Abstract
Background
Attaining the narrow haemoglobin (Hb) range recommended by European Renal Best Practice Guidelines renal anaemia guidelines may be difficult, and whether this leads to better outcomes following dialysis initiation is not known.
Methods
This was an observational study from the Swedish Renal Registry 2012–16, including all patients with non-dialysis-dependent chronic kidney disease (CKD) initiating renal anaemia treatment. We evaluated factors associated with off-target Hb attainment (<10 and >12 g/dL). For those who initiated dialysis, we explored associations between the pre-end-stage kidney disease (pre-ESKD) time in which Hb was within or above range, and pre-ESKD Erythropoietin Resistance Index (ERI) with the 1-year risk of death or major adverse cardiovascular events + (MACE+).
Results
About 5000 patients initiated anaemia treatment, contributing to 25 431 consecutive visits over time. Patients with polycystic kidney disease, diabetic nephropathy and nephrosclerosis, with recent bleeding/transfusion, with higher C-reactive protein or abnormal phosphate had higher odds of maintaining Hb below range. Conversely, patients with older age, CKD Stages 3b–4, pyelonephritis, kidney transplant, iron medication, higher ESA doses or abnormal serum calcium and albumin had higher odds of maintaining Hb above range. A total of 1361 patients initiated dialysis, among whom 220 deaths and 453 MACE+ occurred. A greater time spent with a pre-ESKD Hb >12 g/dL was associated with a lower risk of MACE+ (hazard ratio = 0.76; 95% confidence interval 0.61–0.94) after dialysis initiation, and a lower pre-ESKD Erythropoietin Resistance Index (ERI) was associated with improved survival (1.39; 1.02–1.90).
Conclusions
Our study identified populations that require additional efforts to control their Hb. Our outcome analysis supports the value of pre-ESKD anaemia care while illustrating the problems of ESA hyporesponsiveness in clinical practice.
Keywords: death, ESA hyporesponsiveness, haemoglobin control, MACE+, pre-ESKD
INTRODUCTION
Anaemia is a progressively common complication of chronic kidney disease (CKD) Stages 3–5 [1], primarily attributed to diminished oxygen sensing by the failing kidneys resulting in decreased synthesis of erythropoietin (EPO) and abnormalities affecting iron availability [2]. The current mainstays of renal anaemia treatment are erythropoiesis-stimulating agents (ESAs) and iron supplements [3]. Major clinical trials of ESA use in CKD demonstrated either no benefit or greater harm with normalizing haemoglobin (Hb) compared with lower targets for outcomes including mortality, cardiovascular events and time to dialysis initiation [4–6]. As a result, most guidelines, including the European Renal Best Practice Guidelines (ERBP), recommend a Hb target range of 10–12 g/dL [7]. However, because Hb is highly variable during treatment with ESAs, and multiple factors contribute to ESA hypo-responsiveness [8], attaining such a narrow target range may be challenging. Previous studies have identified predictors of poor Hb in patients with CKD through cross-sectional designs [9–12]. However, predictors may contribute variably over time, justifying the need for regular patient monitoring at the bedside and for longitudinal designs in epidemiological studies.
CKD is a disease continuum, and investigators are gradually adopting a patient perspective in their analytical approaches by evaluating the effect of nephrology care prior to the transition to dialysis on subsequent outcomes. Previous studies have observed that Hb variability [13, 14] or the consistency of pre-end-stage kidney disease (pre-ESKD) anaemia care (i.e. treated versus untreated) [15–17] in the transition period to dialysis predicts subsequent outcomes. Whether attainment of Hb targets or resistance to ESA therapy (which may be a surrogate for inflammation or other comorbidities associated with more severe disease) during pre-ESKD care is associated with outcomes following dialysis initiation is not well studied. We utilized a nationwide cohort of nephrologist-referred patients with CKD receiving anaemia treatment to identify longitudinal predictors of Hb target attainment and to evaluate the association between pre-ESKD Hb target attainment, ESA resistance and outcomes following dialysis initiation.
MATERIALS AND METHODS
Data source
We used data from the Swedish Renal Registry (SRR), a nationwide registry of patients with CKD G3–5 attending routine nephrologist specialist care in Sweden [18, 19]. The SRR collects routine information from stable outpatient nephrologist visits, including attained Hb, use of iron [and type, i.e. intravenous (IV) or oral] and ESA (type and dose). Patients with an incident estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2 should be mandatorily enrolled in the registry, but the registry also encourages the inclusion of patients earlier in the course of the disease (<45 mL/min/1.73 m2) as long as patients are included systematically by the Nephrology clinic. Registrations of subsequent outpatient visits to nephrology care (on average 2–3/year/patient) are thereafter recorded until the start of kidney replacement therapy, death or emigration from the country. Nearly all Nephrology clinics in Sweden (96%) report to the SRR and the estimated national coverage is >75% for nephrologist-referred patients with recognized G4–5 CKD [20]. Via each citizen’s unique personal identification number, the SRR was linked to other government-run registries: the Swedish prescribed drug registry provided complete information on all prescribed drugs dispensed at Swedish pharmacies [21]; the Swedish Patient Registry provided information on all outpatient specialist consultations and hospitalizations occurring in Swedish healthcare from 1997 until end of follow-up for information on comorbidities and non-fatal outcomes [22]; and the Swedish Death Registry added information on date and causes of death [23]. Government-run registries are considered to have no or minimal loss to follow-up. The study was approved by the regional ethical review boards and the Swedish National Board of Welfare.
Patient selection
For this study, we included all adult patients recorded between 2012 and 2016 with eGFR <45 mL/min/1.73 m2 from the initiation of anaemia therapy (ESA, iron or both). We chose this period because it followed the publication of ESA trials and ERBP guidelines. Patients were defined from the index date of first detected anaemia treatment, and data from every subsequent visit after cohort entry was extracted. Individuals who had fewer then two2 visits registered during the non-dialysis phase were excluded, as this was deemed insufficient to evaluate longitudinal determinants of Hb control. We also excluded individuals with a recent or ongoing cancer, defined as a cancer diagnosis within 3 years before index date, and patients whose first patient visit was preceded (within 30 days) by a bleeding episode, or a hospitalization regardless of the cause.
