Abstract
AIM
To evaluate the atherogenic indices and the relationship with visual acuity and bilateral sequential involvement in patients with non-arteritic ischemic optic neuropathy (NAION).
METHODS
A total of 65 patients with NAION and 48 age-sex matched healthy individuals were included in this retrospective study. The demographic characteristics and laboratory findings of the patients and control subjects were obtained from the electronic medical records. The atherogenic indices were calculated using the lipid parameters. The association between visual acuity, bilateral sequential involvement, and atherogenic indices was investigated.
RESULTS
The mean age was 63.8±12.5y in the NAION group and 64.7±10.1y in control group (P=0.707). Although there were no significant differences in terms of total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-c) between two groups (P=0.089, 0.091), all the non-traditional serum lipid ratios were significantly higher in NAION group (P<0.05). In the NAION subgroup analysis, with visual acuity≤20/200 had higher TC/high-density lipoprotein cholesterol (HDL-c), LDL-c/HDL-c, and non-HDL-c/HDL-c values than the patients in the NAION group with visual acuity >20/200 (P=0.032, 0.025, 0.032, respectively). The values for the atherogenic indices were higher in NAION patients with bilateral sequential involvement in comparison to those with unilateral involvement (P=0.271, 0.127, 0.197, 0.128, 0.127, respectively).
CONCLUSION
The study reveals a relationship between NAION and the non-traditional lipid ratios. Atherogenic indices may predict the visual loss severity and second eye involvement in patients with NAION.
Keywords: atherosclerosis, atherogenic index, lipid profile, non-arteritic ischemic optic neuropathy, second eye involvement
INTRODUCTION
Non-arteritic ischemic optic neuropathy (NAION), which is characterized by mono or binocular visual impairment with a sudden and painless onset, is the most common cause of optic neuropathy among the elderly[1]–[2]. Although the incidence of NAION is relatively high, the exact pathological mechanism has not been clearly explained yet. The generally agreed pathogenesis of NAION depends on the acute ischemia due to insufficient circulation of the optic nerve head[2]. Many systemic conditions, including arteriosclerosis, hypertension (HT), diabetes mellitus (DM), hyperlipidemia, hyperhomocysteinemia, relative nocturnal hypotension, and crowded optic disc (“disk at risk”), have been suggested for NAION etiopathogenesis[3]–[6].
The ischemic disorders affecting all the tissues in the body, including the eye and optic nerve, are commonly due to atherosclerosis. Most cases of NAION develop spontaneously in the presence of one or more underlying atherosclerotic risk factors, such as dyslipidemia, smoking, DM, and arterial HT, which are also the risk factors for cardiovascular and cerebral ischemic events. In one series, hypercholesterolemia was present in no less than 40% of the NAION patients, while the incidences of HT, DM, and ischemic heart diseases were significantly higher in those patients than in the general population[7]. It was also shown that the risk of ischemic stroke increased in NAION patients compared to those without NAION[8]–[9]. The study results suggest that NAION can be associated with systemic vascular diseases. The relationships between serum lipid parameters and NAION have been evaluated previously, and increased lipid levels were more often observed in patients with NAION[10]–[11]. Recently, the use of atherogenic indices as markers that have been assessed in various conditions has been suggested to be more discriminative than a single conventional serum lipid panel for predicting the risk of atherosclerotic events[12]–[13]. Considering that 24% to 48% of the NAION patients exhibit second eye involvement within 5-11y of disease onset, it is very important to protect the vision of the eye not affected initially[14]. It has been shown that diabetics and patients who suffered significant vision loss during the first event are at increased risk for bilateral sequential involvement[4].
We hypothesized that higher atherogenic indices could predict the involvement of the second eye and the severity of the visual loss. As far as we know, our study is the first to evaluate the atherogenic indices in NAION patients.
SUBJECTS AND METHODS
Ethical Approval
This retrospective and cross-sectional study was conducted at the Department of Ophthalmology in Ondokuz Mayıs University, Samsun, Turkey. The study protocol was approved by the Institutional Ethics Committee of the Ondokuz Mayıs University and adhered to the tenets of the Declaration of Helsinki. The informed consent was obtained from the subjects.
