Abstract
A brief comprehensive overview is provided of the elements constituting the burden of kidney disease [chronic kidney disease (CKD) and acute kidney injury]. This publication can be used for advocacy, emphasizing the importance and urgency of reducing this heavy and rapidly growing burden. Kidney diseases contribute to significant physical limitations, loss of quality of life, emotional and cognitive disorders, social isolation and premature death. CKD affects close to 100 million Europeans, with 300 million being at risk, and is projected to become the fifth cause of worldwide death by 2040. Kidney disease also imposes financial burdens, given the costs of accessing healthcare and inability to work. The extrapolated annual cost of all CKD is at least as high as that for cancer or diabetes. In addition, dialysis treatment of kidney diseases imposes environmental burdens by necessitating high energy and water consumption and producing plastic waste. Acute kidney injury is associated with further increases in global morbidity, mortality and economic burden. Yet investment in research for treatment of kidney disease lags behind that of other diseases. This publication is a call for European investment in research for kidney health. The innovations generated should mirror the successful European Union actions against cancer over the last 30 years. It is also a plea to nephrology professionals, patients and their families, caregivers and kidney health advocacy organizations to draw, during the Decade of the Kidney (2020–30), the attention of authorities to realize changes in understanding, research and treatment of kidney disease.
Keywords: acute kidney injury, chronic kidney disease, dialysis, epidemiology, environment, health economy, kidney transplantation, mortality, non-communicable diseases, peritoneal dialysis
INTRODUCTION
The social and psychological impact of chronic kidney disease (CKD) is seriously underestimated. The disease and its human and financial burdens are unknown to many, mainly due to unawareness, the intangible nature of how the kidneys function and the difficulty of capturing public attention. Recently a Belgian member of the European Parliament, Hilde Vautmans, appropriately called CKD ‘the most neglected common chronic disease’ [1].
CKD mostly develops slowly, without initial symptoms, and becomes progressively more debilitating at later stages, with little chance for reversal. In the most advanced stages (kidney failure; previously known as end-stage kidney disease), kidney replacement therapy (dialysis or transplantation) is the usual approach to support quality of life and keep the individual alive. Patients can also opt for comprehensive conservative care, assuring their quality of life, but without kidney replacement. Kidney function can also suddenly decline [acute kidney injury (AKI)] [2, 3], which is strongly interconnected with CKD. Patients with CKD are more prone to develop AKI than the general population. In turn, AKI can worsen the course of CKD or become the reason for subsequent incident CKD [4].
This position paper is a call to action prepared by the European Kidney Health Alliance (EKHA), a non-governmental organization advocating for kidney patients at the level of the European Union (EU) and member state healthcare systems [5], and several other stakeholders including patients (see the ‘Acknowledgements’ section). The aim is to draw the attention of the authorities and the public to the urgent need to reduce the collective burden of kidney disease. In contrast to other fields in medicine, such as cardiology and oncology, limited progress has been made with respect to developing novel therapeutics for kidney disease in the last three decades. This inertia must urgently be overcome to generate overdue and long-awaited progress.
This call to action provides data to inform policymakers, administrators, regulators and payers. The media and society, in parallel, can use this text to inform about the multiple burdens associated with kidney disease and the urgent need to acknowledge and address these. However, education is not sufficient and should be coupled, first, with prioritization of diagnosis and, second, with streamlining of care trajectories through a collaborative effort between authorities and stakeholders. Where appropriate, we will make comparisons with other major health advocacy domains to emphasize the need for gearing up financial and intellectual investment in kidney health.
A DEVASTATING DISEASE
Individuals with CKD suffer from countless limitations and symptoms that progressively impact their physical, mental and social functioning (video track with patient testimonies in ref. [6]). Current dialysis and transplant options were rarely developed with a primary focus on the needs and preferences of patients and their care providers.
While kidney disease may have no specific symptoms in a number of patients (especially in the early stages), it contributes to many challenges in others (Table 1). Most of these, e.g. fatigue, sleep disorders and itching, are not fatal, but they worsen progressively and heavily impact global functioning. Comfort is only rarely restored with treatment. Associated complications like cardiovascular disease and infection [7, 8] trigger multiple hospitalizations and surgical interventions, not exceptionally ≥20 per disease course of one patient. Many patients must take >15–20 pills every day [9, 10]. Physical appearance is negatively impacted by scars in the extremities and abdomen from vascular or peritoneal accesses for dialysis treatment. Immunosuppression following transplantation causes hair loss, gum hypertrophy and weight gain. Uncertainty about the future of their dialysis access or kidney graft, as well as restrictions of mobility and social life facilitate development of depression. Pain is common due to complications (bone fractures, nerve lesions, gangrene and infections), but also to therapy [surgery, transcutaneous puncture every other day for haemodialysis (HD) access]. HD is frequently associated with hypotension, muscle cramps, thirst, anaemia, mental changes, headache, vomiting and feeling drained; peritoneal dialysis (PD) is associated with loss of appetite but nevertheless weight gain; and in transplantation, immunosuppression causes muscle weakness, hirsutism, gout, tremors and mood swings. HD also necessitates a number of time-consuming treatments per week for several hours, with additional loss of time due to transport to and from in-centre treatment.
Table 1.
Most important problems perceived by individuals with chronic kidney disease
| Health-related quality of life |
|
| Functional |
|
| Symptoms |
|
| Health behaviour and perception |
|
Children and adolescents with kidney disease encounter growth retardation and limitations in mobility and social and educational development that hamper psychological development. This may be worsened by time spent on dialysis, which mostly takes place in the midst of adult patients [11]. Among older patients, frailty and muscle wasting (sarcopaenia) are common [12].
Unemployment is frequent among adults with chronic diseases. For individuals with advanced CKD and those living with dialysis or transplants, who frequently suffer from several simultaneous comorbidities, unemployment rates of up to 75% have been reported [13–15]. This situation not only affects buying power, but also lifestyle, self-image and mental health.
In summary, CKD causes major and largely underestimated distress, which often causes resistance to treatment, affects all age strata, modifies physical and emotional quality of life and limits socio-economic possibilities.
FAR MORE FREQUENT THAN ASSUMED
Most laypeople without direct exposure assume that kidney disease is limited to dialysis and transplantation. In fact, however, this group constitutes only the tip of the iceberg. The approximate ratio of individuals with CKD not yet requiring dialysis or transplantation compared with those receiving dialysis or transplanted is ˃100:1 [16]. This ratio is higher in countries where access to dialysis or transplantation is limited by reliance on out-of-pocket payments, which restricts access for most who need it. In addition, CKD is not a stand-alone condition but is part of a cluster of non-communicable and communicable (chronic) diseases (Figure 1) that, during their evolution, are frequently complicated by or further complicate kidney disease [17]. The most well-known causes of CKD are hypertension, cardiovascular disease and diabetes, but cancer, liver and autoimmune diseases, as well as various infections and pre-eclampsia, are also linked to CKD. People living with chronic diseases represent one-third of the European adult population and contribute to a large majority of annual European fatalities [18]. People surviving long enough with chronic disease have an increasing risk of developing either CKD or AKI.
