Abstract
Background
Psoriasis is a disease that extends beyond the skin, with profound medical, social, and mental health implications. To our knowledge, no previous studies have specifically investigated the medical and socioeconomic characteristics of women with versus without psoriasis.
Objective
We investigated whether women with psoriasis differed from women without psoriasis with respect to comorbidities, socioeconomic status, healthcare consumption, and drug use, as well as how these characteristics differed according to psoriasis severity.
Methods
In this nationwide, register-based, cross-sectional study, data were collected from Danish registries from 1977 to 2017, linked at the individual level, and identified by International Classification of Diseases codes, prescription data, income and educational information, and contact with public health care services. Psoriasis was defined by either a hospital International Classification of Diseases code for psoriasis or calcipotriol prescription data. Psoriasis severity was stratified based on psoriasis treatment. Age-adjusted logistic regression models were used to estimate the odds ratios (ORs) of outcomes compared with those of women without psoriasis.
Results
A total of 77,143 women (3%) met the criteria for psoriasis. Psoriasis was significantly associated with all investigated outcomes. Women with psoriasis were less likely to have a high income (OR: 0.89; 95% confidence interval [CI], 0.87–0.91), more likely to visit their general practitioner more often (OR: 3.82; 95% CI, 3.70–3.95), and received pain medication more often (OR: 1.57; 95% CI, 1.52–1.62) compared with women without psoriasis.
Conclusion
Psoriasis was significantly associated with all investigated adverse medical and socioeconomic outcomes. Risk of outcomes increased with psoriasis severity. Our study highlights the need for a multidisciplinary collaboration to optimize medical care for women with (especially moderate and severe) psoriasis.
Keywords: Psoriasis, Women, Comorbidities, Socioeconomic status, Healthcare consumption, Drug use
Introduction
Psoriasis is a disease that extends beyond the skin, with profound medical, social, and mental health implications. It is a common chronic immune-mediated inflammatory disease, affecting 2% to 4% of the world’s population (Parisi et al., 2020). Compared with the general population, people with psoriasis are more likely to suffer from cardiometabolic and other immune-mediated inflammatory diseases, as well as mood disorders (Takeshita et al., 2017). Furthermore, they tend to be of lower socioeconomic status, with a subsequent higher utilization of health care services and drug use (Al Sawah et al., 2017, Thomsen et al., 2019).
Several epidemiologic studies have described the medical and socioeconomic characteristics of patients with psoriasis as a homogenous group (Andersen et al., 2019, Groot et al., 2019, Kim et al., 2015, Leisner et al., 2019, Skov et al., 2019, Thomsen et al., 2019, Tzur Bitan et al., 2019). However, few studies consider how patients with different psoriasis severity differ in their medical and socioeconomic characteristics (Egeberg et al., 2016b, Kimball et al., 2018, Mahé et al., 2017, Yeung et al., 2013). In addition, sex differences in psoriasis have been described (Egeberg et al., 2019, Hägg et al., 2017, Skov et al., 2019), and female sex hormones show a modulatory role in psoriasis pathogenesis (Kanda and Watanabe, 2005, Murase et al., 2005), with menarche, pregnancy, and menopause being particular susceptible periods. Apart from offering insight into the baseline risk profile of women with psoriasis according to their disease severity, the findings from our study could uncover potential unmet medical needs that call for a multidisciplinary collaboration to optimize medical care for women with psoriasis.
We aimed to investigate whether women with psoriasis differed from women without psoriasis with respect to comorbidities, socioeconomic status, healthcare consumption, and drug use, as well as how women with different levels of psoriasis severity (mild, moderate, severe) differed in their medical and socioeconomic characteristics.
Methods
Since 1968, high-quality health care data have been routinely collected electronically in Danish nationwide registries for administrative purposes. Each resident has a unique 10-digit identification number that links that person at the individual level to health care records across registries, with lifelong follow-up (Schmidt et al., 2019).
Study design and data sources
We conducted a nationwide, registry-based, cross-sectional study of all adult women (age ≥ 18 years) alive and residing in Denmark on December 31, 2017 (the index date) to assess comorbidities, socioeconomic status, healthcare consumption, and drug use among women with versus without psoriasis (Fig. 1). Data were collected from the Danish registries from 1977 to 2017, identified by International Classification of Diseases, version 8 (ICD-8) codes from 1977 until 1993 and ICD, version 10 (ICD-10) codes thereafter. ICD version 9 coding was never used in Denmark (Schmidt et al., 2014) with regard to prescriptions and contacts with public health care services at any point prior to the index date.
Fig. 1.
Schematic diagram of the cross-sectional study design. A snapshot of the entire female population in Denmark at the time of the index date, defining women with psoriasis (see Methods) and comparing them to women without psoriasis on medical and socioeconomic parameters, looking back until 1977, the year the Danish National Patient Registry was established. Comorbidities were collected between the index date and their individual’s18th birthday. Income was the mean gross annual income during a period of 5 years before the index date. Educational level was collected as highest attained education on the index date. Health care consumption was calculated as the number of hospitalizations, outpatient visits and visits at general practitioner in the year prior to the index date. Drug use was defined as number of redeemed prescriptions in the year before the index date.
The data sources were Danish nationwide registries linked at the individual level:
-
•
The Danish Civil Registration System (established in 1968): date of birth, sex, demographics, migration, and date of death.
-
•
The Danish National Patient Registry (1977): All hospital contacts, date and type of admissions, visits to outpatient clinics, procedures (including in-hospital administration of therapies such as biologics and cancer treatments) and final discharge diagnoses.
-
•
The Danish National Prescription Registry (1995): All redeemed prescriptions; since 2004 information on dose, pack-size, and indication (Wallach Kildemoes et al., 2011).
-
•
The Integrated Database for Labor Market Research (since 1980): civil status, highest obtained educational level, employment status, and income.
Outcomes
We examined immune-mediated, gastrointestinal, cardiometabolic, and psychiatric comorbidities that share inflammatory pathways and immunological mediators with psoriasis to expand the epidemiologic data available. Comorbidities were collected via ICD-10 codes and prescription data (Supplementary Table G). To avoid collecting juvenile comorbidities (that in some cases are no longer present in adulthood), only comorbidities between the patient’s 18th birthday and the index date were collected.
To uncover comorbidity burden in relation to reproductive age, women were stratified into age groups: 18 to 34 years (early reproductive age), 35 to 49 years (late reproductive age), and 50+ years (peri- and postmenopausal age). An age-standardized income index was calculated (0 = lowest income group, 4 = highest income group), based on the mean gross annual income during a period of 5 years prior to the index date. Women without psoriasis were the reference group of the income index. Educational level was categorized according to Statistics Denmark's education classification (Statistics Denmark, 2015).
Healthcare consumption was calculated as the number of events in the year prior to the index: hospitalizations (24-hour admissions), outpatient visits (regardless of specialty), and visits to the general practitioner (GP; in-office and telephone consultations). Drug use was defined as the number of redeemed prescriptions (using Anatomical Therapeutic Chemical Classification System-codes) without indications 1 year prior to the index (Supplementary Table G).
Study population
Psoriasis was defined using two validated methods: an hospital ICD code (ICD-10 L40.0 or L40.9, or ICD-8 696.19; Loft et al., 2019) or by the patient having claimed at least one prescription for the topical calcipotriol (Anatomical Therapeutic Chemical codes D05AX02 or D05AX52; Egeberg and Andersen, 2020) at any time prior to the index date. Women who did not meet the criteria for psoriasis were defined as women without psoriasis. A sensitivity analysis was performed in which psoriasis was defined by ICD code only to determine potential misclassifications using prescription data.
Psoriasis severity strata
Women with psoriasis were stratified according to psoriasis severity (Egeberg et al., 2016a) based on psoriasis treatment at any time prior to the index date. Psoriasis treatment included antipsoriatics with the indication “against psoriasis” and antipsoriatics administered at a department of dermatology to ensure treatment was not administered for a comorbidity:
-
•
Mild psoriasis: topical antipsoriatic drugs, phototherapy, or no treatment.
-
•
Moderate psoriasis: nonbiologic, systemic antipsoriatic drugs (acitretin, ciclosporin, methotrexate).
-
•
Severe psoriasis: Biologic antipsoriatics (adalimumab, brodalumab, certolizumab pegol, etanercept, guselkumab, infliximab, ixekizumab, secukinumab, ustekinumab).
Statistical analyses
Baseline characteristics (age at index date, age at psoriasis diagnosis, psoriasis duration) were described with median and interquartile range (IQR). Comorbidities and socioeconomic status were described with frequencies and percentages, drug use and healthcare consumption with numbers and percentages.
SAS statistical software (version 9.4; SAS Institute Inc., Cary, NC) and STATA software (version 13.0, StataCorp, College Station, TX) were used to calculate age-adjusted odds ratios (ORs) of outcomes by logistic regression. To test for surveillance bias (a nonrandom detection bias), we calculated age-adjusted OR for chlamydia, using highly specific prescription data: single-dose azithromycin, 1 g (Supplemental Table G). A likelihood-ratio test was used to test for linear trends between psoriasis severity strata.
Data on one or two individuals are shown as <3, due to data security requirements. The same statistical methods were performed in the sensitivity analysis (where psoriasis was defined by ICD code only). The 95% confidence intervals (CIs) were reported. Higher odds refers to a statistically significantly higher OR.
