Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited cause of end-stage kidney failure. At present, only one drug, tolvaptan, has been approved for use to slow disease progression, but its use is limited by reduced tolerability and idiosyncratic liver toxicity. Thiazolidinediones were first developed as insulin-sensitizers but also regulate gene transcription in multiple tissues, leading to systemic effects on metabolism, inflammation and vascular reactivity. In this issue, Blazer-Yost et al. report the results of a single-centre Phase 1b double-blind placebo-controlled crossover study of the peroxisome proliferator-activated receptor γ (PPAR-γ) agonist pioglitazone in 18 ADPKD patients. Encouragingly, there were no major safety signals, although evidence of efficacy could not be demonstrated due to the small sample size. We review the preclinical evidence for the use of PPAR-γ agonists in ADPKD and speculate on the likely beneficial and adverse clinical effects of this interesting class of compounds in a future trial.
Keywords: ADPKD, clinical trial, diabetes mellitus, magnetic resonance imaging, polycystic kidney disease
INTRODUCTION
Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited cause of end-stage kidney failure [1]. It has an estimated clinical prevalence of <1 in 2000, although its genetic prevalence could be higher due to many asymptomatic undiagnosed cases in the general population [2]. Around 10% of patients on renal replacement therapy have ADPKD, making it a disease of considerable personal, societal and economic impact. At present, only one drug, tolvaptan, has been approved for use to slow disease progression, but its use is limited by reduced tolerability and idiosyncratic liver toxicity [3]. Therefore alternative drugs (for those intolerant to tolvaptan) and combination approaches (to reduce its side effects and maximize efficacy) are urgently needed.
Thiazolidinediones (TZDs) were first developed as insulin sensitizers to improve glycaemic control in patients with Type 2 diabetes mellitus. They function as agonists of peroxisome proliferator-activated receptor γ (PPAR-γ), which dimerizes with retinoic acid receptor A to form a co-repressor complex regulating the transcription of multiple target genes (Figure 1) [4]. The most widely prescribed TZDs, pioglitazone and rosiglitazone, have been widely used as second-line agents to control glycaemia in diabetic patients. Beyond glycaemic control, anti-proteinuric effects have been observed in diabetic nephropathy and other forms of glomerulonephritis such as immunoglobulin A nephropathy and focal segmental glomerulosclerosis [4]. More recently, their use has become more restricted due to an increased incidence of death and heart failure [5, 6]. TZDs block both epithelial sodium channel–dependent and –independent sodium excretion, leading to oedema as a common side effect of treatment that can in turn exacerbate incipient heart failure [7, 8].
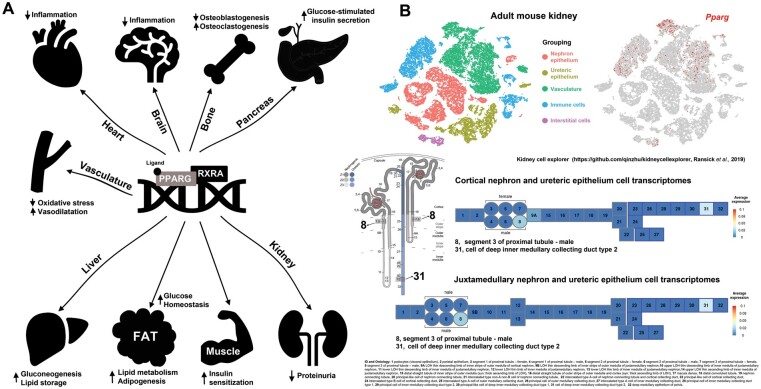
FIGURE 1:
(A) TZDs activate PPARγ–retinoid X receptor functional heterodimers, which in turn regulate gene transcription (PPAR response elements) in multiple tissues, resulting in widespread effects on metabolism, osteogenesis, proteinuria, vascular reactivity and inflammation as reviewed by Mao and Ong [4]. (B) Single-cell Pparg gene expression in mouse adult kidney showing predominant expression in the vasculature and immune cells with detectable low tubular expression in specific segments, i.e. S3 proximal tubule and inner medullary collecting duct (average expression <0.04). The numbered segments correspond to the segmentation reported in the Kidney Cell Explorer (Ontology identification 1–32) [11].