FIGURE 1:
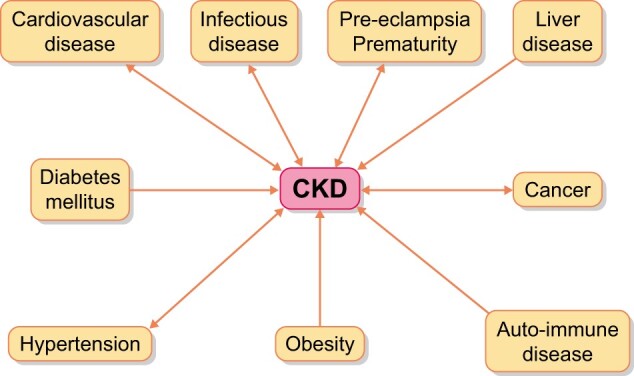
Relationship between other diseases and CKD. The arrows indicate the direction of the interaction. With some diseases, the link is bidirectional. Several of these conditions also have mutual links on their own (e.g. diabetes mellitus and cardiovascular disease), but this is not represented.
CKD is estimated to affect 700–850 million people worldwide, exceeding diabetes, chronic obstructive pulmonary disease and depression [16, 19, 20]. The 2017 Global Burden of Disease Study estimated the prevalence of CKD at ~100 million Europeans (among them 55.7 million people in EU-28 countries) [16]. In accordance with this, the Global Kidney Health Atlas (GKHA) of the International Society of Nephrology (ISN) indicated the prevalence at 10.1% for Western Europe (equalling the global average) and 13.3% for Central and Eastern Europe [21]. However, although screening can easily be accomplished by two simple tests (serum creatinine and urinary albumin), a large majority of those affected remain unaware of their condition [22–24], also precluding prevention of progression and complications, although this is far more cost effective than treating more advanced stages. In addition, even in the presence of pathological parameters that conform with CKD (decreased kidney filtration or increased urinary albumin), the condition is often overlooked by or underestimated by treating physicians.
The number of individuals with CKD will continue to increase, mainly because of ageing of the population, but also due to intrinsic and not yet well-defined factors. With age, the incidence and prevalence of CKD increase exponentially [25, 26], which is mirrored by the year-by-year increase in age of the dialysis population [27]. Also, the risk of AKI increases with age and frailty, and older patients surviving AKI show a higher risk for progression to CKD and often require maintenance dialysis in subsequent months or years [28]. Along with ageing, nutrition, unhealthy lifestyles and environmental factors contribute [29, 30].
The numbers will further increase as advances in healthcare of underlying diseases successfully prolong survival and longevity, thereby allowing CKD to manifest. Some new therapies, e.g. immunotherapy for cancer or cardiac interventions, carry inherent risks of kidney injury [31, 32]. An increasing availability of kidney replacement therapy in lower-income countries will further increase the global population of people living with kidney failure, although these populations in lower-income countries tend to be one or two decades younger than their European counterparts [33].
Briefly, a large portion of the general population is at risk of CKD. However, the large majority of both the general and medical populations is unaware of this risk. Participation in screening, prevention and early treatment is inadequate and should urgently be improved, especially in high-risk populations.
A KILLER DISEASE
Worldwide, CKD mortality for 2017 was estimated by the Global Burden of Disease Study at 1.2 million [more than human immunodeficiency virus (HIV) and tuberculosis and equal to traffic accidents] and a further 1.4 million deaths from cardiovascular disease were attributed to reduced kidney function [16]. The annual mortality of CKD in Europe is estimated at close to 130 000 [16]. Kidney disease rose to become the 10th leading global cause of death in 2019 and the 8th leading cause of death in high-income countries [34] and, concerningly, is projected to become the fifth leading cause of death by 2040, above all cancer types, Alzheimer's, diabetes, HIV and tuberculosis [35]. Over the last 20 years mortality from CKD has not improved, in contrast to most other chronic diseases [16]. The increase in CKD as a cause of death may reflect the rising prevalence of CKD globally related to population aging as well as improving access to diagnosis in lower-income settings, but worryingly may also reflect the relative lack of progress in innovation that is holding kidney disease back compared with other chronic diseases.
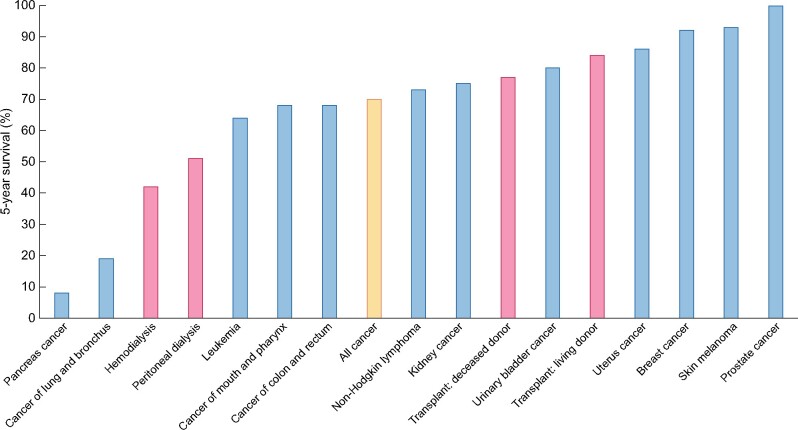
Increased mortality is not limited to advanced CKD but starts rising progressively when kidney filtration function falls to ˂50% of normal, but also with normal filtration in the presence of albuminuria [7, 36]. Additionally, the presence of CKD further increases mortality risk associated with other diseases like cardiovascular disease and diabetes [37]. Premature death for most individuals with CKD not yet in need of dialysis or transplantation is mainly due to high-risk comorbidities (cardiovascular disease, cancer and infectious disease) [7, 8, 38, 39]. Those reaching kidney replacement therapy, especially people living on dialysis, have similar or even worse survival chances than most people diagnosed with cancer [40, 41] (Figure 2). Compared with mortality rates of frequent malignancies, the 5-year mortality of HD patients is only exceeded by the 5-year mortality of pancreas and lung cancer. Mortality from kidney failure also exceeds that of acute myocardial infarction, diabetes, chronic heart failure and stroke [41]. The expected remaining lifetime for advanced CKD versus the general population is more than halved across all age strata [41]. For 20–24-year-old dialysis patients, life expectancy is decreased by ∼70% (∼40 years); not a surprise if one considers that dialysis replaces only a small fraction of the normal function of the kidneys. For individuals of the same age with a kidney graft, the approximate reduction in life expectancy is 25% (∼15 years less) [42].
FIGURE 2:
Percent 5-year survival of kidney replacement treatment modalities (red bars) (HD, PD, transplantation after deceased donation and transplantation after living donation) or 5 years after the diagnosis of cancer (blue bars). Only malignancies with an incidence >3% of all cancers are illustrated. Orange bar: all cancers aggregated. Based on 2016 data [40, 41].
