Abstract
Background
Randomized trials can provide evidence to inform decision-making but this may be limited if the outcomes of importance to patients and clinicians are omitted or reported inconsistently. We aimed to assess the scope and heterogeneity of outcomes reported in trials in peritoneal dialysis (PD).
Methods
We searched the Cochrane Kidney and Transplant Specialized Register for randomized trials in PD. We extracted all reported outcome domains and measurements and analyzed their frequency and characteristics.
Results
From 128 reports of 120 included trials, 80 different outcome domains were reported. Overall, 39 (49%) domains were surrogate, 23 (29%) patient-reported and 18 (22%) clinical. The five most commonly reported domains were PD-related infection [59 (49%) trials], dialysis solute clearance [51 (42%)], kidney function [45 (38%)], protein metabolism [44 (37%)] and inflammatory markers/oxidative stress [42 (35%)]. Quality of life was reported infrequently (4% of trials). Only 14 (12%) trials included a patient-reported outcome as a primary outcome. The median number of outcome measures (defined as a different measurement, aggregation and metric) was 22 (interquartile range 13–37) per trial. PD-related infection was the most frequently reported clinical outcome as well as the most frequently stated primary outcome. A total of 383 different measures for infection were used, with 66 used more than once.
Conclusions
Trials in PD include important clinical outcomes such as infection, but these are measured and reported inconsistently. Patient-reported outcomes are infrequently reported and nearly half of the domains were surrogate. Standardized outcomes for PD trials are required to improve efficiency and relevance.
Keywords: patient-reported outcomes, peritoneal dialysis, outcomes, trials
INTRODUCTION
Peritoneal dialysis (PD) allows for more autonomy and flexibility in treatment compared with center-based hemodialysis. In many countries, the health and quality-of-life outcomes for patients receiving PD are comparable or superior to those for patients receiving hemodialysis [1–3]. However, PD is not without its challenges, including peritonitis and other PD-related infections, which are associated with increased morbidity and mortality and contribute to ˃50% of transfers to hemodialysis [4, 5]. Such complications can also impair a patient’s mental health and quality of life [6, 7]. Additionally, while short-term mortality rates have declined over the years, long-term patient survival rates remain low, with an estimated 11% of patients receiving PD surviving past 10 years [8, 9].
The Standardised Outcomes in Nephrology–Peritoneal Dialysis (SONG-PD) initiative recently established critically important core outcomes to be reported in all clinical trials in PD, as determined by patients, caregivers and health professionals. The core outcomes set includes cardiovascular disease, mortality, technique survival, PD-related infection and life participation [10]. There is a growing body of trial-based evidence in PD. However, the omission of patient-important outcomes can limit their use in supporting decision-making [11, 12] and inconsistent reporting of outcomes across trials can make it difficult to compare the effectiveness of interventions.
The outcomes reported in trials in patients with a kidney transplant [13] or receiving hemodialysis [14] are numerous, heterogeneous and ˃52% of all outcome domains are surrogate endpoints; however, this has not been assessed in trials in patients receiving PD. We aimed to assess the scope and heterogeneity of outcomes reported in randomized controlled trials of interventions for adults receiving PD.
MATERIALS AND METHODS
Selection criteria
We searched the Cochrane Kidney and Transplant Specialized Register using search terms to identify trials involving patients receiving PD up to 5 September 2019. The register is populated from a variety of sources, including CENTRAL, MEDLINE, Embase, hand searches of kidney and transplant journals and the proceedings of major kidney and transplant conferences, the International Clinical Trials Register Search Portal and ClinicalTrials.gov. We included trials published from 2010 to 2019 to obtain a contemporary sample of trials. We excluded trials if less than half of the participant population were patients receiving PD and if the trials were conducted in the pediatric population (<18 years of age), published in languages other than English or used PD for the treatment of conditions other than chronic kidney disease. Post hoc and secondary analyses were included if the original trial report was published between 2010 and 2019.
Data extraction
We extracted the following characteristics from each trial into an Excel (Microsoft, Redmond, WA, USA) spreadsheet: first author, publication year, participating countries, number of centers (single/multiple), sample size [including number of patients on continuous ambulatory PD (CAPD) versus automated PD (APD)], age of participants, trial duration, intervention type, primary outcomes and all discrete outcome measures. A discrete outcome measure was defined as any measurement or event reported separately for all trial arms [14]. If reported, all levels of specification of the outcome measures were extracted, including domain (e.g. PD-related infection), specific measurement (e.g. episodes of peritonitis), specific metric (end value, time to event and change from baseline), method of aggregation (mean, median, percent/proportion and absolute number) and time point of measurement [15].
