Abstract
Background
The iron-based phosphate binder (PB), sucroferric oxyhydroxide (SFOH), is indicated to control serum phosphorus levels in patients with chronic kidney disease on dialysis.
Methods
This non-interventional, prospective, multicentre, cohort study conducted in seven European countries evaluated the safety and effectiveness of SFOH in dialysis patients with hyperphosphataemia in routine practice. Safety outcomes included adverse drug reactions (ADRs) and changes in iron-related parameters. SFOH effectiveness was evaluated by changes-from-baseline (BL) in serum phosphorus and percentage of patients achieving in-target phosphorus levels.
Results
The safety analysis set included 1365 patients (mean observation: 420.3 ± 239.3 days). Overall, 682 (50.0%) patients discontinued the study. Mean SFOH dose during the observation period was 1172.7 ± 539.9 mg (2.3 pills/day). Overall, 617 (45.2%) patients received concomitant PB(s) during SFOH treatment. ADRs and serious ADRs were observed for 531 (38.9%) and 26 (1.9%) patients. Most frequent ADRs were diarrhoea (194 patients, 14.2%) and discoloured faeces (128 patients, 9.4%). Diarrhoea generally occurred early during SFOH treatment and was mostly mild and transient. Small increases from BL in serum ferritin were observed (ranging from +12 to +75 µg/L). SFOH treatment was associated with serum phosphorus reductions (6.3 ± 1.6 mg/dL at BL versus 5.3 ± 1.8 mg/dL at Month 30; ΔBL: −1.0 mg/dL, P < 0.01). Percentage of patients achieving serum phosphorus ≤4.5 mg/dL increased from 12.0% at BL to 34.8% at Month 30, while the percentage achieving serum phosphorus ≤5.5 mg/dL increased from 29.9% to 63.0%.
Conclusions
SFOH has a favourable safety and tolerability profile in a real-world setting, consistent with results of the Phase 3 study. Moreover, SFOH improved serum phosphorus control with a low daily pill burden.
Keywords: chronic kidney disease, end-stage kidney disease, haemodialysis, phosphate binder, peritoneal dialysis
INTRODUCTION
Hyperphosphataemia is a frequent complication among patients with advanced chronic kidney disease (CKD), especially for those with end-stage renal disease [1]. In this population, elevated serum phosphorus is independently associated with increased morbidity and mortality [2–5]. In addition to dietary phosphate restriction and dialytic phosphate removal, most dialysis patients require treatment with oral phosphate binders (PBs) for management of hyperphosphataemia [6]. However, real-world data [7] show 41% of European haemodialysis (HD) patients still have serum phosphorus levels above the range recommended by the Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines (3.5–5.5 mg/dL) [8], while 70% have serum phosphorus levels above normal, the target suggested by the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines [5]. A major reason for poor serum phosphorus control is low adherence to PB therapy, which may occur as a consequence of high pill burden [9, 10].
Sucroferric oxyhydroxide (SFOH, Velphoro®) is a potent [11], iron-based PB with a low daily pill burden approved in the USA [12] and Europe [13] for the control of serum phosphorus levels in patients with CKD on dialysis. Pivotal clinical trials demonstrated that SFOH was well tolerated with a favourable safety profile, and was effective for reducing serum phosphorus [14–16]. In the Phase 3 trial and its extension study, SFOH was non-inferior to sevelamer carbonate for serum phosphorus reduction, but had a substantially lower daily pill burden (3.3 pills/day versus 8.7 pills/day, respectively) over a 1-year treatment period [14, 15]. The most common adverse events (AEs) observed with SFOH were mild transient diarrhoea and stool discolouration.
Because prospective interventional clinical trials of SFOH were performed in specialized centres and enrolled selected dialysis patients, it is of major interest to evaluate daily SFOH use outside the controlled trial setting. This non-interventional post-authorization safety study [Velphoro Evaluation of Real-lIfe saFety, effectIveness and adherencE (VERIFIE)] was conducted to obtain real-world data relating to the longer term (>1 year) safety and effectiveness of SFOH for the treatment of hyperphosphataemia in a large population of patients undergoing HD or peritoneal dialysis (PD) in routine clinical practice. Specific safety objectives of VERIFIE were to assess the potential risk of iron accumulation with SFOH and to evaluate potential masking of gastrointestinal (GI) bleeding by SFOH-induced stool discolouration. Importantly, the real-world effectiveness of SFOH for the reduction of serum phosphorus levels was also evaluated.
MATERIALS AND METHODS
Patients and study design
VERIFIE was a non-interventional, prospective, multicentre cohort study, scheduled to enrol ≥1000 adult dialysis patients in seven European countries.
Study inclusion criteria were as follows: adults (≥18 years) who had provided signed, informed consent and had an indication for SFOH in accordance with the product label [13]; prevalent HD or PD patients (dialysis vintage ≥6 months); and patients who were either treatment-naïve or pre-treated with PBs. Exclusion criteria were prior participation in VERIFIE; current participation in an interventional study; and enrolment in a prior clinical trial of SFOH.