Briefly, an unacceptably high number of individuals die because of CKD and their survival chances are far below those of people without kidney failure and comparable to or worse than people with other chronic diseases. These estimates do not include the burden of AKI, which in Europe alone is associated with an overall in-hospital mortality of ˃23% [43]. In the setting of intensive care, mortality from AKI ranges up to 65% [44], depending on its severity [45], and evolves into CKD in about one-third of its survivors [4].
These data corroborate the ominous impact of kidney disease on the lives of affected patients. More importantly, this negative effect is continuing to worsen over time.
CKD BURDEN IN THE EU
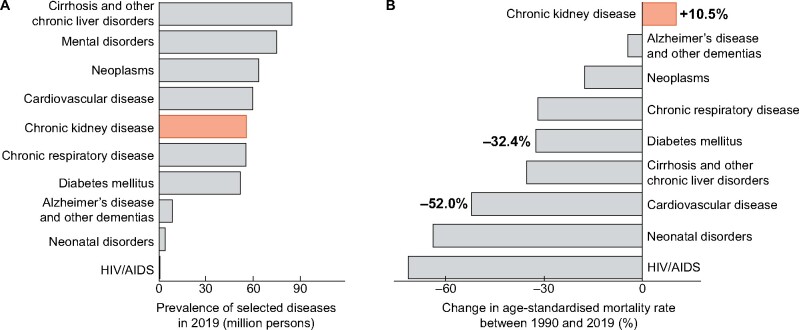
CKD affected ˃55 million people living in the EU and caused almost 130 000 deaths in 2019 (Table 2). In addition, the prevalence was similar to that of most other types of chronic disease, including diabetes, cancer and cardiovascular disease (Figure 3A). Considering age-standardized rates over 29 years, that take into account changes in both age and population structure, mortality of CKD has increased, in contrast to a large array of other communicable and non-communicable diseases (Figure 3B). Importantly, these data do not include AKI, which contributes additional mortality.
Table 2.
Principal metrics of CKD burden in the EU
| Metric | 2019 values |
Change between 1990 and 2019, % |
||
|---|---|---|---|---|
| Number | Rate, per 100 000 population | In all-ages rate | In age-standardized rate | |
| Prevalence |
55 660 588 (52 242 530–59 161 862) |
10 814.2 (10 150.1–11 494.4) |
42.0 (39.3–44.8) |
4.7 (3.5–6.0) |
| Mortality |
126 377 (108 161–136 681) |
24.6 (21.0–26.6) |
99.5 (82.0–111.0) |
10.5 (3.1–16.1) |
All metrics presented as mean and 95% uncertainty interval. Change in all-ages rate considers differences between population size in 1990 and 2019, while change in age-standardized rate considers both differences in population size and population age structure. Source: Global Burden of Disease Study. Data available at https://vizhub.healthdata.org/gbd-compare/.
FIGURE 3:
Comparative burden of selected diseases in the EU. (A) Prevalence numbers 2019. Bars reflect prevalence in million persons. (B) Changes in age-standardized mortality rates. Bars and numeric labels at the bars reflect percentage changes in age-standardized mortality rates between 1990 and 2019. CKD in orange. Source: Global Burden of Disease Study. Data available at https://vizhub.healthdata.org/gbd-compare/.
EXPENSIVE TO TREAT
Kidney replacement therapy (dialysis or transplantation) comes at a high societal cost and the share of global healthcare costs spent on kidney replacement therapy is proportionally 10–20 times higher than the number of patients treated [46]. Costs will rise further due to the projected growth in patient numbers [47].
The most used kidney replacement option, in-centre HD, engenders the highest costs per patient [48], and in Europe, yearly reimbursement per country reaches up to €80 000/patient [49, 50]. Although in countries with a lower gross domestic product, dialysis consumes less in absolute amounts, a larger percentage of the general healthcare budget is spent [50], likely at the expense of other, more cost-effective health investments like screening and prevention [51]. Kidney transplantation is manifestly more cost effective than dialysis, at least in high-income countries [52], but not everybody is an eligible transplant candidate and the transplantation rate is lagging behind in several European countries [53]. Costs of home dialysis (PD and home HD) are intermediate between in-centre HD and kidney transplantation, but these options are also underexploited in Europe [54], in spite of patient preference [55, 56] and better quality of life [57]. Many European countries even offer no specific financial regulations for home HD [50].
Individuals with CKD who are not on dialysis or living with a functioning kidney transplant also represent a substantial source of expenditures, which largely is related to their higher number [58, 59]. Costs per patient increase as CKD becomes more severe [60, 61]. CKD independently augments the cost of many other chronic diseases by a factor of ˃2 [37, 62].
Finding data of aggregated cost for comparisons with other diseases is extremely difficult. Whereas aggregated costs in Europe are available for cancer and diabetes mellitus [63, 64], a similar assessment for CKD necessitates extrapolation of data from several studies, taking into account costs of dialysis, transplantation, CKD that is not dialysed or transplanted and indirect costs (hospitalization, primary care, mental care, transport, etc.) [50, 53, 58, 65–68] (Supplementary data). Adding these up, overall costs for CKD are at least in the same range, if not higher, as those for cancer and diabetes (Figure 4). Inclusion of costs related to AKI would further substantially increase these estimated costs [69, 70]. Also, these data do not include costs of productivity loss due to premature death, sick leave and unemployment and indirect costs due to services provided by family and friends.
FIGURE 4:
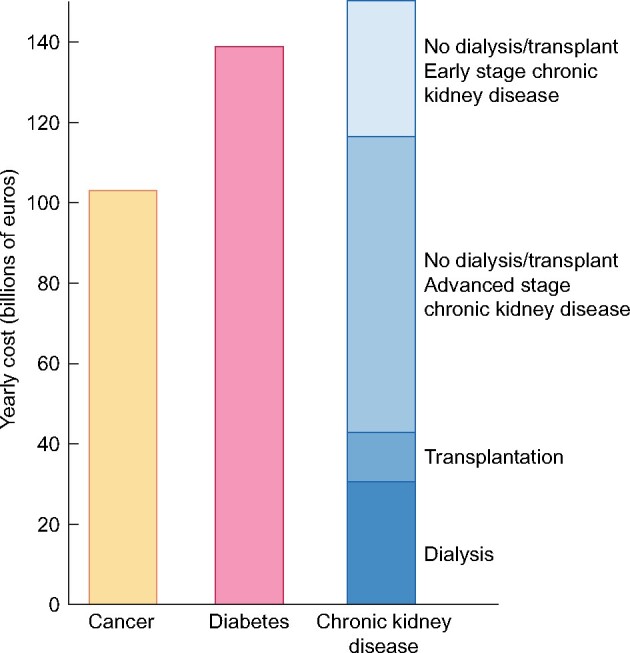
Comparison of aggregated annual healthcare costs for Europe of cancer (yellow), diabetes mellitus (pink) and CKD. Costs of CKD (increasingly dark blue for each of the successive stages) are a composite of early CKD (Stages 1–2 not on dialysis or living with a functioning transplant), more advanced stages of CKD (Stages 3–5 not on dialysis or living with a functioning transplant), transplantation and dialysis. Sources and approaches for calculation: see Supplementary data.