Exposure and outcomes
This study includes two separate cohorts of patients and analyses. In the first cohort, we evaluated all patient visits until death, dialysis or end of follow-up to identify predictors of Hb target attainment. Predictors tested were identified based on biological plausibility, and included demographics, comorbidities (detailed in Supplementary data, Table S1), medications (detailed in Supplementary data, Table S2) and laboratory values. The study outcomes were Hb <10 g/dL and >12 g/dL at each nephrologist visit, and the Hb range 10–12 g/dL was considered the reference category per ERBP guidelines.
The second cohort included patients from the first cohort who had transitioned to dialysis. In this second cohort, we evaluated the association between pre-ESKD Hb attainment, Erythropoietin Resistance Index (ERI) and outcomes during the first year upon initiation of dialysis. The date of dialysis start was the index date. Using all preceding information during pre-ESKD care, we assumed a linear relationship between recorded Hb values (Supplementary data, Figure S1) and calculated the proportion of patient time (in days) until dialysis start in which Hb was maintained within the recommended range of 10–12 g/dL (time in range, TIR) or 12 g/dL (time above range, TAR). Their estimations were as follows:
We did not calculate the proportion of time spent ˂10 g/dL (time below range, TBR), because the proportion of Hb measurements within this range was negligible. The sum of TAR, TIR and TBR equals 100% of the patient’s pre-ESKD recorded time on anaemia treatment.
The Erythropoietin Resistance Index (ERI) was calculated as the weekly weight-adjusted dose of EPO divided by the attained Hb level [24] in all recorded pre-ESKD visits on ESA, and the mean pre-ESKD ERI was computed per patient.
The study outcomes for the second cohort were the occurrence of death (by any cause), and major adverse cardiovascular events + (MACE+). MACE+ was defined as the composite occurrence of non-fatal myocardial infarction, stroke, heart failure or death attributed to cardiovascular disease (CVD), whichever happened first. Outcome definitions are detailed in Supplementary data, Table S3.
Covariates
Demographic data (i.e. age and sex), current medications and clinical data were obtained as entered into the SRR on each visit by the local administrators of each Nephrology unit. Clinical information collected encompasses information on CKD aetiology, body mass index (BMI), routine laboratory biomarkers and CKD-anaemia-specific medications including the use of IV, oral iron and ESA (with doses). Weekly ESA doses for darbepoetin and methoxy polyethylene glycol–epoetin beta were converted to a weekly epoetin equivalent dose derived from the allocated daily doses defined by the World Health Organization Collaborating Centre [25]. Conversion factors of 1:222 and 1:250 were used for the conversion of epoetin:darbepoetin and epoetin:methoxy polyethylene glycol–epoetin beta, respectively. According to the SRR manual, a patient was considered as treated with IV iron for up to 6 months after the last administration of low-frequency, high-dose IV iron. Use of oral iron was identified from pharmacy dispensations as recorded in the Prescribed Drugs Registry up to 3 months before each visit. Comorbid history was defined from relevant diagnoses (International Classification of Diseases 10th revision) or surgical procedure (Nordic Medico-Statistical Committee classification system) before the index date and since 1997, when these classification systems were implemented in Sweden (definitions listed in Supplementary data, Table S1). Use of other relevant medications were defined either as per SRR variables or as per pharmacy dispensations (Anatomical Therapeutic Chemical classification codes) at time of each recorded visit and within 6 months before (definitions listed in Supplementary data, Table S2).
Statistical analyses
Descriptive statistics are presented with continuous variables shown as mean and standard error, if normally distributed, and median and interquartile range (IQR) otherwise.
We used multinomial logistic regression with cluster robust standard errors to estimate the odds ratios (ORs) for Hb target attainment through all repeated patient visits. Predictors were identified on the basis of biological plausibility and updated with values at each visit. In addition, we considered the previous renal anaemia management prescription as a predictor of the current Hb value, as an abnormal Hb value would prompt an adjustment in ESA dose to correct it, and this will impact on the next Hb recorded. Information on some variables was not complete, and the proportion of missingness is reported in Supplementary data, Table S4. We considered these values missing at random, and we used multiple chain equations to generate five imputed datasets with all other covariates as auxiliary covariates in the modelling. The frequency of reporting of outpatient Nephrology visits varies across nephrology units and regions in Sweden. To evaluate the robustness of our results, we repeated our main analysis in patients from the nephrology units that report all outpatient visits into the register.
Next, we used natural cubic splines (with truncated power series as basic functions and knots at 10, 50 and 90% quantiles of distribution) to graphically depict the association between pre-ESKD TIR, TAR and mean ERI and the rate of death or MACE+ during the first year following dialysis initiation. We used Cox regression for the rate of death, Cox regression with competing risk (non-CVD death as competing risk) for the rate of MACE+ associated with categories of TIR, TAR and mean ERI (median value).
All analyses were performed using R version 3.4.3 software (The R Project for Statistical Computing, Vienna, Austria).
RESULTS
Cohort characteristics
After applying inclusion and exclusion criteria, we identified 5000 adult patients with CKD Stages 3b–5 who initiated anaemia treatment in Sweden during 2012–16 (Supplementary data, Figure S2). Characteristics at inclusion are shown in Table 1; patients had a mean age of 69 [standard deviation (SD) 15] years, and 45% were women. Diabetic nephropathy and nephrosclerosis were the main reported causes of CKD, accounting for 25 and 24% of cases, respectively. The majority of patients who initiated anaemia therapy were patients with CKD Stages 4 and 5, and ∼2% were kidney transplant recipients. Hypertension was the most prevalent comorbidity (88% of patients), followed by diabetes (45%) and CVD (43%). As many as 40% of patients initiated iron only, 41% patients initiated ESA only and 19% initiated both ESA and iron.