Sixty-five consecutive NAION patients diagnosed between 2017 and 2020 and 48 healthy volunteers who were matched in age and gender to the patients and had similar vulnerability for common chronic and systemic disorders like DM and HT were enrolled in the study. The control group subjects were recruited among the patients presented to the outpatient clinics of the Department of Ophthalmology for routine ophthalmological examination and volunteered to participate in the study. The demographics (age and gender) and clinical data (ocular findings, systemic diseases, blood parameters, and drug use history) were retrieved from the electronic medical files. A thorough ophthalmological examination that consisted of refraction assessment (KR8900, Topcon, Japan), visual acuity (Snellen chart), slit-lamp biomicroscopy, Goldman applanation tonometry, and dilated fundus examination were performed in all patients and controls. The NAION patients were classified into two groups according to the degree of visual acuity impairment: equal to or better than 20/200 or worse than 20/200 (legal blindness). The criteria for NAION diagnosis included unilateral and sudden loss of vision without pain, the swelling of the optic disc, a relative afferent defect of the pupillae, a defect of the visual field consistent with NAION, and the lack of clinical signs suggesting any other diseases. Unilateral NAION involvement and bilateral sequential involvement were also noted. Blood samples were obtained from the unilateral involvement group during the first attack and from the bilateral sequential involvement group during the second attack.
Blood samples after 12-hour fasting were obtained from the patients and controls. The levels of total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-c), and low-density lipoprotein cholesterol (LDL-c) were assessed via a chemical analyzer system (Roche/Hitachi Cobas C 701 system, Mannheim, Germany). Furthermore, the ratios of TC/HDL-c, TG/HDL-c, LDL-c/HDL-c, non-HDL-c/HDL-c, and the values of non-HDL-c were calculated. The formula used to calculate the non-HDL-c was TC minus HDL-c. The TC/HDL-c ratio was considered to be the index of insulin resistance, and the TG/HDL-c ratio, LDL-c/HDL-c ratio, non-HDL-c/HDL-c ratio, and non-HDL-c were considered to be the atherogenic indices[15]–[19]. The exclusion criteria consisted of acute systemic infection, inflammatory and autoimmune disorders, history of malignancy, renal and hepatic failure, alcohol abuse, retinal disease, any surgery within the three months prior to the study, and use of medical therapies which might affect blood parameters, i.e., corticosteroid, anti-hyperlipidemics, etc.
Statistical Analysis
Statistical Package for the Social Sciences (SPSS) software (V21.0 for Windows; SPSS Inc., Chicago, IL, USA) was used for the statistical analyses. All quantitative variables are expressed as the mean±standard deviation. The normality of distribution was assessed using the Kolmogorov-Smirnov test. The qualitative data with and without normal distribution were compared using the Student's t and Mann-Whitney U tests, respectively. To determine the cut-off value and to quantify the parameter accuracy, a receiver operating characteristic curve (ROC) analysis was performed. A statistical significance was considered with a P-value less than 0.05.
RESULTS
The study was completed with 113 participants, and 65 (58%) of the subjects were female. The mean age of the NAION patients and controls were 63.8±12.5y (range 40-86y) and 64.7±10.1y (range 41-90y), respectively (P=0.707). No statistical differences in the distribution of gender and systemic disorders were present between the groups (P>0.05). Forty (62%) patients in the NAION group had unilateral involvement, and 25 (38%) of them had bilateral sequential involvement. The demographics and clinical features of the participants are shown in Table 1.
Table 1. Demographic characteristics and systemic disorders within the groups.
| Parameters | NAION group (n=65) | Control group (n=48) | P |
| Age mean±SD (y) | 63.8±12.5 | 64.7±10.1 | 0.707 |
| Male/female ratio | 38/27 | 27/21 | 0.814 |
| Hypertension (n) | 12 | 8 | 0.462 |
| Diabetes mellitus (n) | 14 | 11 | 0.581 |
| Cardiovascular disease | 12 | 11 | 0.798 |
| Unilateral/bilateral sequential involvement (n) | 40/25 | - | - |
NAION: Non arteritic ischemic optic neuropathy.