Results
At the index date, the total number of women alive and residing in Denmark was 2,585,484. Of these women, 77,143 (3%) met the criteria for psoriasis and the remaining 2,508,341 (97%) were considered women without psoriasis.
At the index date, women with psoriasis were nearly 10 years older than women without psoriasis, with a median age of 57.8 years (IQR, 43.4–70.3 years) and 48.0 years (IQR, 32.8–63.9 years), respectively. At the time of the first recorded psoriasis diagnosis, the median age was 46.0 years (IQR, 31.0–58.7 years) and the median psoriasis duration (calculated from the first psoriasis diagnosis to the index date) was 9.7 years (IQR, 5.4–17.5 years).
Stratified by psoriasis severity, 90.2% of women were defined as having mild, 8.7% moderate, and 1.1% severe psoriasis. Of these groups, women with severe psoriasis were the youngest at the index, with a median age of 50.6 years (IQR, 38.3–61.4 years), and the youngest at the first recorded psoriasis diagnosis, with a median age of 32.3 years (IQR, 21.3–45.3 years), with the longest psoriasis duration of 15.9 years (IQR, 9.7–21.9 years; Table 1).
Table 1.
Prevalence of comorbidities with age-adjusted ORs in women with versus without psoriasis, stratified for psoriasis severity.
| Women without psoriasis | All women with psoriasis | Mild psoriasis | Moderate psoriasis | Severe psoriasis | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Women at index 2,585,484 (100%) | 2,508,341 | (97.0) | 77,143 | (3.0) | OR | (95% CI) | 69,568 | (2.7) | OR | (95% CI) | 6,698 | (0.3) | OR | (95% CI) | 877 | (0.03) | OR | (95% CI) |
| Age at index, median (IQR) | 48.0 | (32.8–63.9) | 57.8 | (43.4–70.3) | 57.8 | (43.2–70.5) | 58.8 | (46.1–69.7) | 50.6 | (38.3–61.4) | ||||||||
| Age at first registered psoriasis diagnosis, y, median (IQR) | N/A | 46.0 | (31.0–58.7) | 46.2 | (31.1–59.0) | 45.7 | (31.2–57.7) | 32.3 | (21.3–45.3) | |||||||||
| Psoriasis duration, y, median (IQR) | N/A | 9.7 | (5.4–17.5) | 9.3 | (5.2–17.3) | 12.0 | (6.9–18.9) | 15.9 | (9.7–21.9) | |||||||||
| Rheumatoid arthritis, n (%) | 18,185 | (0.7) | 1,153 | (1.5) | 1.65 | (1.55–1.75) | 884 | (1.3) | 1.39 | (1.30–1.49) | 257 | (3.8) | 4.39 | (3.86–4.98) | 12 | (1.4) | 2.06 | (1.16–3.64) |
| 18–34 y | 609 | (0.0) | 25 | (0.0) | NS | NS | NS | |||||||||||
| 35–49 y | 2334 | (0.1) | 140 | (0.2) | NS | NS | NS | |||||||||||
| >50 y | 15,242 | (0.6) | 988 | (1.3) | NS | NS | NS | |||||||||||
| Axial spondylarthrosis, n (%) | 6,713 | (0.3) | 672 | (0.9) | 3.24 | (2.99–3.51) | 537 | (0.8) | 2.86 | (2.62–3.13) | 123 | (1.8) | 6.82 | (5.70–8.17) | 12 | (1.4) | 5.16 | (2.92–9.12) |
| 18–34 y | 1,008 | (0.04) | 82 | (0.1) | NS | NS | NS | |||||||||||
| 35–49 y | 2,453 | (0.1) | 207 | (0.3) | NS | NS | NS | |||||||||||
| >50 y | 3,252 | (0.1) | 383 | (0.5) | NS | NS | NS | |||||||||||
| Psoriatic arthritis, n (%) | 4,062 | (0.2) | 5,639 | (7.3) | 44.70 | (42.88–46.60) | 3,139 | (4.5) | 26.28 | (25.05–27.57) | 2,111 | (31.5) | 256.01 | (240.96–272.01) | 389 | (44.4) | 520.78 | (453.25–598.38) |
| 18–34 y | 280 | (0.01) | 424 | (0.5) | 220 | (0.3) | 148 | (2.2) | 56 | (6.4) | ||||||||
| 35–49 y | 932 | (0.04) | 1,256 | (1.6) | 680 | (1.0) | 466 | (7.0) | 110 | (12.5) | ||||||||
| >50 y | 2,850 | (0.1) | 3,959 | (5.1) | 2,239 | (3.2) | 1;497 | (22.3) | 223 | (25.4) | ||||||||
| Enthesitis, n (%) | 82,046 | (3.3) | 3,827 | (5.0) | 1.39 | (1.34–1.43) | 3,363 | (4.8) | 1.35 | (1.30–1.40) | 414 | (6.2) | 1.73 | (1.57–1.92) | 50 | (5.7) | 1.79 | (1.34–2.38) |
| 18–34 y | 7,902 | (0.3) | 234 | (0.3) | 208 | (0.3) | 19 | (0.3) | 7 | (0.8) | ||||||||
| 35–49 y | 21,432 | (0.9) | 787 | (1.0) | 693 | (1.0) | 80 | (1.2) | 14 | (1.6) | ||||||||
| >50 y | 52,712 | (2.1) | 2,806 | (3.6) | 2,462 | (3.5) | 315 | (4.7) | 29 | (3.3) | ||||||||
| Uveitis, n (%) | 26,293 | (1.0) | 1,457 | (1.9) | 1.47 | (1.39–1.55) | 1,270 | (1.8) | 1.42 | (1.34–1.50) | 163 | (2.4) | 1.90 | (1.63–2.22) | 24 | (2.7) | 2.82 | (1.87–4.23) |
| 18–34 y | 1,947 | (0.1) | 70 | (0.1) | NS | NS | NS | |||||||||||
| 35–49 y | 4,362 | (0.2) | 201 | (0.3) | NS | NS | NS | |||||||||||
| >50 y | 19,984 | (0.8) | 1,186 | (1.5) | NS | NS | NS | |||||||||||
| Ulcerative colitis, n (%) | 22,290 | (0.9) | 1,262 | (1.6) | 1.7 | (1.60–1.80) | 1,115 | (1.6) | 1.66 | (1.57–1.77) | 131 | (2.0) | 2.02 | (1.70–2.40) | 16 | (1.8) | 2.07 | (1.26–3.39) |
| 18–34 y | 3,315 | (0.1) | 115 | (0.1) | 103 | (0.1) | 12 | (0.2) | 0 | (0.0) | ||||||||
| 35–49 y | 6,140 | (0.2) | 312 | (0.4) | 279 | (0.4) | 28 | (0.4) | 5 | (0.6) | ||||||||
| >50 y | 12,835 | (0.5) | 835 | (1.1) | 733 | (1.1) | 91 | (1.4) | 11 | (1.3) | ||||||||
| Crohn’s disease, n (%) | 12,162 | (0.5) | 860 | (1.1) | 2.30 | (2.15–2.47) | 744 | (1.1) | 2.21 | (2.05–2.38) | 105 | (1.6) | 3.24 | (2.67–3.93) | 11 | (1.3) | 2.60 | (1.44–4.72) |
| 18–34 y | 2,738 | (0.1) | 134 | (0.2) | 117 | (0.2) | 13 | (0.2) | 4 | (0.5) | ||||||||
| 35–49 y | 3,800 | (0.2) | 218 | (0.3) | 186 | (0.3) | 28 | (0.4) | 4 | (0.5) | ||||||||
| >50 y | 5,624 | (0.2) | 508 | (0.7) | 441 | (0.6) | 64 | (1.0) | 3 | (0.3) | ||||||||
| Peptic ulcer, n (%) | 305,914 | (12.2) | 14,516 | (18.8) | 1.37 | (1.35–1.40) | 12,711 | (18.3) | 1.32 | (1.30–1.35) | 1,624 | (24.2) | 1.90 | (1.79–2.00) | 181 | (20.6) | 1.95 | (1.65–2.30) |
| 18–34 y | 31,050 | (1.2) | 810 | (1.0) | 709 | (1.0) | 80 | (1.2) | 21 | (2.4) | ||||||||
| 35–49 y | 61,365 | (2.4) | 2,239 | (2.9) | 1,936 | (2.8) | 264 | (3.9) | 39 | (4.4) | ||||||||
| >50 y | 213,499 | (8.5) | 11,467 | (14.9) | 10,066 | (14.5) | 1,280 | (19.1) | 121 | (13.8) | ||||||||
| Diabetes, n (%) | 140,841 | (5.6) | 7,440 | (9.6) | 1.46 | (1.43–1.50) | 6,449 | (9.3) | 1.39 | (1.36–1.43) | 886 | (13.2) | 2.09 | (1.95–2.25) | 105 | (12.0) | 2.42 | (1.97–3.00) |
| 18–34 y | 12,600 | (0.5) | 332 | (0.4) | 291 | (0.4) | 30 | (0.4) | 11 | (1.3) | ||||||||
| 35–49 y | 24,329 | (1.0) | 980 | (1.3) | 841 | (1.2) | 119 | (1.8) | 20 | (2.3) | ||||||||
| >50 y | 103,912 | (4.1) | 6,128 | (7.9) | 5,317 | (7.6) | 737 | (11.0) | 74 | (8.4) | ||||||||
| Hypertension, n (%) | 300,154 | (12.0) | 14,897 | (19.3) | 1.29 | (1.27–1.32) | 13,318 | (19.1) | 1.27 | (1.24–1.29) | 1,431 | (21.4) | 1.51 | (1.42–1.62) | 148 | (16.9) | 1.92 | (1.58–2.34) |
| 18–34 y | 1,494 | (0.06) | 40 | (0.1) | NS | NS | NS | |||||||||||
| 35–49 y | 15,937 | (0.6) | 652 | (0.8) | NS | NS | NS | |||||||||||
| >50 y | 282,723 | (11.3) | 14,205 | (18.4) | NS | NS | NS | |||||||||||
| Myocardial infarction, n (%) | 27,821 | (1.1) | 1,542 | (2.0) | 1.37 | (1.30–1.44) | 1,375 | (2.0) | 1.34 | (1.27–1.42) | 153 | (2.3) | 1.63 | (1.39–1.92) | 14 | (1.6) | 1.88 | (1.10–3.21) |
| 18–34 y | 83 | (0.00) | 3 | (0.0) | NS | NS | NS | |||||||||||
| 35–49 y | 1,220 | (0.05) | 52 | (0.1) | NS | NS | NS | |||||||||||
| >50 y | 2,518 | (0.1) | 1,487 | (1.9) | NS | NS | NS | |||||||||||
| Hypercholesterolemia, n (%) | 368,730 | (14.7) | 19,181 | (24.9) | 1.43 | (1.41–1.46) | 17,015 | (24.5) | 1.38 | (1.36–1.41) | 1,975 | (29.5) | 1.91 | (1.80–2.03) | 191 | (21.8) | 2.11 | (1.76–2.52) |
| 18–34 y | 2,663 | (0.1) | 90 | (0.1) | NS | NS | NS | |||||||||||
| 35–49 y | 17,725 | (0.7) | 768 | (1.0) | NS | NS | NS | |||||||||||
| >50 y | 348,342 | (13.9) | 18,323 | (23.8) | NS | NS | NS | |||||||||||