Table 1.
Baseline characteristics of included patients at the time of their first recorded visit with anaemia treatment
| Covariates | Overall |
|---|---|
| n | 5000 |
| Age, mean (SD), years | 69 (15) |
| Women | 2229 (45) |
| BMI, kg/m2 | 26.6 (23.5–30.8) |
| CKD stages, mL/min/1.73 m2 | |
| G3b 30–44 | 825 (16) |
| G4 15–29 | 2442 (49) |
| G5 <15 | 1733 (35) |
| CKD aetiology | |
| Diabetic nephropathy | 1266 (25) |
| Nephrosclerosis | 1190 (24) |
| Glomerulonephritis | 503 (10) |
| Pyelonephritis | 130 (3) |
| Polycystic kidney disease | 260 (5) |
| Other | 979 (20) |
| Unknown | 672 (13) |
| Kidney transplanted | 78 (2) |
| Comorbidities, n (%) | |
| Hypertension | 4380 (88) |
| Diabetes mellitus | 2253 (45) |
| CVD | 2650 (53) |
| Medications, n (%) | |
| Initial anaemia treatment | |
| Only iron (IV or oral) | 1998 (40) |
| Only ESA | 2066 (41) |
| ESA dose (IU/week) | 4000 (2200–5874) |
| Iron and ESA | 936 (19) |
| ESA dose (IU/week) | 4000 (2800–6000) |
| Statin | 2900 (58) |
| Sodium bicarbonate | 2336 (47) |
| Chemistry, median (IQR) | |
| hsCRP, mg/L | 5.0 (2.0–10.0) |
| Ca2+, mmol/L | 2.3 (2.2–2.4) |
| PO4−, mmol/L | 1.3 (1.2–1.6) |
| PTH, ng/L | 16.4 (10.0–27.0) |
| Albumin, g/L | 37 (34–39) |
Data are presented as mean (SD), median (IQR) or counts (proportion), as appropriate.
Ca2+, calcium; PO4−, phosphate; PTH, parathyroid hormone.
Predictors of Hb below and above ERBP recommended range
Included patients contributed to 25 431 consecutive visits for the analysis of predictors of off target Hb attainment. The majority of Hb measurements (50%) were kept within ERBP recommended range; 39% of measurements were 12 g/dL, mostly between 12 and 13 g/dL (23%); and only 9% were ˂10 g/dL. During follow-up, most visits (n = 14 768, 58%) recorded the use of ESA, of which 7931 visits (31%) recorded the use of ESA in combination with iron. Furthermore, 7072 (28%) visits recorded the use of iron treatment only. The remaining visits (n = 3591, 14%) corresponded to periods that did not require anaemia treatment.
Multivariable predictors of off-target Hb are shown in Table 2. Briefly, men were more likely than women to have Hb values outside target range (both below and above). Patients with age 65–75 years, CKD Stages 3b and 4, pyelonephritis, transplant recipients, having received iron medication or higher EPO doses, or with higher serum calcium and albumin levels were at higher risk of maintaining Hb values above target range. Conversely, patients with polycystic kidney disease, diabetic nephropathy and nephrosclerosis, those having a recent bleeding or receiving transfusion, those with inflammation [C-reactive protein (CRP) >5 mg/dL], and higher phosphate levels were at increased risk of having Hb values below target range.
Table 2.
Multinomial adjusted OR and 95% CIs associated with Hb <10 g/dL and >12 g/dL ERBP recommended target range throughout 25 431 consecutive patients visits
| Covariates | OR (95% CI) |
|
|---|---|---|
| Hb <10 g/dL | Hb >12 g/dL | |
| Age <65 years | Reference | Reference |
| Age 65–75 years | 0.88 (0.76–1.02) | 1.13 (1.04–1.23) |
| Age ≥75 years | 0.85 (0.74–0.98) | 0.98 (0.90–1.06) |
| Men | 1.23 (1.11–1.37) | 1.22 (1.15–1.30) |
| BMI <18.5 kg/m2 | Reference | Reference |
| BMI 18.5–25 kg/m2 | 0.97 (0.68–1.39) | 1.00 (0.81–1.24) |
| BMI ≥25 kg/m2 | 0.86 (0.77–0.97) | 0.99 (0.93–1.06) |
| CKD Stage G5 | Reference | Reference |
| CKD Stage G4 | 0.80 (0.71–0.91) | 1.34 (1.24–1.44) |
| CKD Stage G3b | 0.59 (0.47–0.75) | 1.85 (1.66–2.06) |
| Glomerulonephritis | Reference | Reference |
| Pyelonephritis | 1.32 (0.89–1.95) | 1.34 (1.10–1.62) |
| Diabetic nephropathy | 1.29 (1.03–1.63) | 0.75 (0.66–0.84) |
| Polycystic kidney disease | 1.49 (1.12–1.98) | 0.77 (0.66–0.90) |
| Nephrosclerosis | 1.52 (1.22–1.89) | 0.82 (0.73–0.92) |
| Other aetiology | 1.24 (0.996–1.54) | 0.85 (0.76–0.95) |
| Unknown aetiology | 1.38 (1.09–1.76) | 0.88 (0.78–0.996) |
| Kidney transplanted | 0.78 (0.39–1.59) | 1.83 (1.51–2.22) |
| Diabetes mellitus | 1.08 (0.93–1.25) | 1.02 (0.94–1.10) |
| Hypertension | 0.96 (0.81–1.15) | 1.05 (0.96–1.16) |
