Abstract
Background
Key anatomical factors mean that individuals needing arteriovenous access are unique and have different possibilities for fistula creation. The aim of this article is to describe a new classification system for all patients needing haemodialysis vascular access in the upper extremity with the purpose to simplify sharing the information about suitability for surgical access creation depending on vascular anatomy.
Methods
According to the patient’s vascular anatomy in right and left superior extremities, patients were separated into three arteriovenous access stages (AVAS). The AVAS was validated by three blinded observers using a sample of 70 upper limb arteriovenous maps that were performed using ultrasound on patients referred for vascular access assessment. A sample size calculation was performed and calculated that for three observers, a minimum of 67 maps were required to confirm significant agreement at a Kappa value of 0.9 (95% confidence interval 0.75–0.99).
Results
The Kappa value for inter-rater reliability using Fleiss’ Kappa coefficient was 0.94 and all patients fitted into the AVAS classification system.
Conclusion
The AVAS classification system is a simplified way to share information about vascular access options based on a patient’s vascular anatomy with high inter-rater reliability.
Keywords: arteriovenous fistula, arteriovenous graft, classification, haemodialysis, vascular access
INTRODUCTION
Haemodialysis is either a temporary therapy before kidney transplantation or permanent renal replacement therapy for patients with end-stage kidney disease. It is dependent on arteriovenous access (AVA), which allows cannulation and delivery of minimum blood flow of 300 mL/min through a dialysis machine [1]. Generally, native autogenous AVA (fistula) is recommended due to improved patency and fewer postoperative complications in comparison with arteriovenous grafts [2, 3]. There are several options for surgical AVA creation. The first-line option is the creation of the forearm AVA, most commonly radiocephalic fistulas; other forearm options include ‘Snuff box’ fistulas at the wrist and radio-basilic fistulas. The next option is an upper arm AVA, which includes different types of Gracz’s fistula at the elbow [4]. The creation of brachio-basilic AVA, plus transposition of the basilica vein if required, is the last conventional autogenous option in the upper arm. For patients who do not have suitable vessels to create cephalic or basilic vein fistulas, there are various options for arteriovenous grafts or special types of access in the lower extremity, neck or chest.
Each patient needing AVA is unique and has different possibilities for fistula creation that depend on many factors as well the anatomical condition of inflow arteries and outflow veins, the so-called ‘arteriovenous access map’. A clinically useful classification of end-stage vascular access failure has previously been described. However, that system focused on patients that had exhausted access options [5]. The aim of this article is to describe an easy-to-use classification system based on vascular anatomy for all patients needing surgical creation of haemodialysis vascular access in the upper extremity and to test the system for inter-rater reliability.
Anatomical factors affecting AVA creation
Venous quality should be clinically examined by an experienced clinician with and without a venous tourniquet in a warm room in order to ensure maximum vasodilatation [2]. Duplex ultrasound (DUS) of the vessels is an important non-invasive diagnostic method; a randomized trial demonstrated a primary failure rate of 25% without pre-operative DUS versus a failure rate of 6% with DUS [6, 7]. DUS is therefore recommended as a diagnostic tool before AVA creation to evaluate vessel diameters and localize any obstructive or stenotic lesions that may be present [2]. A generally accepted cut-off for internal arterial and vein diameter for successful autogenous AVA in the wrist or forearm is 2.0 mm. A relatively straight venous course is also beneficial, and the vein should lie <6 mm from the skin surface unless a transposition procedure is being performed [1]. For elbow AVA, as well as for first-stage brachiobasilic AVA, a minimum arterial and venous diameter of 3 mm is sufficient [2]. For a one-stage brachiobasilic AVA in the upper arm, a diameter of 3 mm is preferred for the basilic vein [2]. Confirmed central vein occlusion or central vein stenosis is a significant risk factor for failure of AVA to mature, and the creation of AVA is generally less successful in these circumstances [3, 8]. Furthermore, peripheral vessels damage such as phlebitis and calcified non-compressible arteries are other significant barriers for successful AVA creation [3].