Transnational health-economic assessments based on different studies from different countries might be skewed due to differences in population, environment, definitions and timing. However, a German study [59] enables a comparison between expenditures for CKD and cancer on a single-country basis [63]. Here also, both expenditures were similar (€25.5 billion/year for cancer versus €24.2 billion for CKD), although the study did not include early CKD stages and transplantation. These data thus corroborate our findings.
Thus it is fair to assume that costs for kidney care are at least in the same range as those for cancer or diabetes. Unfortunately, aggregated European data for CKD healthcare costs are lacking. Therefore the creation of a registry of individual incidence and prevalence data for dialysis and transplantation covering all EU countries, also tracking costs and quality of care, is needed. In addition, a comprehensive tracking system of prevalence of not dialysed or transplanted CKD and associated costs is desirable, especially for the later stages.
These costs could be reduced by promoting prevention and less costly therapeutic strategies (generic drugs and home dialysis) and reimbursement systems for real costs, but also by pursuing more fair and transparent price setting for drugs and technical equipment.
GAPS IN DELIVERY OF KIDNEY CARE ACROSS EUROPE
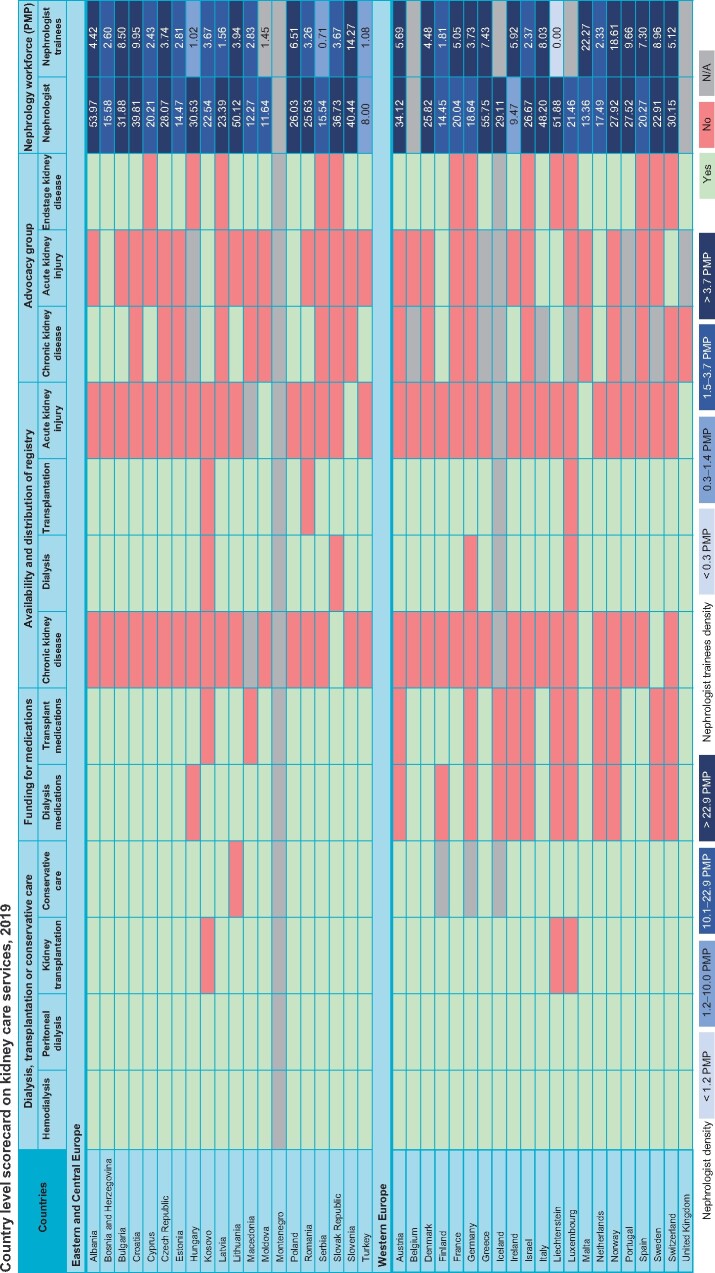
The ISN survey on country-level capacity for kidney care services was published in 2017 and 2019 as the first and second edition of the GKHA (ISN-GKHA) [71, 72]. The atlas highlighted significant barriers regarding delivery of optimal kidney care across countries and regions. Europe as a continent generally performed better than other parts of the world. However, there was significant variability in workforce distribution (Figure 5; Supplementary data, Figures S1 and S2) and other essential services; in particular, significant limitations in surveillance systems and advocacy tools for both AKI and CKD (Figure 5). In addition, significant gaps were noted in the ability to fund services for patients with CKD and provision of dialysis and transplantation, especially in Central and Eastern Europe (Supplementary data, Figures S3 and S4). Equitable funding services are pivotal to reduce the major health consequences of kidney disease.
FIGURE 5:
Country-level score card on availability of kidney care services across Europe comparing data from the ISN-GKHA for the year 2019. Central and Eastern Europe (above) and Western Europe (below). Available services: green; unavailable services: red; N/A: not available. Funding for medications: public funding that is free at the point of delivery exclusive of private medical insurance or other sources. Advocacy group: organizations or foundations advocating the case of kidney disease at national or regional level. Nephrology workforce: total number of nephrologists and trainees in nephrology in a country per million population. Source: GKHA (https://www.theisn.org/initiatives/global-kidney-health-atlas/).
AN ENVIRONMENTAL CHALLENGE
All treatments for CKD leave a considerable carbonfootprint and cause substantial pollution due to frequent therapeutic interventions, hospitalizations, use of consumables, intake of large numbers of drugs requiring energy for production and transport of goods and people. In addition, dialysis generates lots of plastic waste [73, 74] and consumes enormous amounts of water, corresponding to ˃169 billion litres per year worldwide. Only 35% of water consumption is used for dialysis per se, whereas 65% is reverse osmosis reject water that goes directly to the drain despite being perfectly drinkable [73].
Climate changes also impact kidney health by promoting risk factors for AKI [dehydration due to drought and heatwaves, infectious and parasitic diseases after floods (leptospirosis, dysentery and malaria), extension of the spreading areas of tropical diseases (dengue and malaria)] and problems with interruption of dialysis, as storms, floods and hurricanes gain in frequency and severity [75]. Thus the ecological burden of kidney disease is substantial while disturbed ecology, in turn, threatens kidney health. All of these aspects are in urgent need of solutions.
THE COVID-19 CRISIS: STRESSING THE NEED TO FOCUS ON KIDNEY DISEASE
The current coronavirus disease 2019 (COVID-19) pandemic is emblematic of the failure, or at least a delay, in global recognition of the importance of kidney disease [76]. Initially COVID-19 was seen as a pulmonary and infectious problem, while other chronic diseases remained under the radar for some time. The other conditions that came initially into focus as risk factors for severe disease were cardiovascular disease, diabetes and hypertension [77, 78], all of which are prime causes of CKD. The recognition that kidney disease (acute and chronic) is a leading risk factor for death came later. Ultimately it appears that the population with CKD, especially those living with dialysis or transplants, is among the highest-risk groups for hospitalization and mortality [79–83]. Large-scale European data only became available late, after the end of the first wave, explaining why, for early data, Europe had to rely on the US Centers for Disease Control (CDC) [84] and China [77], as the CDC of the EU does not report on chronic diseases.