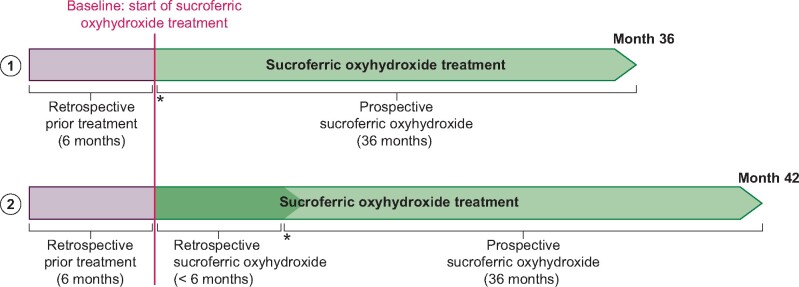
Patients newly prescribed SFOH or who had been treated with SFOH up to 6 months prior to study entry were eligible. The study design is displayed in Figure 1. The scheduled study duration was 3 years, with a recruitment phase of 2 years. The planned prospective observation period per patient ranged from 12 to 36 months.
FIGURE 1:
VERIFIE study design. (1) Enrolment option for patients with SFOH treatment initiation at the time of inclusion into the study (treatment-naïve patients): treatment-naïve patients have a prospective observation period of up to 36 months. Retrospective data covering a period of 6 months prior to SFOH treatment start are collected. (2) Enrolment option for patients with SFOH treatment start up to 6 months prior to inclusion into the study (pre-treated patients): in pre-treated patients, SFOH treatment is documented for up to 42 months (for patients pre-treated with SFOH for >3 months and up to 6 months) including retrospective documentation of SFOH treatment up to 6 months and a prospective observation period of up to 36 months. Additionally, retrospective data covering a period of 6 months prior to SFOH treatment start are collected. Asterisks indicate inclusion into the study.
Ethical considerations
The study was conducted in accordance with the Declaration of Helsinki [17], and the protocol was approved by Institutional Review Boards within each participating country. All participants provided written informed consent. An external study Steering Committee monitored study progress and ensured that all predefined objectives were met. The study was registered on ClinialTrials.gov (NCT02687594).
Study outcomes
Primary endpoints were the incidence and proportion of adverse drug reactions (ADRs) and medical events of special interest (MESIs; all AEs indicative of GI bleeding, diarrhoea and iron accumulation irrespective of their relationship to SFOH). Physicians’ evaluation of the potential masking effect of the stool discolouration due to SFOH treatment on GI bleeding diagnosis, iron-related parameters [ferritin, transferrin saturation (TSAT) and haemoglobin], and fatal events were also assessed. Secondary endpoints included evaluation of laboratory parameters, including phosphorus (to evaluate SFOH effectiveness) and other CKD–mineral bone disorder (CKD-MBD) parameters, mean daily SFOH dose and dose changes and adherence to SFOH therapy.
Study assessments and data collection
Study data were collected at the initial visit, baseline (BL; i.e. SFOH treatment initiation), and Months 1, 3, 6, 12, 18, 24, 30 and 36 after treatment initiation. The number of observational time points for each patient depended on their length of follow-up.
Investigators collected patient demographics, medical history and comorbidities for the 6 months before SFOH initiation from medical records or the initial and/or BL visit. Routine measurements and assessments (e.g. laboratory parameters) were collected by investigators during patients’ routine practice visits. Data relating to the presence or absence of prior and concomitant use of other PBs and intravenous (IV) or oral iron supplements were collected.
Investigators assessed the potential impact of the stool discolouration due to SFOH on the timing of diagnosis of GI bleeding (i.e. the potential masking effect and diagnostic delay). Details were collected via a physician questionnaire.
Patient-reported adherence to SFOH therapy was measured using a shortened version (four questions) of the Morisky adherence questionnaire (Supplementary data, Table S1) [18]. Data were collected at BL (for prior PB use) and at each follow-up visit (for SFOH use). For patients who completed the questionnaire, responses were aggregated into a single measure of treatment adherence by scoring answers of ‘No’ with 1 and ‘Yes’ with 0. Four adherence categories were generated with the resulting sum scores: 4 (most adherent patients), 3, 2 and 0–1 (least adherent patients).
Analysis populations
All primary safety endpoint analyses were performed on the ‘safety analysis set’, which included patients who received ≥1 SFOH dose and who had ≥1 post-BL safety assessment available. Analyses of serum phosphorus levels and treatment adherence were performed on the ‘full analysis set’, which included all patients who received ≥1 SFOH dose, and who had ≥1 BL and ≥1 post-BL serum phosphorus value available.
Statistical analysis
Sample size calculations were based on the expected occurrence of GI bleeding events, because evaluation of masking of GI bleeding was a primary outcome of interest. A one-sample Chi-square test with a 0.05, two-sided significance level was used for the calculation. Based on data from the Dialysis Outcomes and Patient Patterns Study (DOPPS) reporting a GI bleeding incidence of up to 5% in real-world dialysis patients [19], the study duration (up to 36 months), and potential dropout rate (up to 50%), a conservative calculation found n = 1000 patients was sufficient to achieve the study target of 900–1000 patient-years exposure.
For the primary endpoint analyses, crude incidence rates of ADRs, MESIs and fatal events with 95% confidence intervals (CIs) were calculated. Exposure-adjusted incidence rates (EAIRs) per patient-year were also calculated as the number of patients with a specific event, divided by total follow-up time for all patients.
For the effectiveness analysis, mean ± standard deviation (SD) serum phosphorus values and changes from BL were summarized at each study assessment for the full analysis set, along with the proportion of patients achieving serum phosphorus goal, based on KDOQI and KDIGO guideline recommendations (≤5.5 and ≤4.5 mg/dL, respectively). A subgroup analysis was also performed to evaluate the effectiveness of SFOH monotherapy versus SFOH administered in combination with other PB(s).
