Abstract
Background
Acquired pulmonary vein stenosis (PVS) is an infrequent complication of atrial fibrillation ablation that is often misdiagnosed due to predominant respiratory symptoms. It can result in pulmonary venous hypertension, with varying presentations, ranging from shortness of breath to haemoptysis.
Case summary
We report two patients with a history of paroxysmal atrial fibrillation treated with radiofrequency ablation and pulmonary vein (PV) isolation, who subsequently developed PVS. Case 1 initially presented with indolent symptoms of shortness of breath and cough. He was initially diagnosed with and treated for pneumonia. In contrast, Case 2 presented with massive haemoptysis, requiring intubation and intensive care unit admission. Both patients were eventually diagnosed with PVS by computed tomography. They were treated with PV angioplasty and stenting.
Discussion
While previously limited to the congenital heart disease population, PVS is occurring more frequently now in adult patients as a complication of ablation procedures. It is most effectively treated with angioplasty and stent implantation but has a high rate of recurrence.
Keywords: Congenital heart disease, Pulmonary vein stenosis, Pulmonary vein stenting, Case report, Ablation complication, Haemoptysis
Learning points
Pulmonary vein stenosis is a rare complication of atrial fibrillation ablation with varying clinical presentations, ranging from recurrent pleural effusions and pulmonary infections to life-threatening haemoptysis.
Balloon angioplasty with pulmonary vein stenting is the most effective treatment.
Introduction
Pulmonary venous hypertension causes a myriad of pulmonary findings such as pleural effusion formation, recurrent pneumonias, and haemoptysis.1 Massive haemoptysis secondary to pulmonary vein stenosis (PVS) is a rare, but life-threatening occurrence. Progressive reduction in pulmonary vein (PV) cross-sectional area leads to an increase in pulmonary venous pressure, venous congestion, and a decrease in pulmonary perfusion.2
Here, we describe two widely differing presentations of PVS secondary to radiofrequency ablation/PV isolation for atrial tachyarrhythmia. It is crucial to consider PVS in the workup of pulmonary symptoms in patients with a history of atrial fibrillation ablation.3
Timeline
| Case 1 | |
|---|---|
| Month 0 | Patient underwent atrial fibrillation ablation with pulmonary vein isolation |
| Month 18 | Initial presentation with cough, fever, and shortness of breath |
| Month 21 | Second presentation with cough, fever, and shortness of breath. Patient diagnosed with recurrent pneumonia; pleural effusion identified on X-ray |
| Month 22 | Outpatient thoracentesis |
| Month 22 | Contrast-enhanced CT scan demonstrated pulmonary vein stenosis |
| Month 23 | Pulmonary vein angioplasty |
| Month 25 | Pulmonary vein angioplasty with stenting |
|
| |
| Case 2 | |
|
| |
| Month 0 | Patient underwent atrial fibrillation ablation with pulmonary vein isolation |
| Month 17 | Patient underwent surgery with septal myectomy, pulmonary vein ablation, left atrial appendage clipping |
|
Month 25 Day 1 |
Initial presentation with small volume hemoptysis |
|
Month 25 Day 8 |
Repeat presentation with large volume hemoptysis. Patient emergently intubated in emergency room and admitted to ICU |
|
Month 25 Day 9 |
Initial non-contrast-enhanced CT scan demonstrated opacification in left lower lobe with unclear source of bleeding |
|
Month 25 Day 11 |
Patient transferred to our facility; pulmonary vein stenosis identified on contrast-enhanced CT scan |
|
Month 25 Day 13 |
Pulmonary vein angioplasty with stenting |
Case presentations
Case 1
A 33-year-old man with Ebstein anomaly and atrial septal defect (latter repaired at age 4) and paroxysmal atrial fibrillation presented to the emergency room with 2 weeks cough and intermittent fever. The patient underwent radiofrequency ablation with PV isolation 21 months prior. He had visited the emergency room with similar symptoms several months previously and was treated for presumed community-acquired pneumonia.
On presentation, the patient was febrile to 38.7˚C with otherwise normal vitals. X-ray was significant for a large left-sided pleural effusion with an opacity at the left lung base. He was again prescribed a course of azithromycin and discharged home from the emergency room.