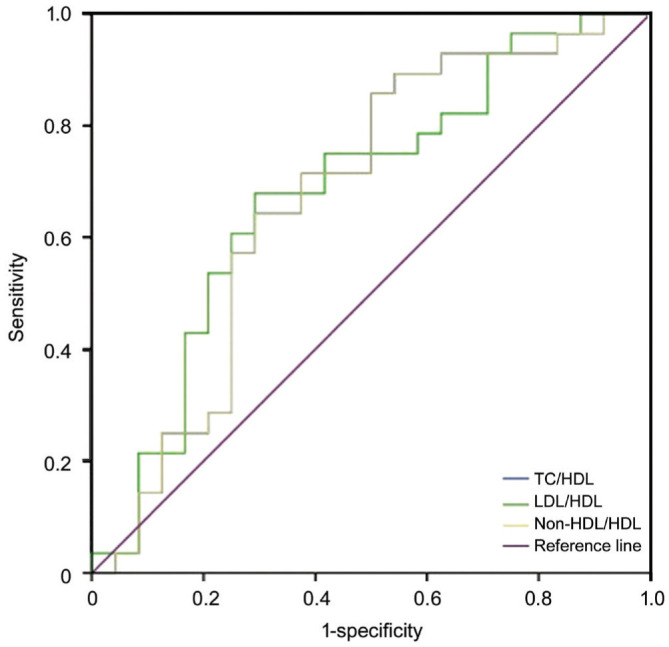
The median values of TG and non-HDL-c in the NAION group were significantly higher, while the HDL-c values were significantly lower compared to those of the controls (P<0.05). Although not statistically significant, the median values of TC and LDL-c in the NAION patients tended to be higher than the controls (P=0.089 and P=0.091, respectively). The non-conventional serum lipid ratios, including TC/HDL-c, TG/HDL-c, LDL-c/HDL-c, and non-HDL-c/HDL-c in the NAION patients, were significantly higher than the controls as shown in Table 2. In the subgroup analysis, the NAION patients with visual acuity ≤20/200 had higher TC/HDL-c, LDL-c/HDL-c, and non-HDL-c/HDL-c values than the NAION patients with visual acuity >20/200 (P=0.032, 0.025, 0.032, respectively, Table 3). No such significant differences in conventional serum lipids were found between the two subgroups (P>0.05). ROC curve analysis was performed to assess the diagnostic values of the atherogenic indices for differentiating the NAION group from the control group and to predict the visual acuity in the NAION patients. The optimal cut-off values of the atherogenic indices in the NAION subgroups are shown in Tables 4 and 5, and the areas under the ROC curve are displayed in Figures 1 and 2.
Table 2. Serum lipid profiles and lipid ratios of the participants.
| Parameters | NAION group | Control group | P |
| TC (mg/dL) | 196.5 (124.5-338.9) | 184.7 (114.2-259.3) | 0.089 |
| TG (mg/dL) | 171.6 (58.0-466.0) | 120.5 (55.2-241.8) | <0.001 |
| LDL-c (mg/dL) | 116.6 (49.1-212.1) | 106.7 (55.0-152.3) | 0.091 |
| HDL-c (mg/dL) | 45.5 (26.3-91.0) | 54.3 (27.8-95.8) | 0.001 |
| Non-HDL-c (mg/dL) | 151.0 (86.9-280.1) | 130.3 (85.1-186.0) | 0.002 |
| TC/HDL-c | 4.4 (2.1-7.1) | 3.6 (2.1-6.2) | <0.001 |
| TG/HDL-c | 4.1 (0.9-9.2) | 2.5 (0.6-6.0) | <0.001 |
| LDL-c/HDL-c | 2.6 (0.9-5.3) | 2.1 (0.9-4.2) | <0.001 |
| Non-HDL-c/HDL-c | 3.4 (1.1-6.1) | 2.6 (1.1-5.2) | <0.001 |
NAION: Non arteritic ischemic optic neuropathy; TC: Total cholesterol; TG: Triglyceride; LDL-c: Low density lipoprotein; HDL-c: High density lipoprotein.