Analysis
Outcome measures from all included trials were classified into outcome domains by two reviewers (K.E.M. and Y.C.). The list of outcome domains and measures was reviewed and discussed by the SONG-PD Steering Group to assess the appropriateness of grouping of outcomes and domain names. One reviewer (K.E.M.) classified all outcome domains into one of three categories: clinical (medical event or comorbidity diagnosed by the clinician) [16], surrogate (biochemical, imaging or other marker used as a substitute for a clinical outcome) [17] or patient-reported (outcomes that are reported directly by patients regarding how they function or feel in relation to a health condition and its therapy, without interpretation by a healthcare professional or anyone else) [18]. The classifications were agreed upon by two reviewers (Y.C. and A.T.). We ascertained the frequency of reporting across trials for each outcome domain. We conducted a detailed analysis of outcome measures for two selected frequently reported outcome domains in each category—clinical, surrogate and patient-reported. We assessed the measurement, metric, method of aggregation and time points. We performed descriptive analyses using R version 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria; http://www.R-project.org/).
RESULTS
Trial characteristics
We identified 128 reports from 120 trials involving 9799 participants (Figure 1). Nine (8%) trials were multinational. Of the remaining 111 trials, 78 (65%) were conducted in Asia, 18 (15%) in Europe, 8 (7%) in North America, 4 (3%) in Oceania and 3 (3%) in South America. The median duration of trials was 6 [interquartile range (IQR) 3–12] months. The median sample size of trials was 64 (IQR 32–101) participants. Thirty-one (26%) trials assessed types of PD solutions and 45 (37%) assessed other pharmacological interventions. Eighteen (15%) evaluated a surgical intervention, 13 (11%) assessed a dietary intervention, 4 (3%) assessed PD modalities and 9 (8%) assessed other interventions (including psychological, technological, exercise, nurse-led case management and bioimpedance analysis). Of the 80 trials that reported the PD modality of participants, 50 (63%) included only patients receiving CAPD, 22 (28%) included patients receiving either CAPD or APD and 8 (10%) included only patients receiving APD. Trial characteristics are provided in Table 1.
FIGURE 1:
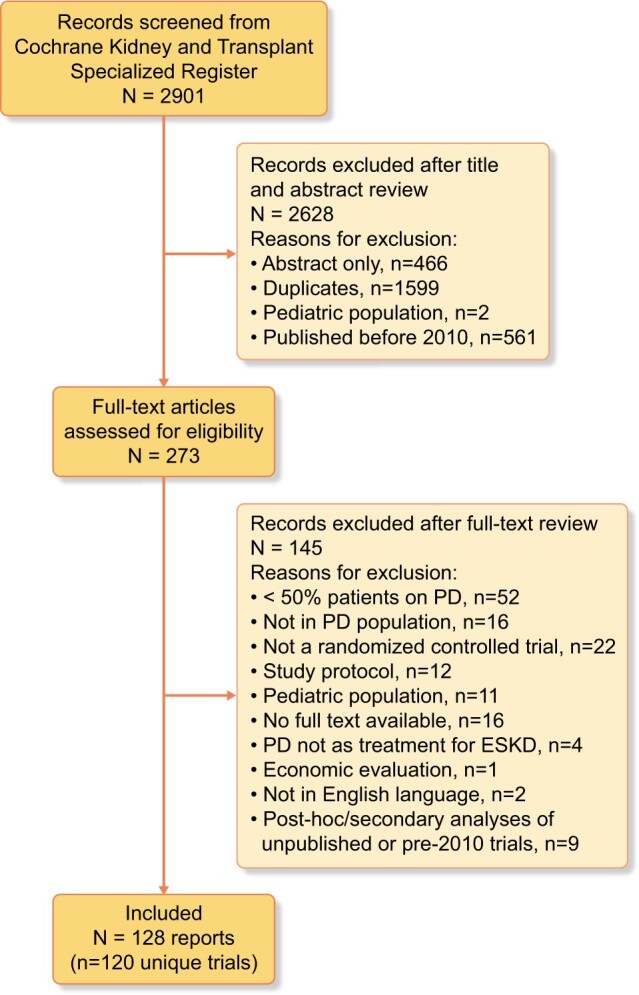
Search results.
Table 1.