In view of the frequent cytokine storms in critically ill COVID-19 patients [85], a substantial number of AKI cases were to be expected, but insights into the real epidemiology and the negative prognostic impact of AKI lagged behind, again with the first data coming from outside Europe [86, 87]. The world was scaling up access to ventilators and developing triage guidelines for access to intensive care but remained unprepared for the rapid demand for acute dialysis [88].
In addition, a large number of significant problems affecting patients with kidney disease often went unnoticed outside nephrology circles: decreased transplantation rates from both deceased and living donors due to a necessary focus shift of intensive care units (ICUs) to COVID care or with the intent to decrease infectious risk [89, 90] increased the risk of transplant candidates dying on the waiting list; a shortage of dialysis supplies and protective material [88]; postponement of arteriovenous fistula creation and PD catheter insertions as non-essential interventions [91]; severe COVID-19 outbreaks in the closed communities of in-centre HD units [92], while patients on home dialysis were relatively protected [93]; and heavy workload and infection risk for personnel in nephrology units with the potential of causing burnout [94].
An additional difficulty was created by the almost systematic exclusion of patients with CKD from trials of drug therapies and vaccinations for COVID-19, forcing the nephrology community into off-label use while remaining in the dark about therapeutic efficacy [82, 95, 96]. This is illustrative of a more global concern as individuals with CKD are in general excluded from clinical studies. This is likely one of the reasons for the disappointing progress in fighting CKD. The COVID-19 vaccine being almost exclusively available in higher-income settings is also a matter of major concern [97].
Briefly, the population with kidney disease was one of the most heavily affected risk groups by COVID-19, but the identification of this risk was delayed, partly due to insufficient awareness, attention and data capture.
LOW INVESTMENT IN INNOVATION
In view of the high burden of kidney disease and the paradoxical low investment in innovation [98], a common effort to find novel solutions is a primary need for all concerned. However, the basic concept of HD has barely changed since the original prototype developed by Willem (Pim) Kolff in 1942. Likewise, all other kidney replacement options, as well as the pharmacological approaches to delay progression to kidney failure, have not progressed at the same pace as those for diseases like cancer, HIV, cardiovascular disease or diabetes. The advent of sodium–glucose cotransporter-2 inhibitors has been the first innovation to delay progression of CKD in decades [99, 100], but many other therapies to modulate kidney function remain insufficiently explored.
It would be interesting to compare the EU efforts through research programmes like Horizon 2020 or Horizon Europe, which address kidney health as a primary target, with those addressing other diseases. Concerningly, kidney health does not even figure in the list of priority areas for EU health research and innovation [101] (Supplementary Table 1). Indirect information offers reasons for concern. In response to an editorial stressing the importance of research investment in accordance with patient needs [102], global investment in HIV was ˃30 times higher than that for chronic diseases (including CKD), despite the huge contrast in disability-adjusted life years lost, which were almost 20 times lower for HIV [103]. A UK analysis comparing research expenditures for cancer, coronary heart disease, Alzheimer's and stroke versus their cost, showed a marked discrepancy, with more support for cancer compared with the other disorders [104]. According to the US National Institutes of Health, despite much recent effort (see below), kidney health still receives less research support than many other disorders [105]. It is clear that we do not suggest that there should be less investment in other diseases, but we are convinced that more investment in CKD is urgently needed.
In conclusion, in the case of kidney disease, there is an imperative need to match cost and burden of disease with research and innovation investment. We also stress the need for transparency in European research funding allocation to allow comparisons of investment in different diseases. In view of the current budgetary constraints, we also advocate that if therapies are the result of publicly funded research and innovation, those should not incur unreasonable societal costs or be bought and shelved [98]. Between 2005 and 2018, costs for oncologic drugs more than tripled [63], although the price does not appear to be related to clinical benefit [106]. The skyrocketing price of orphan drugs has recently been a major matter of concern [107]. More transparency and consistency of oversight are required to achieve fair pricing and equitable access to innovative therapies [108].
NEED FOR A CHANGE OF PARADIGM
The most pressing actions required and the most alarming facts about kidney disease are summarized in Table 3. All stakeholders (organizations, patients and professionals) should intensify advocacy about the urgent need to reduce the burden of kidney disease among broad layers of society, including administrators, regulators, policymakers, healthcare workers and the general population. Patients play a critical role by communicating their difficulties, distress and concerns and by defending their case.
Table 3.
The most important actions required and the most imminent threats
| Actions required |
|
| Most imminent threats |
|
In addition, in view of the staggering inertia in developing new therapeutic options for kidney disease (chronic and acute), we plead for a conceptual change in the paradigms regarding kidney therapies and research. The EU has a unique opportunity to play a leading and structuring role, motivating harmonized approaches among the member states. This approach is the only way to avoid complete dependency on other countries when it comes to therapeutic innovations.
In the USA, joint action between the American Association of Kidney Patients (AAKP), the authorities and nephrology professionals culminated in the Kidney Health Initiative, a public–private partnership to stimulate innovative drug and device development for kidney health [47, 109]. One of the outcomes was the signing in July 2019 of an executive order to fundamentally change clinical kidney care [110] and also a call for innovation by the AAKP during a ‘Decade of the Kidney’ [111].
Kidney disease, and especially dialysis and transplantation, have, next to health, important implications for economics, safety, ecology, education, research and innovation, which all are important EU competencies [112]. Kidney health is linked to all EU-supported Sustainable Development Goals (SDGs) of the World Health Organization [113–115]. Consistent with the aims of the SDGs, the EU and the nephrology community have a responsibility to make people’s lives better. We advocate that the European Commission and Parliament and the EU member states take a leading role in fighting kidney disease, mirroring the successful EU achievements over the last 30 years in cancer [116], especially because of the many parallels between the conditions, as illustrated in this text. Unfortunate as it is, COVID-19 may serve as an eye-opener that a change of paradigm is needed.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
CONFLICT OF INTEREST STATEMENT
C.W. has received honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, GlaxoSmithKline, Ely Lilly and MSD. R.V. is an advisor to B. Braun Avitum, Baxter Healthcare, Kibow, Jafron, Debiotech, Fresenius Medical Care and the Dutch Kidney Foundation. All other authors have declared no conflicts of interest.
Supplementary Material
ACKNOWLEDGEMENTS
The authors represent the following international organizations related to kidney health: • European Kidney Health Alliance (R.V., N.L. and F.W.), • European Kidney Patient Federation (D.G.), • European Renal Association–European Dialysis and Transplant Association (C.W. and R.G.), • European Dialysis and Transplantation Nurses Association–European Renal Care Association (E.N.), • Dutch Kidney Foundation (T.O.) and • ISN (A.B. and V.L.). We thank Sophanny Tiv, Kidney Health Research Group, University of Alberta, Edmonton, AB, Canada, for help with the figures and Nico Starink, a student at the University of British Columbia, Vancouver, BC, Canada and Eveline Scheres, Transition Manager EKHA and Buiten de Lijnen, The Netherlands for their inspiring suggestions.
