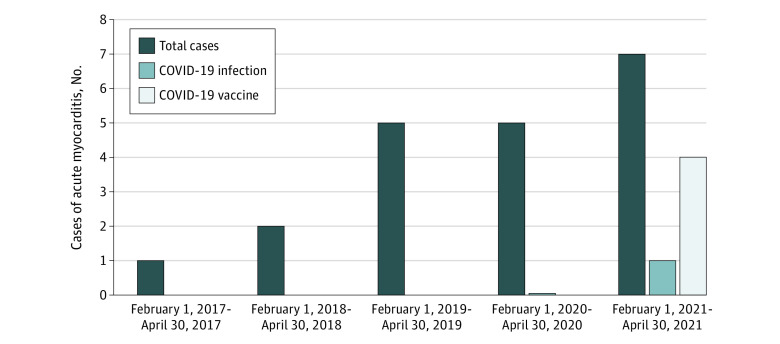
This study describes 4 patients who presented with acute myocarditis after mRNA COVID-19 vaccination.
Key Points
Question
Is COVID-19 vaccination linked to the occurrence of myocarditis?
Findings
In this study of 7 patients with acute myocarditis, 4 occurred within 5 days of COVID-19 vaccination between February 1 and April 30, 2021. All 4 patients had received the second dose of a messenger RNA (mRNA) vaccine, presented with severe chest pain, had biomarker evidence of myocardial injury, were hospitalized, and had cardiac magnetic resonance imaging findings typical of myocarditis.
Meaning
Although causality cannot be established, the findings raise the possibility of an association between mRNA COVID-19 vaccination and acute myocarditis.
Abstract
Importance
Vaccine-associated myocarditis is an unusual entity that has been described for the smallpox vaccine, but only anecdotal case reports have been described for other vaccines. Whether COVID-19 vaccination may be linked to the occurrence of myocarditis is unknown.
Objective
To describe a group of 7 patients with acute myocarditis over 3 months, 4 of whom had recent messenger RNA (mRNA) COVID-19 vaccination.
Design, Setting, and Participants
All patients referred for cardiovascular magnetic resonance imaging at Duke University Medical Center were asked to participate in a prospective outcomes registry. Two searches of the registry database were performed: first, to identify patients with acute myocarditis for the 3-month period between February 1 and April 30 for 2017 through 2021, and second, to identify all patients with possible vaccine-associated myocarditis for the past 20 years. Once patients with possible vaccine-associated myocarditis were identified, data available in the registry were supplemented by additional data collection from the electronic health record and a telephone interview.
Exposures
mRNA COVID-19 vaccine.
Main Outcomes and Measures
Occurrence of acute myocarditis by cardiovascular magnetic resonance imaging.
Results
In the 3-month period between February 1 and April 30, 2021, 7 patients with acute myocarditis were identified, of which 4 occurred within 5 days of COVID-19 vaccination. Three were younger male individuals (age, 23-36 years) and 1 was a 70-year-old female individual. All 4 had received the second dose of an mRNA vaccine (2 received mRNA-1273 [Moderna], and 2 received BNT162b2 [Pfizer]). All presented with severe chest pain, had biomarker evidence of myocardial injury, and were hospitalized. Coincident testing for COVID-19 and respiratory viruses provided no alternative explanation. Cardiac magnetic resonance imaging findings were typical for myocarditis, including regional dysfunction, late gadolinium enhancement, and elevated native T1 and T2.
Conclusions and Relevance
In this study, magnetic resonance imaging findings were found to be consistent with acute myocarditis in 7 patients; 4 of whom had preceding COVID-19 vaccination. Further investigation is needed to determine associations of COVID-19 vaccination and myocarditis.
Introduction
Vaccine-associated myocarditis is an unusual entity that has been described for the smallpox vaccine,1 but otherwise only anecdotal case reports have been described for other vaccines. Among 416 629 adults receiving live measles, mumps, and rubella; varicella; oral polio; or yellow fever viral vaccinations in the Vaccine Safety Datalink, there were no patients with myocarditis in the 42 days following vaccination.2 There are a few case reports of myocarditis following the seasonal influenza vaccine in otherwise healthy adults,3,4 but a causal relationship is difficult to establish and the case reports could have been due to chance.