Mean ± SD values and changes from BL in iron parameters and CKD-MBD parameters were summarized for the safety analysis set and full analysis set populations, respectively. Mean ± SD daily SFOH dose and associated pill burden (number of pills/day) were calculated for patients in the safety analysis set. Another subgroup analysis stratifying patients in the safety analysis set according to concomitant IV or oral iron was performed to evaluate the effect of SFOH therapy on serum ferritin levels, independent of potential confounding from concomitant iron supplement use.
All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). P-values were generated from an exploratory post hoc analysis.
RESULTS
Patients
The final study analysis was performed 36 months after the first patient, first visit (data collection ended: 6 April 2019). In total, 1365 and 1322 patients were included in the safety analysis set and full analysis set populations, respectively (Supplementary data, Figure S1). Numbers of patients, centres and patients per centre are shown in Supplementary data, Table S2.
BL characteristics of patients in the safety analysis set are shown in Table 1. The majority were male (66.5%) and were undergoing HD (87.8%). Most patients (62.3%) had received other PB(s) prior to starting SFOH. During the study, 45.2% of patients received other concomitant PB(s) in addition to SFOH treatment (Supplementary data, Table S3).
Table 1.
BL demographic and clinical characteristics
| Parameter | Safety analysis set |
|---|---|
| (n = 1365) | |
| Age, years | 61.5 ± 14.9 |
| Male, n (%) | 908 (66.5) |
| Body weight, kg | 77.1 ± 18.2 |
| BMI, kg/m2 | 27.2 ± 5.8 |
| Primary cause of CKD, n (%) | |
| Diabetes | 265 (19.4) |
| Hypertension | 281 (20.6) |
| Other | 788 (57.7) |
| Missing | 2 (0.1) |
| Dialysis modality, n (%) | |
| HD | 1198 (87.8) |
| PD | 160 (11.7) |
| Missing information on dialysis modality | 7 (0.5) |
| Dialysis vintage, years | 4.3 ± 5.7 |
| Prior treatment with other PB(s), n (%) | 850 (62.3) |
| Sevelamer | 300 (35.3) |
| Calcium-based PB | 160 (18.8) |
| Calcium-based/sevelamer | 130 (15.3) |
| Lanthanum | 110 (12.9) |
| Calcium-based/lanthanum | 51 (6.0) |
| Sevelamer/lanthanum | 31 (3.6) |
| Aluminium-based | 23 (2.7) |
| Sevelamer/aluminium-based | 12 (0.9) |
| Calcium-based/sevelamer/lanthanum | 10 (1.2) |
| Lanthanum/aluminium-based | 8 (0.9) |
| Calcium-based/sevelamer/aluminium-based | 7 (0.8) |
| Calcium-based/aluminium-based | 4 (0.5) |
| Calcium-based/lanthanum/aluminium-based | 2 (0.2) |
| Calcium-based/sevelamer/lanthanum/ aluminium-based | 2 (0.2) |
Values are the n (%) or mean ± SD.
BMI, body mass index.
In total, 682 patients (50.0%) in the safety analysis set prematurely withdrew from the study (Table 2). The most common reasons for premature withdrawal among these patients were discontinuation of SFOH for unspecified reasons (34.3%), ADRs or MESIs (21.5%), death (17.2%) and kidney transplantation (13.6%).
Table 2.
Reasons for withdrawals and premature discontinuations from the study
| n (%) | Safety analysis set |
|---|---|
| (n = 1365) | |
| Patients who withdrew or discontinued study prematurely | 682 (100.0) |
| ADR/MESI | 147 (21.5) |
| Withdrawal of consent | 10 (1.5) |
| Lost to follow-up | 38 (5.6) |
| Treatment with SFOH discontinued permanentlya | 234 (34.3) |
| Administrative problems | 1 (0.15) |
| Death | 117 (17.2) |
| AE (other than ADR/MESI/fatal event) | 5 (0.7) |
| Kidney transplantation | 93 (13.6) |
| Lack of efficacy | 1 (0.15) |
| Other PB therapy | 1 (0.15) |
| Parathyroidectomy | 3 (0.4) |
| Participation in different study | 1 (0.15) |
| Inclusion/exclusion criteria not met | 2 (0.3) |
| Patient’s decision | 19 (2.8) |
| Physician’s decision | 2 (0.3) |
| Patient’s poor compliance | 5 (0.7) |
| Product complaint | 2 (0.3) |
| Treatment start of SFOH >6 months prior to study inclusion | 1 (0.15) |
Specific reasons recorded for 220 patients: ADR, MESI, fatal event (n = 90); serum phosphorus controlled (n = 17); AE (n = 9); hospitalization (n = 2); kidney transplantation (n = 26); lost to follow-up (n = 1); other PB (n = 5); parathyroidectomy (n = 1); patient decision (n = 50); physician decision (n = 2); patient’s poor compliance (n = 17).
SFOH treatment duration
The mean ± SD observation period for patients in the safety analysis set was 420.3 ± 239.3 days. A post hoc analysis showed that mean ± SD exposure duration to SFOH (excluding off-treatment days) was 1.12 ± 0.65 patient-years, and that 58.7% of patients received treatment with SFOH for ≥12 months (Supplementary data, Table S4).
Primary endpoint analyses
In total, 531 patients (38.9%) in the safety analysis set reported ≥1 ADR during treatment with SFOH (Table 3). The most common ADRs were GI disorders, which were reported for 31.9% of patients, mainly diarrhoea (14.2%) and discoloured faeces (9.4%). Serious ADRs were reported for 26 patients (1.9%); the most common were GI disorders (1.0%) (Supplementary data, Table S5).
Table 3.