Footnotes
The ‘Decade of the Kidney’ is a global initiative of the American Association of Kidney Patients, launched in 2019.
REFERENCES
- 1.https://www.youtube.com/watch?v=fP- cmDZy7ac
- 2. Hoste EAJ, Kellum JA, Selby NM. et al. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol 2018; 14: 607–625 [DOI] [PubMed] [Google Scholar]
- 3. Lameire NH, Bagga A, Cruz D. et al. Acute kidney injury: an increasing global concern. Lancet 2013; 382: 170–179 [DOI] [PubMed] [Google Scholar]
- 4. Chawla LS, Kimmel PL.. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int 2012; 82: 516–524 [DOI] [PubMed] [Google Scholar]
- 5.European Kidney Health Alliance. http://ekha.eu/
- 6.Nierstichting. Webinar on Need for Innovation in Renal Replacement Therapy (RRT). https://nierstichting.nl/professionals/webinar/
- 7. Vanholder R, Massy Z, Argiles A. et al. Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephrol Dial Transplant 2005; 20: 1048–1056 [DOI] [PubMed] [Google Scholar]
- 8. Matsushita K, Coresh J, Sang Y. et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol 2015; 3: 514–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chiu YW, Teitelbaum I, Misra M. et al. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol 2009; 4: 1089–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parker K, Nikam M, Jayanti A. et al. Medication burden in CKD-5D: impact of dialysis modality and setting. Clin Kidney J 2014; 7: 557–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Snauwaert E, Van Biesen W, Raes A. et al. A plea for more uremic toxin research in children with chronic kidney disease. Pediatr Nephrol 2018; 33: 921–924 [DOI] [PubMed] [Google Scholar]
- 12. Ortiz A, Sanchez-Nino MD.. Sarcopenia in CKD: a roadmap from basic pathogenetic mechanisms to clinical trials. Clin Kidney J 2019; 12: 110–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Danuser B, Simcox A, Studer R. et al. Employment 12 months after kidney transplantation: an in-depth bio-psycho-social analysis of the Swiss Transplant Cohort. PLoS One 2017; 12: e0175161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dukic M, Zibar L. Employment in patients with renal replacement therapy. SEEMed J 2019; 3: 11–20 [Google Scholar]
- 15. Julian MJ, Molinuevo TJ, Sanchez GJ.. Employment in the patient with chronic kidney disease related to renal replacement therapy. Nefrologia 2012; 32: 439–445 [DOI] [PubMed] [Google Scholar]
- 16. GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020; 395: 709–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zoccali C, Vanholder R, Massy ZA. et al. The systemic nature of CKD. Nat Rev Nephrol 2017; 13: 344–358 [DOI] [PubMed] [Google Scholar]
- 18.Busse R, Blümel M, Scheller-Kreinsen D et al. Tackling chronic disease in Europe. Strategies, interventions and challenges. Observatory study series 20. https://www.euro.who.int/__data/assets/pdf_file/0008/96632/E93736.pdf
- 19. Jager KJ, Kovesdy C, Langham R. et al. A single number for advocacy and communication-worldwide more than 850 million individuals have kidney diseases. Nephrol Dial Transplant 2019; 34: 1803–1805 [DOI] [PubMed] [Google Scholar]
- 20. Bello AK, Johnson DW, Feehally J. et al. Global Kidney Health Atlas (GKHA): design and methods. Kidney Int Suppl (2011) 2017; 7: 145–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.International Society of Nephrology. CKD early identification & intervention toolkit. https://www.theisn.org/focus/ckd?gclid=CjwKCAjwsan5BRAOEiwALzomX_3BfsC4QPZXCsl5HUVO5M-_90j2_FXANANQsI6nq2Dgjel_T5inLBoC0zYQAvD_BwE#health-atlas
- 22. Plantinga LC, Tuot DS, Powe NR.. Awareness of chronic kidney disease among patients and providers. Adv Chronic Kidney Dis 2010; 17: 225–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Whaley-Connell A, Shlipak MG, Inker LA. et al. Awareness of kidney disease and relationship to end-stage renal disease and mortality. Am J Med 2012; 125: 661–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saunders MR, Kim SD, Patel N. et al. Hospitalized patients frequently unaware of their chronic kidney disease. J Hosp Med 2015; 10: 619–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Drey N, Roderick P, Mullee M. et al. A population-based study of the incidence and outcomes of diagnosed chronic kidney disease. Am J Kidney Dis 2003; 42: 677–684 [DOI] [PubMed] [Google Scholar]
- 26. Raymond NT, Zehnder D, Smith SC. et al. Elevated relative mortality risk with mild-to-moderate chronic kidney disease decreases with age. Nephrol Dial 2007; 22: 3214–3220 [DOI] [PubMed] [Google Scholar]
- 27. Canaud B, Tong L, Tentori F. et al. Clinical practices and outcomes in elderly hemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Clin J Am Soc Nephrol 2011; 6: 1651–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ishani A, Xue JL, Himmelfarb J. et al. Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 2009; 20: 223–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bowe B, Artimovich E, Xie Y. et al. The global and national burden of chronic kidney disease attributable to ambient fine particulate matter air pollution: a modelling study. BMJ Glob Health 2020; 5: e002063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vanholder R, Van Laecke S, Glorieux G. et al. Deleting death and dialysis: conservative care of cardio-vascular risk and kidney function loss in chronic kidney disease (CKD). Toxins 2018; 10: 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cortazar FB, Marrone KA, Troxell ML. et al. Clinicopathological features of acute kidney injury associated with immune checkpoint inhibitors. Kidney Int 2016; 90: 638–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Valle JA, McCoy LA, Maddox TM. et al. Longitudinal risk of adverse events in patients with acute kidney injury after percutaneous coronary intervention: insights from the national cardiovascular data registry. Circ Cardiovasc Interv 2017; 10: e004439. [DOI] [PubMed] [Google Scholar]
- 33. Harris DCH, Davies SJ, Finkelstein FO. et al. Increasing access to integrated ESKD care as part of universal health coverage. Kidney Int 2019; 95(4S): S1–S33 [DOI] [PubMed] [Google Scholar]
- 34.World Health Organization. The top 10 causes of death. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death
- 35. Foreman KJ, Marquez N, Dolgert A. et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018; 392: 2052–2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Matsushita K, van der Velde M, Astor BC. et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010; 375: 2073–2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wan EYF, Yu EYT, Chin WY. et al. Burden of CKD and cardiovascular disease on life expectancy and health service utilization: a cohort study of Hong Kong Chinese hypertensive patients. J Am Soc Nephrol 2019; 30: 1991–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stengel B. Chronic kidney disease and cancer: a troubling connection. J Nephrol 2010; 23: 253–262 [PMC free article] [PubMed] [Google Scholar]
- 39. Wang HE, Gamboa C, Warnock DG. et al. Chronic kidney disease and risk of death from infection. Am J Nephrol 2011; 34: 330–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.National Cancer Institute. SEER Cancer Statistics Review (CSR) 1975–2014. https://seer.cancer.gov/csr/1975_2014