In late December 2020, COVID-19 vaccination began in the US, and on April 7, 2021, vaccination was opened to all adults 16 years or older in North Carolina. Here, we report cardiac magnetic resonance (CMR) imaging findings in 4 patients consistent with acute myocarditis at our institution; all 4 had recent vaccination for COVID-19. All presented with severe chest pain associated with biomarker evidence of myocardial injury and were hospitalized. Included are data on clinical presentation and results from in-hospital testing. To provide context, we also report the prevalence of acute myocarditis by CMR imaging at our institution during the same 3-month period for each of the past 5 years.
Methods
All patients referred for CMR imaging at Duke University Medical Center are asked to participate in a prospective CMR imaging outcomes registry, which contains clinical data, finalized clinical CMR imaging reports, and full Digital Imaging and Communications in Medicine image data sets.5 The registry has been approved by the institutional review board at Duke University Medical Center, and all patients sign informed consent prior to participating.
We performed 2 searches of the registry. First, to identify patients with acute myocarditis for February, March, and April of 2017 through 2021, a search for the term myocarditis was performed within the report summary and limited to the relevant time period. Second, to identify all patients with possible vaccine-associated myocarditis for the past 20 years, we searched for the terms vaccine or vaccination in the report summary and the history fields without limitation in the time period. A second independent reviewer performed the same searches and confirmed the counts. Once patients with possible vaccine-associated myocarditis were identified, data available in the registry were supplemented with information from the electronic health record and telephone interview.
CMR imaging was performed on 1.5-T or 3-T scanners. A standard protocol for myocarditis was used, which included cine, T1 and T2 mapping, and late gadolinium enhancement (LGE).6,7 Native T1 and T2 times were measured on pixelwise maps with regions drawn to match areas with and without LGE. Cutoffs for abnormally elevated T1 and T2 were based on 2 SDs above the respective means in a healthy population imaged on the same scanners.
Results
In the 3-month period between February 1 and April 30, 2021, we identified 7 patients with acute myocarditis of whom 4 had recent COVID-19 vaccination (Figure 1). A search of the entire registry identified a single additional patient with myocarditis diagnosed by CMR imaging within days following quadrivalent influenza vaccination. The patient was a member of the military, and this was his second episode; he had documented myocarditis associated with smallpox vaccination 4 years earlier.
Figure 1. Patients With Cardiac Magnetic Resonance Imaging Findings Consistent With Acute Myocarditis.
The total number of patients with acute myocarditis is highest for 2021 with 4 associated with recent COVID-19 vaccination. Only 1 patient had myocarditis associated with acute COVID-19 infection.
The clinical characteristics of the 4 patients with myocarditis following COVID-19 vaccination are shown in the Table. Data on race and ethnicity were not available. Three were younger male individuals (age, 23-36 years) and 1 was a 70-year-old female individual. All 4 had received the second dose of a messenger RNA (mRNA) vaccine (2 received mRNA-1273 [Moderna], and 2 received BNT162b2 [Pfizer-BioNTech]) between 1 and 5 days before hospitalization. None had a viral prodrome or prior COVID-19 infection. COVID-19 and respiratory virus polymerase chain reaction test results were negative in 3 and 2 patients, respectively, who underwent testing. None had acute pulmonary disease on chest radiograph.
Table. Characteristics of Patients Who Received COVID-19 Vaccination.