Characteristics of included trials (N = 120)
| Trial characteristic | Trials, n (%) |
|---|---|
| Year of publication | |
| 2010–14 | 63 (53) |
| 2015–19 | 57 (48) |
| Duration of trial (months)a | |
| <6 | 57 (48) |
| 6–11 | 17 (14) |
| 12–23 | 26 (22) |
| 24–35 | 16 (13) |
| ≥36 | 3 (3) |
| Study site | |
| Single center | 83 (69) |
| Multiple centers | 37 (31) |
| Sample size (n) | |
| 1–50 | 49 (41) |
| 51–100 | 40 (33) |
| 101–200 | 24 (20) |
| >200 | 7 (6) |
| PD modalityb | |
| CAPD only | 50 (63) |
| APD only | 8 (10) |
| CAPD and APD | 22 (28) |
| Location | |
| Asia | 78 (65) |
| Europe | 18 (15) |
| North America | 8 (7) |
| Oceania | 4 (3) |
| South America | 3 (3) |
| Multinational studies | 9 (8) |
| Intervention type | |
| Pharmacological | 76 (63) |
| Surgical | 18 (15) |
| Diet | 13 (11) |
| PD modality/regimen | 4 (3) |
One trial did not report duration or follow-up length.
It excludes surgical trials and trials that did not specify PD modality (CAPD or APD).
Outcome measures and domains
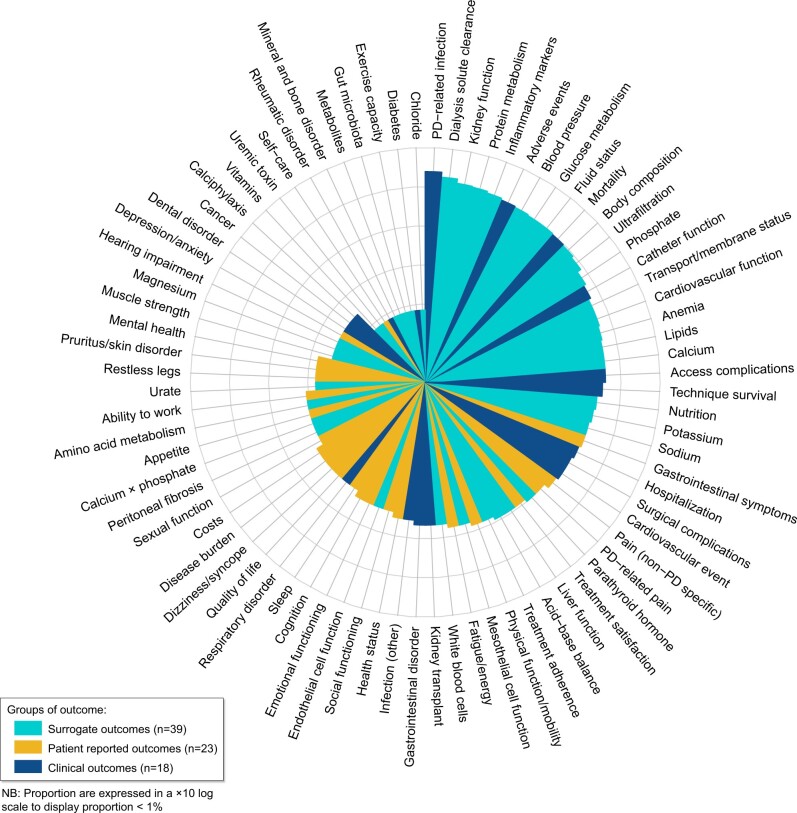
In total, 3024 outcome measures were reported across the 120 trials. The median number of outcome measures was 22 (IQR 13–37) per trial. The outcome measures were classified into 80 outcome domains, of which 39 (49%) were surrogate, 23 (29%) were patient-reported and 18 (22%) were clinical outcomes. The proportion of trials reporting each outcome domain is provided in Figure 2. The top five most frequently reported outcome domains were PD-related infection [59 trials (49%)], dialysis solute clearance [51 (42%)], kidney function [45 (38%)], protein metabolism [44 (37%)] and inflammatory markers/oxidative stress [42 (35%)]. Sixty outcome domains (75%) were reported in ˂20% of trials. Adverse events, mortality and technique survival were reported in 40 (33%), 37 (31%) and 22 (18%) trials, respectively. The number of trials reporting at least one surrogate outcome domain was 94 (78%); 79 (66%) trials reported at least one clinical outcome and 41 (34%) trials reported at least one patient-reported outcome.
FIGURE 2:
Number of trials reporting each outcome domain (total 120 trials, 80 outcome domains).