| Depression, n (%) | 35,597 | (1.4) | 1,848 | (2.4) | 1.41 | (1.35–1.48) | 1,599 | (2.3) | 1.35 | (1.28–1.42) | 216 | (3.2) | 1.91 | (1.66–2.18) | 33 | (3.8) | 2.82 | (1.99–4.00) |
| 18–34 y | 3,131 | (0.1) | 107 | (0.1) | NS | NS | NS | |||||||||||
| 35–49 y | 7,787 | (0.3) | 321 | (0.4) | NS | NS | NS | |||||||||||
| >50 y | 24,679 | (1.0) | 1,420 | (1.8) | NS | NS | NS | |||||||||||
| Anxiety, n (%) | 19,485 | (0.8) | 783 | (1.0) | 1.24 | (1.16–1.33) | 674 | (1.0) | 1.18 | (1.10–1.28) | 92 | (1.4) | 1.68 | (1.36–2.06) | 17 | (1.9) | 2.51 | (1.56–4.06) |
| 18–34 y | 19,485 | (0.8) | 783 | (1.0) | NS | NS | NS | |||||||||||
| 35–49 y | 3,531 | (0.1) | 88 | (0.1) | NS | NS | NS | |||||||||||
| >50 y | 5,916 | (0.2) | 187 | (0.2) | NS | NS | NS | |||||||||||
CI, confidence interval; IQR, interquartile range; OR, odds ratio; N/A, not available; NS, not shown.
Mild psoriasis: Antipsoriatic topicals, phototherapy or no treatment.
Moderate psoriasis: Nonbiologic systemic antipsoriatic drugs (acitretin, ciclosporin, methotrexate) with the indication “against psoriasis”.
Severe psoriasis: Biologic antipsoriatics (adalimumab, brodalumab, certolizumab pegol, etanercept, guselkumab, infliximab, ixekizumab, secukinumab, ustekinumab) administered at a department of dermatology.
See corresponding Fig. 2.
Comorbidities
After age adjustment, women with psoriasis had higher odds of all investigated comorbidities compared with women without psoriasis. Women with psoriasis had 65% higher odds for seropositive rheumatoid arthritis (OR: 1.65; 95% CI, 1.55–1.75), more than three times higher odds for axial spondylarthritis (OR: 3.24; 95% CI, 2.99–3.51), 2.3 times higher odds for Crohn’s disease (OR: 2.30; 95% CI, 2.15–2.47), and 44.7 times higher odds for psoriatic arthritis (OR: 44.70; 95% CI, 42.88–46.60) compared with women without psoriasis (Fig. 2). The latter OR was outlying in Fig. 2 and therefore excluded from the figure.
Fig. 2.
Age-adjusted odds ratios (ORs) for comorbidities in women with psoriasis compared to women without psoriasis. Error bars are 95% confidence intervals. Mild psoriasis: antipsoriatic topicals, phototherapy, or no treatment. Moderate psoriasis: nonbiologic systemic antipsoriatic drugs (acitretin, ciclosporin, methotrexate) with the indication “against psoriasis.” Severe psoriasis: biologic antipsoriatics (adalimumab, brodalumab, certolizumab pegol, etanercept, guselkumab, infliximab, ixekizumab, secukinumab, ustekinumab) administered at a department of dermatology. Psoriatic arthritis is left out of this figure due to high and outlying OR; see corresponding Table 1.
A stepwise increase in OR point estimates for comorbidities was observed across mild, moderate, and severe psoriasis, except for rheumatoid arthritis, axial spondylarthritis, and Crohn’s disease, where women with moderate psoriasis had higher ORs for disease (Fig. 2; Table 1). Stratified by age, there was an increase in comorbid disease prevalence with age in both women with and without psoriasis. Still, women with moderate and severe psoriasis in the age groups of 18 to 34 years (early reproductive age) and 35 to 49 years (late reproductive age) showed higher a prevalence compared with women without psoriasis.
Socioeconomic status
Compared with women without psoriasis, those with psoriasis had lower odds of being in the highest income group (OR: 0.89; 95% CI, 0.87–0.91), having more higher education (up to 19–23 years of schooling; OR: 0.88; 95% CI, 0.86–0.91), and were more frequently divorced (OR: 1.13; 95% CI, 1.10–1.15). Women with moderate psoriasis had lowest odds of being in the highest income group (OR: 0.64; 95% CI, 0.60–0.70), and women with severe psoriasis had the lowest odds of having more higher education (OR: 0.55; 95% CI, 0.39–0.76) and the highest odds for being divorced (OR: 1.25; 95% CI, 1.03–1.52) compared with those without psoriasis (Table 2).
Table 2.
Prevalence and age-adjusted ORs for income, educational level, and marital status at index in women with versus without psoriasis (background population).
| Women without psoriasis | All women with psoriasis | Mild psoriasis | Moderate psoriasis | Severe psoriasis | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Women at index 2,585,484 (100%) | 2,508,341 | (97.0) | 77,143 | (3.0) | OR | (95% CI) | 69,568 | (2.7) | OR | (95% CI) | 6,698 | (0.3) | OR | (95% CI) | 877 | (0.03) | OR | (95% CI) |
| Income group,* n (%) | ||||||||||||||||||
| Lowest (0) | 512,784 | (20.4) | 4,314 | (5.6) | 0.21 | (0.21–0.22) | 4,025 | (5.8) | 0.22 | (0.22–0.23) | 234 | (3.5) | 0.12 | (0.10–0.13) | 55 | (6.3) | 0.23 | (0.17–0.31) |
| Below average (1) | 500,142 | (19.9) | 16,951 | (22.0) | 0.86 | (0.84–0.87) | 15,300 | (22.0) | 0.87 | (0.85–0.89) | 1,486 | (22.2) | 0.76 | (0.70–0.81) | 165 | (18.8) | 0.72 | (0.59–0.88) |
| Average (2) | 497,415 | (19.8) | 19,682 | (25.5) | 1 (ref) | 17,498 | (25.2) | 1 (ref) | 1,956 | (29.2) | 1 (ref) | 228 | (26.0) | 1 (ref) | ||||
| Above average (3) | 498,510 | (19.9) | 18,588 | (24.1) | 0.94 | (0.92–0.96) | 16,588 | (23.8) | 0.95 | (0.93–0.97) | 1,748 | (26.1) | 0.89 | (0.84–0.95) | 252 | (28.7) | 1.10 | (0.92–1.32) |
| Highest (4) | 499,490 | (19.9) | 17,608 | (22.8) | 0.89 | (0.87–0.91) | 16,157 | (23.2) | 0.92 | (0.90–0.94) | 1,274 | (19.0) | 0.64 | (0.60–0.70) | 177 | (20.2) | 0.77 | (0.64–0.94) |
| Highest educational level, n (%) | ||||||||||||||||||
| Primary school (10–11 y) | 621,255 | (24.8) | 21,074 | (27.3) | 0.93 | (0.91–0.95) | 18,669 | (26.8) | 0.90 | (0.88–0.92) | 2,154 | (32.2) | 1.27 | (0.19–1.36) | 251 | (28.6) | 1.14 | (0.94–1.37) |
| High school and vocational school (14 y) | 883,940 | (35.2) | 30,683 | (39.8) | 1.07 | (1.05–1.09) | 27,528 | (39.6) | 1.05 | (1.03–1.07) | 2,786 | (41.6) | 1.30 | (1.22–1.39) | 369 | (42.1) | 1.17 | (0.99–1.39) |
| Short higher education (17 y) | 584,703 | (23.3) | 18,654 | (24.2) | 1 (ref) | 17,056 | (24.5) | 1 (ref) | 1,390 | (20.8) | 1 (ref) | 208 | (23.7) | 1 (ref) | ||||
| Long higher education (19–23 y) | 220,683 | (8.8) | 5,609 | (7.3) | 0.88 | (0.86–0.91) | 5,289 | (7.6) | 0.91 | (0.89–0.94) | 277 | (4.1) | 0.59 | (0.52–0.67) | 43 | (4.9) | 0.55 | (0.39–0.76) |
| Missing | 197,760 | (7.9) | 1,123 | (1.5) | ||||||||||||||
| Marital status at index, n (%) | ||||||||||||||||||
| Married | 1,072,860 | (42.8) | 37,594 | (48.7) | 1 (ref) | 33,900 | (48.7) | 1 (ref) | 3,297 | (49.2) | 1 (ref) | 397 | (45.3) | 1 (ref) | ||||
| Divorced | 295,420 | (11.8) | 12,230 | (15.9) | 1.13 | (1.10–1.15) | 10,947 | (15.7) | 1.12 | (1.09–1.14) | 1,145 | (17.1) | 1.20 | (1.12–1.28) | 138 | (15.7) | 1.25 | (1.03–1.52) |
| Widow | 203,756 | (8.1) | 8,703 | (11.3) | 0.80 | (0.78–0.82) | 7,876 | (11.3) | 0.80 | (0.78–0.82) | 774 | (11.6) | 0.77 | (0.71–0.84) | 53 | (6.0) | 0.66 | (0.48–0.89) |
| Unmarried | 934,261 | (37.2) | 18,581 | (24.1) | 0.83 | (0.82–0.85) | 16,810 | (24.2) | 0.84 | (0.82–0.85) | 1,482 | (22.1) | 0.79 | (0.73–0.84) | 289 | (33.0) | 0.89 | (0.74–1.06) |
| Missing | 2,079 | (0,1) | 35 | (0,0) | ||||||||||||||
CI, confidence interval; IQR, interquartile range; OR, odds ratio; ref, reference
*Income group already indexed by age, so ORs (unadjusted for age) were calculated.