| CVD | 1.06 (0.95–1.20) | 0.97 (0.91–1.04) |
| Recent transfusion | 1.26 (1.10–1.45) | 0.96 (0.88–1.05) |
| Recent bleeding | 1.20 (1.05–1.37) | 1.02 (0.95–1.09) |
| Iron medication use | 0.96 (0.87–1.07) | 1.06 (1.002–1.13) |
| Non-use of ESA | Reference | Reference |
| ESA <3600 IU/week | 0.72 (0.63–0.83) | 0.87 (0.81–0.94) |
| ESA 3600–6400 IU/week | 0.95 (0.82–1.10) | 1.18 (1.08–1.29) |
| ESA ≥6400 IU/week | 1.07 (0.91–1.25) | 1.35 (1.21–1.50) |
| Statin | 0.86 (0.77–0.96) | 1.04 (0.98–1.11) |
| Sodium bicarbonate | 0.85 (0.76–0.95) | 0.99 (0.93–1.06) |
| hsCRP <5 mg/dL | Reference | Reference |
| hsCRP ≥5 mg/dL | 1.16 (1.04–1.30) | 0.91 (0.86–0.97) |
| Ca2+ (mmol/L) low tertile | 1.36 (1.20–1.54) | 0.82 (0.76–0.88) |
| Ca2+ (mmol/L) mid tertile | Reference | Reference |
| Ca2+ (mmol/L) high tertile | 0.81 (0.69–0.94) | 1.21 (1.13–1.30) |
| PO4− (mmol/L) low tertile | 0.88 (0.76–1.02) | 1.27 (1.19–1.37) |
| PO4− (mmol/L) mid tertile | Reference | Reference |
| PO4− (mmol/L) high tertile | 1.33 (1.17–1.51) | 0.90 (0.83–0.98) |
| PTH (ng/L) low tertile | 1.07 (0.93–1.24) | 1.00 (0.93–1.08) |
| PTH (ng/L) mid tertile | Reference | Reference |
| PTH (ng/L) high tertile | 1.11 (0.98–1.26) | 0.99 (0.92–1.07) |
| Albumin (per g/dL higher) | 0.93 (0.92–0.94) | 1.05 (1.04–1.05) |
| Previous Hb (per g/dL higher) | 0.97 (0.97–0.98) | 1.05 (1.05–1.06) |
Bold text indicates statistically significant higher or lower odds. Ca2+, calcium; PO4−, phosphate; PTH, parathyroid hormone.
We observed similar results when we evaluated the subset of patients followed at the nephrology units that report all performed visits into the register (Supplementary data, Table S5).
Pre-ESKD Hb target attainment, ERI and outcomes following dialysis initiation
Of the initial 5000 included patients, 1361 (27%) initiated chronic dialysis during the observation period. Characteristics of these individuals at the time of dialysis initiation are described in Table 3. Mean (SD) age of these patients was 65 (15) years, and 41% were women. The most common comorbidities were hypertension (93%), diabetes mellitus (49%) and heart failure (27%). There were 6587 recorded patient visits recorded to compute pre-ESKD TIR, TAR and mean ERI. The median (IQR) pre-ESKD TIR was 56% (31–79%), median (IQR) TAR 11% (0–43%), with their distributions shown in Supplementary data, Figure S3. The median (IQR) ERI was 0.52 (0.35–0.78) IU/kg/week/g/dL, and lower ERI was associated with higher categories of attained Hb (P for trend <0.001; Figure 1).
Table 3.
Baseline characteristics of included patients at the time of initiation of chronic dialysis
| Covariate | Overall |
|---|---|
| n | 1361 |
| Age, years | 65 (15) |
| Haemodialysis (%) | 846 (62) |
| Peritoneal dialysis (%) | 515 (38) |
| Women (%) | 553 (41) |
| BMI, kg/m2 | 26.4 (23.5, 30.3) |
| Year of dialysis start (%) | |
| 2012–13 | 425 (31) |
| 2014–15 | 308 (23) |
| 2016–17 | 628 (46) |
| CKD aetiology | |
| Diabetic nephropathy | 433 (32) |
| Nephrosclerosis | 232 (17) |
| Glomerulonephritis | 180 (13) |
| Pyelonephritis | 31 (2) |
| Polycystic kidney disease | 120 (9) |
| Other | 239 (18) |
| Unknown | 126 (9) |
| Comorbidities, n (%) | |
| Diabetes mellitus | 667 (49) |
| Hypertension | 1262 (93) |
| Myocardial infarction | 261 (19) |
| Heart failure | 369 (27) |
| Cerebrovascular disease | 224 (16) |
| Peripheral vascular disease | 227 (17) |
| Atrial fibrillation | 203 (15) |
| Stroke | 147 (11) |
| Medications, n (%) | |
| ESA | 1256 (92) |
| Iron | 919 (68) |
| ACEIs and ARBs | 933 (69) |
| β-blockers | 1041 (76) |
| Calcium channel blockers | 1099 (81) |
| Statin | 805 (59) |
| Phosphate binders | 1134 (83) |
| Sodium bicarbonate | 1042 (77) |
| Characteristics of their pre-ESKD period | |
| ERI from all pre-ESKD visits, IU/kg/week/g/dL | 0.5 (0.4–0.8) |
| Slope of eGFR decline, mL/min/1.73 m2/year | −4.0 (0.2) |
| Days observed during pre-ESKD | 463 (264–788) |
Data are presented as mean (SD), median (IQR) or counts (proportion), as appropriate.
ACEIs and ARBs, angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers.
