Laboratory of Preventive Medicine, School of Public Health, Nanchang University, Nanchang, Jiangxi, China.
Xue-Xin Cheng, Biological Resource Center, The Second Affiliated Hospital of Nanchang University, No.1 of Minde Road, Nanchang, 330006, China; Jiangxi Medical College, Nanchang University, Nanchang, Jiangxi, China.
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68: 394–424. [DOI] [PubMed] [Google Scholar]
- 2. Dekker E, Tanis PJ, Vleugels JLA, et al. Colorectal cancer. Lancet 2019; 394: 1467–1480. [DOI] [PubMed] [Google Scholar]
- 3. Jung G, Hernandez-Illan E, Moreira L, et al. Epigenetics of colorectal cancer: biomarker and therapeutic potential. Nat Rev Gastroenterol Hepatol 2020; 17: 111–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim SY, Kim TW. Current challenges in the implementation of precision oncology for the management of metastatic colorectal cancer. ESMO Open 2020; 5: e000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Merlano MC, Granetto C, Fea E, et al. Heterogeneity of colon cancer: from bench to bedside. ESMO Open 2017; 2: e000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zheng C, Jiang F, Lin H, et al. Clinical characteristics and prognosis of different primary tumor location in colorectal cancer: a population-based cohort study. Clin Transl Oncol 2019; 21: 1524–1531. [DOI] [PubMed] [Google Scholar]
- 7. You XH, Jiang YH, Fang Z, et al. Chemotherapy plus bevacizumab as an optimal first-line therapeutic treatment for patients with right-sided metastatic colon cancer: a meta-analysis of first-line clinical trials. ESMO Open 2020; 5: e000605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. You XH, Wen C, Xia ZJ, et al. Primary tumor sidedness predicts bevacizumab benefit in metastatic colorectal cancer patients. Front Oncol 2019; 9: 723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang CB, Shahjehan F, Merchea A, et al. Impact of tumor location and variables associated with overall survival in patients with colorectal cancer: a mayo clinic colon and rectal cancer registry study. Front Oncol 2019; 9: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100: 57–70. [DOI] [PubMed] [Google Scholar]
- 11. Tuomisto AE, Makinen MJ, Vayrynen JP. Systemic inflammation in colorectal cancer: underlying factors, effects, and prognostic significance. World J Gastroenterol 2019; 25: 4383–4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang K, Karin M. Tumor-elicited inflammation and colorectal cancer. Adv Cancer Res 2015; 128: 173–196. [DOI] [PubMed] [Google Scholar]
- 13. Friis S, Riis AH, Erichsen R, et al. Low-dose aspirin or nonsteroidal anti-inflammatory drug use and colorectal cancer risk: a population-based, case-control study. Ann Intern Med 2015; 163: 347–355. [DOI] [PubMed] [Google Scholar]
- 14. Kuo CN, Pan JJ, Huang YW, et al. Association between nonsteroidal anti-inflammatory drugs and colorectal cancer: a population-based case-control study. Cancer Epidemiol Biomarkers Prev 2018; 27: 737–745. [DOI] [PubMed] [Google Scholar]
- 15. Hua X, Phipps AI, Burnett-Hartman AN, et al. Timing of aspirin and other nonsteroidal anti-inflammatory drug use among patients with colorectal cancer in relation to tumor markers and survival. J Clin Oncol 2017; 35: 2806–2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li SQ, Jiang YH, Lin J, et al. Albumin-to-fibrinogen ratio as a promising biomarker to predict clinical outcome of non-small cell lung cancer individuals. Cancer Med 2018; 7: 1221–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gao QF, Qiu JC, Huang XH, et al. The predictive and prognostic role of a novel ADS score in esophageal squamous cell carcinoma patients undergoing esophagectomy. Cancer Cell Int 2018; 18: 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang L, Chen QG, Li SQ, et al. Preoperative fibrinogen to prealbumin ratio as a novel predictor for clinical outcome of hepatocellular carcinoma. Future Oncol 2019; 15: 13–22. [DOI] [PubMed] [Google Scholar]
- 19. Zhang J, Li SQ, Liao ZH, et al. Prognostic value of a novel FPR biomarker in patients with surgical stage II and III gastric cancer. Oncotarget 2017; 8: 75195–75205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen QG, Zhang L, Sun F, et al. Elevated FPR confers to radiochemoresistance and predicts clinical efficacy and outcome of metastatic colorectal cancer patients. Aging (Albany NY) 2019; 11: 1716–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Greene FL, Page DL, Fleming ID, et al. AJCC Cancer staging handbook. 6th ed. New York: Springer, 2002. [Google Scholar]