Overall quality of life, reported as the total score of quality-of-life scales including the Kidney Disease Quality of Life Instrument (KDQOL), 36-Item Short Form Health Survey (SF-36) and European Quality of Life 5-Dimensions (EQ-5D) questionnaire, was reported in five (4%) trials. Gastrointestinal symptoms were the most frequent patient-reported outcome [17 trails (14%)], followed by non-PD-specific pain [14 trials (12%)] and PD-related pain [12 trials (10%)]. The least frequent patient-reported outcome was self-care, being reported in only one trial. A complete list of the included patient-reported outcomes with examples of outcome measures is provided in Table 2.
Table 2.
Patient-reported outcome domains and measures included in PD trials
| Outcome domain | Trials, n (%) | Examples of measures |
|---|---|---|
| Gastrointestinal symptoms | 17 (14) | Number of events of constipation |
| Pain (non-PD-specific) | 14 (12) | SF-36 pain score, mean |
| PD-related pain | 12 (10) | Visual analog scale for pain |
| Treatment satisfaction | 11 (9) | Number of patients reporting satisfaction with treatment |
| Physical function/mobility | 10 (8) | SF-36 physical functioning scale |
| Fatigue/energy | 9 (8) | Number and proportion of patients reporting fatigue |
| Health status | 7 (6) | SF-36 general health score, mean |
| Cognition | 6 (5) | KDQOL cognitive function, mean |
| Disease burden | 6 (5) | KDQOL burden of kidney disease, mean |
| Emotional functioning | 6 (5) | SF-36 emotional wellbeing, mean |
| Social functioning | 6 (5) | SF-36 social function score, mean |
| Costs | 5 (4) | Per-patient cost, dollars |
| Dizziness/syncope | 5 (4) | Number and proportion of patients reporting dizziness |
| Sleep | 5 (4) | KDQOL sleep score, mean |
| Ability to work | 4 (3) | KDQOL work status, mean |
| Appetite | 4 (3) | Number of patients reporting lack of appetite |
| Quality of life | 4 (3) | SF-36 total score, mean |
| Sexual function | 4 (3) | KDQOL sexual function, mean |
| Mental health | 3 (3) | SF-36 mental health score, mean |
| Pruritus/skin disorder | 3 (3) | Number of patients reporting skin itchiness |
| Restless legs | 3 (3) | Number and proportion of patients reporting restless legs |
| Depression/anxiety | 2 (2) | Hospital anxiety and depression scale depression score, mean |
| Self-care | 1 (1) | EQ-5D looking after self-score |
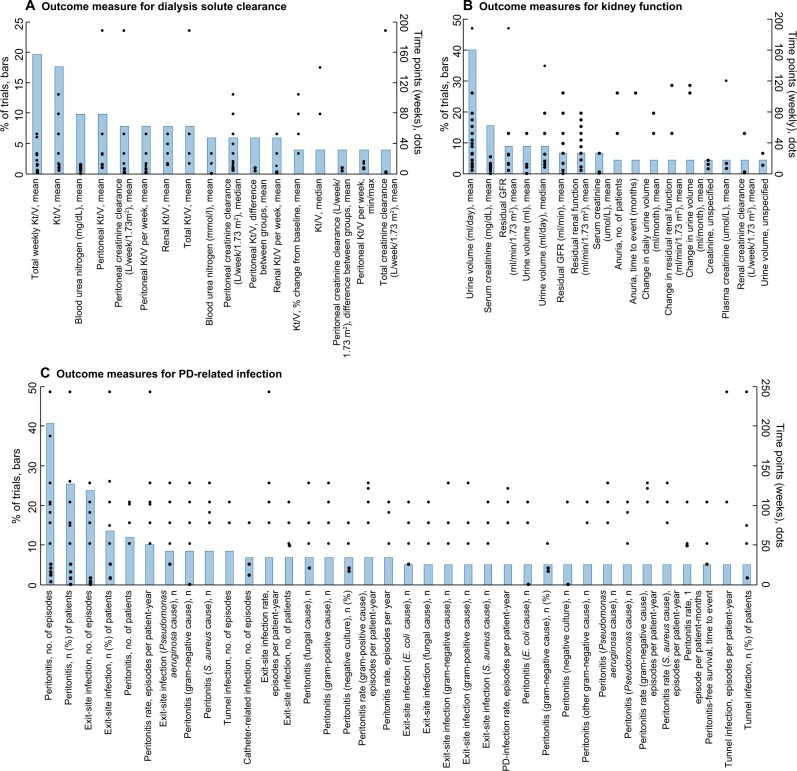
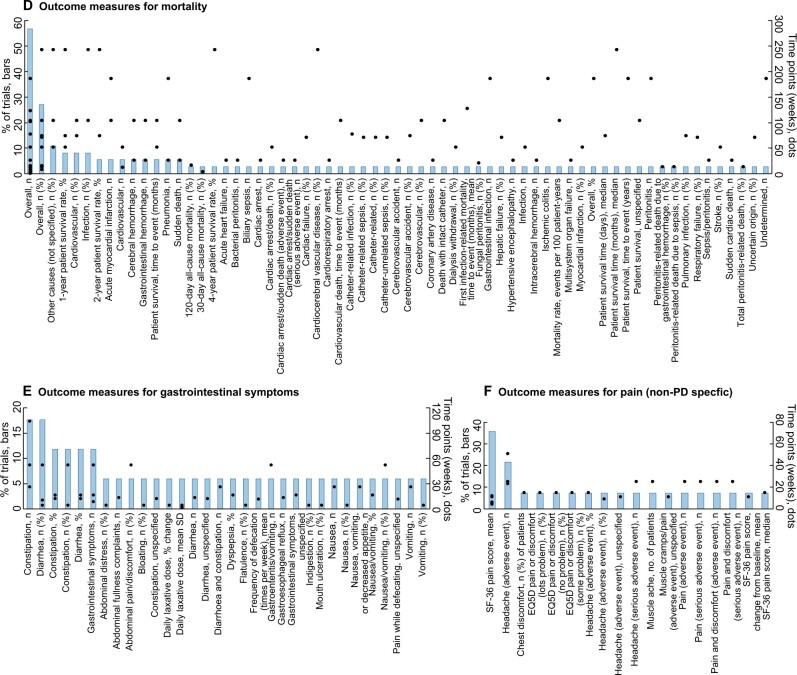
The range of specific outcome measures for six outcome domains (two selected outcome domains each for surrogate, clinical and patient-reported outcomes) and the time points of measurement are provided in Figure 3A–F. The two most frequent surrogate outcomes were dialysis solute clearance and kidney function, which had 84 and 73 different measures used across all trials, respectively (Figure 3A and B). For clinical outcomes, PD-related infection and mortality had 383 and 65 different outcome measures, respectively (Figure 3C and D). For patient-reported outcomes, gastrointestinal symptoms and pain (non-PD-specific) had 32 and 18 different outcome measures, respectively (Figure 3E and F).