At a subsequent outpatient visit, lung ultrasound demonstrated a persistent pleural effusion for which he underwent a therapeutic thoracentesis with the removal of 1.3 L serosanguinous fluid. His symptoms of cough and shortness of breath returned within 1 week. Therefore, a thoracoscopy with pleural biopsy and tunnelled pleural catheter placement was performed. Biopsy revealed non-specific inflammation. Follow-up contrast-enhanced CT scan after the procedure revealed a small loculated left pleural effusion, volume loss at the periphery of the left lower lobe, and a new finding of marked narrowing of the left PVs.
Given these findings, a cardiac catherization was performed. Intra-procedural trans-oesophageal echocardiography revealed severe stenosis of the common trunk of the left upper and lower PVs. Balloon angioplasty of the stenotic ostium was performed with a dual kissing balloon technique. Mean pre-procedural Doppler gradient on echocardiography was 7 mmHg, which decreased to 3.8 mmHg post-procedure (Figure 1). Following the procedure, he was started on aspirin, clopidogrel, and apixaban to maintain stent patency.
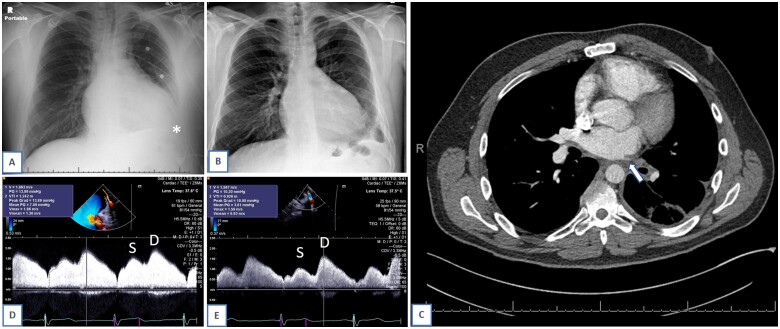
Figure 1:
Case 1. Chest X-ray demonstrating pleural effusion at time of initial presentation, asterisk (A). Chest X-ray, following pulmonary vein stenting procedure (B). Computed tomography angiography on which pulmonary vein stenosis diagnosed, arrow. Computed tomography also notable for left pleural effusion and subsegmental atelectasis in left lower lobe. (C). Pulsed wave Doppler of pulmonary vein, mean gradient 7 mmHg pre-procedure. Systolic and diastolic components marked on slide (D). Pulsed wave Doppler, mean gradient 3.8 mmHg post-procedure. Systolic and diastolic components marked on slide (E).
The patient’s recovery was complicated by recurrence of symptoms several weeks later. Repeat catheterization demonstrated restenosis of the left pulmonary venous antrum. In the second procedure, the lesion was pre-dilated, and a 10 mm diameter by 20 mm long bare-metal stent was placed from the PV ostium to the point of bifurcation. The patient was continued on apixaban and clopidogrel to maintain stent patency and for recurrent paroxysmal atrial fibrillation. Over the following 5 years, he had two small-sized haemoptysis episodes related to PV stent restenosis (Figure 2). Symptoms were alleviated after repeat angioplasty. While the patient’s CHA2DS2VASc Score was 0 and HASBLED Score was 2, he was continued on apixaban post-procedure to maintain stent patency.
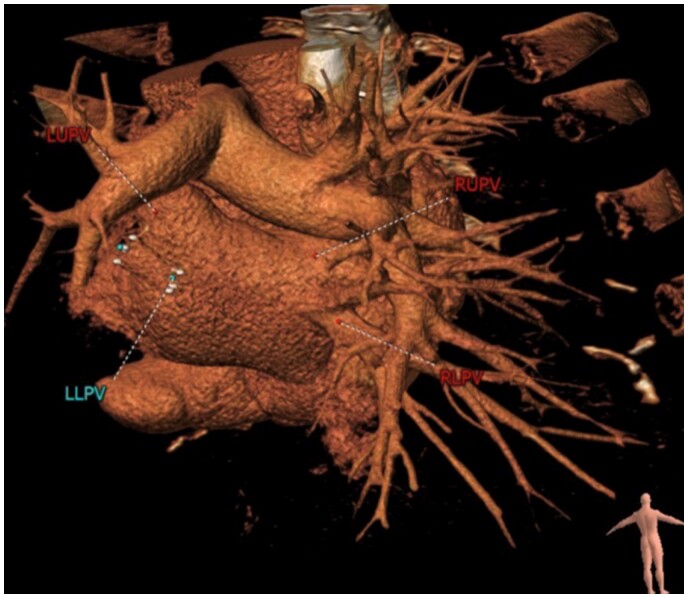
Figure 2:
Case 1. Computed tomography reconstruction, demonstrating restenosis of left-sided pulmonary veins following initial angioplasty.