ADRs occurring in ≥1% of patients by system organ class and preferred term
| System organ class | Safety analysis set |
|
|---|---|---|
| (n = 1365) | ||
| Preferred term | Patients, n (%) | EAIRa per year (95% CI) |
| Patients with at least one ADRb | 531 (38.9) | 0.461 (0.422–0.502) |
| GI disorders | 436 (31.9) | 0.355 (0.322–0.390) |
| Diarrhoea | 194 (14.2) | 0.133 (0.115–0.153) |
| Faeces discoloured | 128 (9.4) | 0.090 (0.075–0.107) |
| Abnormal faeces | 48 (3.5) | 0.032 (0.023–0.042) |
| Constipation | 40 (2.9) | 0.026 (0.018–0.035) |
| Abdominal pain | 38 (2.8) | 0.024 (0.017–0.034) |
| Nausea | 36 (2.6) | 0.023 (0.016–0.032) |
| Faeces soft | 20 (1.5) | 0.013 (0.008–0.020) |
| Vomiting | 17 (1.2) | 0.011 (0.006–0.017) |
| Dyspepsia | 16 (1.2) | 0.010 (0.006–0.017) |
| Injury, poisoning and procedural complications | 59 (4.3) | 0.039 (0.030–0.050) |
| Off-label use | 29 (2.1) | 0.019 (0.013–0.027) |
| General disorders and administration site conditions | 56 (4.1) | 0.037 (0.028–0.048) |
| Drug ineffective | 26 (1.9) | 0.017 (0.011–0.025) |
| Treatment non-compliance | 15 (1.1) | 0.010 (0.005–0.016) |
| Product issues | 24 (1.8) | 0.015 (0.010–0.023) |
| Product taste abnormal | 23 (1.7) | 0.015 (0.009–0.022) |
The EAIR is defined as the number of patients with a specific event divided by the total follow-up time for all patients in years.
All ADRs were coded based on MedDRA version 22.0 terminology into system organ class and preferred terms.
MedDRA: Medical Dictionary for Regulatory Activities.
Overall, 250 patients (18.3%) had at least one MESI during the study (Table 4); most of these were GI disorders. Thirty-eight patients (2.8%) had GI bleeding during SFOH treatment (46 events). Most of these patients (n = 32, 84.2%) had specific risk factors for GI bleeding, including medication, such as anticoagulant therapy (n = 24, 63.2%), history of GI bleeding (n = 10, 26.3%), medical conditions/disease with increased bleeding risk (n = 7, 18.4%) or other reasons (n = 2, 5.3%). Documented clinical reports were available for 40 GI bleeding events: for 36, no delay in GI bleeding diagnosis due to SFOH-related stool discolouration was reported, while for four events (10.0%), stool discolouration with SFOH was reported as causing an insignificant delay, without affecting patient health.
Table 4.
MESI reported for two or more patients by system organ class and preferred term
| System organ class Preferred term | Safety analysis set (n = 1365) Patients, n (%) | EAIRa per year (95% CI) |
|---|---|---|
| Patients with at least one MESIb | 250 (18.3) | 0.176 (0.155–0.199) |
| GI disorders | 249 (18.2) | 0.175 (0.154–0.199) |
| Diarrhoea | 217 (15.9) | 0.151 (0.131–0.172) |
| GI haemorrhage | 18 (1.3) | 0.012 (0.007–0.018) |
| Rectal haemorrhage | 6 (0.4) | 0.004 (0.001–0.008) |
| Haematemesis | 4 (0.3) | 0.003 (0.001–0.007) |
| Melena | 3 (0.2) | 0.002 (0.000–0.006) |
| Haematochezia | 2 (0.15) | 0.001 (0.000–0.005) |
| Metabolism and nutrition disorders | 2 (0.15) | 0.001 (0.000–0.005) |
| Iron overload | 2 (0.15) | 0.001 (0.000–0.005) |
The EAIR is defined as the number of patients with a specific event divided by the total follow-up time for all patients in years.
All MESI were coded based on MedDRA version 22.0 terminology into System Organ Class and Preferred Terms. MedDRA: Medical Dictionary for Regulatory Activities.
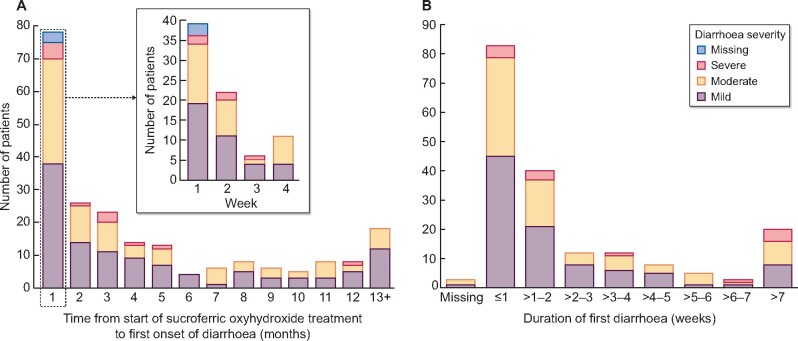
Diarrhoea was reported for 217 patients (15.9%); in 194 (89.4%) patients, it was assessed by the investigators as being related to SFOH treatment. The first event of diarrhoea generally occurred early during SFOH treatment (Figure 2A) and severity was mild (53.0%), moderate (40.1%) or severe (5.5%). A documented degree of severity was missing for three (1%) patients. For 186 patients, the first diarrhoea event was reported as recovered/resolved; for the majority of these subjects (66.1%), diarrhoea resolved within 2 weeks of onset (Figure 2B).