In this issue, Blazer-Yost et al. report the results of a single-centre Phase 1b double-blind placebo-controlled crossover study of the PPAR-γ pioglitazone in 18 ADPKD patients (ClinicalTrials.gov NCT02697617) [9]. A low dose (15 mg) of pioglitazone or placebo was given for 12 months each with a transition to the opposite arm after a short washout period. The primary endpoints were to monitor the known side effects of PPAR-γ agonists and included incident episodes of fluid retention, heart failure, liver toxicity and hypoglycaemia. Secondary endpoints were to assess changes in total kidney volume (TKV), blood pressure (BP) and kidney function (estimated glomerular filtration rate determined with the Chronic Kidney Disease Epidemiology Collaboration equation).
Fifteen patients (83%) completed the 2-year study with 3 dropouts (17%) due to unspecified personal reasons (two patients at Months 3 or 12) or pregnancy (one patient at Month 6): all 18 patients were included in the safety analysis. Encouragingly, there were no major safety signals, i.e. episodes of heart failure or liver toxicity. Unexpectedly, total body water as assessed by bioimpedance analysis was significantly reduced during pioglitazone treatment. One patient had asymptomatic recurrent episodes of unexplained non-fasting hypoglycaemia (<4 mmol/L).
Unfortunately, the secondary endpoints were not met due to a smaller sample size than anticipated. The authors had calculated that a minimum of 22 recruits to complete both arms were required to demonstrate a treatment effect of 33% with pioglitazone on an estimated annual TKV growth of 6%. Despite ‘aggressive recruitment’, this was not achieved, thus rendering the study underpowered for efficacy. Nonetheless, pioglitazone was associated with a trend towards a reduced rate of TKV growth compared with placebo (4.3 ± 6.3% versus 7.85 ± 7.68%; P = 0.15). A greater decrease in TKV (−3.5%) was detected, although a higher rate of annual TKV growth (8%) was present in this cohort compared with previous studies (6%): all study participants fell within a rapid disease progression classification (Mayo Imaging Class 1C–E). Similarly, a non-significant reduction in diastolic BP was detected with pioglitazone (83 versus 86 mmHg; P = 0.08), consistent with previous non-ADPKD trials [10]. The authors did not test higher doses of pioglitazone due to concerns about likely dose-dependent side effects and after extrapolation from their preclinical data suggested maximal efficacy from a low dose in rodent models (Table 1).
Table 1.
Preclinical studies of TZDs in PKD rodent models
| References | Drug and dosing (mg/kg/day) | Model and gender (M/F) | Age and treatment duration | Reduction in %KW (Y/N/NA) | Effect on BP (Y/N/NA) | Adverse events | Extrarenal effects |
|---|---|---|---|---|---|---|---|
| Muto et al. [12] | Pioglitazone (80) | Pkd1 null (M, F) | E 7.5 (2 days) | NA | NA | None | Improved survival; decreased oedema, cardiac defects |
| Pioglitazone (40) | Pkd1 hets (M, F) | Week 16 (6 M) | NA | N (10 M) | None | Improved aortic EDD | |
| Raphael et al. [13] | Pioglitazone (5) | PC-Pkd1KO (Aqp2Cre) mice (M, F) | PN 1 (20 weeks) | N | Y (1 M) | None | Improved survival; increased BW |
| Dai et al. [14] | Rosiglitazone (10) | Han: SPRD (Cy/+) rat (M) | Week 3 (8 weeks, 6–18 M) | Y (8 weeks) | Y (6 M) | None | Improved survival; increased heart and decreased liver weights >6 M |
| Blazer- Yost et al. [15] | Pioglitazone (4, 20) | PCK rat (M, F) | Week 3 (7 weeks) | Y (M) at 7 weeks both doses | NA | None | Decreased fractional liver weights at low dose |
| Pioglitazone (20) | PCK rat (F) | Week 4 (14 weeks) | Y (F) | NA | None | Decreased fractional liver weights | |
| Yoshihara et al. [16] | Pioglitazone (10) | PCK rat (M, F) | Week 4 (16 weeks) | Y | NA | None | Decreased fractional liver weights |
| Flaig et al. [17] | Rosiglitazone (4, 0.4, 0.04) | PCK rat (F) | Week 4 (24 days) | Y (0.04 mg/kg group only) | NA | Mortality in 4 mg/kg group due to cholangitis | No effect on liver or heart weights |
| Pioglitazone (2, 0.2) | Wpk rat (M, F) | PN 5 (14 days) | Y (0.2 mg/kg group only) | NA | None | Decreased heart weights in 0.2 mg/kg group | |
| Kanhai et al. [18] | Pioglitazone (30) | iKspCre-Pkd1del mice (PN18–19 induced) (M, F) | Week 5 (9–11 weeks) | N | NA | None | NA |
M, male; F, female; Y, yes; N, no; NA, not assessed; EDD, endothelium-dependent dilatation; BW, body weight; E, embryonic; PN, post-natal.