FIGURE 1:
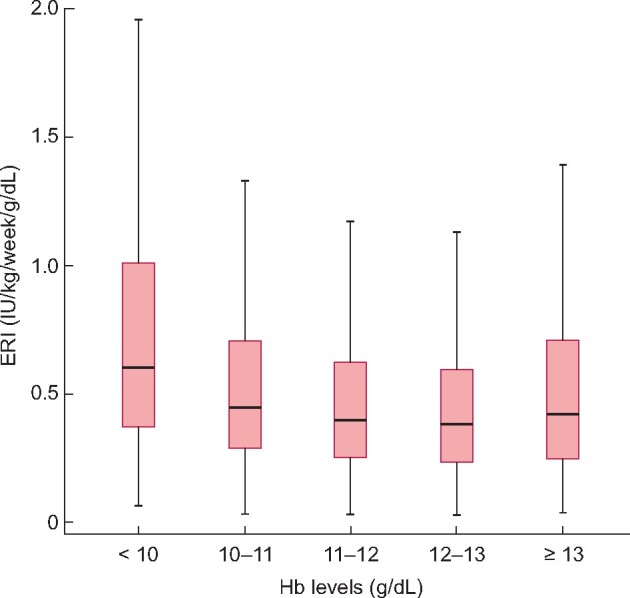
ERI distribution across different Hb levels during pre-ESKD recorded visits for patients initiating chronic dialysis.
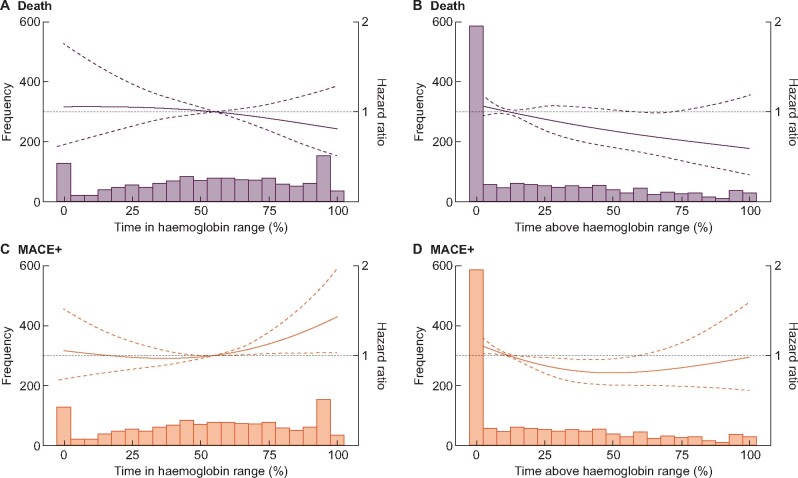
There were 220 deaths recorded during the first year of dialysis. On a continuous scale (Figure 2A and B), we did not observe any association between pre-ESKD TIR and death, but a trend towards lower risk of death was observed as TAR increased. In categorical analyses, patients above median pre-ESKD TIR (56%) [hazard ratio (HR) = 0.96; 95% confidence interval (CI) 0.69–1.33] or TAR (11%) (HR = 0.81; 95% CI 0.59–1.11) were not at a different risk of death compared with patients below these thresholds.
FIGURE 2:
Multivariable-adjusted [adjusted for age, sex, BMI, initial dialysis therapy (haemodialysis or peritoneal dialysis), calendar year of dialysis start, diabetes, hypertension, myocardial infarction, stroke, peripheral vascular disease, heart failure, atrial fibrillation, ACEi/ARBs, beta-blockers, calcium blockers, ESA use, iron medication use, statins, phosphate binders, sodium bicarbonate, person-months with renal anaemia during their pre-ESKD phase and slope of eGFR decline during their pre-ESKD phase.] associations between pre-ESKD TIR, TAR and the rate (hazard) of death and MACE+ during the first year after initiation of dialysis. ACEIs and ARBs, angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers.
There were 453 MACE+ events recorded during the first year of dialysis. On a continuous scale (Figure 2C and D), we did not observe a clear association between TIR, TAR and the risk of MACE+. On a categorical scale, patients with TIR above the median (56%) were at a 26% higher relative risk of MACE+ that did not reach statistical significance (HR = 1.26; 95% CI 0.99–1.58). Patients with TAR above the median (11%) were at a statistically significantly 24% lower risk of MACE+ (HR = 0.76; 95% CI 0.61–0.94) compared with patients with TAR <11%.
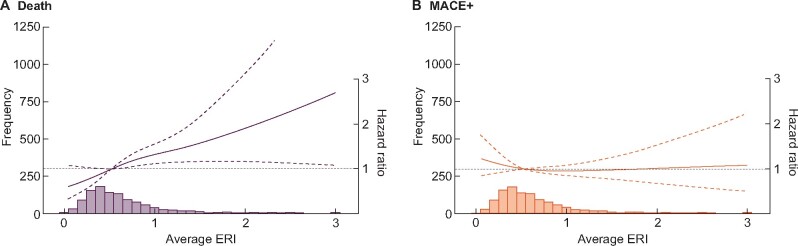
On a continuous scale, higher pre-ESKD mean ERI were associated with an increased risk of death, but no association was found between ERI and MACE+ (Figure 3). On a categorical scale, patients with a pre-ESKD ERI above the mean had a significant higher risk of death (HR = 1.39; 95% CI 1.02–1.90), but no effect on risk of MACE + (HR = 0.87; 95% CI 0.70–1.08) compared with patients below this threshold.
FIGURE 3:
Multivariable-adjusted [adjusted for age, sex, BMI, initial dialysis therapy (haemodialysis or peritoneal dialysis), calendar year of dialysis start, diabetes, hypertension, myocardial infarction, stroke, peripheral vascular disease, heart failure, atrial fibrillation, ACEi/ARBs, beta-blockers, calcium blockers, ESA use, iron medication use, statins, phosphate binders, sodium bicarbonate, person-months with renal anaemia during their pre-ESKD phase and slope of eGFR decline during their pre-ESKD phase] associations between pre-ESKD mean ERI and the rate (hazard) of death and MACE+ during the first year after initiation of dialysis. ACEIs and ARBs, angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers.