FIGURE 2:
Characteristics of diarrhoea during treatment with SFOH. (A) Time from start of treatment to first onset of diarrhoea (safety analysis set, subgroup of patients with reported diarrhoea; n = 217). (B) Duration of first diarrhoea event (safety analysis set, patients with diarrhoea with outcome; n = 186), where outcome = ‘recovered/resolved’ or ‘recovered/resolved with sequelae’.
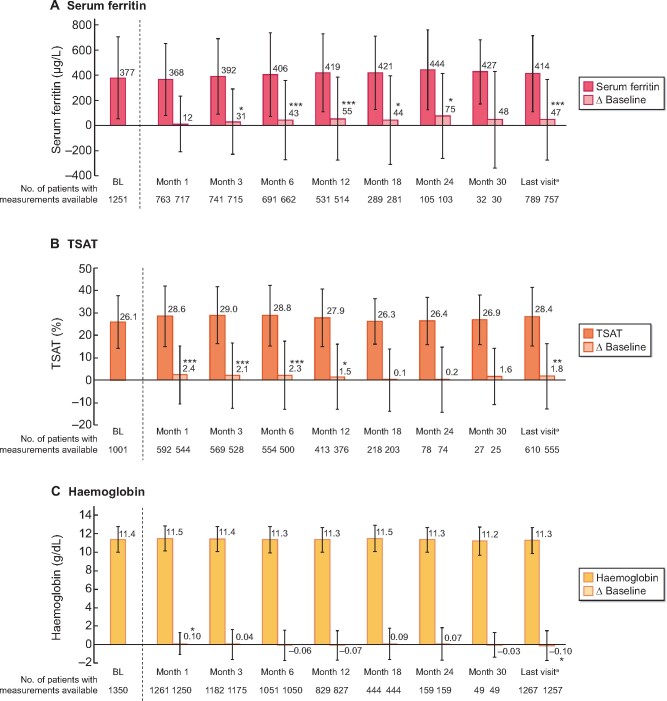
Two patients, both of whom received concomitant IV iron therapy, had iron accumulation (iron overload, defined as a MESI) during the observation period; in one, events were considered related to SFOH treatment. Patients in the safety analysis set displayed small but statistically significant (P < 0.05) increases from BL in mean serum ferritin at most time points (Figure 3A). Small increases in TSAT levels were observed, whereas haemoglobin values remained unchanged (Figures 3B and C). In the safety analysis set, 800 patients (58.6%) received IV or oral iron supplementation therapy prior to SFOH initiation, whereas 880 (64.5%) received concomitant IV or oral iron during SFOH treatment. A subgroup analysis showed mean serum ferritin levels increased significantly from BL among patients who received any concomitant iron therapy during SFOH treatment, but generally decreased among patients who did not receive any iron therapy (Supplementary data, Figure S2).
FIGURE 3:
Mean ± SD values of iron-related parameters and changes versus BL during the observation period (safety analysis set; n = 1365). (A) Serum ferritin, (B) TSAT and (C) haemoglobin. *P < 0.05, **P < 0.01 and ***P < 0.001 versus BL. aTo account for the effect of premature discontinuations, data for all patients at the last completed observation time point were summarized by a final follow-up: ‘last visit’. Data for Month 36 not shown due to low number of patients with follow-up data at this time point. Bars show mean values and whiskers represent SDs.
In total, 119 patients (8.7%) in the safety analysis set had fatal events during the observation period. None of the fatal events was assessed by the investigator as being related to SFOH treatment.
A subgroup analysis showed the safety and tolerability profile of SFOH was similar for patients undergoing HD (n = 1198) or PD (n = 160) during the study. There were no major differences in the type or frequency of ADRs or serious ADRs reported for HD and PD patients (data not shown).
Secondary endpoint analyses
Serum phosphorus control
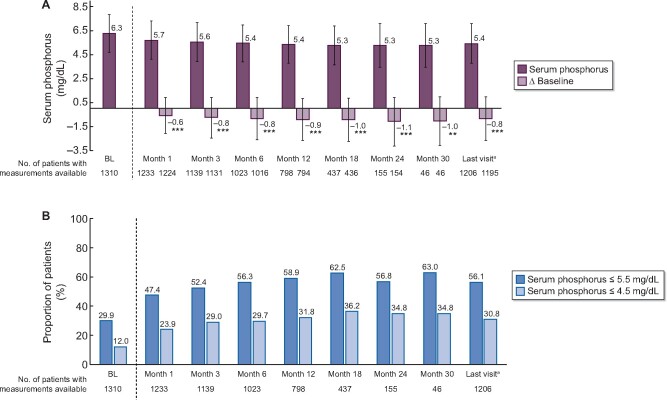
Among patients in the full analysis set, serum phosphorus decreased significantly during SFOH treatment (6.3 ± 1.6 mg/dL at BL to 5.7 ± 1.6 mg/dL at Month 1; P < 0.001 and 5.3 ± 1.8 mg/dL at Month 30; P < 0.01) (Figure 4A). The proportion of patients with serum phosphorus ≤5.5 mg/dL increased from 29.9% at BL to 47.4–63.0% during the observation period (Figure 4B). The proportion with serum phosphorus ≤4.5 mg/dL also increased, from 12.0% at BL to 23.9–36.2% during SFOH treatment.
FIGURE 4:
Serum phosphorus control during the observation period (full analysis set; n = 1322). (A) Mean ± SD phosphorus concentrations and changes from BL over time. **P < 0.01, ***P < 0.001 versus BL. (B) Proportion of patients with serum phosphorus ≤5.5 and ≤4.5 mg/dL. aTo account for the effect of premature discontinuations, data for all patients at the last completed observation time point were summarized by a final follow-up: ‘last visit’. Data for Month 36 not shown due to low number of patients with follow-up data at this time point. In (A), bars show mean values and whiskers represent SDs.