Mild psoriasis: Antipsoriatic topicals, phototherapy or no treatment.
Moderate psoriasis: Nonbiologic systemic antipsoriatic drugs (acitretin, ciclosporin, methotrexate) with the indication “against psoriasis”.
Severe psoriasis: Biologic antipsoriatics (adalimumab, brodalumab, certolizumab pegol, etanercept, guselkumab, infliximab, ixekizumab, secukinumab, ustekinumab) administered at a department of dermatology.
See corresponding Fig. 2.
Healthcare consumption
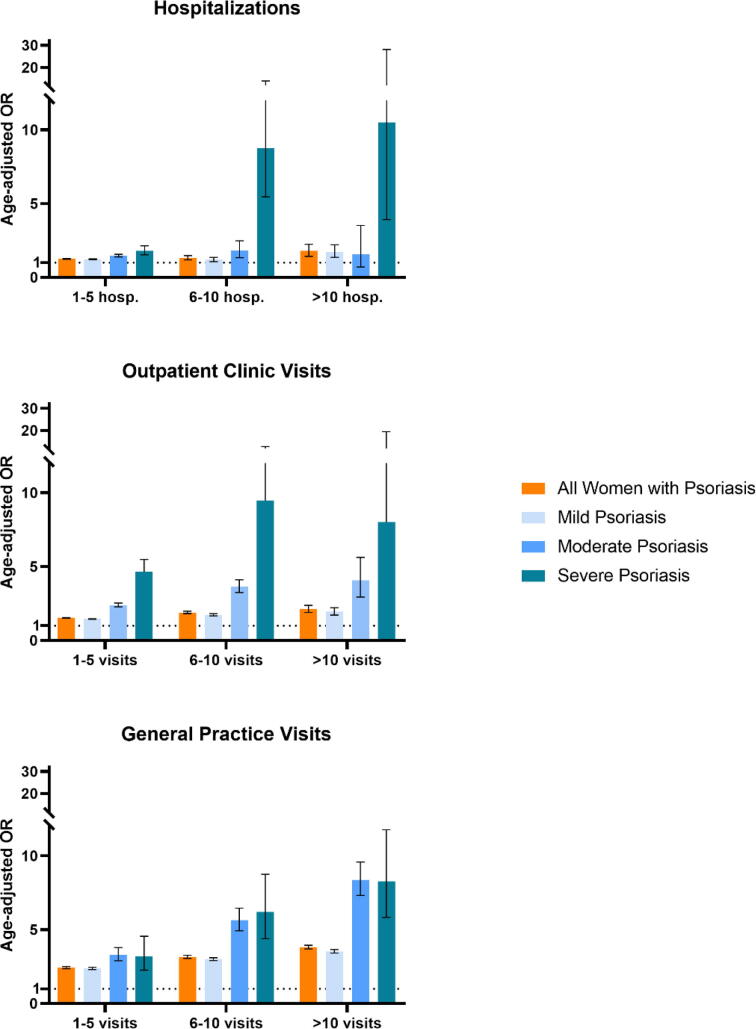
One year prior to the index date, women with psoriasis had higher odds for frequently being hospitalized (>10 hospitalizations; OR, 1.79; 95% CI, 1.43–2.25), visiting outpatient clinics (>10 visits; OR: 2.12; 95% CI, 1.89–2.38), and visiting their GP (>10 visits; OR: 3.82; 95% CI, 3.70–3.95) compared with those without psoriasis (Table 3). A stepwise increase in OR point estimates for hospitalizations and outpatient visits was observed across psoriasis severity, and women with moderate psoriasis had the highest odds of visiting their GP (Fig. 3; Table 3).
Table 3.
Prevalence and age-adjusted ORs for hospitalizations, visits at outpatient clinics, and general practice 1 year prior to index in women with versus without psoriasis.
| Women without psoriasis | All women with psoriasis | Mild psoriasis | Moderate psoriasis | Severe psoriasis | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Women at index 2,585,484 (100%) | 2,508,341 | (97.0) | 77,143 | (3.0) | OR | (95% CI) | 69,568 | (2.7) | OR | (95% CI) | 6,698 | (0.3) | OR | (95% CI) | 877 | (0.03) | OR | (95% CI) |
| Hospitalizations, n (%) | ||||||||||||||||||
| 0 hospitalizations | 2,208,659 | (88.1) | 64,772 | (84.0) | 1 (ref) | 58,628 | (84.3) | 1 (ref) | 5,455 | (81.4) | 1 (ref) | 689 | (78.6) | 1 (ref) | ||||
| 1–5 hospitalizations | 291,994 | (11.6) | 11,950 | (15.5) | 1.26 | (1.23–1.28) | 10,588 | (15.2) | 1.23 | (1.21–1.26) | 1,196 | (17.9) | 1.48 | (1.39–1.57) | 166 | (18.9) | 1.81 | (1.53–2.15) |
| 6–10 hospitalizations | 6,482 | (0.3) | 342 | (0.4) | 1.32 | (1.19–1.48) | 283 | (0.4) | 1.21 | (1.07–1.36) | 41 | (0.6) | 1.83 | (1.34–2.49) | 18 | (2.1) | 8.75 | (5.46–14.03) |
| >10 hospitalizations | 1,206 | (0.0) | 79 | (0.1) | 1.79 | (1.43–2.25) | 69 | (0.1) | 1.73 | (1.36–2.21) | 6 | (0.1) | 1.58 | (0.71–3.53) | 4 | (0.5) | 10.50 | (3.92–28.13) |
| Outpatient clinic visits, n (%) | ||||||||||||||||||
| 0 visits | 1,380,594 | (55.0) | 31,402 | (40.7) | 1 (ref) | 29,197 | (42.0) | 1 (ref) | 2,022 | (30.2) | 1 (ref) | 183 | (20.9) | 1 (ref) | ||||
| 1–5 visits | 1,072,622 | (42.8) | 42,726 | (55.4) | 1.54 | (1.51–1.56) | 37,793 | (54.3) | 1.46 | (1.44–1.48) | 4,302 | (64.2) | 2.40 | (2.28–2.54) | 631 | (71.9) | 4.65 | (3.93–5.49) |
| 6–10 visits | 50,032 | (2.0) | 2,708 | (3.5) | 1.90 | (1.83–1.98) | 2,314 | (3.3) | 1.74 | (1.67–1.82) | 336 | (5.0) | 3.66 | (3.25–4.11) | 58 | (6.6) | 9.48 | (7.02–12.79) |
| >10 visits | 5,093 | (0.2) | 307 | (0.4) | 2.12 | (1.89–2.38) | 264 | (0.4) | 1.96 | (1.73–2.22) | 38 | (0.6) | 4.07 | (2.95–5.63) | 5 | (0.6) | 8.02 | (3.29–19.54) |
| General practice visits, n (%) | ||||||||||||||||||
| 0 visits | 445,390 | (17.8) | 4,634 | (6.0) | 1 (ref) | 4,361 | (6.3) | 1 (ref) | 237 | (3.5) | 1 (ref) | 36 | (4.1) | 1 (ref) | ||||
| 1–5 visits | 983,966 | (39.2) | 26,437 | (34.3) | 2.43 | (2.36–2.51) | 24,346 | (5.0) | 2.38 | (2.30–2.46) | 1,839 | (27.5) | 3.32 | (2.90–3.80) | 252 | (28.7) | 3.21 | (2.27–4.56) |
| 6–10 visits | 635,624 | (25.3) | 23,760 | (30.8) | 3.16 | (3.06–3.27) | 21,299 | (30.6) | 3.01 | (2.91–3.11) | 2,152 | (32.1) | 5.64 | (4.93–6.46) | 309 | (35.2) | 6.20 | (4.39–8.76) |
| >10 visits | 443,361 | (17.7) | 22,312 | (28.9) | 3.82 | (3.70–3.95) | 19,562 | (28.1) | 3.54 | (3.42–3.66) | 2,470 | (36.9) | 8.38 | (7.32–9.59) | 280 | (31.9) | 8.29 | (5.84–11.78) |
CI, confidence interval; OR, odds ratio; ref, reference.