mean (range)
Table 3. Serum lipid profiles and lipid ratios of the NAION group according to the visual acuity.
| Visual acuity | ≤20/200 | >20/200 | P |
| TC (mg/dL) | 199.9 (125.8-338.9) | 192.4 (124.5-280.3) | 0.521 |
| TG (mg/dL) | 173.2 (69.8-339.6) | 166.6 (58.0-466.0) | 0.287 |
| LDL-c (mg/dL) | 121.6 (59.3-212.1) | 109.7 (49.1-190.0) | 0.215 |
| HDL-c (mg/dL) | 43.5 (28.0-66.3) | 49.4 (26.3-91.0) | 0.199 |
| Non-HDL-c (mg/dL) | 156.3 (86.9-280.1) | 142.9 (90.7-240.9) | 0.177 |
| TC/HDL-c | 4.6 (2.7-7.0) | 4.1 (2.1-7.1) | 0.032 |
| TG/HDL-c | 4.0 (1.3-8.0) | 4.1 (0.9-9.2) | 0.102 |
| LDL-c/HDL-c | 2.8 (1.4-5.3) | 2.3 (0.9-4.1) | 0.025 |
| Non-HDL-c/HDL-c | 3.6 (1.7-6.0) | 3.1 (1.1-6.1) | 0.032 |
NAION: Non arteritic ischemic optic neuropathy; TC: Total cholesterol; TG: Triglyceride; LDL-c: Low density lipoprotein; HDL-c: High density lipoprotein.
mean (range)
Table 4. ROC analyse of each aterogenic indices to differentiate NAION from the control group.
| Parameters | Cut-off value | Sensitivity (%) | Specificity (%) | AUC |
| Non-HDL-c (mg/dL) | 132.2 | 71 | 52 | 0.66 |
| TC/HDL-c | 3.67 | 79 | 60 | 0.74 |
| TG/HDL-c | 2.54 | 74 | 60 | 0.71 |
| LDL-c/HDL-c | 1.99 | 77 | 54 | 0.69 |
| Non-HDL-c/HDL-c | 2.67 | 79 | 60 | 0.74 |
NAION: Non arteritic ischemic optic neuropathy; TC: Total cholesterol; TG: Triglyceride; LDL-c: Low density lipoprotein; HDL-c: High density lipoprotein.
Table 5. ROC analyses of aterogenic indices according to visual acuity subgroups in NAION patients.
| Parameters | Cut-off value | Sensitivity (%) | Specificity (%) | AUC |
| TC/HDL-c | 3.77 | 85 | 50 | 0.67 |
| LDL-c/HDL-c | 2.35 | 75 | 58 | 0.68 |
| Non-HDL-c/HDL-c | 2.98 | 71 | 63 | 0.67 |
NAION: Non arteritic ischemic optic neuropathy; TC: Total cholesterol; TG: Triglyceride; LDL-c: Low density lipoprotein; HDL-c: High density lipoprotein.
Figure 1. The ROC curve analysis for the diagnostic values of atherogenic indices to differentiate the NAION group from the control group.
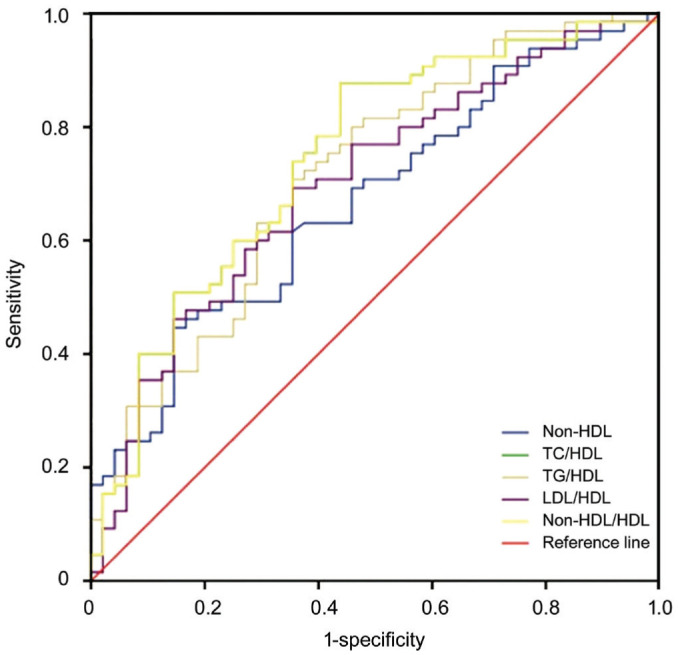
Figure 2. The ROC curve analysis for the prediction of visual acuity in NAION patients.