DISCUSSION
Few studies have evaluated Hb predictors in cross-sectional designs [9–12], and we believe this is the only analysis to date that evaluates Hb target attainment longitudinally through the patient’s pre-ESKD journey. As many as 50% of the patient visits in our study reported a Hb within the recommended 10–12 g/dL range, a proportion that agrees with cross-sectional evidence from the dialysis population of the UK [10] or the USA [26]. A high proportion of the remaining patient visits (39%) in our study had Hb measurements above the target range, which also agrees with previous reports of anaemia prevalence in CKD [9–12]. This may reflect the difficulties in adjusting ESA doses in order to achieve narrow Hb targets, and/or it may represent the belief that patients with good response to ESAs—who may easily achieve higher Hb levels—have improved survival. In this sense, our finding that the immediately previous Hb value, iron use and ESA dose are important for predicting Hb both below and above target range is a reflection of such process [27, 28].
Patients with CKD Stage 5 were more likely to maintain Hb in range than patients with CKD Stage 3b or 4, a difference that we speculate may be explained by the more frequent nephrologist consultation and better care at later CKD stages including better Hb monitoring and management. Likewise, patients with a functioning kidney transplant and attending nephrology check-ups were more likely to be above range, which may reflect a more aggressive management of post-transplant anaemia [29, 30]. Men were more likely than women to have Hb outside range, which aligns with and expands a recent report of dialysis patients [31]. This difference may be explained by the lower Hb that healthy women have compared with men [32], together with a poorer response to ESAs [33, 34] and a need for higher ESA doses in women to achieve similar haematocrits as men [33].
This analysis both revealed new predictors of Hb control and confirmed factors previously reported. Our observations that pyelonephritis predicts high Hb values and that polycystic kidney disease, diabetic nephropathy and nephrosclerosis predict low Hb values are possibly novel. While it is accepted that renal anaemia manifests at a lower eGFR in patients with polycystic kidney disease [35], this is a selection of patients in whom anaemia developed and treatment was initiated. We found no prior studies evaluating anaemia management in patients of this aetiology. Alterations in laboratory biomarkers also predicted Hb target attainment in our study: elevated high-sensitivity CRP (hsCRP) predicted the likelihood to have low Hb values, which aligns with our understanding of inflammation as a key factor for resistance to ESA [36]. In agreement with our results, abnormalities in phosphorus and calcium have been associated with abnormal Hb and altered response to ESA [37, 38]. It is not known whether the associations are mediated through some other component of the CKD–mineral bone disorder syndrome, direct effects of these electrolytes on red blood cells [39, 40] or both. Collectively, our results credibly illustrate the multiple conditions that may affect Hb control, and identify many of the factors that lead to ESA hypo-responsiveness. As a clinical application, knowledge of patient phenotypes with difficulties in attaining Hb targets may allow the implementation of corrective measures, through more stringent monitoring of Hb, intensified or alternative therapeutic strategies.
Early studies have addressed the value of treating anaemia during the transition to dialysis [16, 17]. Recently, Wetmore et al. [41] found that compared with patients with less consistent or no pre-ESKD ESA treatment, patients who received more consistent pre-ESKD ESA treatment had lower risks for death 1 year after the initiation of dialysis. Complementing those studies, we hypothesized that the maintenance of Hb within the recommended target ranges during pre-ESKD care would associate with better outcomes after dialysis initiation. While in our study the time spent with Hb within the recommended ERBP range was not associated with better outcome, we observed that individuals who more frequently maintained Hb above the recommended range (higher TAR) seemed to experience lower MACE risk compared with those with lower TAR. This may sound counterintuitive, given the results from pivotal trials in which targeting higher Hb values with ESA led to increased death/MACE risk [5, 6, 42]. However, our analysis did not evaluate only Hb targets, but achieved Hb levels, which is the result of the strategies used to reach the targets and is influenced by patient’s adherence and response to treatment, comorbidities, frailty and lifestyle behaviours. We note that most achieved Hb measurements above range in our study were between 12 and 13 mg/L, and we speculate that a patient more often having Hb within this range is likely someone responding well to ESA treatment. In support of this, our study observed lower ERI with higher attained Hb in this range. It is well established that patients with ESA-resistant renal anaemia have a poorer prognosis than those without [24, 43, 44], and failure to account for individual patients’ response to ESA has been proposed [45] to explain the divergence between observational studies comparing the mortality risk of long-acting versus short-acting ESA [46] and meta-analyses of randomized controlled trials [47]. We expand this evidence by showing a higher risk of death for patients with consistently low pre-ESKD ESA responsiveness (high mean pre-ESKD ERI). Our findings on the importance of ESA resistance support a post hoc analysis of the CHOIR study demonstrating that ESA dose level, rather than actual Hb level attained, contributes to poor outcome [48], and expands observational analyses in patients on dialysis showing that the time spent with off-target Hb values, rather than Hb variability, explains associations with death [49].
Our study had both strengths and limitations. Key strengths are the complete longitudinal coverage of nephrology-referred patients across a broad CKD spectrum in a nationwide registry with detailed information about diagnosis, laboratory data and treatments. The possibility of linking those records to other healthcare resources allowed us to obtain extensive data on comorbidities, other medications and outcomes. Our main limitation is that we can only evaluate the nephrology visits that are reported to the SRR. Furthermore, while inclusion of patients with CKD 4 in the SRR is mandatory and has virtually complete national coverage, patients with CKD Stage 3b are recorded by the physicians on a voluntary basis and therefore the coverage is likely to be less complete. Thus, it is not known whether non-registered patients or visits differ meaningfully from those who are registered. We recognize that the number of patients initiating dialysis in our study was low, which limits the strength of our conclusions. We acknowledge the lack of information on important haematological covariates including iron dose, ferritin and transferrin saturation, and that causality between the associations reported cannot be inferred from this or any other observational study.