- 22. Cuccurullo V, Mansi L. AJCC cancer staging handbook: from the AJCC cancer staging manual (7th edition). Eur J Nucl Med Mol Imaging 2011; 38: 408. [Google Scholar]
- 23. Sun F, Peng HX, Gao QF, et al. Preoperative circulating FPR and CCF score are promising biomarkers for predicting clinical outcome of stage II-III colorectal cancer patients. Cancer Manag Res 2018; 10: 2151–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li SQ, You XH, Sun F, et al. Albumin to fibrinogen ratio and fibrinogen to pre-albumin ratio are economical, simple and promising prognostic factors for solid malignancy. J Thorac Dis 2019; 11(Suppl. 15): S2036–S2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jennewein C, Tran N, Paulus P, et al. Novel aspects of fibrin(ogen) fragments during inflammation. Mol Med 2011; 17: 568–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Luyendyk JP, Schoenecker JG, Flick MJ. The multifaceted role of fibrinogen in tissue injury and inflammation. Blood 2019; 133: 511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kwaan HC, Lindholm PF. Fibrin and fibrinolysis in cancer. Semin Thromb Hemost 2019; 45: 413–422. [DOI] [PubMed] [Google Scholar]
- 28. Fanali G, di Masi A, Trezza V, et al. Human serum albumin: from bench to bedside. Mol Aspects Med 2012; 33: 209–290. [DOI] [PubMed] [Google Scholar]
- 29. Haskins IN, Baginsky M, Amdur RL, et al. Preoperative hypoalbuminemia is associated with worse outcomes in colon cancer patients. Clin Nutr 2017; 36: 1333–1338. [DOI] [PubMed] [Google Scholar]
- 30. Crusz SM, Balkwill FR. Inflammation and cancer: advances and new agents. Nat Rev Clin Oncol 2015; 12: 584–596. [DOI] [PubMed] [Google Scholar]
- 31. Brennan CA, Garrett WS. Gut microbiota, inflammation, and colorectal cancer. Annu Rev Microbiol 2016; 70: 395–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang J, Roberts TM, Shivdasani RA. Targeting PI3K signaling as a therapeutic approach for colorectal cancer. Gastroenterology 2011; 141: 50–61. [DOI] [PubMed] [Google Scholar]
- 33. Grossi V, Peserico A, Tezil T, et al. p38alpha MAPK pathway: a key factor in colorectal cancer therapy and chemoresistance. World J Gastroenterol 2014; 20: 9744–9758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Isidro RA, Appleyard CB. Colonic macrophage polarization in homeostasis, inflammation, and cancer. Am J Physiol Gastrointest Liver Physiol 2016; 311: G59–G73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yu T, Guo F, Yu Y, et al. Fusobacterium nucleatum promotes chemoresistance to colorectal cancer by modulating autophagy. Cell 2017; 170: 548–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359211022886 for The value of circulating fibrinogen-to-pre-albumin ratio in predicting survival and benefit from chemotherapy in colorectal cancer by Hou-Qun Ying, Fan Sun, Yu-Cui Liao, Dan Cai, Ying Yang and Xue-Xin Cheng in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-2-tam-10.1177_17588359211022886 for The value of circulating fibrinogen-to-pre-albumin ratio in predicting survival and benefit from chemotherapy in colorectal cancer by Hou-Qun Ying, Fan Sun, Yu-Cui Liao, Dan Cai, Ying Yang and Xue-Xin Cheng in Therapeutic Advances in Medical Oncology
Supplemental material, sj-pdf-3-tam-10.1177_17588359211022886 for The value of circulating fibrinogen-to-pre-albumin ratio in predicting survival and benefit from chemotherapy in colorectal cancer by Hou-Qun Ying, Fan Sun, Yu-Cui Liao, Dan Cai, Ying Yang and Xue-Xin Cheng in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tif-4-tam-10.1177_17588359211022886 for The value of circulating fibrinogen-to-pre-albumin ratio in predicting survival and benefit from chemotherapy in colorectal cancer by Hou-Qun Ying, Fan Sun, Yu-Cui Liao, Dan Cai, Ying Yang and Xue-Xin Cheng in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tif-5-tam-10.1177_17588359211022886 for The value of circulating fibrinogen-to-pre-albumin ratio in predicting survival and benefit from chemotherapy in colorectal cancer by Hou-Qun Ying, Fan Sun, Yu-Cui Liao, Dan Cai, Ying Yang and Xue-Xin Cheng in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tif-6-tam-10.1177_17588359211022886 for The value of circulating fibrinogen-to-pre-albumin ratio in predicting survival and benefit from chemotherapy in colorectal cancer by Hou-Qun Ying, Fan Sun, Yu-Cui Liao, Dan Cai, Ying Yang and Xue-Xin Cheng in Therapeutic Advances in Medical Oncology