FIGURE 3:
(A) Frequency of outcome measures (definitions and time points) of dialysis solute clearance reported in >4% of trials (17 of 84 outcomes measures shown). (B) Frequency of outcome measures (definitions and time points) of kidney function reported in >4% of trials (17 of 73 outcomes measures shown). (C) Frequency of outcome measures (definitions and time points) of PD-related infection reported in >5% of trials (36 of 383 outcomes measures shown). (D) Frequency of outcome measures (definitions and time points) among trials reporting mortality (37 trials, 65 outcomes measures). (E) Frequency of outcome measures (definitions and time points) among trials reporting gastrointestinal symptoms (17 trials, 32 outcomes measures). (F) Frequency of outcome measures (definitions and time points) among trials reporting pain (non-PD-specific) (14 trials and 18 outcomes measures).
When assessing the number of trials that report outcomes previously identified as important to patients and caregivers [24], only three outcomes (PD-related infection, mortality and blood pressure) were reported in at least 30% of trials. Three outcomes (flexibility with time, ability to travel and impact on family) were not reported in any trials. The proportion of trials reporting the top 10 highest ranked outcomes for patients and caregivers is provided in Supplementary data, Table S1.
Characteristics of primary outcomes
Sixty-six (55%) trials explicitly reported the primary outcome(s) in the published trial report. Fifty-four (45%) did not explicitly report the primary outcome(s). Of these 54, primary outcomes were obtained by referring to the trial registration websites for five trials. No trial registration number was provided in the reports of the remaining 49 trials, hence the primary outcome(s) for these trials were extracted based on implicit reporting.
Across the 120 trials, 18 (15%) trials specified one unique primary outcome, while 102 (85%) trials specified several primary outcomes. The outcomes specified as primary corresponded to 57 different outcome domains, of which 28 (49%) were surrogate, 19 (33%) were patient-reported and 10 (18%) were clinical. The most frequently reported primary outcomes across trials were PD-related infection [25 trials (21%)], kidney function [16 trials (13%)], catheter function [15 trials (13%)], inflammatory markers/oxidative stress [14 trials (12%)], cardiovascular function [11 trials (9%)] and dialysis solute clearance [11 trials (9%)]. When considering the proportion of the trials that reported primary outcomes from each of the three categories (surrogate, clinical and patient-reported), it was found that 83 (69%) trials included at least one primary surrogate outcome, 40 (33%) trials included at least one primary clinical outcome and 14 (12%) trials included at least one primary patient-reported outcome. The proportion of trials reporting each primary outcome domain is provided in Supplementary data, Table S2.