- 41. 2016. USRDS annual data report: ESRD in the United States. Am J Kidney Dis 2017; 69 (Suppl 1): S215–S658 [Google Scholar]
- 42. Kramer A, Boenink R, Noordzij M. et al. The ERA-EDTA registry annual report 2017: a summary. Clin Kidney J 2020; 13: 693–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Susantitaphong P, Cruz DN, Cerda J. et al. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol 2013; 8: 1482–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bellomo R, Ronco C, Mehta RL. et al. Acute kidney injury in the ICU: from injury to recovery: reports from the 5th Paris International Conference. Ann Intensive Care 2017; 7: 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kellum JA, Sileanu FE, Bihorac A. et al. Recovery after acute kidney injury. Am J Respir Crit Care Med 2017; 195: 784–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vanholder R, Annemans L, Brown E. et al. Reducing the costs of chronic kidney disease while delivering quality health care: a call to action. Nat Rev Nephrol 2017; 13: 393–409 [DOI] [PubMed] [Google Scholar]
- 47. Himmelfarb J, Vanholder R, Mehrotra R. et al. The current and future landscape of dialysis. Nat Rev Nephrol 2020; 16: 573–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vanholder R, Davenport A, Hannedouche T. et al. Reimbursement of dialysis: a comparison of seven countries. J Am Soc Nephrol 2012; 23: 1291–1298 [DOI] [PubMed] [Google Scholar]
- 49. van der Tol A, Lameire N, Morton RL. et al. An international analysis of dialysis services reimbursement. Clin J Am Soc Nephrol 2019; 14: 84–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. van der Tol A, Stel VS, Jager KJ. et al. A call for harmonization of European kidney care: dialysis reimbursement and distribution of kidney replacement therapies. Nephrol Dial Transplant 2020; 35: 979–986 [DOI] [PubMed] [Google Scholar]
- 51. Howard K, White S, Salkeld G. et al. Cost-effectiveness of screening and optimal management for diabetes, hypertension, and chronic kidney disease: a modeled analysis. Value Health 2010; 13: 196–208 [DOI] [PubMed] [Google Scholar]
- 52. Haller M, Gutjahr G, Kramar R. et al. Cost-effectiveness analysis of renal replacement therapy in Austria. Nephrol Dial Transplant 2011; 26: 2988–2995 [DOI] [PubMed] [Google Scholar]
- 53. Vanholder R, Stel VS, Jager KJ. et al. How to increase kidney transplant activity throughout Europe–an advocacy review by the European Kidney Health Alliance. Nephrol Dial Transplant 2019; 34: 1254–1261 [DOI] [PubMed] [Google Scholar]
- 54. Kramer A, Pippias M, Noordzij M. et al. The European Renal Association–European Dialysis and Transplant Association (ERA-EDTA) registry annual report 2015: a summary. Clin Kidney J 2018; 11: 108–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rubin HR, Fink NE, Plantinga LC. et al. Patient ratings of dialysis care with peritoneal dialysis vs hemodialysis. JAMA 2004; 291: 697–703 [DOI] [PubMed] [Google Scholar]
- 56. Ludlow MJ, Lauder LA, Mathew TH. et al. Australian consumer perspectives on dialysis: first national census. Nephrology (Carlton) 2012; 17: 703–709 [DOI] [PubMed] [Google Scholar]
- 57. Wu AW, Fink NE, Marsh-Manzi JV. et al. Changes in quality of life during hemodialysis and peritoneal dialysis treatment: generic and disease specific measures. J Am Soc Nephrol 2004; 15: 743–753 [DOI] [PubMed] [Google Scholar]
- 58. Kerr M, Bray B, Medcalf J. et al. Estimating the financial cost of chronic kidney disease to the NHS in England. Nephrol Dial Transplant 2012; 27(Suppl 3): iii73–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gandjour A, Armsen W, Wehmeyer W. et al. Costs of patients with chronic kidney disease in Germany. PLoS One 2020; 15: e0231375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Honeycutt AA, Segel JE, Zhuo X. et al. Medical costs of CKD in the Medicare population. J Am Soc Nephrol 2013; 24: 1478–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Elshahat S, Cockwell P, Maxwell AP. et al. The impact of chronic kidney disease on developed countries from a health economics perspective: a systematic scoping review. PLoS One 2020; 15: e0230512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Cortaredona S, Ventelou B.. The extra cost of comorbidity: multiple illnesses and the economic burden of non-communicable diseases. BMC Med 2017; 15: 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hofmarcher T, Lindgren P, Wilking N. et al. The cost of cancer in Europe 2018. Eur J Cancer 2020; 129: 41–49 [DOI] [PubMed] [Google Scholar]
- 64. Williams R, Karuranga S, Malanda B. et al. Global and regional estimates and projections of diabetes-related health expenditure: results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 2020; 162: 108072. [DOI] [PubMed] [Google Scholar]
- 65. Mohnen SM, van Oosten MJM, Los J. et al. Healthcare costs of patients on different renal replacement modalities - analysis of Dutch health insurance claims data. PLoS One 2019; 14: e0220800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.European Renal Association–European Dialysis and TransplantAssociation. ERA-EDTA Registry annual report 2009. https://era-edta-reg.org/files/annualreports/AnnRep2009.pdf
- 67. Hill NR, Fatoba ST, Oke JL. et al. Global prevalence of chronic kidney disease – a systematic review and meta-analysis. PLoS One 2016; 11: e0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Jommi C, Armeni P, Battista M. et al. The cost of patients with chronic kidney failure before dialysis: results from the IRIDE observational study. Pharm Open 2018; 2: 459–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kerr M, Bedford M, Matthews B. et al. The economic impact of acute kidney injury in England. Nephrol Dial Transplant 2014; 29: 1362–1368 [DOI] [PubMed] [Google Scholar]
- 70. Silver SA, Long J, Zheng Y. et al. Cost of acute kidney injury in hospitalized patients. J Hosp Med 2017; 12: 70–76 [DOI] [PubMed] [Google Scholar]
- 71. Bello AK, Levin A, Tonelli M. et al. Assessment of global kidney health care status. JAMA 2017; 317: 1864–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bello AK, Levin A, Lunney M. et al. Status of care for end stage kidney disease in countries and regions worldwide: international cross sectional survey. BMJ 2019; 367: l5873. [DOI] [PubMed] [Google Scholar]
- 73. Agar JW. Green dialysis: the environmental challenges ahead. Semin Dial 2015; 28: 186–192 [DOI] [PubMed] [Google Scholar]
- 74. Blankestijn PJ, Bruchfeld A, Capasso G. et al. Lancet countdown paper: what does it mean for nephrology? Nephrol Dial Transplant 2019; 34: 4–6 [DOI] [PubMed] [Google Scholar]
- 75. Barraclough KA, Blashki GA, Holt SG. et al. Climate change and kidney disease—threats and opportunities. Kidney Int 2017; 92: 526–530 [DOI] [PubMed] [Google Scholar]
- 76. Vanholder R, Lameire N.. COVID-19 and policy changes for kidney disease: the need for a ‘decade of the kidney’. Nephrol Dial Transplant 2021; 36: 8–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Guan WJ, Ni ZY, Hu Y. et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382: 1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wu Z, McGoogan JM.. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA 2020; 323: 1239–1242 [DOI] [PubMed] [Google Scholar]