Classification of AVA patients according to vessel anatomy
The arteriovenous access stage (AVAS) is a surgical classification system that is based purely on anatomical considerations and is suitable for use in all renal patients, including those with previous access attempts and central venous access. Measurements of arteries and veins are performed using ultrasound and without the use of a tourniquet. Regarding the possibility of AVA creation, according to the patient’s vascular anatomy in right and left superior extremities, it is suggested to divide patients into three stages, called the AVAS.
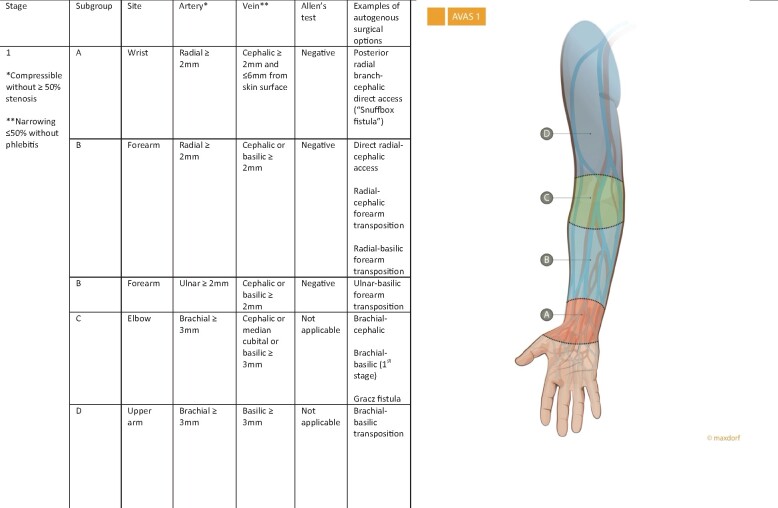
The first stage—AVAS 1
AVAS stage one (AVAS 1) indicates the possibility of autogenous AVA in different parts of the upper extremity. Stage one is divided into four subgroups (A, B, C and D), which describe the anatomical location for possible AVA. The ideal patient for AVA is assigned as stage one (AVAS 1) with all four subgroups (AVAS 1-ABCD), indicating that in this patient AVA could be created in all parts of the upper extremity [wrist (A), forearm (B), elbow (C) and upper arm (D), Figure 1]. AVAS 1-ABCD therefore indicates a candidate for all types of autogenous (primary options) and for secondary options (implantation of synthetic grafts) for AVA creation in the forearm and upper arm.
FIGURE 1:
AVAS 1 indicates the possibility of autogenous AVA in different parts of the upper extremity. A negative Allen’s test is demonstration of a complete palmar arch and intact collateral blood flow to the hand.
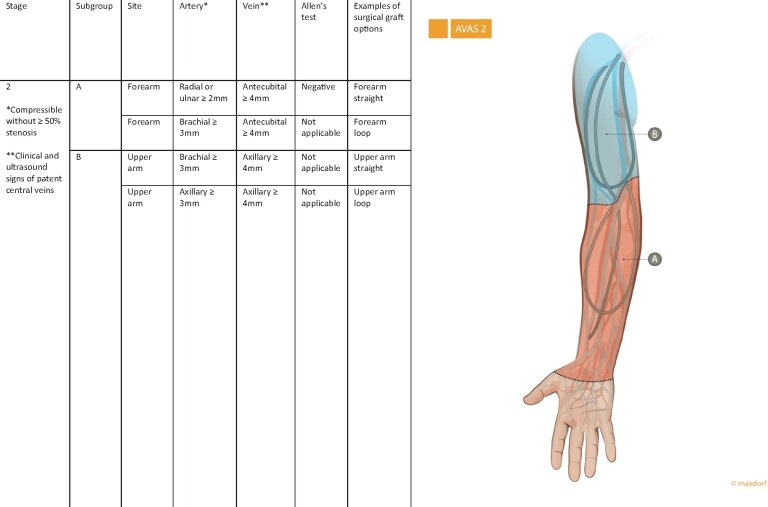
The second stage—AVAS 2
AVAS stage two (AVAS 2) is reserved for patients in whom conventional autogenous AVA is not possible and for whom the only option is an arteriovenous graft. AVAS 2 is divided according to the type of graft location (AVAS 2-AB). AVAS 2-A indicates the feasibility of an arteriovenous graft located in the forearm with an outflow vein in the elbow. AVAS 2-B indicates the feasibility of a graft located in the upper arm with an outflow vein in the axillary vein (Figure 2).