DISCUSSION
Across trials in PD, the outcomes reported were wide-ranging and inconsistent. With 80 outcome domains reported across 120 trials, most (60 domains) of these outcome domains were reported in ˂20% of trials. Approximately half of the outcome domains were surrogate endpoints and 7 of the 10 most frequently reported outcome domains were surrogate endpoints. The most frequently reported clinical outcomes were PD-related infection, adverse events and mortality, which were reported in 49%, 33% and 31% of trials, respectively. Although 23 (29%) of the total outcome domains were patient-reported, these were infrequently and inconsistently used. Moreover, there was a vast array of outcome measures within the outcome domains, with 3024 outcome measures being used across all outcome domains.
Many of the trial reports did not explicitly specify what the primary outcome was, which made it difficult to ascertain the main objective of the trial. This issue is confounded by the fact that many of the trials without explicitly stated primary outcomes were also not registered in trial registries, despite the fact that trial registration has been recommended and implemented in policy since 2005 by the International Committee of Medical Journal Editors, World Health Organization and US Food and Drug Administration [19]. This leads to concerns regarding outcome reporting bias and a lack of transparency and understanding of the study context [20]. When observing the range of primary outcomes both explicitly and implicitly reported, it was found that many trials included multiple primary outcomes, of which many were surrogates, which again causes concern as to the primary objective of these trials. Furthermore, only 12% of trials included a primary outcome that was patient-reported, indicating that trial outcomes may lack relevance to patients and end users. However, despite the small range of clinical outcome domains that featured as primary endpoints (18% of the primary outcome domains were clinical), one-third of all trials reported a primary outcome that was clinical, and the most commonly used primary outcome across trials was PD-related infection.
PD-related infection was the most frequently reported outcome domain (49% trials). Within this domain, peritonitis was the most frequently reported type of infection. Despite both the 2010 and 2016 International Society for Peritoneal Dialysis (ISPD) guidelines for PD-related infections recommending the reporting of peritonitis as a rate [21, 22], the most frequently used measure was the total number of episodes of peritonitis during the trial period. The 2016 ISPD guideline suggests that peritonitis should be reported in a standardized manner as the number of episodes per patient-year, however, this measure was used in only 10% of trials that reported a PD-related infection. A recently published systematic review explored in more detail the outcomes and definitions for peritonitis in trials and observational studies [23]. Similarly, the review found that there was wide variability in both the reporting of and definitions used for peritonitis, which highlights the need for a standardized definition and greater uptake of ISPD-recommended methods of reporting peritonitis.
While PD-related infection is a critically important outcome for patients receiving PD, their caregivers and health professionals [24, 25] and is frequently reported in trials, other outcomes of critical importance to stakeholders including catheter function, technique survival and fatigue are less frequent, appearing in only 23%, 18% and 8% of trials, respectively. Instead, trials in PD frequently report surrogate endpoints such as dialysis solute clearance, kidney function, protein metabolism and inflammatory markers/oxidative stress. The use of surrogate endpoints is common because of feasibility, as shorter duration and smaller sample sizes would be needed to achieve adequate power [26]. However, the relevance of these outcomes in clinical settings is questionable. In the case of dialysis solute clearance, Kt/V is widely utilized as a marker of dialysis adequacy in clinical practice and research, despite the fact that trials have been unable to demonstrate any clear association between peritoneal small solute clearance and patient outcomes [27–29].
Patients receiving PD may experience symptoms including fatigue, itching, cramping, poor sleep, loss of appetite, constipation and vomiting [30, 31]. Of the 23 patient-reported outcome domains reported by trials in this review, gastrointestinal symptoms were the most frequently reported; however, they were reported in only 14% of trials. Overall, our findings show that patient-reported outcomes are infrequently reported across trials in PD. However, even fewer were found to be reported in trials in patients receiving hemodialysis, kidney transplant recipients and children with chronic kidney disease [13, 14, 32]. This may be explained by the limited number of validated tools for measuring patient-reported outcomes in this population, or because trialists may perceive patient-reported outcomes as time and resource intensive [33]. We suggest that further research should be undertaken to establish validated and feasible patient-reported outcome measures to enhance the assessment and reporting of patients’ symptoms and quality of life in PD trials.