Hospitalizations: Whole day admission.
Outpatient clinic visits: Visits to outpatient clinic, regardless of specialty.
General practice visits: In-office consultations and electronic consultations.
Mild psoriasis: Antipsoriatic topicals, phototherapy or no treatment.
Moderate psoriasis: Nonbiologic systemic antipsoriatic drugs (acitretin, ciclosporin, methotrexate) with the indication “against psoriasis.”
Severe psoriasis: Biologic antipsoriatics (adalimumab, brodalumab, certolizumab pegol, etanercept, guselkumab, infliximab, ixekizumab, secukinumab, ustekinumab) administered at a department of dermatology.
See corresponding Fig. 3.
Fig. 3.
Age-adjusted odds ratios (ORs) for hospitalizations and visits at outpatient clinics and general practice 1 year before index in women with psoriasis compared to women without psoriasis. Error bars are 95% confidence intervals. Hospitalizations: whole day admission. Outpatient clinic visits: visits to outpatient clinic, regardless of specialty. General practice visits: in-office and electronic consultations. Mild psoriasis: antipsoriatic topicals, phototherapy, or no treatment. Moderate psoriasis: nonbiologic systemic antipsoriatic drugs (acitretin, ciclosporin, methotrexate) with the indication “against psoriasis.” Severe psoriasis: niologic antipsoriatics (adalimumab, brodalumab, certolizumab pegol, etanercept, guselkumab, infliximab, ixekizumab, secukinumab, ustekinumab) administered at a department of dermatology. See corresponding Table 3.
Drug use
Compared with women without psoriasis, those with psoriasis had, expectedly, higher odds of redeeming all psoriasis therapies and regularly redeeming prescription pain medication (6–10 redeemed prescriptions; OR, 1.57; 95% CI, 1.52–1.62) and antidepressants (6–10 redeemed prescriptions; OR: 1.39; 95% CI, 1.33–1.44; Table 4). Overall, women with severe psoriasis had the highest ORs of all investigated drugs (Fig. 4; Table 4). Women with psoriasis had higher odds of redeeming all investigated pain medications (opioids, acetylsalicylic acid, nonsteroidal antiinflammatory drugs and paracetamol; Fig. 4; Supplementary Table A).
Table 4.
Prevalence and age-adjusted ORs for redeemed prescriptions and administered therapy at a dermatological department 1 year prior to index in women with versus without psoriasis.
| Women without psoriasis | All women with psoriasis | Mild psoriasis | Moderate psoriasis | Severe psoriasis | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Women at index 2,585,484 (100%) | 2,508,341 | (97.0) | 77,143 | (3.0) | OR | (95% CI) | 69,568 | (2.7) | OR | (95% CI) | 6,698 | (0.3) | OR | (95% CI) | 877 | (0.03) | OR | (95% CI) |
| Topical antipsoriatics, n (%) | ||||||||||||||||||
| 0 redeemed | 2,281,814 | (91.0) | 47,432 | (61.5) | 1 (ref) | 43,850 | (63.0) | 1 (ref) | 3,170 | (47.3) | 1 (ref) | 412 | (47.0) | 1 (ref) | ||||
| 1–5 redeemed | 223,101 | (8.9) | 27,545 | (35.7) | 5.51 | (5.43–5.60) | 24,073 | (34.6) | 5.20 | (5.12–5.29) | 3,062 | (45.7) | 9.17 | (8.72–9.64) | 410 | (46.8) | 10.41 | (9.07–11.95) |
| 6–10 redeemed | 2,914 | (0.1) | 1,747 | (2.3) | 24.88 | (23.42–26.44) | 1,365 | (2.0) | 20.74 | (19.42–22.15) | 343 | (5.1) | 71.07 | (63.12–80.01) | 39 | (4.4) | 78.59 | (56.32–109.67) |
| >10 redeemed | 512 | (0.02) | 419 | (0.5) | 33.49 | (29.38–38.17) | 280 | (0.4) | 23.68 | (20.43–27.45) | 123 | (1.8) | 141.01 | (115.27–172.51) | 16 | (1.8) | 186.43 | (111.95–310.45) |
| Hospital-administered phototherapy, n (%) | ||||||||||||||||||
| 0 redeemed | 2,507,788 | (100.0) | 76,939 | (99.7) | 1 (ref) | 69,462 | (99.8) | 1 (ref) | 6,618 | (98.8) | 859 | (97.9) | ||||||
| 1–5 redeemed | 151 | (0.01) | 27 | (0.03) | 6.64 | (4.39–10.03) | NS | 2.74 | (1.44–5.21) | NS | NS | |||||||
| 6–10 redeemed | 97 | (0.00) | 20 | (0.03) | 7.36 | (4.53–11.96) | NS | 4.86 | (2.66–8.89) | NS | NS | |||||||
| >10 redeemed | 305 | (0.01) | 157 | (0.2) | 16.38 | (13.47–19.91) | NS | 9.51 | (7.45–12.14) | NS | NS | |||||||
| Systemic antipsoriatic drugs, n (%) | ||||||||||||||||||
| 0 redeemed | 2,494,868 | (99.5) | 72,979 | (94.6) | 1 (ref) | NS | NS | NS | ||||||||||
| 1–5 redeemed | 13,110 | (0.5) | 3,893 | (5.0) | 8.36 | (8.06–8.68) | NS | NS | NS | |||||||||
| 6–10 redeemed | 338 | (0.01) | 261 | (0.3) | 24.02 | (20.40–28.28) | NS | NS | NS | |||||||||
| >10 redeemed | 25 | (0.00) | 10 | (0.0) | 12.58 | (6.01–26.34) | NS | NS | NS | |||||||||
| Biologic antipsoriatic drugs, n (%) | ||||||||||||||||||
| 0 redeemed | 2,508,209 | (100.0) | 76,355 | (99.0) | 1 (ref) | 69,568 | (100.0) | 6,686 | (99.8) | 101 | (11.5) | |||||||
| 1–5 redeemed | 104 | (0.00) | 654 | (0.8) | 232.55 | (188.84–286.37) | 0 | (0.00) | NS | NS | ||||||||
| 6–10 redeemed | 22 | (0.00) | 124 | (0.2) | 193.97 | (122.95–306.00) | 0 | (0.00) | NS | NS | ||||||||
| >10 redeemed | 6 | (0.00) | 10 | (0.01) | 60.99 | (21.78–170.81) | 0 | (0.00) | NS | NS | ||||||||
| Pain medications, n (%) | ||||||||||||||||||
| 0 redeemed | 1,738,191 | (69.3) | 42,918 | (55.6) | 1 (ref) | 39,573 | (56.9) | 1 (ref) | 2,910 | (43.4) | 1 (ref) | 435 | (49.6) | 1 (ref) | ||||
| 1–5 redeemed | 604,335 | (24.1) | 25,227 | (32.7) | 1.44 | (1.42–1.47) | 22,316 | (32.1) | 1.38 | (1.35–1.40) | 2,593 | (38.7) | 2.25 | (2.13–2.38) | 318 | (36.3) | 2.23 | (1.92–2.58) |
| 6–10 redeemed | 94,417 | (3.8) | 5,090 | (6.6) | 1.57 | (1.52–1.62) | 4,326 | (6.2) | 1.43 | (1.38–1.48) | 694 | (10.4) | 3.35 | (3.07–3.66) | 70 | (8.0) | 3.35 | (2.57–4.37) |
| >10 redeemed | 71,398 | (2.8) | 3,908 | (5.1) | 1.53 | (1.48–1.58) | 3,353 | (4.8) | 1.40 | (1.35–1.46) | 501 | (7.5) | 3.09 | (2.80–3.42) | 54 | (6.2) | 3.47 | (2.58–4.67) |
| Antidepressants, n (%) | ||||||||||||||||||
| 0 redeemed | 2,263,672 | (90.2) | 66,316 | (86.0) | 1 (ref) | 60,046 | (86.3) | 1 (ref) | 5,555 | (82.9) | 1 (ref) | 715 | (81.5) | 1 (ref) | ||||
| 1–5 redeemed | 179,378 | (7.2) | 7719 | (10.0) | 1.28 | (1.25–1.31) | 6796 | (9.8) | 1.24 | (1.21–1.28) | 810 | (12.1) | 1.58 | (1.47–1.71) | 113 | (12.9) | 1.98 | (1.62–2.42) |
| 6–10 redeemed | 46,329 | (1.8) | 2208 | (2.9) | 1.39 | (1.33–1.44) | 1929 | (2.8) | 1.34 | (1.28–1.40) | 240 | (3.6) | 1.77 | (1.56–2.02) | 39 | (4.4) | 2.64 | (1.91–3.66) |
| >10 redeemed | 18,962 | (0.8) | 900 | (1.2) | 1.13 | (1.06–1.21) | 797 | (1.1) | 1.11 | (1.03–1.19) | 93 | (1.4) | 1.35 | (1.10–1.66) | 10 | (1.1) | 1.64 | (0.87–3.07) |
CI, confidence interval; OR, odds ratio; NS, not shown; ref, reference.