The NAION patients with bilateral sequential involvement had higher non-HDL-c, TC/HDL-c, TG/HDL-c, LDL-c/HDL-c, and non-HDL-c/HDL-c values than those with the unilateral involvement, but the differences were not statistically significant (P=0.271, 0.127, 0.197, 0.128, 0.127, respectively).
DISCUSSION
NAION, which is the most common ischemic optic neuropathy type, can be due to a variety of risk factors. Some of the risk factors are more accepted and well documented, while others are anecdotal case reports. Numerous studies have shown that arterial HT, DM, and atherosclerosis are established risk factors for NAION occurrence[20]. Since the risk factors for systemic vascular diseases are also risk factors for NAION, the question as to whether NAION indicates the presence of a systemic vascular disease arises. The investigation of the relationships between NAION and systemic vascular disorders might provide some clues for clarifying this disease. Previous research results were inconsistent for the relative risk of cerebral and cardiovascular events after the occurrence of NAION. The NAION patients were found to be at increased risk for ischemic heart disease and ischemic stroke compared to patients without NAION[8]–[9]. On the other hand, Hasanreisoğlu et al[21] concluded that the risks of cardiovascular and cerebrovascular events in patients with NAION were not different than those in the general population. Furthermore, a prospective study with 406 NAION patients did not reveal any increased risks for cerebrovascular events or cardiac diseases[7].
The impact of dyslipidemia on the vascular system is well known, and atherosclerosis constitutes a common point in the pathogenesis of both systemic vascular diseases and NAION. Dyslipidemia shows its effect by decreasing antioxidant defense and causing endothelial dysfunction, likely via interference with nitric oxide, which is a potent vasodilator and has antiatherogenic effects. The serum TC, TG, and LDL-c levels were elevated, while the HDL-c levels were low in the process of vascular calcification[22]. Circulating LDL-c is the major source of lipids that accumulate in atherosclerotic plaques, and HDL-c plays a protective role in atherosclerosis and arterial stiffness[23]. The formula of TC minus HDL-c, which is used to calculate the non-HDL-c, is considered as a measurement of the cholesterol in the LDL-c and very-low-density lipoprotein (VLDL-c) particles. The non-HDL-c compared to LDL-c was mentioned to be a better determinant of arterial stiffness[24]–[25]. In recent years, there has been a consensus that non-conventional serum lipid ratios compared to single conventional lipid parameters are better in discriminating the atherogenic events. The TC/HDL-c and LDL-c/HDL-c ratios were reported as powerful markers that indicate the presence of cardiovascular diseases and atherosclerosis[26]. Moreover, in clinical practice, the ratio of non-HDL-c/HDL-c compared to LDL-c, HDL-c, and non-HDL-c is a better, convenient, and economic marker that indicates the risks of coronary artery disease, arterial stiffness, and insulin resistance[27]. Furthermore, TG/HDL-c is a more effective marker in comparison to the remaining single lipid parameters for predicting cardiovascular disorders and the resistance to insulin[28]–[29].