In conclusion, low-dose pioglitazone (15 mg) was found to be safe when given to a small cohort of ADPKD patients with early but rapidly progressive disease over 12 months. The risk of hypoglycaemia appeared to be mitigated at this dose, although exposure to a larger population with a wider range of age and kidney function could yet unmask a higher incident rate. The lack of increased oedema and heart failure was reassuring given concerns regarding cardiac safety signals unmasked in clinical trials of PPAR-γ agonists in diabetic patients [6].
The lack of efficacy on the main exploratory outcome of annual TKV growth was likely related to an insufficient sample size, although the additional possibility that a suboptimal dose was used cannot be excluded. The authors designed their study based on data suggesting that a lower dose of pioglitazone was more effective than a higher dose in rodent PKD models, arguing that the basis for this is a specific effect on cystic fibrosis transmembrane conductance regulator(CFTR) messenger RNA [19]. Despite this premise, it is apparent that a consistent effect of PPAR-γ agonists has not been found in all PKD rodent models tested so far, including orthologous ones (Table 1). In addition, both pleiotropic systemic and renal effects of these compounds beyond CFTR are likely given the widespread tissue and limited nephron expression of PPAR-γ (Figure 1).
The stage is now set for a Phase 2 parallel-arm multicentre trial of pioglitazone in ADPKD. With no options to slow disease progression in the last 150 years, new treatment possibilities for ADPKD are now rapidly emerging, including drug-repurposing approaches [20, 21]: PPAR-γ agonists could yet find a new lease on life in the treatment of ADPKD.
ACKNOWLEDGEMENTS
Z.M. acknowledges funding from the National Natural Science Foundation of China (82070705 and 81770670). A.C.M.O. acknowledges support from the Medical Research Council (UK), Kidney Research UK and the Sheffield Kidney Research Foundation.
FUNDING
A.C.M.O. has received research funding from ONO and Otsuka, lecture fees from Otsuka and consultancy fees from Sanofi-Genzyme, Galapagos and Mironid as a member of scientific advisory boards and steering committes outside the scope of this work. All grants and fees have been paid to his institution.