A subgroup analysis, stratifying patients by concomitant PB(s) use during the study, showed that subjects who received SFOH in combination with other PB(s) had higher BL serum phosphorus than those who received SFOH monotherapy (Supplementary Figure S3). The absolute reductions in serum phosphorus during SFOH treatment were comparable in both subgroups. The proportion of patients achieving serum phosphorus goal (≤5.5 mg/dL) at BL was higher among those receiving SFOH monotherapy than those receiving concomitant PB(s). The proportion with serum phosphorus ≤5.5 mg/dL increased to a similar extent in both groups during SFOH treatment, remaining higher in the SFOH monotherapy group (Supplementary data, Figure S4).
SFOH exposure and daily pill burden
In the safety analysis set, mean ± SD initial daily SFOH dose at BL was 1047.2 ± 486.3 mg (2.1 pills/day), and increased to 1204.6 ± 618.5 mg (2.4 pills/day) by the last daily dose. The mean SFOH daily dose during the overall observation period was 1172.7 ± 539.9 mg (2.3 pills/day).
A subgroup analysis showed the mean daily SFOH dose during the observation period was similar for patients who received SFOH with or without concomitant PB therapy [1228.0 ± 598.8 mg (2.5 pills/day) and 1126.7 ± 481.0 mg (2.3 pills/day), respectively].
Adherence to SFOH therapy
Drug adherence scores based on the Morisky adherence questionnaire for patients in the full analysis set who completed all four questions are shown in Figure 5. The most frequent score category at all post-BL visits up until Month 12 of SFOH therapy was 4 (‘very adherent’), observed for >48.0% of patients. The proportion of very adherent patients initially increased from 44.5% at BL (for those receiving prior PB therapy) to 56.1% at Month 1, before decreasing again slightly to 48.7% at Month 3 and remaining constant at subsequent time points. Across all post-BL study visits, ˂14% of SFOH-treated patients had a score of 0–1 (not adherent at all).
FIGURE 5:
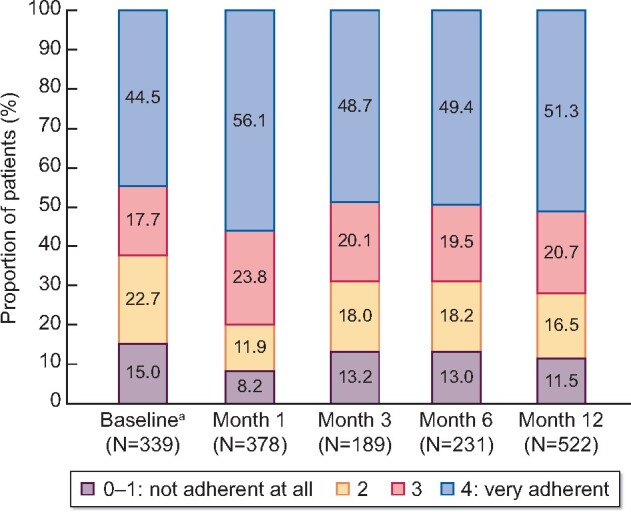
Drug adherence score based on Morisky questionnaire (full analysis set; subgroup of patients who fully completed the questionnaire). The drug adherence score based on Morisky adherence questionnaire is calculated as sum of ‘No’ answers if complete assessments were given for all four questions. aAt baseline (SFOH treatment start) information regarding previous PB use other than SFOH was collected.
Other CKD-MBD parameters
There were no major changes in serum concentrations of calcium, parathyroid hormone and 25-hydroxyvitamin D during SFOH treatment (Supplementary data, Table S6).
DISCUSSION
In this real-world study evaluating the clinical use of SFOH in a large cohort of European HD and PD patients, no new safety signals were identified. The safety profile of SFOH was in line with that expected from clinical trials [14–16]. In addition, SFOH improved serum phosphorus control, with a larger proportion of patients, as compared with BL, achieving in-target serum phosphorus levels during treatment.
A primary objective of the study was to evaluate the longer term real-world safety of SFOH in dialysis patients. The mean duration of patients’ exposure to SFOH exceeded 1 year (1.12 patient-years), and the study met its goal of obtaining at least 1000 patient-years of SFOH exposure.
The BL characteristics of the VERIFIE patient population were similar to European dialysis patients from other large observational studies, such as COSMOS [20], apart from a slightly younger age in VERIFIE (62 versus 64 years) and a somewhat longer dialysis vintage (4.3 versus 3.2 years). It is important to acknowledge that the VERIFIE study cohort comprises a selected population of patients who were prescribed SFOH as part of routine practice and therefore it may not be fully representative of the wider European dialysis population.
Most frequent ADRs during the study were GI disorders, mainly diarrhoea and discoloured faeces. This is consistent with Phase 3 clinical study findings, during which 24 and 16% of SFOH-treated patients, respectively, reported diarrhoea and discoloured faeces as treatment-emergent AEs [12].
Diarrhoea was reported by 15.9% of patients during this study and its characteristics (timing, severity and duration) were consistent with Phase 3 study observations. In the VERIFIE population, diarrhoea tended to occur early during SFOH treatment, was predominantly mild or moderate in severity, and for most patients it resolved within 2 weeks.