Topical antipsoriatics: Corticosteroids (dermatological preparations) antipsoriatics (for topical use).
Hospital-administered phototherapy: Ultraviolet light therapies.
Systemic antipsoriatic drugs: Methotrexate, ciclosporin, acitretin.
Biologic antipsoriatic drugs: Adalimumab, brodalumab, certolizumab pegol, etanercept, guselkumab, infliximab, ixekizumab, secukinumab, ustekinumab.
Pain medications: Paracetamol (acetaminophen), nonsteroidal antiinflammatory drugs, acetylsalicylic acid, opioids.
Antidepressants: ATC-code N06A (nonselective monoamine reuptake inhibitors, selective serotonin reuptake inhibitors, monoamine oxidase inhibitors, nonselective monoamine oxidase A inhibitors, other). Detailed information of ATC-codes in Supplemental Table 1.
Mild psoriasis: Antipsoriatic topicals, phototherapy or no treatment.
Moderate psoriasis: Nonbiologic systemic antipsoriatic drugs (acitretin, ciclosporin, methotrexate) with the indication “against psoriasis”.
Severe psoriasis: Biologic antipsoriatics (adalimumab, brodalumab, certolizumab pegol, etanercept, guselkumab, infliximab, ixekizumab, secukinumab, ustekinumab) administered at a department of dermatology.
See corresponding Fig. 4.
Fig. 4.
Age-adjusted odds ratios (ORs) for redeemed prescriptions for antidepressants, all pain medications, opioids, and paracetamol (acetaminophen) 1 year before index in women with psoriasis compared with women without psoriasis. Error bars are 95% confidence intervals. Antidepressants (ACT-code N06A): nonselective monoamine reuptake inhibitors, selective serotonin reuptake inhibitors, monoamine oxidase inhibitors, nonselective monoamine oxidase A inhibitors, other antidepressants. Pain medications: opioids (ATC-code N02A), acetylsalicylic acid (ATC-code N02BA), nonsteroidal antiinflammatory drugs (ATC-code M01A), paracetamol (acetaminophen; ATC-code N02BE). Mild psoriasis: antipsoriatic topicals, phototherapy, or no treatment. Moderate psoriasis: nonbiologic systemic antipsoriatic drugs (acitretin, ciclosporin, methotrexate) with the indication “against psoriasis.” Severe psoriasis: biologic antipsoriatics (adalimumab, brodalumab, certolizumab pegol, etanercept, guselkumab, infliximab, ixekizumab, secukinumab, ustekinumab) administered at a department of dermatology. Acetylsalicylic acid and nonsteroidal antiinflammatory drugs are left out of this figure due to micro data. See corresponding Table 4 and Supplementary Table A.
Sensitivity analysis
The sensitivity analysis (defining psoriasis by ICD code only) showed that, compared with women without psoriasis, women with a ICD code for psoriasis had even higher odds of the investigated comorbidities, even lower odds of having a high income and long higher education, and higher odds of being divorced than women with psoriasis in the main analysis (defined by ICD code and psoriasis prescription data). Additionally, women with an ICD code for psoriasis had even higher odds of healthcare consumption and drug use than women with psoriasis in the main analysis (Supplementary Tables B-F).
Surveillance bias
To examine whether the observed associations in our study could be explained by surveillance bias, we tested the association between psoriasis and chlamydia (a negative outcome that is likely to be diagnosed equally in both women with and without psoriasis with no plausible mechanistic link to psoriasis; European Network of Centres for Pharmacoepidemiology and Pharmacovigilance, 2020). Psoriasis was significantly associated with chlamydia compared with women without psoriasis (OR: 1.39; 95% CI, 1.35–1.43), which suggests some degree of surveillance bias.
Trend test
Increasing psoriasis severity was significantly associated with increasing ORs for the following outcomes (p for trend <.001): enthesitis, uveitis, diabetes, hypertension, myocardial infarction, depression, anxiety, hospitalizations, and use of antidepressants and acetylsalicylic acid.
Discussion
In this nationwide, cross-sectional study, women with psoriasis had a higher prevalence of comorbidities, lower socioeconomic status, higher healthcare consumption, and higher drug use compared with women without psoriasis, with significant dose–response associations between increasing psoriasis severity and certain outcomes. Thus, our findings are in line with and expand upon the results of previous studies (Ahlehoff et al., 2011, Blegvad et al., 2019, Egeberg et al., 2016b, Takeshita et al., 2017).
Comorbidities
A recent Danish study demonstrated that immune-mediated inflammatory diseases are frequent in patients with psoriasis and are mostly diagnosed before psoriasis (Andersen et al., 2019). The design of our cross-sectional study did not account for whether the comorbidity came before or after the psoriasis diagnosis, which would have shed light on the causal relationship between psoriasis and comorbidities. Danish patients with psoriasis reported limited access to family planning and pregnancy information in a recent survey (Schreiber et al., 2020). These findings, together with the higher prevalence of comorbidities among women in the early and late reproductive age groups in women with moderate and severe psoriasis found in our study, stress the need for close family planning counseling by physicians.
Socioeconomic status
A high comorbidity burden has a substantial impact on the socioeconomic status of patients with psoriasis (Al Sawah et al., 2017, Duvetorp et al., 2019, Han et al., 2011). A recent Danish study found that patients with psoriasis had lower employment rates than controls (Thomsen et al., 2019). In our study, women with moderate psoriasis showed the lowest odds for being in the highest income group, which might be due to unemployment, underemployment, or missed work days due to sick days. However, we are unable to draw conclusions from these results because we did not specify employment status and public transfer income when indexing the income groups.
Previous studies have found that biologic treatment improved work productivity and reduced sick days and healthcare consumption (Boggs et al., 2014, Vender et al., 2012); yet, our study was not designed to assess treatment effects. Furthermore, the sensitivity analysis showed that women with moderate psoriasis had the highest ORs for most comorbidities. Together with lower income, more visits to a GP, and high drug use, this collectively suggests that women with moderate psoriasis perhaps are undertreated, with unmet medical needs across several medical specialties.
Healthcare consumption
We speculate that the high ORs for outpatient visits observed among women with severe psoriasis could be explained by antipsoriatic biologic treatments being administered by dermatological outpatient clinics in Denmark. Women with psoriasis were almost four times more likely to visit their GP > 10 times 1 year prior to the index date than women without psoriasis. This illustrates that women with psoriasis are more likely to seek medical care and possibly also more likely to get tested for and diagnosed with other diseases (also known as surveillance bias; Haut and Pronovost, 2011), which in turn could lead to higher healthcare consumption and subsequent drug use. Our findings confirm a recent Danish study that found multimorbidity and socioeconomic status to be a predictor of high healthcare consumption (Frølich et al., 2019).
Drug use
In line with a previous study, women with psoriasis had a higher use of antidepressants (Han et al., 2011). The antidepressants included in our study had indications for depression, anxiety, and neuropathic and nonmalignant pain. The observed significant dose–response association between psoriasis severity and depression, anxiety, and their use of antidepressants could indicate that women with moderate-to-severe psoriasis are particularly mentally vulnerable.
Women with moderate and severe psoriasis had a two- to three-fold higher use of opioids than women without psoriasis. This may partly be explained by the their higher ORs of rheumatoid arthritis, axial spondylarthritis, and psoriatic arthritis, although opioids are not the first drug of choice for pain management and are contraindicated for long-term use (Geenen et al., 2018, Gossec et al., 2016, The Capital Region of Denmark, 2020, Van Der Heijde et al., 2017).
Lastly, persistent opioid use has been associated with low socioeconomic status, higher divorce frequency, and higher somatic and psychiatric comorbidity burden (Mellbye et al., 2014, Svendsen et al., 2014).
Sensitivity analysis
Defined by hospital-diagnosed psoriasis (ICD code only), women with psoriasis showed slightly worse ORs for medical and socioeconomic parameters compared with the women with psoriasis in the main analysis (defined by either prescription data or ICD code).
Severity strata
In many studies, moderate and severe psoriasis is considered one entity, often defined by receiving systemic treatment (i.e., nonbiologic or biologic antipsoriatic treatment; Al Sawah et al., 2017, Bröms et al., 2018, Egeberg et al., 2017, Yang et al., 2011). In contrast, the present study’s baseline characteristics of women with moderate psoriasis differed markedly from women with severe psoriasis. When compared, the latter group was 8.2 years younger at index, 13.2 years younger at first psoriasis diagnosis, and had had psoriasis for 3 years longer. This heterogenicity argues that they are two distinct groups, which also becomes evident in the study results. Until recently, the lack of a clear international consensus definition of mild, moderate, and severe psoriasis muddles the interpretation, comparability, and generalizability of clinical and epidemiological psoriasis research.