| Characteristic | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
|---|---|---|---|---|
| Age, y | 36 | 23 | 70 | 24 |
| Sex | Male | Male | Female | Male |
| Comorbidities | ||||
| Hypertension | No | No | Yes | No |
| Diabetes | No | No | No | No |
| Hypercholesterolemia | No | No | Yes | No |
| Cigarette smoking | No | No | Yes | No |
| History of CAD or MI | No | No | No | No |
| Prior myocarditis | No | No | No | No |
| Prior COVID-19 infection | No | No | No | No |
| Vaccine received | mRNA-1273 | BNT162b2 | mRNA-1273 | BNT162b2 |
| Doses received | 2 | 2 | 2 | 2 |
| Symptoms prior to vaccination (last dose) | ||||
| Viral prodromea | No | No | No | No |
| Symptoms within 24 h of vaccination | ||||
| Injection site discomfort | Yes | Yes | No | Yes |
| Fatigue/muscle ache | Yes | Yes | No | Yes |
| Fever/chills | Yes | Yes | No | Yes |
| Headache | No | No | No | Yes |
| Nausea | No | No | No | No |
| Chest pain | No | No | Yes | No |
| Hospitalization | ||||
| Interval after vaccination, d | 3 | 5 | 1 | 2 |
| Symptoms leading to hospitalization | ||||
| Chest pain | Yes | Yes | Yes | Yes |
| Chest pain severity | Severe | Severe | Severe | Severe |
| Symptom onset | <12 h of Hospitalization | <12 h of Hospitalization | <12 h of Hospitalization | <24 h of Hospitalization |
| Shortness of breath | Yes | Yes | Yes | No |
| Diaphoresis | No | No | Yes | No |
| Syncope/presyncope | No | Yes | No | No |
| Palpitations | No | No | No | Yes |
| In-hospital testing | ||||
| Electrocardiogram | Diffuse ST elevation | Lateral | Anterolateral | Diffuse ST elevation |
| PR depression | ST elevation | ST elevation | PR depression | |
| Peak troponin, ng/L | hs-Tn T: 230 (abnormal) | hs-Tn I: 7452 (abnormal) | Tn I: 2.34 (abnormal) | hs-Tn T: 698 (abnormal) |
| CRP, mg/dL | 6.32 (Abnormal) | 2.2 (Abnormal) | NP | 6.08 (Abnormal) |
| ESR, mm/h | 6 | 30 (Abnormal) | NP | 12 |
| ProBNP, pg/mL | NP | 780 (Abnormal) | 5194 (Abnormal) | 65 |
| WBC, /µL | 10 200 | 10 800 | 16 700 (Abnormal) | 13 500 (Abnormal) |
| COVID-19 testingb | Negativec | Negatived | NP | Negativec |
| Respiratory virus PCRe | Negative | Negative | NP | NP |
| Chest radiography | Normal | Normal | Normal | Normal |
| Chest CT | Negative for PE | Negative for PE | NP | NP |
| Coronary angiography | NP | NP | Normal coronaries | NP |
| Cardiac MRI | ||||
| Interval after vaccination, d | 3 | 5 | 3 | 3 |
| LVEF, % | 53 | 58 | 40 | 59 |
| Regional wall motion abnormality | Yes | Yes | Yes | Yes |
| Pericardial effusion | Trace | Small | Small | Trace |
| Pericardial thickness | Normal | Normal | Normal | Normal |
| LGE present | Yes | Yes | Yes | Yes |
| Location | Apical lateral | Multiple | Multiple | Lateral |
| Myocardial pattern | Epicardial | Epicardial | Patchy, diffuse | Epicardial, patchy |
| Pericardial enhancement | No | No | No | No |
| Native T1 | ||||
| In region of LGE | Abnormal | Abnormal | Abnormal | Abnormal |
| In region without LGE | Normal | Normal | Normal | Normal |
| Native T2 | ||||
| In region of LGE | Not imaged | Abnormal | Abnormal | Abnormal |
| In region without LGE | Normal | Normal | Normal | Normal |
| Therapy | ||||
| Corticosteroids | No | Yes | No | No |
| Colchicine | Yes | Yes | No | Yes |
| NSAIDs | Yes | No | No | Yes |
Abbreviations: CAD, coronary artery disease; CRP, C-reactive protein; CT, computed tomography; ESR, erythrocyte sedimentation rate; hs-Tn, high-sensitivity troponin; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MRI, magnetic resonance imaging; NP, not performed; NSAIDs, nonsteroidal anti-inflammatory drugs; PCR, polymerase chain reaction; PE, pulmonary emboli; proBNP, pro–brain-type natriuretic peptide; WBC, white blood cell count.
SI conversion factors: To convert CRP to milligrams per liter, multiply by 10; ESR to millimeters per hour, multiply by 1; troponin to micrograms per liter, multiply by 1; WBC to ×109 per liter, multiply by 0.001.
Upper respiratory tract symptoms, fever, muscle aches.
Performed within 2 days after the onset of chest pain in those who underwent testing.
Isothermal COVID-19 test was performed (Abbott ID Now; lower detection limit of 125 genome equivalent/mL).