Case 2
A 43-year-old male with prior history of pulmonary sarcoidosis, obstructive hypertrophic cardiomyopathy, and paroxysmal atrial fibrillation presented with haemoptysis. The patient was diagnosed with obstructive hypertrophic cardiomyopathy 15 years prior, had a single-chamber ICD placed for primary prevention, and underwent PV isolation 2 years prior to presentation. He had recurrent syncopal episodes from left ventricular outflow tract obstruction and underwent septal myectomy, mitral valvuloplasty, left atrial appendage clipping, and epicardial radiofrequency ablation 8 months prior to presentation. Myectomy pathology revealed myocyte hypertrophy and granulomas consistent with cardiac sarcoidosis.
The patient first began coughing up blood-tinged sputum 1 week prior to admission but was thought to have bronchitis at two urgent care encounters. Over the course of the week prior to admission, he developed progressive shortness of breath and increased frequency and quantity of blood in his sputum. On presentation, he was afebrile, normotensive, with normal work of breathing. In the emergency room, he had several episodes of massive haemoptysis, each over 100 mL, and was emergently intubated for airway protection. Immediate bronchoscopy demonstrated active bleeding with large clots in the left lower lobe. Prothrombin complex concentrate was administered to mitigate the effect of rivaroxaban, which he had been taking prior to admission for atrial fibrillation (CHA2DS2VASc Score 3, HAS-BLED Score 2). Initial non-contrast CT and repeated bronchoscopies suggested primarily left lung involvement without a clear source of bleeding.
The patient was transferred to our institution after several days with limited control of the haemoptysis. Repeat contrast-enhanced CT angiography did not reveal active bleeding, but the left upper and left lower PVs were poorly visualized, increasing clinical suspicion for PVS as the underlying cause of haemoptysis (Figure 3). Cardiac catheterization revealed complete obstruction of the left upper PV, with evidence of collateralization to left lower PV circulation, suggesting chronicity. Balloon angioplasty and stenting of the left lower PV was performed with a 9 mm diameter by 26 mm long bare-metal stent, reducing the PV mean pressure difference from 15 mmHg to 4 mmHg via cardiac catheterization measurements. The left upper PV was inaccessible. He was started on heparin post-procedure to maintain stent patency.
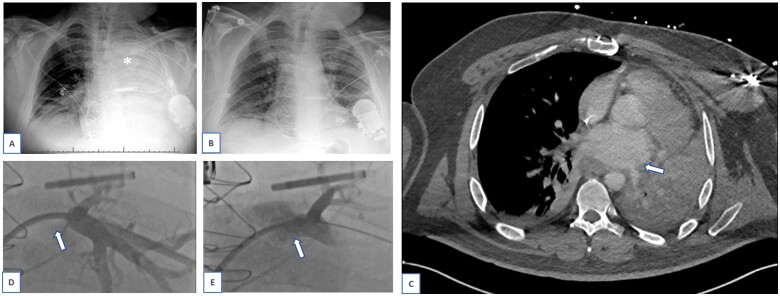
Figure 3:
Case 2. Chest X-ray at time of presentation to our centre, demonstrating complete collapse of left lung, asterisk (A). Chest X-ray several days after pulmonary vein stenting procedure (B). Computed tomography angiography on which pulmonary vein stenosis diagnosed, arrow (C). Pulmonary vein angiography via trans-septal access, before stent was placed. Left upper pulmonary vein is occluded, arrow (D). Pulmonary vein angiography via trans-septal access, post-stent placement. The branch veins are incompletely opacified due to brisk antegrade flow of contrast. Left upper pulmonary vein is stented, arrow (E).