Strengths and limitations
Important strengths of this cross-sectional study include the high quality and completeness of the Danish nationwide registries (Schmidt et al., 2019) and the use of validated psoriasis definitions (Egeberg and Andersen, 2020, Loft et al., 2019).
The International Psoriasis Council recently released a consensus statement on the recategorization of psoriasis severity, rejecting mild, moderate, and severe categories in favor of two categories: candidates for topical therapy and candidates for systemic therapy, assessed by clinical criteria (Strober et al., 2020).
A limitation of our study is that the registries used do not provide access to clinical measurements, such as body surface area, Psoriasis Area Severity Index, or location of involvement, to confirm psoriasis severity. Nonetheless, receiving antipsoriatic systemic therapy and biologics, could be considered as (and work as a surrogate marker for) failure of topical therapy, which is how we defined moderate and severe psoriasis in this study. In summary, the distinct differences between our population of women with moderate and severe psoriasis challenges the newly established consensus on psoriasis severity; however, to make future epidemiological research uniform, we propose that the redefined categorizations should be followed.
Danish psoriasis treatment guidelines recommend that patients be treated with and fail on antipsoriatic topicals, phototherapy, methotrexate, acitretin or ciclosporin before they can be considered for biologics (Danish Dermatological Society, 2019, Danish Health Authority, 2016). Collectively, this may overestimate the number of women with moderate psoriasis and underestimate the number with clinically severe psoriasis and consequently skew our findings.
We tested for surveillance bias (nonrandom detection bias) by calculating age-adjusted OR for the highly specific prescription data for chlamydia treatment. The magnitude of the OR suggested surveillance bias; however, it cannot alone explain the study's findings. We speculate that psoriasis itself plays a causative role.
Conclusion
Both the main and sensitivity analysis showed that women with psoriasis were more likely to have the investigated comorbidities; they were of lower socioeconomic status, with higher healthcare consumption, and higher use of prescription pain medication and antidepressants, which may partially be explained by a possible surveillance bias. Stratified by psoriasis severity, our data showed a stepwise increase across mild, moderate, and severe psoriasis in the OR point estimates for most outcomes and with significant dose–response associations for certain outcomes. Our study expands on the body of existing evidence on psoriasis and its profound medical and socioeconomical consequences, as well as calls for changes to be made in our clinical approach with a need for multidisciplinary collaboration to optimize medical care for women with (especially moderate and severe) psoriasis.
Conflicts of interest
Cæcilie Bachdal Johansen has received research funding from and attended advisory board meetings for UCB. Alexander Egeberg has received research funding from Pfizer, Eli Lilly, Novartis, AbbVie, Janssen Pharmaceuticals, the Danish National Psoriasis Foundation, the Simon Spies Foundation, and the Kgl Hofbundtmager Aage Bang Foundation and has received honoraria as consultant and/or speaker from AbbVie, Almirall, LEO Pharma, Samsung Bioepis Co., Ltd., Pfizer, Eli Lilly and Company, Novartis, Galderma, Dermavant, UCB, Mylan, Bristol-Myers Squibb, and Janssen Pharmaceuticals. Espen Jimenez-Solem has received research funding from Eli Lilly and Janssen. Ida Vittrup has received salary from a research grant from Regeneron Pharmaceuticals. Lone Skov has been a paid speaker for AbbVie, Eli Lilly, Novartis, Sanofi, and LEO Pharma and has been a consultant or has served on advisory boards with AbbVie, Janssen Cilag, Novartis, Eli Lilly, LEO Pharma, UCB, Almirall, and Sanofi; she has served as an investigator for AbbVie, Sanofi, Janssen Cilag, Boehringer Ingelheim, AstraZenica, Eli Lilly, Novartis, Pfizer, Regeneron, and LEO Pharma and has received research and educational grants from Novartis, Sanofi, Janssen Cilag, and LEO Pharma. Simon Francis Thomsen is or recently was a speaker and/or advisor for and/or has received research funding from Abbvie, AstraZeneca, Celgene, Eli Lilly, Janssen, LEO Pharma, Novartis, Pierre Fabre, Roche, Sanofi, and UCB.
Funding
This work was supported by a research grant from UCB. UCB contributed with interpretation of finalized study results; however, it had no access to raw data and did not participate in data collection or analysis.
Study approval
The author(s) confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijwd.2020.11.004.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Ahlehoff O., Gislason G.H., Charlot M., Jørgensen C.H., Lindhardsen J., Olesen J.B. Psoriasis is associated with clinically significant cardiovascular risk: A Danish nationwide cohort study. J Intern Med. 2011;270(2):147–157. doi: 10.1111/j.1365-2796.2010.02310.x. [DOI] [PubMed] [Google Scholar]
- Andersen Y.M.F., Wu J., Thyssen J.P., Egeberg A. Chronologic order of appearance of immune-mediated inflammatory diseases relative to diagnosis of psoriasis. J Am Acad Dermatol. 2019;81(6):1283–1291. doi: 10.1016/j.jaad.2019.04.033. [DOI] [PubMed] [Google Scholar]
- Blegvad C., Nybo Andersen A.M., Adam A., Zachariae C., Skov L. Psoriasis as a predictor of cardiometabolic comorbidity in women: A study based on the Danish national birth cohort. Acta Derm Venereol. 2019;99(3):274–278. doi: 10.2340/00015555-3090. [DOI] [PubMed] [Google Scholar]
- Boggs R.L., Kárpáti S., Li W., Williams T., Pedersen R., Mallbris L. Employment is maintained and sick days decreased in psoriasis/psoriatic arthritis patients with etanercept treatment. BMC Dermatol. 2014;14(1):2–7. doi: 10.1186/1471-5945-14-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bröms G., Haerskjold A., Granath F., Kieler H., Pedersen L., Berglind I.A. Effect of maternal psoriasis on pregnancy and birth outcomes: A population-based cohort study from Denmark and Sweden. Acta Derm Venereol. 2018;98(8):728–734. doi: 10.2340/00015555-2923. [DOI] [PubMed] [Google Scholar]
- Danish Dermatological Society. Guidelines for treatment of psoriasis with 2nd generation immunomodulatory therapy [Internet]. 2019 [cited xxx]. Available from: https://dds.nu/wp-content/uploads/2019/12/Guideline-DDS-2.-generations-immunomodulatorisk-behandling-final-oktober-2019.pdf.
- Danish Health Authority. National clinical guideline for psoriasis. 2016.
- Duvetorp A., Østergaard M., Skov L., Seifert O., Tveit K.S., Danielsen K. Quality of life and contact with healthcare systems among patients with psoriasis and psoriatic arthritis: Results from the NORdic PAtient survey of Psoriasis and Psoriatic arthritis (NORPAPP) Arch Dermatol Res. 2019;311(5):351–360. doi: 10.1007/s00403-019-01906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeberg A., Andersen Y.M.F. Use of topical calcipotriol for identification of patients with psoriasis in administrative healthcare data—A validation study. J Eur Acad Dermatology Venereol. 2020;34(2):e90–e91. doi: 10.1111/jdv.15991. [DOI] [PubMed] [Google Scholar]
- Egeberg A., Andersen Y.M.F., Thyssen J.P. Prevalence and characteristics of psoriasis in Denmark: Findings from the Danish skin cohort. BMJ Open. 2019;9(3):1–7. doi: 10.1136/bmjopen-2018-028116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeberg A., Mallbris L., Gislason G.H., Skov L., Hansen P.R. Risk of multiple sclerosis in patients with psoriasis: A Danish nationwide cohort study. J Invest Dermatol. 2016;136(1):93–98. doi: 10.1038/JID.2015.350. [DOI] [PubMed] [Google Scholar]
- Egeberg A., Mallbris L., Warren R.B., Bachelez H., Gislason G.H., Hansen P.R. Association between psoriasis and inflammatory bowel disease: A Danish nationwide cohort study. Br J Dermatol. 2016;175(3):487–492. doi: 10.1111/bjd.14528. [DOI] [PubMed] [Google Scholar]
- Egeberg A., Thyssen J.P., Jensen P., Gislason G.H., Skov L. Risk of myocardial infarction in patients with psoriasis and psoriatic arthritis: A nationwide cohort study. Acta Derm Venereol. 2017;97(7):819–824. doi: 10.2340/00015555-2657. [DOI] [PubMed] [Google Scholar]
- European Network of Centres for Pharmacoepidemiology and Pharmacovigilance. ENCePP guide on methodological standards in pharmacoepidemiology [Internet]. 2020 [cited 2020 Aug 18]. Available from: http://www.encepp.eu/standards_and_guidances/methodologicalGuide4_2_2_5.shtml.