To conclude, this nationwide study of nephrologist-referred patients with non-dialysis-dependent CKD stages treated for renal anaemia identified populations in which efforts to control Hb may require additional or alternative treatment. A greater time spent in a pre-ESKD Hb target range >12 g/dL and a lower pre-ESKD ERI predicted improved survival after dialysis initiation, supporting the value of pre-ESKD anaemia care and illustrating the problems of addressing ESA hyporesponsiveness in clinical practice.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
Supplementary Material
FUNDING
This study was supported by an institutional grant from AstraZeneca to Karolinska Institutet. In addition, we acknowledge grant support from the Swedish Research Council (grant number 2019-01059) and the Swedish Heart and Lung Foundation.
CONFLICT OF INTEREST STATEMENT
M.E. reports speaker or advisory board fees from AstraZeneca, Astellas Pharma and Vifor Pharma. G.J. is employed by AstraZeneca. J.J.C. reports funding from Astellas and Vifor Pharma outside the submitted work, and speaker or advisory board fees from Baxter, AstraZeneca and Astellas Pharma. P.B., A.S. and Y.X. have no conflicts of interest to report.
REFERENCES
- 1. Locatelli F, Pisoni RL, Combe C. et al. Anaemia in haemodialysis patients of five European countries: association with morbidity and mortality in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 2004; 19: 121–132 [DOI] [PubMed] [Google Scholar]
- 2. Del Vecchio L, Locatelli F.. New treatment approaches in chronic kidney disease-associated anaemia. Expert Opin Biol Ther 2014; 14: 687–696 [DOI] [PubMed] [Google Scholar]
- 3. Macdougall IC. Anaemia of chronic kidney disease. Medicine 2007; 35: 457–460 [Google Scholar]
- 4. Drüeke TB, Locatelli F, Clyne N. et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 2006; 355: 2071–2084 [DOI] [PubMed] [Google Scholar]
- 5. Pfeffer MA, Burdmann EA, Chen CY. et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 2009; 361: 2019–2032 [DOI] [PubMed] [Google Scholar]
- 6. Singh AK, Szczech L, Tang KL. et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 2006; 355: 2085–2098 [DOI] [PubMed] [Google Scholar]
- 7. Locatelli F, Aljama P, Canaud B. et al. ; on behalf of the Anaemia Working Group of European Renal Best Practice (ERBP). Target haemoglobin to aim for with erythropoiesis-stimulating agents: a position statement by ERBP following publication of the Trial to Reduce Cardiovascular Events with Aranesp® Therapy (TREAT) Study. Nephrol Dial Transplant 2010; 25: 2846–2850 [DOI] [PubMed] [Google Scholar]
- 8. Ifudu O. Patient characteristics determining rHuEPO dose requirements. Nephrol Dial Transplant 2002; 17 (Suppl 5): 38–41 [DOI] [PubMed] [Google Scholar]
- 9. Pisoni RL, Bragg-Gresham JL, Young EW. et al. Anemia management and outcomes from 12 countries in the dialysis outcomes and practice patterns study (DOPPS). Am J Kidney Dis 2004; 44: 94–111 [DOI] [PubMed] [Google Scholar]
- 10. Evans K, Pyart R, Steenkamp R. et al. UK renal registry: 20th annual report of the renal association. Nephron 2018; 139: 1–12 [DOI] [PubMed] [Google Scholar]
- 11. Cases-Amenós A, Martínez-Castelao A, Fort-Ros J. et al. Prevalencia de anemia y su manejo clínico en la enfermedad renal crónica estadios 3-5 no en diálisis en Cataluña: estudio MICENAS I. Nefrología (Madrid) 2014; 34: 189–198 [Google Scholar]
- 12. Ryu SR, Park SK, Jung JY. et al. The prevalence and management of anemia in chronic kidney disease patients: result from the Korean cohort study for outcomes in patients with chronic kidney disease (KNOW-CKD). J Korean Med Sci 2017; 32: 249–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kleine CE, Soohoo M, Ranasinghe ON. et al. Association of pre-end-stage renal disease hemoglobin with early dialysis outcomes. Am J Nephrol 2018; 47: 333–342 [DOI] [PubMed] [Google Scholar]
- 14. Sumida K, Diskin CD, Molnar MZ. et al. Pre-end-stage renal disease hemoglobin variability predicts post-end-stage renal disease mortality in patients transitioning to dialysis. Am J Nephrol 2017; 46: 397–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Target guideline 5: target haemoglobin concentration for the treatment of the anaemia of chronic renal failure. Nephrol Dial Transplant 1999; 14 (Suppl 5): 11 [Google Scholar]
- 16. Xue JL, St Peter WL, Ebben JP. et al. Anemia treatment in the pre-ESRD period and associated mortality in elderly patients. Am J Kidney Dis 2002; 40: 1153–1161 [DOI] [PubMed] [Google Scholar]
- 17. Fink J, Blahut S, Reddy M. et al. Use of erythropoietin before the initiation of dialysis and its impact on mortality. Am J Kidney Dis 2001; 37: 348–355 [DOI] [PubMed] [Google Scholar]
- 18. Evans M, Carrero JJ, Bellocco R. et al. Initiation of erythropoiesis-stimulating agents and outcomes: a nationwide observational cohort study in anaemic chronic kidney disease patients. Nephrol Dial Transplant 2017; 32: 1892–1901 [DOI] [PubMed] [Google Scholar]
- 19. Evans M, Suttorp MM, Bellocco R. et al. Trends in haemoglobin, erythropoietin-stimulating agents and iron use in Swedish chronic kidney disease patients between 2008 and 2013. Nephrol Dial Transplant 2016; 31: 628–635 [DOI] [PubMed] [Google Scholar]
- 20. Donal E, Thebault C, Lund LH. et al. Heart failure with a preserved ejection fraction additive value of an exercise stress echocardiography. Eur Heart J Cardiovasc Imaging 2012; 13: 656–665 [DOI] [PubMed] [Google Scholar]