FIGURE 2:
AVAS 2 is reserved for patients in whom conventional autogenous AVA is not possible and the only option is an arteriovenous graft. A negative Allen’s test is demonstration of a complete palmar arch and intact collateral blood flow to the hand.
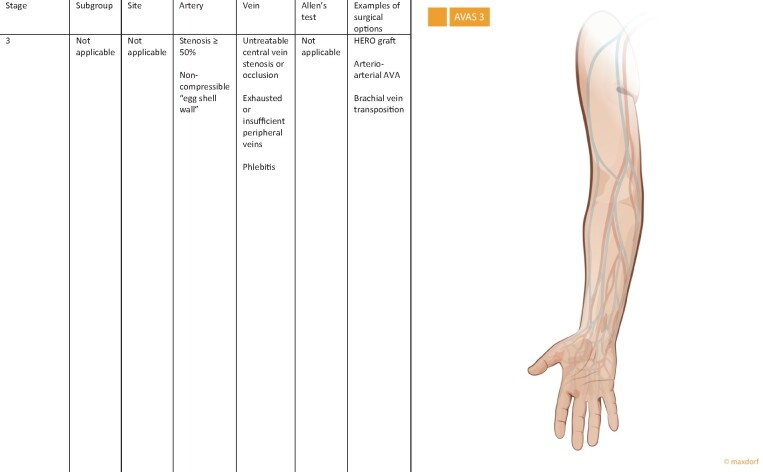
The third stage—AVAS 3
AVAS stage three (AVAS 3) is reserved for patients without the possibility for conventionally created autogenous or prosthetic options for access creation due to insufficient venous, arterial system or its combination. Patients with untreatable central vein stenosis or occlusion, and/or exhausted venous system, and/or insufficient arterial system are assigned to category AVAS 3 (Figure 3). These patients have to be referred as an urgent kidney transplant recipient, or in the case of untreatable central vein occlusion, the Haemodialysis Reliable Outflow (HeRO) graft could be used for dialysis [9]. Arterio-arterial AVA is another possibility [10], as well as catheter placement into the vena cava, or AVA in lower extremity where a high rate of complication has been described [2]. This stage also includes patients with brachio-brachial vein access needing in the first step anastomosis between one of the brachial veins with brachial artery and after the maturation phase, brachial vein transposition [11].
FIGURE 3:
AVAS 3 is reserved for patients without the possibility for conventionally created autogenous or prosthetic options for access creation due to insufficient venous, arterial system or its combination. A negative Allen’s test is demonstration of a complete palmar arch and intact collateral blood flow to the hand.
For each patient, the AVAS consists of two parts that indicate the right (R) and left (L) extremity. For example, AVAS R1-ABCD, AVAS L3 means ideal vessel anatomy for AVA creation on the right and unsuitable anatomy for AVA on the left extremity, respectively.
Validation of AVAS
The AVAS was validated using a random sample of upper limb arteriovenous maps performed using ultrasound (Sonosite M-turbo, UK) on patients referred for vascular access assessment. Blinded observers that were independent of the mapping process classified the full AVAS, including the subgroup for each map. The data were treated as categorical. A sample size calculation was performed and calculated that for three observers, a minimum of 67 maps were required to confirm significant agreement at a Kappa value of 0.9 (95% confidence interval 0.75–0.99). Three blinded observers (two from the Czech Republic and one from the UK) rated a sample of 70 upper limb arteriovenous maps with AVAS with a Kappa value of 0.94 for inter-rater reliability using Fleiss’ Kappa coefficient. All patients fitted into the classification system. Statistical analysis was performed on R version 3.4.0.
DISCUSSION
Haemodialysis is the prevalent dialysis modality for over 2 million people worldwide [12]. Although strongly advocated in clinical guidelines, timing the formation and development of a functional AVA for haemodialysis is logistically challenging and associated with a high failure rate [13]. An aging population and the associated prevalence of comorbidities, including obesity, diabetes and peripheral vascular disease, result in less vascular adaptability and higher failure rates [14]. For pre-emptively placed autogenous fistulas, a recently published US study reported a maturation rate of 79% [15]. However, in elderly patients, the rate of fistula failure can be as high as 45% [16]. The clinical implications of AVA failure are severe, with a registry-based study from France reporting that non-functional AVA at haemodialysis initiation was associated with an increase of 10% in overall mortality risk, compared with functional AVA [17].