- Frølich A., Ghith N., Schiøtz M., Jacobsen R., Stockmarr A. Multimorbidity, healthcare utilization and socioeconomic status: A register-based study in Denmark. PLoS One. 2019;14(8):1–15. doi: 10.1371/journal.pone.0214183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geenen R., Overman C.L., Christensen R., Åsenlöf P., Capela S., Huisinga K.L. EULAR recommendations for the health professional’s approach to pain management in inflammatory arthritis and osteoarthritis. Ann Rheum Dis. 2018;77(6):797–807. doi: 10.1136/annrheumdis-2017-212662. [DOI] [PubMed] [Google Scholar]
- Gossec L., Smolen J.S., Ramiro S., De Wit M., Cutolo M., Dougados M. European League Against Rheumatism (EULAR) recommendations for the management of psoriatic arthritis with pharmacological therapies: 2015 update. Ann Rheum Dis. 2016;75(3):499–510. doi: 10.1136/annrheumdis-2015-208337. [DOI] [PubMed] [Google Scholar]
- Groot J., Nybo Andersen A.M., Adam A., Tind Nielsen T.E., Blegvad C., Skov L. Associations between maternal socioeconomic position and psoriasis: A cohort study among the offspring of the Danish National Birth Cohort. Br J Dermatol. 2019;180(2):321–328. doi: 10.1111/bjd.17091. [DOI] [PubMed] [Google Scholar]
- Hägg D., Sundström A., Eriksson M., Schmitt-Egenolf M. Severity of psoriasis differs between men and women: A study of the clinical outcome measure psoriasis area and severity index (PASI) in 5438 Swedish register patients. Am J Clin Dermatol. 2017;18(4):583–590. doi: 10.1007/s40257-017-0274-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han C., Lofland J.H., Zhao N., Schenkel B. Increased prevalence of psychiatric disorders and health care-associated costs among patients with moderate-to-severe psoriasis. J Drugs Dermatol. 2011;10(8):843–850. [PubMed] [Google Scholar]
- Haut E.R., Pronovost P.J. Surveillance bias in outcomes reporting. JAMA. 2011;305(23):2462–2463. doi: 10.1001/jama.2011.822. [DOI] [PubMed] [Google Scholar]
- Van Der Heijde D., Ramiro S., Landewé R., Baraliakos X., Van Den Bosch F., Sepriano A. 2016 update of the ASAS-EULAR management recommendations for axial spondyloarthritis. Ann Rheum Dis. 2017;76(6):978–991. doi: 10.1136/annrheumdis-2016-210770. [DOI] [PubMed] [Google Scholar]
- Schreiber K., Johansen C., Jensen U.F., Egeberg A., Thomsen S.F., Hansen A.L. PARE0024 awareness about family planning and pregnancy expectation among patients with chronic inflammatory disease of the skin or joints. Ann Rheum Dis. 2020;79(Suppl. 1) doi: 10.1007/s40744-021-00348-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda N., Watanabe S. Regulatory roles of sex hormones in cutaneous biology and immunology. J Dermatol Sci. 2005;38(1):1–7. doi: 10.1016/j.jdermsci.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Kim G.E., Seidler E., Kimball A.B. A measure of chronic quality of life predicts socioeconomic and medical outcomes in psoriasis patients. J Eur Acad Dermatology Venereol. 2015;29(2):249–254. doi: 10.1111/jdv.12503. [DOI] [PubMed] [Google Scholar]
- Kimball A.B., Augustin M., Gordon K.B., Krueger G.G., Pariser D., Fakharzadeh S. Correlation of psoriasis activity with socioeconomic status: Cross-sectional analysis of patients enrolled in the Psoriasis Longitudinal Assessment and Registry (PSOLAR) Br J Dermatol. 2018;179(4):984–986. doi: 10.1111/bjd.16737. [DOI] [PubMed] [Google Scholar]
- Leisner M.Z., Riis J.L., Schwartz S., Iversen L., Østergaard S.D., Olsen M.S. Psoriasis and risk of mental disorders in Denmark. JAMA Dermatol. 2019;155(6):745–747. doi: 10.1001/jamadermatol.2019.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loft N.D., Andersen C.H., Halling-Overgaard A.S., Thyssen J.P., Skov L., Egeberg A. Validation of psoriasis diagnoses in the Danish national patient register. Acta Derm Venereol. 2019;99(11):1037–1038. doi: 10.2340/00015555-3278. [DOI] [PubMed] [Google Scholar]
- Mahé E., Beauchet A., Reguiai Z., Maccari F., Ruer-Mulard M., Chaby G. Socioeconomic inequalities and severity of plaque psoriasis at a first consultation in dermatology centers. Acta Derm Venereol. 2017;97(5):632–638. doi: 10.2340/00015555-2625. [DOI] [PubMed] [Google Scholar]
- Mellbye A., Karlstad O., Skurtveit S., Borchgrevink P.C., Fredheim O.M.S. Co-morbidity in persistent opioid users with chronic non-malignant pain in Norway. Eur J Pain. 2014;18(8):1083–1093. doi: 10.1002/j.1532-2149.2014.00449.x. [DOI] [PubMed] [Google Scholar]
- Murase J.E., Chan K.K., Garite T.J., Cooper D.M., Weinstein G.D. Hormonal effect on psoriasis in pregnancy and post partum. Arch Dermatol. 2005;141(5):601–606. doi: 10.1001/archderm.141.5.601. [DOI] [PubMed] [Google Scholar]
- Parisi R., Iskandar I.Y.K., Kontopantelis E., Augustin M., Griffiths C.E.M., Ashcroft D.M. National, regional, and worldwide epidemiology of psoriasis: systematic analysis and modelling study. BMJ. 2020;369 doi: 10.1136/bmj.m1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Sawah S., Foster S.A., Goldblum O.M., Malatestinic W.N., Zhu B., Shi N. Healthcare costs in psoriasis and psoriasis sub-groups over time following psoriasis diagnosis. J Med Econ. 2017;20(9):982–990. doi: 10.1080/13696998.2017.1345749. [DOI] [PubMed] [Google Scholar]
- Schmidt M., Pedersen L., Sørensen H.T. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29:541–549. doi: 10.1007/s10654-014-9930-3. [DOI] [PubMed] [Google Scholar]
- Schmidt M., Schmidt S.A.J., Adelborg K., Sundbøll J., Laugesen K., Ehrenstein V. The Danish health care system and epidemiological research: From health care contacts to database records. Clin Epidemiol. 2019;11:563–591. doi: 10.2147/CLEP.S179083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skov L., Thomsen S.F., Kristensen L.E., Dodge R., Hedegaard M.S., Kjellberg J. Cause-specific mortality in patients with psoriasis and psoriatic arthritis. Br J Dermatol. 2019;180(1):100–107. doi: 10.1111/bjd.16919. [DOI] [PubMed] [Google Scholar]
- Statistics Denmark. DISCED-15 [Internet]. 2015 [cited xxx]. Available from: http://www.dst.dk/extranet/uddannelsesklassifikation/DISCED-15.pdf.
- Strober B., Ryan C., van de Kerkhof P., van der Walt J., Kimball A.B., Barker J. Recategorization of psoriasis severity: Delphi consensus from the International Psoriasis Council. J Am Acad Dermatol. 2020;82(1):117–122. doi: 10.1016/j.jaad.2019.08.026. [DOI] [PubMed] [Google Scholar]
- Svendsen K., Fredheim O.M., Romundstad P., Borchgrevink P.C., Skurtveit S. Persistent opioid use and socio-economic factors: A population-based study in Norway. Acta Anaesthesiol Scand. 2014;58(4):437–445. doi: 10.1111/aas.12281. [DOI] [PubMed] [Google Scholar]
- Takeshita J., Grewal S., Langan S.M., Mehta N.N., Ogdie A., Van Voorhees A.S. Psoriasis and comorbid diseases part I. Epidemiology. J Am Acad Dermatol. 2017;76(3):377–390. doi: 10.1016/j.jaad.2016.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Capital Region of Denmark. Pharmacological treatment of non-malignant pain in rheumatic diseases [Internet]. 2020 [cited 2020 Aug 18]. Available from: https://vip.regionh.dk/VIP/Admin/GUI.nsf/Desktop.html.
- Thomsen S.F., Skov L., Dodge R., Hedegaard M.S., Kjellberg J. Socioeconomic costs and health inequalities from psoriasis: A cohort study. Dermatology. 2019;235(5):372–379. doi: 10.1159/000499924. [DOI] [PubMed] [Google Scholar]
- Tzur Bitan D., Krieger I., Comaneshter D., Cohen A.D., Feingold D. The association between the socioeconomic status and anxiety–depression comorbidity in patients with psoriasis: A nationwide population-based study. J Eur Acad Dermatology Venereol. 2019;33(8):1555–1561. doi: 10.1111/jdv.15651. [DOI] [PubMed] [Google Scholar]
- Vender R., Lynde C., Ho V., Chau D., Poulin-Costello M. Work productivity and healthcare resource utilization outcomes for patients on etanercept for moderate-to-severe plaque psoriasis: Results from a 1-year, multicentre, open-label, single-arm study in a clinical setting. Appl Health Econ Health Policy. 2012;10(5):343–353. doi: 10.1007/BF03261868. [DOI] [PubMed] [Google Scholar]
- Wallach Kildemoes H., Toft Sørensen H., Hallas J. The Danish national prescription registry. Scand J Public Health. 2011;39(7):38–41. doi: 10.1177/1403494810394717. [DOI] [PubMed] [Google Scholar]
- Yang Y.W., Chen C.S., Chen Y.H., Lin H.C. Psoriasis and pregnancy outcomes: A nationwide population-based study. J Am Acad Dermatol. 2011;64(1):71–77. doi: 10.1016/j.jaad.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Yeung H., Takeshita J., Mehta N.N., Kimmel S.E., Ogdie A., Margolis D.J. Psoriasis severity and the prevalence of major medical comorbidity: A population-based study. JAMA Dermatol. 2013;149(10):1173–1179. doi: 10.1001/jamadermatol.2013.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.