Real-time PCR COVID-19 test was performed (Cepheid Xpert Xpress SARS-CoV-2; lower detection limit of 131 copies/mL).
Influenza A, influenza B, respiratory syncytial virus.
All 4 patients had abnormal electrocardiogram results and elevated troponin levels. The older female individual underwent coronary angiography, which revealed no atherosclerosis. CMR imaging was performed between 3 and 5 days after vaccination. All had regional wall motion abnormalities on cine imaging, and ejection fraction ranged between 40% and 59%. LGE was present in a nonischemic pattern consistent with myocarditis in all 4 patients (Figure 2). Both native T1 and T2 were elevated in the regions with LGE, consistent with acute injury, except in 1 patient who did not have a T2 map acquired at a slice location that included an area with LGE. Pericardial thickness was normal in all, and no patients had pericardial LGE.
Figure 2. Cardiac Magnetic Resonance Imaging in Patients With Acute Myocarditis Following COVID-19 Vaccination.
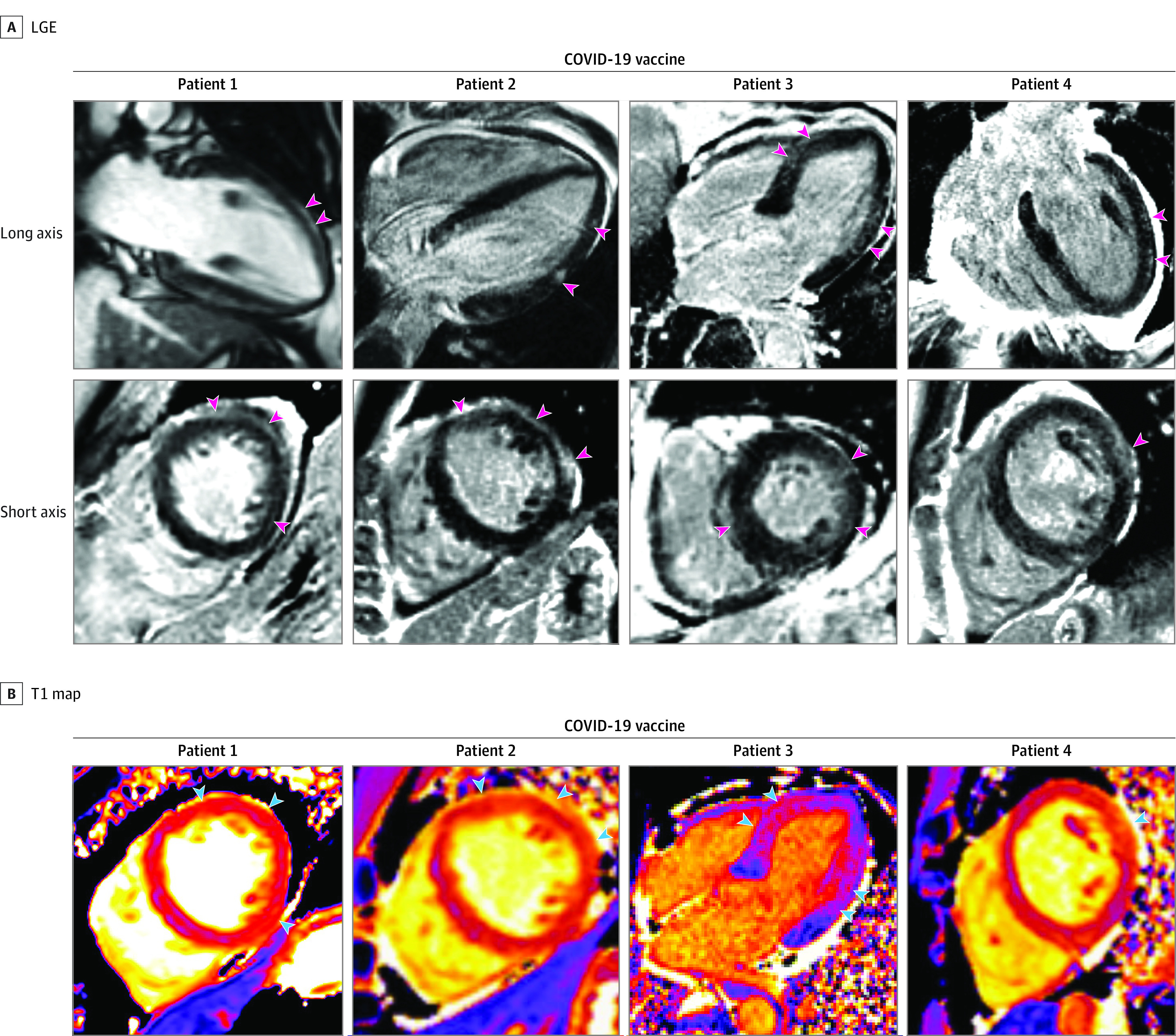
Late gadolinium enhancement (LGE) images and T1 maps are shown in 4 patients who recently received COVID-19 vaccine. Patients 1, 2, and 4 demonstrate epicardial LGE, and patient 3 demonstrates patchy, diffuse LGE (pink arrowheads), which are consistent with myocarditis. T1 maps demonstrate abnormal (elevated) native T1 in the regions with LGE (blue arrowheads).