Several research studies have investigated the relationship between serum lipid parameters and NAION. In most of those, the parameters were found to be high[10]–[11]; however, other studies did not confirm those results[30]–[31]. In the present study, we found significantly higher TG and lower HDL-c levels in NAION patients, while no such differences in TC and LDL-c levels were found between the study groups. In contrast, all the non-conventional lipid ratios were found to be significantly higher in the NAION patients in comparison to the controls. Our results support the hypothesis that these markers, compared to single conventional lipid profiles, are better in discriminating the atherosclerotic events. In another ongoing study conducted by our research group, we found increased arterial stiffness in NAION patients in comparison to the control group, a result that has not been published previously. The increased non-HDL-c values and non-HDL-c/HDL-c ratio in the NAION group also support the results regarding the arterial stiffness in the former study. In the subgroup analysis, although no significant differences were found in conventional serum lipids, the NAION patients with visual acuity ≤20/200 compared to those with >20/200 had higher values of TC/HDL-c, LDL-c/HDL-c, and non-HDL-c/HDL-c, which were more powerful markers of atherosclerosis and arterial stiffness. This could be explained by the increased severity of atherosclerosis and its effect on the microvascular system that supplies the optic nerve.
It is also possible that reduced levels of serum lipids could be protective on the remaining eye. The incidence of the involvement of the second eye varies widely in the literature, i.e., ranging between 19% and 38% in 5y after the onset of NAION, between 24% and 48% within 5-11y of the onset[14],[32]. In our series, 38% of the patients had bilateral NAION. Patients with bilateral sequential involvement had higher non-HDL-c, TC/HDL-c, TG/HDL-c, LDL-c/HDL-c, and non-HDL-c/HDL-c values compared to those with unilateral involvement; however, the differences were not statistically significant. The relatively small sample size of NAION patients with bilateral involvement may have prevented the detection of statistically significant differences in those parameters in comparison to the patients with unilateral involvement. As far as we know, the current study is the first that investigated the relationship between atherogenic indices and NAION. However, the retrospective and the single-center design of the study with a relatively small sample size could be considered as the limitations.
In conclusion, the values of the atherogenic indices in the NAION patients compared to controls were significantly higher. We also found significantly higher values in some of the atherogenic indices in NAION patients with severe vision loss. It is of utmost importance to minimize the risk factors in order to decrease the risk of NAION occurrence in the remaining eye or the progression of loss of vision in the already affected eye. Regulation of lipid metabolism, in which the atherogenic indices are taken into consideration, may provide additional benefits to NAION patients. Further larger studies are needed to identify the effectiveness of using atherogenic indices to predict visual outcome and second eye involvement.
Acknowledgments
Conflicts of Interest: Koçak N, None; Yeter V, None; Turunç M, None; Bayrambaş M, None; Eraydın B, None; Güngör İ, None.
REFERENCES
- 1.Keren S, Zanolli M, Dotan G. Visual outcome following bilateral non-arteritic anterior ischemic optic neuropathy: a systematic review and meta-analysis. BMC Ophthalmol. 2017;17(1):155. doi: 10.1186/s12886-017-0543-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Birer S, Arda H, Kilic D, Baskol G. Systemic oxidative stress in non-arteritic anterior ischemic optic neuropathy. Eye (Lond) 2019;33(7):1140–1144. doi: 10.1038/s41433-019-0388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buono LM, Foroozan R, Sergott RC, Savino PJ. Nonarteritic anterior ischemic optic neuropathy. Curr Opin Ophthalmol. 2002;13(6):357–361. doi: 10.1097/00055735-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Newman NJ, Scherer R, Langenberg P, Kelman S, Feldon S, Kaufman D, Dickersin K, Ischemic Optic Neuropathy Decompression Trial Research Group The fellow eye in naion: report from the ischemic optic neuropathy decompression trial follow-up study. Am J Ophthalmol. 2002;134(3):317–328. doi: 10.1016/s0002-9394(02)01639-2. [DOI] [PubMed] [Google Scholar]