- 79. Williamson EJ, Walker AJ, Bhaskaran K. et al. Factors associated with COVID-19-related death using open SAFELY. Nature 2020; 584: 430–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Clark A, Jit M, Warren-Gash C. et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Glob Health 2020; 8: e1003–e1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Jager KJ, Kramer A, Chesnaye NC. et al. Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int 2020; 98: 1540–1548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.ERA-EDTA Council, ERACODA Working Group. Chronic kidney disease is a key risk factor for severe COVID-19: a call to action by the ERA-EDTA. Nephrol Dial Transplant 2021; 36: 87–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Gansevoort RT, Hilbrands LB.. CKD is a key risk factor for COVID-19 mortality. Nat Rev Nephrol 2020; 16: 705–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Centers for Disease Control and Prevention. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 — United States, February 12–March 28, 2020. https://www.cdc.gov/mmwr/volumes/69/wr/mm6913e2.htm
- 85. Batlle D, Soler MJ, Sparks MA. et al. Acute kidney injury in COVID-19: emerging evidence of a distinct pathophysiology. J Am Soc Nephrol 2020; 31: 1380–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Pei G, Zhang Z, Peng J. et al. Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol 2020; 31: 1157–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Richardson S, Hirsch JS, Narasimhan M. et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 2020: 323: 2052–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Goldfarb DS, Benstein JA, Zhdanova O. et al. Impending shortages of kidney replacement therapy for COVID-19 patients. Clin J Am Soc Nephrol 2020; 15: 880–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Manara AR, Mumford L, Callaghan CJ. et al. Donation and transplantation activity in the UK during the COVID-19 lockdown. Lancet 2020; 396: 465–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Alasfar S, Avery RK.. The impact of COVID-19 on kidney transplantation. Nat Rev Nephrol 2020; 16: 568–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Brown EA, Perl J.. Increasing peritoneal dialysis use in response to the COVID-19 pandemic: will it go viral? J Am Soc Nephrol 2020; 31: 1928–1930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Watnick S, McNamara E.. On the frontline of the COVID-19 outbreak: keeping patients on long-term dialysis safe. Clin J Am Soc Nephrol 2020; 15: 710–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Quintaliani G, Reboldi G, Di Napoli A. et al. Exposure to novel coronavirus in patients on renal replacement therapy during the exponential phase of COVID-19 pandemic: survey of the Italian Society of Nephrology. J Nephrol 2020; 33: 725–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sever MS, Ortiz A, Maggiore U. et al. Mass disasters and burnout in nephrology personnel: from earthquakes and hurricanes to COVID-19 pandemic. Clin J Am Soc Nephrol 2021; doi: 10.2215/CJN.08400520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Combe C, Kirsch AH, Alfano G. et al. At least 156 reasons to prioritise COVID-19 vaccination in patients receiving in-centre haemodialysis. Nephrol Dial Transplant 2021; 36: 571–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.British Renal Society. Clinical efficay of vaccine in HD patients. https://britishrenal.us13.list-manage.com/track/click?u=ba16832efb8ed279407c624e8&id=8b21384214&e=7c5781d482
- 97. Lucero-Prisno DE, Ogunkola IO, Imo UF. et al. Who will pay for the COVID-19 vaccines for Africa? Am J Trop Med Hyg 2021; doi: 10.4269/ajtmh.20-1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Wieringa FP, Sheldon M.. The Kidney Health Initiative innovation roadmap for renal replacement therapies: building the yellow brick road, while updating the map. Artif Organs 2020; 44: 111–122 [DOI] [PubMed] [Google Scholar]
- 99. Verma S, McMurray JJV.. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia 2018; 61: 2108–2117 [DOI] [PubMed] [Google Scholar]
- 100. Wanner C, Marx N.. SGLT2 inhibitors: the future for treatment of type 2 diabetes mellitus and other chronic diseases. Diabetologia 2018; 61: 2134–2139 [DOI] [PubMed] [Google Scholar]
- 101.European Commission. Why the EU supports health research and innovation. https://ec.europa.eu/info/research-and-innovation/research-area/health-research-and-innovation_en
- 102. The Lancet Diabetes Endocrinology. Diabetes and NCD research: meeting the needs of the patient. Lancet Diabet Endocrinol 2016; 4: 873. [DOI] [PubMed] [Google Scholar]
- 103. Allen L. Non-communicable disease funding. Lancet Diabetes Endocrinol 2017; 5: 92. [DOI] [PubMed] [Google Scholar]
- 104. Luengo-Fernandez R, Leal J, Gray A.. UK research spend in 2008 and 2012: comparing stroke, cancer, coronary heart disease and dementia. BMJ Open 2015; 5: e006648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.National Institutes of Health. Estimates of Funding for Various Research, Condition, and Disease Categories (RCDC). https://report.nih.gov/categorical_spending.aspx
- 106. Vivot A, Jacot J, Zeitoun JD. et al. Clinical benefit, price and approval characteristics of FDA-approved new drugs for treating advanced solid cancer, 2000–2015. Ann Oncol 2017; 28: 1111–1116 [DOI] [PubMed] [Google Scholar]
- 107.America's Health Insurance Plans. The Rise of Orphan Drugs. https://www.ahip.org/wp-content/uploads/IB_OrphanDrugs-1004.pdf
- 108. Moon S, Mariat S, Kamae I. et al. Defining the concept of fair pricing for medicines. BMJ 2020; 368: l4726. [DOI] [PubMed] [Google Scholar]
- 109. Zoccali C, Vanholder R, Wagner CA. et al. Funding kidney research as a public health priority: challenges and opportunities. Nephrol Dial Transplant 2020; doi: 10.1093/ndt/gfaa163 [DOI] [PubMed] [Google Scholar]
- 110.American Nephrology Nurses Association. Advancing American Kidney Health: News & Updates. https://www.annanurse.org/article/advancing-american-kidney-health
- 111.American Association of Kidney Patients. Decade of the Kidney. https://aakp.org/decade-of-the-kidney/
- 112.European Union. FAQ EU competences and Commission powers. https://europa.eu/citizens-initiative/faq-eu-competences-and-commission-powers_en
- 113. Luyckx VA, Tonelli M, Stanifer JW.. The global burden of kidney disease and the sustainable development goals. Bull World Health Org 2018; 96: 414–422D [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.European Commission. EU holistic approach to sustainable development. https://ec.europa.eu/info/strategy/international-strategies/sustainable-development-goals/eu-holistic-approach-sustainable-development_en
- 115. Luyckx VA, Al-Aly Z, Bello AK. et al. Sustainable development goals relevant to kidney health: an update on progress. Nat Rev Nephrol 2021; 17: 15–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.European Commission. EU Action on Cancer. https://ec.europa.eu/health/sites/health/files/major_chronic_diseases/docs/30years_euaction_cancer_en.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.