There is currently no anatomical classification system in use for patients requiring surgically created AVA for haemodialysis in the upper extremity. The AVAS classification could be used in clinical practice to replace long and heterogeneous descriptions of patient’s arteriovenous map, and thus simplify sharing the information about the patient’s condition for AVA creation between nephrologists, interventional nephrologists/radiologists and vascular access surgeons. Furthermore, the proposed classification system could be used in patients in whom ligation of the access or treatment of access thrombosis is being considered, e.g. patients with useable AVA who have received a functioning kidney transplant, patients with a functioning transplant presenting with fistula thrombosis, patients with symptomatic aneurysmal access or patients with risk of cardiac failure due to high flow AVA. In these patients, a high AVAS [2, 3] indicates clearly the potential problems with new AVA creation in the future and would alert clinicians to potentially avoid ligation or to aggressively pursue de-clotting of the current AVA if at all possible.
A limitation of the AVAS is that factors known to predispose to primary forearm AVA failure have not been considered, e.g. older age, female gender, presence of diabetes and cardiac failure [18–21]. It also does not take into consideration that patients with forearm eczema or extensive solar keratosis and older patients with particularly thin and fragile skin who are not suitable for forearm fistula and may be better suited to upper arm fistula [22]. Finally, there are always patients where the utility of a surgical classifications system of this nature is lacking. Examples include patients presenting in acute renal failure (so-called ‘crash landers’) or those with short life expectancy due to malignancy or severe cardiovascular disease. These individuals do not have independent anatomical contraindications for AVA creation; however, the use of haemodialysis catheter placement is generally accepted as the first option in these particular settings, and as such the AVAS may not be immediately useful.
Finally, stratification of the patient according to AVAS will facilitate the evaluation of the patient for research purposes and allow for a more meaningful audit of AVA patency rates and performance against suggested standards. The AVAS classification is not designed to replace personalized and patient-centred decision-making on vascular access creation. However, in individual cases, the AVAS classification is a simplified way of sharing the information about suitability for access creation depending on vascular anatomy that has excellent inter-rater reliability. It is also useful for standardizing the measurements that should be taken during arteriovenous mapping. On a larger scale, the AVAS may be used to better define vascular access populations, allowing for a more meaningful comparison of unit-level results, in terms of key performance indicators, e.g. the percentage of patients starting dialysis with an AVA versus a central line. It could also be repeated at different time points for monitoring the performance of units with regards to vein preservation.
Before the AVAS classification is adopted internationally and taken up in clinical guidelines, it should be validated prospectively in a larger population of patients across multiple international centres and assessed by key stakeholders (e.g. the Vascular Access Society of Britain and Ireland). Co-production with patient partners would also enable the translation of AVAS into terminology that is more easily understood and communicated to patients.