- 5.Balducci N, Morara M, Veronese C, Barboni P, Casadei NL, Savini G, Parisi V, Sadun AA, Ciardella A. Optical coherence tomography angiography in acute arteritic and non-arteritic anterior ischemic optic neuropathy. Graefes Arch Clin Exp Ophthalmol. 2017;255(11):2255–2261. doi: 10.1007/s00417-017-3774-y. [DOI] [PubMed] [Google Scholar]
- 6.Gaier ED, Wang M, Gilbert AL, Rizzo JF, Cestari DM, Miller JB. Quantitative analysis of optical coherence tomographic angiography (OCT-A) in patients with non-arteritic anterior ischemic optic neuropathy (NAION) corresponds to visual function. PLoS One. 2018;13(6):e0199793. doi: 10.1371/journal.pone.0199793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hayreh SS, Joos KM, Podhajsky PA, Long CR. Systemic diseases associated with nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 1994;118(6):766–780. doi: 10.1016/s0002-9394(14)72557-7. [DOI] [PubMed] [Google Scholar]
- 8.Lee YC, Wang JH, Huang TL, Tsai RK. Increased risk of stroke in patients with nonarteritic anterior ischemic optic neuropathy: a nationwide retrospective cohort study. Am J Ophthalmol. 2016;170:183–189. doi: 10.1016/j.ajo.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Kim MS, Jeong HY, Cho KH, Oh SW, Byun SJ, Woo SJ, Yang HK, Hwang JM, Park KH, Kim CK, Park SJ. Nonarteritic anterior ischemic optic neuropathy is associated with cerebral small vessel disease. PLoS One. 2019;14(11):e0225322. doi: 10.1371/journal.pone.0225322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talks SJ, Chong NHV, Gibson JM, Dodson PM. Fibrinogen, cholesterol and smoking as risk factors for non-arteritic anterior ischaemic optic neuropathy. Eye (Lond) 1995;9(1):85–88. doi: 10.1038/eye.1995.13. [DOI] [PubMed] [Google Scholar]
- 11.Deramo VA, Sergott RC, Augsburger JJ, Foroozan R, Savino PJ, Leone A. Ischemic optic neuropathy as the first manifestation of elevated cholesterol levels in young patients. Ophthalmology. 2003;110(5):1041–1046. discussion 1046. doi: 10.1016/S0161-6420(03)00079-4. [DOI] [PubMed] [Google Scholar]
- 12.Song P, Xu L, Xu J, Zhang HQ, Yu CX, Guan QB, Zhao M, Zhang X. Atherogenic index of plasma is associated with body fat level in type 2 diabetes mellitus patients. Curr Vasc Pharmacol. 2018;16(6):589–595. doi: 10.2174/1570161116666180103125456. [DOI] [PubMed] [Google Scholar]
- 13.Cure E, Icli A, Ugur Uslu A, et al. Atherogenic index of plasma may be strong predictor of subclinical atherosclerosis in patients with Behçet disease. Z Rheumatol. 2017;76(3):259–266. doi: 10.1007/s00393-016-0141-z. [DOI] [PubMed] [Google Scholar]
- 14.Beck RW, Hayreh SS. Role of aspirin in reducing the frequency of second eye involvement in patients with non-arteritic anterior ischaemic optic neuropathy. Eye (Lond) 2000;14(Pt 1):118. doi: 10.1038/eye.2000.34. [DOI] [PubMed] [Google Scholar]
- 15.Ren X, Chen ZA, Zheng S, Han T, Li Y, Liu W, Hu Y. Association between triglyceride to HDL-C ratio (TG/HDL-C) and insulin resistance in Chinese patients with newly diagnosed type 2 diabetes mellitus. PLoS One. 2016;11(4):e0154345. doi: 10.1371/journal.pone.0154345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nogay NH. Assessment of the correlation between the atherogenic index of plasma and cardiometabolic risk factors in children and adolescents: might it be superior to the TG/HDL-C ratio? J Pediatr Endocrinol Metab. 2017;30(9):947–955. doi: 10.1515/jpem-2016-0479. [DOI] [PubMed] [Google Scholar]