CONFLICT OF INTEREST STATEMENT
The results presented in this article have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1. Ong AC, Devuyst O, Knebelmann B. et al. Autosomal dominant polycystic kidney disease: the changing face of clinical management. Lancet 2015; 385: 1993–2002 [DOI] [PubMed] [Google Scholar]
- 2. Lanktree MB, Haghighi A, Guiard E. et al. Prevalence estimates of polycystic kidney and liver disease by population sequencing. J Am Soc Nephrol 2018; 29: 2593–2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Torres VE, Chapman AB, Devuyst O. et al. Tolvaptan in later-stage autosomal dominant polycystic kidney disease. N Engl J Med 2017; 377: 1930–1942 [DOI] [PubMed] [Google Scholar]
- 4. Mao Z, Ong AC.. Peroxisome proliferator-activated receptor gamma agonists in kidney disease—future promise, present fears. Nephron Clin Pract 2009; 112: c230–c241 [DOI] [PubMed] [Google Scholar]
- 5. Dormandy JA, Charbonnel B, Eckland DJ. et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive study (PROspective pioglitAzone clinical trial in macro vascular events): a randomised controlled trial. Lancet 2005; 366: 1279–1289 [DOI] [PubMed] [Google Scholar]
- 6. Nissen SE, Wolski K.. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 2007; 356: 2457–2471 [DOI] [PubMed] [Google Scholar]
- 7. Guan Y, Hao C, Cha DR. et al. Thiazolidinediones expand body fluid volume through PPARγ stimulation of ENaC-mediated renal salt absorption. Nat Med 2005; 11: 861–866 [DOI] [PubMed] [Google Scholar]
- 8. Vallon V, Hummler E, Rieg T. et al. Thiazolidinedione-induced fluid retention is independent of collecting duct alphaENaC activity. J Am Soc Nephrol 2009; 20: 721–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Blazer-Yost BL, Bacallao RL, Erickson BJ. et al. A randomized phase 1b cross-over study of safety of low-dose pioglitazone for treatment of autosomal dominant polycystic kidney disease. Clin Kidney J 2021; 14: 1738–1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Qayyum R, Adomaityte J.. A meta-analysis of the effect of thiazolidinediones on blood pressure. J Clin Hypertens (Greenwich) 2006; 8: 19–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ransick A, Lindstrom NO, Liu J. et al. Single-cell profiling reveals sex, lineage, and regional diversity in the mouse kidney. Dev Cell 2019; 51: 399–413.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Muto S, Aiba A, Saito Y. et al. Pioglitazone improves the phenotype and molecular defects of a targeted Pkd1 mutant. Hum Mol Genet 2002; 11: 1731–1742 [DOI] [PubMed] [Google Scholar]
- 13. Raphael KL, Strait KA, Stricklett PK. et al. Effect of pioglitazone on survival and renal function in a mouse model of polycystic kidney disease. Am J Nephrol 2009; 30: 468–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dai B, Liu Y, Mei C. et al. Rosiglitazone attenuates development of polycystic kidney disease and prolongs survival in Han:SPRD rats. Clin Sci (Lond) 2010; 119: 323–333 [DOI] [PubMed] [Google Scholar]
- 15. Blazer-Yost BL, Haydon J, Eggleston-Gulyas T. et al. Pioglitazone attenuates cystic burden in the PCK rodent model of polycystic kidney disease. PPAR Res 2010; 2010: 274376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yoshihara D, Kurahashi H, Morita M. et al. PPAR-γ agonist ameliorates kidney and liver disease in an orthologous rat model of human autosomal recessive polycystic kidney disease. Am J Physiol Renal Physiol 2011; 300: F465–F474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Flaig SM, Gattone VH, Blazer-Yost BL.. Inhibition of cyst growth in PCK and Wpk rat models of polycystic kidney disease with low doses of peroxisome proliferator-activated receptor γ agonists. J Transl Int Med 2016; 4: 118–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kanhai AA, Bange H, Verburg L. et al. Renal cyst growth is attenuated by a combination treatment of tolvaptan and pioglitazone, while pioglitazone treatment alone is not effective. Sci Rep 2020; 10: 1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nofziger C, Brown KK, Smith CD. et al. PPARγ agonists inhibit vasopressin-mediated anion transport in the MDCK-C7 cell line. Am J Physiol Renal Physiol 2009; 297: F55–F62 [DOI] [PubMed] [Google Scholar]
- 20. Chang M-Y, Ong ACM.. Targeting new cellular disease pathways in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant 2018; 33: 1310–1316 [DOI] [PubMed] [Google Scholar]
- 21. Malas TB, Leonhard WN, Bange H. et al. Prioritization of novel ADPKD drug candidates from disease-stage specific gene expression profiles. EBioMedicine 2020; 51: 102585. [DOI] [PMC free article] [PubMed] [Google Scholar]