CONCLUSION
The AVAS classification is a simplified way of sharing the information about suitability for access creation depending on vascular anatomy that demonstrates high inter-rater reliability. It can be used to highlight future problems with new access creation in patients considered for fistula ligation or de-clotting and to facilitate the evaluation of the patients for research as well as audit purposes.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1.Vascular Access Work Group. Clinical practice guidelines for vascular access. Am J Kidney Dis 2006; 48 (Suppl 1): S176–S247 [DOI] [PubMed] [Google Scholar]
- 2. Schmidli J, Widmer MK, Basile C. et al. Editor's Choice - Vascular Access: 2018 Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 2018; 55: 757–818 [DOI] [PubMed] [Google Scholar]
- 3. Lok CE, Huber TS, Lee T. et al. KDOQI Clinical Practice Guideline for Vascular Access: 2019 Update. Am J Kidney Dis 2020; 75: S1–S164 [DOI] [PubMed] [Google Scholar]
- 4. Gracz KC, Ing TS, Soung LS. et al. Proximal forearm fistula for maintenance hemodialysis. Kidney Int 1977; 11: 71–75 [DOI] [PubMed] [Google Scholar]
- 5. Al Shakarchi J, Nath J, McGrogan D. et al. End-stage vascular access failure: can we define and can we classify? Clin Kidney J 2015; 8: 590–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mihmanli I, Besirli K, Kurugoglu S. et al. Cephalic vein and hemodialysis fistula: surgeon's observation versus color Doppler ultrasonographic findings. J Ultrasound Med 2001; 20: 217–222 [DOI] [PubMed] [Google Scholar]
- 7. Georgiadis GS, Charalampidis DG, Argyriou C. et al. The necessity for routine pre-operative ultrasound mapping before arteriovenous fistula creation: a meta-analysis. Eur J Vasc Endovasc Surg 2015; 49: 600–605 [DOI] [PubMed] [Google Scholar]
- 8. Mickley V. Central vein obstruction in vascular access. Eur J Vasc Endovasc Surg 2006; 32: 439–444 [DOI] [PubMed] [Google Scholar]
- 9. Al Shakarchi J, Houston JG, Jones RG. et al. A review on the Hemodialysis Reliable Outflow (HeRO) graft for haemodialysis vascular access. Eur J Vasc Endovasc Surg 2015; 50: 108–113 [DOI] [PubMed] [Google Scholar]
- 10. Khafagy T, Regal S, ElKassaby M. et al. Early results of brachial arterio-arterial prosthetic loop (AAPL) for hemodialysis. Eur J Vasc Endovasc Surg 2016; 51: 867–871 [DOI] [PubMed] [Google Scholar]
- 11. Dorobantu LF, Stiru O, Iliescu VA. et al. The brachio-brachial arteriovenous fistula: a new method in patients without a superficial venous system in the upper limb. J Vasc Access 2006; 7: 87–89 [DOI] [PubMed] [Google Scholar]
- 12. Agarwal AK, Haddad NJ, Vachharajani TJ. et al. Innovations in vascular access for hemodialysis. Kidney Int 2019; 95: 1053–1063 [DOI] [PubMed] [Google Scholar]
- 13. Murea M, Geary RL, Davis RP. et al. Vascular access for hemodialysis: a perpetual challenge. Semin Dial 2019; 32: 527–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nanami M, Suemitsu K, Nagasawa Y. et al. Current topics in vascular access: superficialization of arteriovenous fistula. Contrib Nephrol 2019; 198: 1–11 [DOI] [PubMed] [Google Scholar]
- 15. Arhuidese IJ, Orandi BJ, Nejim B. et al. Utilization, patency, and complications associated with vascular access for hemodialysis in the United States. J Vasc Surg 2018; 68: 1166–1174 [DOI] [PubMed] [Google Scholar]
- 16. Hod T, Patibandla BK, Vin Y. et al. Arteriovenous fistula placement in the elderly: when is the optimal time? J Am Soc Nephrol 2015; 26: 448–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alencar de Pinho N, Coscas R, Metzger M. et al. ; on behalf of the French REIN registry. Vascular access conversion and patient outcome after hemodialysis initiation with a nonfunctional arteriovenous access: a prospective registry-based study. BMC Nephrol 2017; 18: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McGrogan DG, Maxwell AP, Khawaja AZ. et al. Current tools for prediction of arteriovenous fistula outcomes. Clin Kidney J 2015; 8: 282–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hod T, Desilva RN, Patibandla BK. et al. Factors predicting failure of AV ‘fistula first’ policy in the elderly. Hemodial Int 2014; 18: 507–515 [DOI] [PubMed] [Google Scholar]
- 20. Miller CD, Robbin ML, Allon M.. Gender differences in outcomes of arteriovenous fistulas in hemodialysis patients. Kidney Int 2003; 63: 346–352 [DOI] [PubMed] [Google Scholar]
- 21. Ng YY, Wu SC, Hung YN. et al. Effect of demographic characteristics and timing of vascular access maturation on patency in Chinese incident haemodialysis patients. Nephrol Dial Transplant 2009; 24: 3447–3453 [DOI] [PubMed] [Google Scholar]
- 22. Sidawy AN, Spergel LM, Besarab A. et al. The Society for Vascular Surgery: clinical practice guidelines for the surgical placement and maintenance of arteriovenous hemodialysis access. J Vasc Surg 2008; 48: 2S–25S [DOI] [PubMed] [Google Scholar]