He was eventually extubated 9 days post-procedure and safely transitioned to apixaban prior to discharge. Repeated chest X-rays have shown re-inflation of the left lung. Thirty-six weeks after discharge, he is doing well without recurrence of haemoptysis.
Discussion
Until recently, PVS had been most commonly described in the paediatric population. Congenital PVS is associated with anomalous PV connection, atrial and ventricular septal defects, and complex heterotaxy syndromes.1 Acquired PVS is a rare complication of atrial fibrillation ablation with PV isolation that is often missed due to predominant pulmonary symptoms.
Presentation includes shortness of breath, haemoptysis, recurrent symptoms suggestive of pneumonia, and progression to right-sided heart failure. Transcatheter balloon angioplasty and stent placement is the mainstay treatment, though with high recurrence rates.4,5 Antiproliferative agents such as imatinib and bevacizumab are under investigation.
The advent of new treatment modalities for atrial fibrillation has led to an increase in the incidence of acquired PVS.3,6 A multi-centre trial conducted at 13 sites found mild or moderate PV stenosis in 8.2% of ablated PVs at 3 months post-procedure, though all patients were asymptomatic.7 It is an infrequent complication of atrial fibrillation ablation procedures, previously estimated to occur following 0.3–3.4% of cases,8 though patients typically only undergo PV imaging when symptomatic.7 Aetiology is attributed to adventitial inflammation and collagen deposition at the site of ablation.9,10
Pulmonary vein anatomy varies widely in the general population, with up to 20% of people having three right-sided PVs, rather than two.11 In up to 25% of patients, the left PVs drain into a common ostium.11 Prior case studies have also demonstrated an association between severity of symptoms and number of PVs involved.1 This likely occurs due to an increase in outflow obstruction of pulmonary venous flow, leading to an increase in pulmonary venous hypertension. Having only one vein occluded can lead to compensatory collateralization,12 as was seen in Case 2, with increased pulmonary venous pressures developing only when the other vessel becomes occluded as well.
Imaging such as an echocardiogram, cardiac CT and magnetic resonance imaging (MRI), and ventilation-perfusion scans have previously been used as diagnostic modalities. Signs of congestion can be seen on chest X-ray but are non-specific. While ventilation–perfusion scans are superior in characterizing blood flow, CT, and MRI can be used to evaluate the size and calibre of PVs, including the exact site and length of stenosis.1,13 Trans-oesophageal echocardiography can also be used to visualize PVs but often is not the initial test of choice, unless there is high clinical suspicion.
Management of PVS typically involves angioplasty to increase the calibre of the veins.14 However, the restenosis rate is high, and patients may require additional interventions for recurrent symptoms in the future.8 Stent implantation has been associated with better outcomes than balloon angioplasty, with one study estimating a relative risk reduction of 53%.5 In another study, the restenosis rate following PVS intervention was 57% for balloon angioplasty alone and 27% for balloon angioplasty with stenting.8 A different study reported restenosis rates of 72% for balloon angioplasty alone and 33% for balloon angioplasty with stenting.14 However, stent implantation also requires the initiation of antiplatelets or anticoagulants, which lead to an increased bleeding risk. Careful risk/benefit analysis is necessary. In some cases of massive haemoptysis that is not relieved by PV angioplasty, partial pneumonectomy may be necessary.15 Clinicians should maintain a high index of suspicion for PVS in cases of recurrent, unexplained pulmonary symptoms, especially in patients with a history of radiofrequency ablation procedures.3
Lead author biography

Nina (Gertsvolf) Talmor, MD, is an Internal Medicine resident physician at New York University Langone Health Center in New York, NY, USA. She will be applying to Cardiology Fellowship in 2021 and plans to pursue a career in interventional cardiology. Her research interests include complications occurring during or after angiography, sex differences in cardiovascular disease, and adult congenital heart disease management.
Acknowledgements
The authors thank Samuel Maidman MD, Jonathan Lee MD, Sunil Saharan MD, and Anthony Andriotis MD for treating the patients presented in this case report and their critical reading of the manuscript.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patients in line with COPE guidelines.
Conflict of interest: Dr. Massera reports consulting fees from Bristol Meyers Squibb.