The proportion of patients with GI bleeding (2.8%) was low in comparison with other real-world dialysis patient cohorts, such as the DOPPS, for which annual incidence rates of up to 5% have been reported [19]. There were no reports of clinically significant delays in GI bleeding diagnosis due to stool discoloration masking the GI bleeding. SFOH-related stool discoloration was reported as causing an insignificant delay in GI bleeding diagnosis in a small number of patients (n = 4, 0.3%), without this delay affecting their outcome. It is also important to note that patients receiving dialysis are typically under close clinical surveillance, undergoing evaluation of haematological laboratory parameters. Therefore, it is unusual for upper GI bleeding to have discoloured faeces as the predominant and sole clinical manifestation. Furthermore, lower GI bleeding is characterized by the passage of bright red blood per rectum, which is therefore unlikely to be masked by SFOH.
A small increase in mean serum ferritin was observed during SFOH treatment (ΔBL: +12 µg/L to +75 µg/L), which was consistent with findings from the Phase 3 study and its extension [14, 15, 21]. A subgroup analysis in the present study suggested the serum ferritin increase was driven by concomitant IV or oral iron use, because ferritin values did not increase in patients without concomitant oral or IV iron use. Two patients had MESIs of iron overload during this study, both of whom received concomitant treatments that included IV iron. One patient had an iron utilization disorder. The available information from these two cases does not support a change of the safety profile in terms of potential iron accumulation with SFOH.
The number of deaths that occurred during the VERIFIE study was relatively low (n = 119) in comparison with other observational studies [20].
SFOH effectively lowered serum phosphorus levels over 30 months of treatment, with a low daily pill burden. Increases in the proportion of patients achieving serum phosphorus goals based on KDOQI and KDIGO guidelines (≤5.5 and ≤4.5 mg/dL, respectively), was evident from Month 1, continued until Month 18 and thereafter was maintained for the remainder of the observation period. The reductions in mean serum phosphorus among patients treated with SFOH in VERIFIE were not as pronounced as those previously reported in the Phase 3 trial and its extension study [14, 15]. However, the Phase 3 study population had higher mean serum phosphorus levels at BL, likely due to their mandatory 2- to 4-week washout of other PB(s) before starting SFOH. Patients in VERIFIE generally received other PB therapy prior to recruitment.
Consistent with the Phase 3 study [14, 15], the mean daily pill burden of SFOH was low (2.3 SFOH pills/day). The mean SFOH dose at BL (2.1 SFOH pills/day) was lower than the starting dose recommended in the product label (3 pills/day) [13], indicating that clinicians in real-world practice tend to initiate SFOH therapy at a lower dose.
Almost half of patients in VERIFIE (n = 617, 45.2%) received concomitant PB(s) in addition to SFOH treatment. The use of multiple PBs by European dialysis patients is common in real-world clinical practice; in the COSMOS cohort, ∼22% of patient-years were prescribed combinations of different PB therapies [22]. A subgroup analysis showed reductions in serum phosphorus among patients receiving concomitant PB therapy, as well as those receiving SFOH monotherapy. However, serum phosphorus control (percentage of patients with ≤5.5 mg/dL) was better in the SFOH monotherapy group, probably due to these patients having lower mean BL serum phosphorus. Overall, our results, demonstrating clinically significant serum phosphorus reduction with SFOH, are important, considering that it can be more difficult to demonstrate real-world effectiveness than to achieve efficacy in a clinical trial setting.
Treatment adherence to SFOH during the study was analysed based on four questions from the Morisky questionnaire. The results indicated that adherence to SFOH therapy was good and remained consistent during the observation period (post-BL), with between 49 and 56% of patients ‘very adherent’ (score of 4) to SFOH up to Month 12, and ˂14% patients ‘not adherent at all’ (score of 0–1). It is important to note this analysis had limitations. Since the number of patients who fully completed the questionnaire was relatively low, only a small proportion from the full analysis set (<40%) were included in the analysis, and the follow-up was restricted to 12 months. In addition, because only four questions from the full Morisky questionnaire were used, the adherence scores described in the results are not validated and therefore should be interpreted with caution.
Limitations of the VERIFIE study included its non-interventional observational design, which may have been a source of selection bias. Furthermore, the study was open label with no randomization, or matched comparator population. Finally, polypharmacy and concomitant PB use may have influenced outcomes, and data relating to daily doses/pill burden of concomitant PBs or iron supplements were not collected.
In conclusion, SFOH had a favourable safety and tolerability profile consistent with that observed in the Phase 3 trial and its extension study [14, 15]. Moreover, SFOH was shown to improve phosphorus control with a low daily pill burden in a real-world setting.
SUPPLEMENTARY DATA
Supplementary data are available at ckj online.
FUNDING
The VERIFIE study was sponsored by Vifor Fresenius Medical Care Renal Pharma (Glattbrugg, Switzerland). Medical writing assistance was provided by AXON Communications (London, UK), and funded by Vifor Fresenius Medical Care Renal Pharma.