We conducted a systematic and detailed review of outcomes reported in all trials in PD published since 2010. We searched the Cochrane Kidney and Transplant Specialized Register, which is populated from a variety of key databases and sources. However, we acknowledge some potential limitations. We studied outcomes reported in published trials and could not ascertain outcomes that may have been measured but were not reported. Trials published in a language other than English were excluded; however, we expected that the inclusion of these trials would have further demonstrated heterogeneity of outcomes and outcome measures.
The outcomes reported in trials of patients receiving PD are numerous and largely comprised of surrogate outcomes. In comparison, important clinical and patient-reported outcomes are infrequently reported. Outcome measures are inconsistent and heterogeneous, which makes it difficult to compare the effectiveness of interventions across trials. Implementation of a standardized core outcome set for trials in PD will improve the reporting of outcomes in trials that are important and relevant to all stakeholders to better support shared decision-making and improve patient care and outcomes for patients receiving PD.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
FUNDING
K.E.M. is supported by a National Health and Medical Research Council (NHMRC) Postgraduate Scholarship (APP1151343). D.W.J. is supported by an NHMRC Practitioner Fellowship. Y.C. is supported by an NHMRC Early Career Fellowship. J.I.S. is supported by grant K23DK103972 from the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases. A.T. is supported by an NHMRC Fellowship (APP1106716). The funding organization had no role in the preparation, review or approval of the manuscript.
AUTHORS’ CONTRIBUTIONS
Research design was performed by all authors. Data collection was performed by K.E.M., Y.C., B.S., J.S., A.K. and A.T. Data analysis was performed by all authors. Each author contributed important intellectual content during manuscript drafting or revision and agrees to be personally accountable for the individual’s own contributions and to ensure that questions pertaining to the accuracy or integrity of any portion of the work, even one in which the author was not directly involved, are appropriately investigated and resolved, including with documentation in the literature if appropriate.
CONFLICT OF INTEREST STATEMENT
None declared. The results presented in this article have not been published previously in whole or part except in abstract format.
Supplementary Material
REFERENCES
- 1. Zazzeroni L, Pasquinelli G, Nanni E. et al. Comparison of quality of life in patients undergoing hemodialysis and peritoneal dialysis: a systematic review and meta-analysis. Kidney Blood Press Res 2017; 42: 717–727 [DOI] [PubMed] [Google Scholar]
- 2. Lozier MR, Sanchez AM, Lee JJ. et al. Comparison of cardiovascular outcomes by dialysis modality: a systematic review and meta-analysis. Perit Dial Int 2019; 39: 306–314 [DOI] [PubMed] [Google Scholar]
- 3. Elsayed ME, Morris AD, Li X. et al. Propensity score matched mortality comparisons of peritoneal and in-centre haemodialysis: systematic review and meta-analysis. Nephrol Dial Transplant 2020; doi: 10.1093/ndt/gfz278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cho Y, Johnson DW.. Peritoneal dialysis-related peritonitis: towards improving evidence, practices, and outcomes. Am J Kidney Dis 2014; 64: 278–289 [DOI] [PubMed] [Google Scholar]
- 5. Chen JHC, Johnson DW, Hawley C. et al. Association between causes of peritoneal dialysis technique failure and all-cause mortality. Sci Rep 2018; 8: 3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baillie J, Lankshear A.. Patients’ and relatives’ experiences of peritonitis when using peritoneal dialysis. J Ren Care 2015; 41: 177–186 [DOI] [PubMed] [Google Scholar]
- 7. Jacquet S, Trinh E.. The potential burden of home dialysis on patients and caregivers: a narrative review. Can J Kidney Health Dis 2019; 6:2054358119893335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kendrick J, Teitelbaum I.. Strategies for improving long-term survival in peritoneal dialysis patients. Clin J Am Soc Nephrol 2010; 5: 1123–1131 [DOI] [PubMed] [Google Scholar]
- 9. Teixeira JP, Combs SA, Teitelbaum I.. Peritoneal dialysis: update on patient survival. Clin Nephrol 2015; 83: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Manera KE, Johnson DW, Craig JC. et al. Establishing a core outcome set for peritoneal dialysis: report of the SONG-PD (Standardized Outcomes in Nephrology–Peritoneal Dialysis) Consensus Workshop. Am J Kidney Dis 2020; 75: 404–412 [DOI] [PubMed] [Google Scholar]