- 17.Dobiášová M. Atherogenic impact of lecithin-cholesterol acyltransferase and its relation to cholesterol esterification rate in HDL (FER(HDL)) and AIP [log(TG/HDL-C)] biomarkers: the butterfly effect? Physiol Res. 2017;66(2):193–203. doi: 10.33549/physiolres.933621. [DOI] [PubMed] [Google Scholar]
- 18.Kiyosue A. Nonfasting TG/HDL-C ratio seems a good predictor of MACE in CAD patients with statin therapy. Could it be a treatment target? J Cardiol. 2018;71(1):8–9. doi: 10.1016/j.jjcc.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 19.Arsenault BJ, Rana JS, Stroes ES, et al. Beyond low-density lipoprotein cholesterol: respective contributions of non-high-density lipoprotein cholesterol levels, triglycerides, and the total cholesterol/high-density lipoprotein cholesterol ratio to coronary heart disease risk in apparently healthy men and women. J Am Coll Cardiol. 2009;55(1):35–41. doi: 10.1016/j.jacc.2009.07.057. [DOI] [PubMed] [Google Scholar]
- 20.Hayreh SS. Non-arteritic anterior ischaemic optic neuropathy and phosphodiesterase-5 inhibitors. Br J Ophthalmol. 2008;92(12):1577–1580. doi: 10.1136/bjo.2008.149013. [DOI] [PubMed] [Google Scholar]
- 21.Hasanreisoğlu M, Robenshtok E, Ezrahi D, Stiebel-Kalish H. Do patients with non-arteritic ischemic optic neuritis have increased risk for cardiovascular and cerebrovascular events? Neuroepidemiology. 2013;40(3):220–224. doi: 10.1159/000342155. [DOI] [PubMed] [Google Scholar]
- 22.Emerging Risk Factors Collaboration. Di Angelantonio E, Sarwar N, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mattace-Raso FUS, van der Cammen TJM, Hofman A, et al. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113(5):657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 24.Holewijn S, den Heijer M, Swinkels DW, H Stalenhoef AF, de Graaf J. Apolipoprotein B, non-HDL cholesterol and LDL cholesterol for identifying individuals at increased cardiovascular risk. J Intern Med. 2010;268(6):567–577. doi: 10.1111/j.1365-2796.2010.02277.x. [DOI] [PubMed] [Google Scholar]
- 25.Denke MA. Weighing in before the fight: low-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol versus apolipoprotein B as the best predictor for coronary heart disease and the best measure of therapy. Circulation. 2005;112(22):3368–3370. doi: 10.1161/CIRCULATIONAHA.105.588178. [DOI] [PubMed] [Google Scholar]
- 26.Barter P, Gotto AM, LaRosa JC, Maroni J, Szarek M, Grundy SM, Kastelein JJ, Bittner V, Fruchart JC, Treating to New Targets Investigators HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007;357(13):1301–1310. doi: 10.1056/NEJMoa064278. [DOI] [PubMed] [Google Scholar]
- 27.Kim SW, Jee JH, Kim HJ, et al. Non-HDL-cholesterol/HDL-cholesterol is a better predictor of metabolic syndrome and insulin resistance than apolipoprotein B/apolipoprotein A1. Int J Cardiol. 2013;168(3):2678–2683. doi: 10.1016/j.ijcard.2013.03.027. [DOI] [PubMed] [Google Scholar]
- 28.Sultani R, Tong DC, Peverelle M, Lee YS, Baradi A, Wilson AM. Elevated triglycerides to high-density lipoprotein cholesterol (TG/HDL-C) ratio predicts long-term mortality in high-risk patients. Heart Lung Circ. 2020;29(3):414–421. doi: 10.1016/j.hlc.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 29.Young KA, Maturu A, Lorenzo C, et al. The triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio as a predictor of insulin resistance, β-cell function, and diabetes in Hispanics and African Americans. J Diabetes Complications. 2019;33(2):118–122. doi: 10.1016/j.jdiacomp.2018.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jacobson DM. Nonarteritic anterior ischemic optic neuropathy. Arch Ophthalmol. 1997;115(11):1403. doi: 10.1001/archopht.1997.01100160573008. [DOI] [PubMed] [Google Scholar]
- 31.McCulley TJ, Lam BL, Feuer WJ. A comparison of risk factors for postoperative and spontaneous nonarteritic anterior ischemic optic neuropathy. J Neuro Ophthalmol. 2005;25(1):22–24. doi: 10.1097/00041327-200503000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Beck RW, Hayreh SS, Podhajsky PA, Tan ES, Moke PS. Aspirin therapy in nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 1997;123(2):212–217. doi: 10.1016/s0002-9394(14)71038-4. [DOI] [PubMed] [Google Scholar]