The hospital courses for all 4 were uneventful without evidence of arrhythmias or heart failure, and treatment was conservative with nonsteroidal anti-inflammatory drug and colchicine, with 1 receiving corticosteroids. All were discharged within 2 to 4 days of hospitalization.
Discussion
We identified at our institution 4 patients with acute myocarditis occurring within days of mRNA COVID-19 vaccination. Although a causal relationship cannot be established, we note that none had a viral prodrome or had coincident testing (including COVID-19 polymerase chain reaction and respiratory virus polymerase chain reaction) that revealed an alternative explanation. None had COVID-19 infection in the prior year, suggesting that myocarditis from subsequent multisystem inflammatory syndrome is also unlikely.8 Additionally, the diagnosis of acute myocarditis was straightforward. The presenting symptom was acute onset of severe chest pain, and myocardial injury was detected by elevated troponin levels in all patients. CMR imaging abnormalities were observed across multiple techniques (regions with wall motion abnormalities on cine imaging–matched regions with LGE and regions with abnormal native T1 and T2) and cannot be attributed to image artifacts. Moreover, the concordant findings were typical for acute myocarditis. Hence, it is possible that these 4 cases of acute myocarditis represent a rare, potential adverse event linked to mRNA COVID-19 vaccination.
On January 7, 2021, the first patients at our institution began receiving COVID-19 vaccines, and vaccination was opened to all people 16 years and older on April 7, 2021. The first patient at our institution with myocarditis following COVID-19 vaccination was observed in the middle of February 2021, in line with the timing of the second vaccination dose. As of April 30, 2021, there were 561 197 individuals in North Carolina living in the 6 counties surrounding our institution who had been fully vaccinated against COVID-19 infection, representing 33% of the population (statewide, 99.1% received an mRNA vaccine).9 Since we have identified 4 patients with myocarditis following vaccination, this indicates that if COVID-19 vaccination is associated with myocarditis, it is quite rare. Nonetheless, these 4 patients represent the majority of patients with acute myocarditis identified in the past 3 months at our institution, and this led to the highest total number of patients with acute myocarditis compared with the same 3-month period for the past 5 years (Figure 1). Additionally, we identified only those patients with severe unremitting chest pain who sought medical attention. Those with mild or moderate chest pain might not seek medical attention, and it is possible that subclinical myocarditis may occur and could be detected by active surveillance, as has been described with smallpox vaccination.10
Fortunately, the hospital courses of the 4 patients with myocarditis following COVID-19 vaccination were uneventful, and they were discharged within 2 to 4 days. In brief follow-up, none required rehospitalization (1 was seen in the emergency department for chest pain with negative troponin and pro–brain-type natriuretic peptide levels and was discharged), suggesting that the early prognosis could be benign. This is consistent with the study by Ammirati et al11 that showed that the cardiac mortality plus transplant rate was 0% at 5 years in patients with an uncomplicated presentation of acute myocarditis. Conversely, patients with a complicated presentation (left ventricular ejection fraction, <50% with sustained ventricular arrhythmias or a low cardiac output syndrome requiring inotropes or mechanical circulatory support) had a cardiac mortality plus transplant rate of 14.7% at 5 years. Further study is needed to determine the range of clinical presentations and outcomes for patients with myocarditis following mRNA vaccines.