CONFLICT OF INTEREST STATEMENT
M.G.V. has received fees or research support from Vifor Fresenius Medical Care Renal Pharma, Fresenius Medical Care, Medice, Amgen, Otsuka, Kyowa Kirin, Cablon Medical and Shire. I.N.B. has received honoraria or research support from Fresenius Medical Care, Calliditas Therapeutics, Novartis Hellas, Genesis Pharma Hellas and NovoNordisk A/S. A.L.M.d.F. has nothing to disclose. P.A.K. has received honoraria and travel support from Fresenius, Vifor and Pharmacosmos, and IIT grants for the Salford renal research programme from Astellas, BergenBio and Vifor. M.K. has received consulting and lecture honoraria from Amgen, FMC, Medice, Sanofi, Shire, VFMCRP and Vifor Pharma. P.M. has received honoraria from Vifor and Sandoz. M.S.-G. is an employee of Fresenius Medical Care and has share options/ownership in Fresenius Medical Care. A.D. is an employee of Fresenius Medical Care. S.W. is an employee of Vifor Fresenius Medical Care Renal Pharma. A.P. is an employee of Vifor Fresenius Medical Care Renal Pharma. L.H.F. is an employee of Fresenius Medical Care Renal Therapies Group. J.R. was president of the Steering Committee for VERIFIE study. C.W. has honoraria for advisory board services from Vifor and Steering Committee participation from Fresenius Medical Care. J.B.C.-A. has received research support, fee and travel support from Kyowa Kirin, Vifor Fresenius Medical Care and Amgen. D.F. has received lecture and advisory board fees from Fresenius Kabi and Vifor, lecture fees from Sanofi, Amgen and Eli Lilly, and research grants from Fresenius Medical Care. The results presented in this article have not been published previously in whole or part, except in abstract format.
Supplementary Material
REFERENCES
- 1. Barreto FC, Barreto DV, Massy ZA. et al. Strategies for phosphate control in patients with CKD. Kidney Int Rep 2019; 4: 1043–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Block GA, Hulbert-Shearon TE, Levin NW. et al. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis 1998; 31: 607–617 [DOI] [PubMed] [Google Scholar]
- 3. Block GA, Klassen PS, Lazarus JM. et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 2004; 15: 2208–2218 [DOI] [PubMed] [Google Scholar]
- 4. Ganesh SK, Stack AG, Levin NW. et al. Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol 2001; 12: 2131–2138 [DOI] [PubMed] [Google Scholar]
- 5. Kidney Disease: Improving Global Outcomes CKD-MBD Working Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int 2017; 113 (Suppl): 1–59 [Google Scholar]
- 6. Hutchison AJ, Smith CP, Brenchley PE.. Pharmacology, efficacy and safety of oral phosphate binders. Nat Rev Nephrol 2011; 7: 578–589 [DOI] [PubMed] [Google Scholar]
- 7. Fernández-Martín JL, Carrero JJ, Benedik M. et al. COSMOS: the dialysis scenario of CKD-MBD in Europe. Nephrol Dial Transplant 2013; 28: 1922–1935 [DOI] [PubMed] [Google Scholar]
- 8. National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 2003; 42 (Suppl): S1–S201 [PubMed] [Google Scholar]
- 9. Arenas MD, Malek T, Gil MT. et al. Challenge of phosphorus control in hemodialysis patients: a problem of adherence? J Nephrol 2010; 23: 525–534 [PubMed] [Google Scholar]
- 10. Fissell RB, Karaboyas A, Bieber BA. et al. Phosphate binder pill burden, patient-reported non-adherence, and mineral bone disorder markers: findings from the DOPPS. Hemodial Int 2016; 20: 38–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wilhelm M, Gaillard S, Rakov V. et al. The iron-based phosphate binder PA21 has potent phosphate binding capacity and minimal iron release across a physiological pH range in vitro. Clin Nephrol 2014; 81: 251–258 [DOI] [PubMed] [Google Scholar]
- 12.Fresenius Medical Care North America. Velphoro (Sucroferric Oxyhydroxide) Prescribing Information. 2014. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/205109s006lbl.pdf (18 February 2020, date last accessed)
- 13.Vifor Fresenius Medical Care Renal Pharma France. Velphoro (Sucroferric Oxyhydroxide) Summary of Product Characteristics.https://www.medicines.org.uk/emc/product/3532/smpc (18 February 2020, date last accessed)
- 14. Floege J, Covic AC, Ketteler M. et al. ; on behalf of the Sucroferric Oxyhydroxide Study Group. Effects of the iron-based phosphate binder, sucroferric oxyhydroxide, in dialysis patients. Nephrol Dial Transplant 2015; 30: 1037–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Floege J, Covic AC, Ketteler M. et al. A phase III study of the efficacy and safety of a novel iron-based phosphate binder in dialysis patients. Kidney Int 2014; 86: 638–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wüthrich RP, Chonchol M, Covic A. et al. Randomized clinical trial of the iron-based phosphate binder PA21 in hemodialysis patients. Clin J Am Soc Nephrol 2013; 8: 280–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013; 310: 2191–2194 [DOI] [PubMed] [Google Scholar]
- 18. Morisky DE, Ang A, Krousel-Wood M, Ward HJ.. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens 2008; 10: 348–354 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19. Al Salmi I, Larkina M, Wang M. et al. Missed hemodialysis treatments: international variation, predictors, and outcomes in the dialysis outcomes and practice patterns study (DOPPS). Am J Kidney Dis 2018; 72: 634–643 [DOI] [PubMed] [Google Scholar]
- 20. Fernández-Martin JL, Dusso A, Martínez-Camblor P. et al. ; COSMOS group. Serum phosphate optimal timing and range associated with patients survival in haemodialysis: the COSMOS study. Nephrol Dial Transplant 2019; 34: 673–681 [DOI] [PubMed] [Google Scholar]
- 21. Covic AC, Floege J, Ketteler M. et al. Iron-related parameters in dialysis patients treated with sucroferric oxyhydroxide. Nephrol Dial Transplant 2017; 32: 1330–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cannata-Andía JB, Fernández-Martin JL, Locatelli F, et al. Use of phosphate-binding agents is associated with a lower risk of mortality. Kidney Int 2013; 84(5): 998–1008 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.