- 21. Wettermark B, Hammar N, Fored CM. et al. The new Swedish prescribed drug register–opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf 2007; 16: 726–735 [DOI] [PubMed] [Google Scholar]
- 22. Ludvigsson JF, Andersson E, Ekbom A. et al. External review and validation of the Swedish national inpatient register. BMC Public Health 2011; 11: 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brooke HL, Talback M, Hornblad J. et al. The Swedish cause of death register. Eur J Epidemiol 2017; 32: 765–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. López-Gómez JM, Portolés JM, Aljama P.. Factors that condition the response to erythropoietin in patients on hemodialysis and their relation to mortality. Kidney Int Suppl 2008; 74: S75–S81 [DOI] [PubMed] [Google Scholar]
- 25.World Health Organization. Collaborating Center for Drug Statistics Methodology. Guidelines for ATC Classification and DDD assignment.2019. https://www.whocc.no/ (2019, date last accessed)
- 26.US Renal Data System. Annual data report. https://www.usrds.org/ (2018, date last accessed)
- 27. Fishbane S, Nissenson AR.. Anemia management in chronic kidney disease. Kidney Int 2010; 78: S3–S9 [DOI] [PubMed] [Google Scholar]
- 28. Coyne DW. Influence of industry on renal guideline development. Clin J Am Soc Nephrol 2007; 2: 3–7 [DOI] [PubMed] [Google Scholar]
- 29. Djamali A, Samaniego M, Muth B. et al. Medical care of kidney transplant recipients after the first posttransplant year. Clin J Am Soc Nephrol 2006; 1: 623–640 [DOI] [PubMed] [Google Scholar]
- 30. Molnar MZ, Mucsi I, Macdougall IC. et al. Prevalence and management of anaemia in renal transplant recipients: data from ten European centres. Nephron Clin Pract 2011; 117: c127–c134 [DOI] [PubMed] [Google Scholar]
- 31. Weigert A, Drozdz M, Silva F. et al. Influence of gender and age on haemodialysis practices: a European multicentre analysis. Clin Kidney J 2020; 13: 217–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ferrucci L, Maggio M, Bandinelli S. et al. Low testosterone levels and the risk of anemia in older men and women. Arch Intern Med 2006; 166: 1380–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Madore F, Lowrie EG, Brugnara C. et al. Anemia in hemodialysis patients: variables affecting this outcome predictor. J Am Soc Nephrol 1997; 8: 1921–1929 [DOI] [PubMed] [Google Scholar]
- 34. Ifudu O, Uribarri J, Rajwani I. et al. Gender modulates responsiveness to recombinant erythropoietin. Am J Kidney Dis 2001; 38: 518–522 [DOI] [PubMed] [Google Scholar]
- 35. de Almeida EA, Alho I, Marques F. et al. Haemoglobin and erythropoietin levels in polycystic kidney disease. Nephrol Dial Transplant 2007; 23: 412–413 [DOI] [PubMed] [Google Scholar]
- 36. Smrzova J, Balla J, Bárány P.. Inflammation and resistance to erythropoiesis-stimulating agents—what do we know and what needs to be clarified? Nephrol Dial Transplant 2005; 20: viii2–viii7 [DOI] [PubMed] [Google Scholar]
- 37. Diskin CJ, Stokes TJ, Dansby LM. et al. Can acidosis and hyperphosphataemia result in increased erythropoietin dosing in haemodialysis patients? Nephrology (Carlton) 2006; 11: 394–399 [DOI] [PubMed] [Google Scholar]
- 38. Kimata N, Akiba T, Pisoni RL. et al. Mineral metabolism and haemoglobin concentration among haemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant 2005; 20: 927–935 [DOI] [PubMed] [Google Scholar]
- 39. Lichtman MA, Miller DR.. Erythrocyte glycolysis, 2, 3-diphosphoglycerate and adenosine triphosphate concentration in uremic subjects: relationship to extracellular phosphate concentration. J Lab Clin Med 1970; 76: 267–279 [PubMed] [Google Scholar]
- 40. Kaestner L, Bogdanova A, Egee S.. Calcium channels and calcium-regulated channels in human red blood cells. Adv Exp Med Biol 2020; 1131: 625–648 [DOI] [PubMed] [Google Scholar]
- 41. Wetmore JB, Li S, Yan H. et al. Predialysis anemia management and outcomes following dialysis initiation: a retrospective cohort analysis. PLoS One 2018; 13: e0203767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Besarab A, Bolton WK, Browne JK. et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 1998; 339: 584–590 [DOI] [PubMed] [Google Scholar]
- 43. Evans M, Bower H, Cockburn E. et al. Contemporary management of anaemia, erythropoietin resistance and cardiovascular risk in patients with advanced chronic kidney disease: a nationwide analysis. Clin Kidney J 2020; 13: 821–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rosner MH, Bolton WK.. The mortality risk associated with higher hemoglobin: is the therapy to blame? Kidney Int 2008; 74: 695–697 [DOI] [PubMed] [Google Scholar]
- 45. Hanafusa N, Tsuchiya K.. Equivalent doses matter, rather than types. J Am Soc Nephrol 2019; 30: 1772–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sakaguchi Y, Hamano T, Wada A. et al. Types of erythropoietin-stimulating agents and mortality among patients undergoing hemodialysis. J Am Soc Nephrol 2019; 30: 1037–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wilhelm-Leen ER, Winkelmayer WC.. Mortality risk of darbepoetin alfa versus epoetin alfa in patients with CKD: systematic review and meta-analysis. Am J Kidney Dis 2015; 66: 69–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McCullough PA, Barnhart HX, Inrig JK. et al. Cardiovascular toxicity of epoetin-alfa in patients with chronic kidney disease. Am J Nephrol 2013; 37: 549–558 [DOI] [PubMed] [Google Scholar]
- 49. Gilbertson DT, Ebben JP, Foley RN. et al. Hemoglobin level variability: associations with mortality. Clin J Am Soc Nephrol 2008; 3: 133–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.