Limitations
Several caveats should be considered. First, there is no control group, and it is not possible to compare rates of acute myocarditis between those randomly assigned to receive vaccination vs no vaccination. Second, given the media attention, there could be recall or referral bias after COVID-19 vaccination compared with other vaccine exposures, and any temporal link between vaccination and myocarditis could just be due to chance. Third, although tests for COVID-19 infection and respiratory viruses were undertaken in some of the patients, these tests are neither foolproof nor comprehensive. Finally, there is no serological data, which could provide evidence of an excessive response to vaccination or prior subclinical COVID-19 infection (as determined by antinucleocapsid antibodies).
Conclusions
The findings from the present report raise the possibility of an association between mRNA COVID-19 vaccination and acute myocarditis. Since most individuals in North Carolina have received mRNA vaccines, it remains unknown if acute myocarditis will be observed following non-mRNA vaccines. Additionally, whether patients who experience acute myocarditis following COVID-19 vaccination should receive subsequent booster vaccinations for COVID-19 variants (or annual vaccinations for prophylaxis, if needed) is an open question, and the risk vs benefit profile will have to be considered carefully.
References
- 1.Eckart RE, Love SS, Atwood JE, et al. ; Department of Defense Smallpox Vaccination Clinical Evaluation Team . Incidence and follow-up of inflammatory cardiac complications after smallpox vaccination. J Am Coll Cardiol. 2004;44(1):201-205. doi: 10.1016/j.jacc.2004.05.004 [DOI] [PubMed] [Google Scholar]
- 2.Kuntz J, Crane B, Weinmann S, Naleway AL; Vaccine Safety Datalink Investigator Team . Myocarditis and pericarditis are rare following live viral vaccinations in adults. Vaccine. 2018;36(12):1524-1527. doi: 10.1016/j.vaccine.2018.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng MP, Kozoriz MG, Ahmadi AA, Kelsall J, Paquette K, Onrot JM. Post-vaccination myositis and myocarditis in a previously healthy male. Allergy Asthma Clin Immunol. 2016;12:6. doi: 10.1186/s13223-016-0114-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim YJ, Bae JI, Ryoo SM, Kim WY. Acute fulminant myocarditis following influenza vaccination requiring extracorporeal membrane oxygenation. Acute Crit Care. 2019;34(2):165-169. doi: 10.4266/acc.2017.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heitner JF, Kim RJ, Kim HW, et al. Prognostic value of vasodilator stress cardiac magnetic resonance imaging: a multicenter study with 48 000 patient-years of follow-up. JAMA Cardiol. 2019;4(3):256-264. doi: 10.1001/jamacardio.2019.0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kramer CM, Barkhausen J, Bucciarelli-Ducci C, Flamm SD, Kim RJ, Nagel E. Standardized cardiovascular magnetic resonance imaging (CMR) protocols: 2020 update. J Cardiovasc Magn Reson. 2020;22(1):17. doi: 10.1186/s12968-020-00607-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schulz-Menger J, Bluemke DA, Bremerich J, et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson. 2013;15:35. doi: 10.1186/1532-429X-15-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morris SB, Schwartz NG, Patel P, et al. Case series of multisystem inflammatory syndrome in adults associated with SARS-CoV-2 infection: United Kingdom and United States, March-August 2020. MMWR Morb Mortal Wkly Rep. 2020;69(40):1450-1456. doi: 10.15585/mmwr.mm6940e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.North Carolina Department of Health and Human Services . COVID-19 North Carolina dashboard. Accessed April, 30 2021. https://covid19.ncdhhs.gov/dashboard
- 10.Engler RJ, Nelson MR, Collins LC Jr, et al. A prospective study of the incidence of myocarditis/pericarditis and new onset cardiac symptoms following smallpox and influenza vaccination. PLoS One. 2015;10(3):e0118283. doi: 10.1371/journal.pone.0118283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ammirati E, Cipriani M, Moro C, et al. ; Registro Lombardo delle Miocarditi . Clinical presentation and outcome in a contemporary cohort of patients with acute myocarditis: multicenter Lombardy registry. Circulation. 2018;138(11):1088-1099. doi: 10.1161/CIRCULATIONAHA.118.035319 [DOI] [PubMed] [Google Scholar]