Funding: None declared.
References
- 1. Latson LA, Prieto LR.. Congenital and acquired pulmonary vein stenosis. Circulation 2007;115:103–108. [DOI] [PubMed] [Google Scholar]
- 2. Pazos-López P, García-Rodríguez C, Guitián-González A, Paredes-Galán E, Álvarez-Moure M, Rodríguez-Álvarez M. et al. Pulmonary vein stenosis: etiology, diagnosis and management. World J Cardiol 2016;8:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saad EB, Rossillo A, Saad CP, Martin DO, Bhargava M, Erciyes D. et al. Pulmonary vein stenosis after radiofrequency ablation of atrial fibrillation: functional characterization, evolution, and influence of the ablation strategy. Circulation 2003;108:3102–3107. [DOI] [PubMed] [Google Scholar]
- 4. Braun S, Platzek I, Zöphel K, Weise M, Kolditz M, Halank M. et al. Haemoptysis due to pulmonary venous stenosis. Eur Respir Rev 2014;23:170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fender EA, Widmer RJ, Mahowald MK, Hodge DO, Packer DL, Holmes DR Jr.. Recurrent pulmonary vein stenosis after successful intervention: prognosis and management of restenosis. Catheter Cardiovasc Interv 2020;95:954–958. [DOI] [PubMed] [Google Scholar]
- 6. Robbins IM, Colvin EV, Doyle TP, Kemp WE, Loyd JE, McMahon WS. et al. Pulmonary vein stenosis after catheter ablation of atrial fibrillation. Circulation 1998;98:1769–1775. [DOI] [PubMed] [Google Scholar]
- 7. Samuel M, Khairy P, Mongeon FP, Andrade JG, Gomes S, Galvan Z. et al. Pulmonary vein stenosis after atrial fibrillation ablation: insights from the ADVICE trial. Can J Cardiol 2020;36:1965–1974. [DOI] [PubMed] [Google Scholar]
- 8. Fender EA, Widmer RJ, Hodge DO, Cooper GM, Monahan KH, Peterson LA. et al. Severe pulmonary vein stenosis resulting from ablation for atrial fibrillation: presentation, management, and clinical outcomes. Circulation 2016;134:1812–1821. [DOI] [PubMed] [Google Scholar]
- 9. Dong J, Vasamreddy CR, Jayam V, Dalal D, Dickfeld T, Eldadah Z. et al. Incidence and predictors of pulmonary vein stenosis following catheter ablation of atrial fibrillation using the anatomic pulmonary vein ablation approach: results from paired magnetic resonance imaging. J Cardiovasc Electrophysiol 2005;16:845–852. [DOI] [PubMed] [Google Scholar]
- 10. Ito K, Kato K, Tanaka H.. Experience using drug-coated balloon venoplasty for acquired pulmonary vein stenosis after radiofrequency ablation. J Cardiol Cases 2021;23:3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Klimek-Piotrowska W, Hołda MK, Piątek K, Koziej M, Hołda J.. Normal distal pulmonary vein anatomy. PeerJ 2016;4:e1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hilbert S, Paetsch I, Bollmann A, Jahnke C.. Pulmonary vein collateral formation as a long-term result of post-interventional pulmonary vein stenosis. Eur Heart J 2016;37:2474. [DOI] [PubMed] [Google Scholar]
- 13. Widmer RJ, Holmes DR Jr. Chapter 23 - Pulmonary vein stenosis: management and outcomes. In: Rihal CS, Raphael CE(eds). Handbook of Structural Heart Interventions. Elsevier; 2021:267-278.e1.
- 14. Prieto LR, Schoenhagen P, Arruda MJ, Natale A, Worley SE.. Comparison of stent versus balloon angioplasty for pulmonary vein stenosis complicating pulmonary vein isolation. J Cardiovasc Electrophysiol 2008;19:673–678. [DOI] [PubMed] [Google Scholar]
- 15. Lee JY, Chon GR, Park JH, Kang BJ, Shim TS, Jo KW.. Massive hemoptysis due to pulmonary vein stenosis following catheter ablation for atrial fibrillation. Respir Care 2015;60:e52–5. [DOI] [PubMed] [Google Scholar]