- 11. Black N. Patient reported outcome measures could help transform healthcare. BMJ 2013; 346: f167. [DOI] [PubMed] [Google Scholar]
- 12. Greenhalgh J. The applications of PROs in clinical practice: what are they, do they work, and why? Qual Life Res 2009; 18: 115–123 [DOI] [PubMed] [Google Scholar]
- 13. Sautenet B, Tong A, Chapman JR. et al. Range and consistency of outcomes reported in randomized trials conducted in kidney transplant recipients: a systematic review. Transplantation 2018; 102: 2065–2071 [DOI] [PubMed] [Google Scholar]
- 14. Sautenet B, Tong A, Williams G. et al. Scope and consistency of outcomes reported in randomized trials conducted in adults receiving hemodialysis: a systematic review. Am J Kidney Dis 2018; 72: 62–74 [DOI] [PubMed] [Google Scholar]
- 15. Saldanha IJ, Dickersin K, Wang X. et al. Outcomes in Cochrane Systematic Reviews addressing four common eye conditions: an evaluation of completeness and comparability. PLoS One 2014; 9: e109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Velentgas P, Dreyer NA, Wu AW. et al. Outcome definition and measurement. In: Velentgas P, Dreyer NA, Nourjah P (eds). Developing a Protocol for Observational Comparative Effectiveness Research: A User’s Guide. Rockville, MD: Agency for Healthcare Research and Quality, ; 2013 [PubMed] [Google Scholar]
- 17. Aronson JK. Biomarkers and surrogate endpoints. Br J Clin Pharmacol 2005; 59: 491–494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.US Food and Drug Administration. Guidance for Industry: Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. Rockville, MD: US Food and Drug Administration; , 2009 [Google Scholar]
- 19. Zarin DA, Tse T, Williams RJ. et al. Update on trial registration 11 years after the ICMJE policy was established. N Engl J Med 2017; 376: 383–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cho Y, Davies SJ, Johnson DW.. Raising the standard of trial registration, conduct, and reporting. Perit Dial Int 2020; 40: 112–114 [DOI] [PubMed] [Google Scholar]
- 21. Li PK, Szeto CC, Piraino B. et al. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int 2010; 30: 393–423 [DOI] [PubMed] [Google Scholar]
- 22. Li PKT, Szeto CC, Piraino B. et al. ISPD peritonitis recommendations: 2016 update on prevention and treatment. Perit Dial Int 2016; 36: 481–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sahlawi MA, Wilson G, Stallard B. et al. Peritoneal dialysis-associated peritonitis outcomes reported in trials and observational studies: a systematic review. Perit Dial Int 2020; 40: 132–140 [DOI] [PubMed] [Google Scholar]
- 24. Manera KE, Johnson DW, Craig JC. et al. Patient and caregiver priorities for outcomes in peritoneal dialysis. Clin J Am Soc Nephrol 2019; 14: 74–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Manera KE, Tong A, Craig JC. et al. An international Delphi survey helped develop consensus-based core outcome domains for trials in peritoneal dialysis. Kidney Int 2019; 96: 699–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boissel JP, Collet JP, Moleur P. et al. Surrogate endpoints: a basis for a rational approach. Eur J Clin Pharmacol 1992; 43: 235–244 [DOI] [PubMed] [Google Scholar]
- 27. Paniagua R, Amato D, Vonesh E. et al. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol 2002; 13: 1307–1320 [DOI] [PubMed] [Google Scholar]
- 28. Perl J, Dember LM, Bargman JM. et al. The use of a multidimensional measure of dialysis adequacy—moving beyond small solute kinetics. Clin J Am Soc Nephrol 2017; 12: 839–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bargman JM. We use Kt/V urea as a measure of adequacy of peritoneal dialysis. Semin Dial 2016; 29: 258–259 [DOI] [PubMed] [Google Scholar]
- 30. Figueiredo AE, Goodlad C, Clemenger M. et al. Evaluation of physical symptoms in patients on peritoneal dialysis. Int J Nephrol 2012; 2012: 305424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kosmadakis G, Albaret J, da Costa Correia E. et al. Gastrointestinal disorders in peritoneal dialysis patients. Am J Nephrol 2018; 48: 319–325 [DOI] [PubMed] [Google Scholar]
- 32. Chong LSH, Sautenet B, Tong A. et al. Range and heterogeneity of outcomes in randomized trials of pediatric chronic kidney disease. J Pediatr 2017; 186: 110–117.e11 [DOI] [PubMed] [Google Scholar]
- 33. Morton RL, Lioufas N, Dansie K. et al. Use of patient-reported outcome measures and patient-reported experience measures in renal units in Australia and New Zealand: a cross-sectional survey study. Nephrology 2020; 25: 14–21 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.