Abstract
The pro-inflammatory cytokine interleukin (IL)-6 has been associated with outcomes in small pulmonary arterial hypertension (PAH) cohorts composed largely of patients with severe idiopathic PAH (IPAH). It is unclear whether IL-6 is a marker of critical illness or a mechanistic biomarker of pulmonary vascular remodelling. We hypothesised that IL-6 is produced by pulmonary vascular cells and sought to explore IL-6 associations with phenotypes and outcomes across diverse subtypes in a large PAH cohort.
IL-6 protein and gene expression levels were measured in cultured pulmonary artery smooth muscle cells (PASMCs) and endothelial cells (PAECs) from PAH patients and healthy controls. Serum IL-6 was measured in 2017 well-characterised PAH subjects representing each PAH subgroup. Relationships between IL-6 levels, clinical variables, and mortality were analysed using regression models.
Significantly higher IL-6 protein and gene expression levels were produced by PASMCs than by PAECs in PAH (p<0.001), while there was no difference in IL-6 between cell types in controls. Serum IL-6 was highest in PAH related to portal hypertension and connective tissue diseases (CTD-PAH). In multivariable modelling, serum IL-6 was associated with survival in the overall cohort (hazard ratio 1.22, 95% CI 1.08–1.38; p<0.01) and in IPAH, but not in CTD-PAH. IL-6 remained associated with survival in low-risk subgroups of subjects with mild disease.
IL-6 is released from PASMCs, and circulating IL-6 is associated with specific clinical phenotypes and outcomes in various PAH subgroups, including subjects with less severe disease. IL-6 is a mechanistic biomarker, and thus a potential therapeutic target, in certain PAH subgroups.
Introduction
Pulmonary arterial hypertension (PAH) is a progressive disease characterised by abnormal cellular proliferation, pulmonary vascular remodelling and increased pulmonary vascular resistance [1, 2]. Perivascular inflammation with lymphocyte and macrophage infiltration has been observed across PAH subtypes [3], and preclinical studies support direct involvement of inflammatory mechanisms in PAH pathobiology [4–7]. Cytokines known to drive abnormal proliferation of pulmonary vascular cells are easily measurable in human serum. Thus, pro-inflammatory cytokines may serve as mechanistic biomarkers that provide insights into phenotypic differences in PAH pathobiology, disease severity and survival across different disease subtypes [8, 9].
Interleukin (IL)-6 is a circulating pro-inflammatory cytokine [10]. Among tissue sources, the lung has the second highest expression of IL-6 at the RNA level [11]. The predominant cellular source of IL-6 in the pulmonary vasculature is unclear, although the membrane-bound IL-6 receptor is upregulated in pulmonary artery smooth muscle cells (PASMCs) in patients with idiopathic PAH (IPAH) [12]. Previous studies have shown that IL-6 is elevated in PAH and independently associated with indices of right ventricular function [5, 13–15]. However, prior investigations of IL-6 as a prognostic biomarker have found inconsistent associations with mortality [13, 15].
Most prior studies of IL-6 in PAH have been undertaken in small, single-centre cohorts composed primarily of IPAH patients with severe disease, calling into question whether IL-6 is reflective of PAH or critical illness, and whether associations with clinical outcomes are generalisable to other PAH subtypes. In one small PAH cohort, IL-6 was an independent predictor of mortality in a subgroup of patients with normal brain natriuretic peptide levels (<180 pg·mL−1) [16], suggesting a prognostic value in mild disease.
To date, there has been no large-scale, multicentre study of associations between IL-6 levels and patient phenotypes and outcomes across diverse PAH clinical subgroups, including patients with mild PAH. Whether IL-6 reflects cellular processes occurring in the pulmonary vasculature or merely reflects severe disease remains uncertain. Given the diverse array of mechanisms known to contribute to PAH pathogenesis [2], markers identifying specific pathobiology in particular subgroups of patients may inform clinical phenotyping and could link phenotypes to tailored PAH-specific therapies. This study addresses several knowledge gaps by investigating the cellular sources of IL-6 in the pulmonary vasculature at both the protein and RNA level, and by examining relationships between IL-6 levels, detailed clinical metrics, and outcomes in a multicentre, deeply phenotyped, heterogeneous PAH cohort.
Methods
Cohort data collection
This study was conducted in accordance with the Declaration of Helsinki and approved by the Johns Hopkins University institutional review board (NA_00069663, Baltimore, MD, USA). Samples and clinical data were obtained from the National Institutes of Health and National Heart, Lung, and Blood Institute PAH Biobank (www.pahbiobank.org), which includes data aggregated from 34 enrolment centres across North America. Specimen collection was approved by the institutional review board at each centre, and informed consent was obtained for all subjects prior to their enrolment. Eligible enrolees are patients with World Health Organization group 1 PAH. Clinical data are extracted from the electronic medical records of each patient, and de-identified data are managed by the PAH Biobank. Patients provide whole-blood specimens via venipuncture at enrolment, which are stored as serum in secure freezers at Cincinnati Children’s Hospital Medical Center (Cincinnati, OH, USA). PAH Biobank specimens and data from enrolees aged ⩾21 years (n=2017) and serum samples from adult controls without PAH from Vanderbilt University (Nashville, TN, USA) (n=60) were studied. Serum IL-6 levels were measured using a commercial electrochemiluminescence immunoassay (ELISA) in a 96-well plate-based format (Meso Scale Discovery, Gaithersburg, MD, USA). The average lower limit of detection was 0.152 pg·mL−1.
Cell line data collection
Cell lines were obtained from the Cardiovascular Medical Research and Education Fund Pulmonary Hypertension Breakthrough Initiative (PHBI), which included PASMCs and pulmonary artery endothelial cells (PAECs) from transplanted patients with severe PAH (n=22) or from non-transplanted donors (n=11) [11, 17, 18]. Cells were maintained in normal culture conditions, and IL-6 levels from the conditioned media for each cell type were measured by ELISA. Cells were subjected to RNA extraction when they reached 80–90% confluence in order to perform RNA sequencing (RNAseq). IL-6 gene expression levels were measured in fragments per kilobase of exon model per million reads mapped and compared across cell types. Full methodology for performance of cell culture, RNAseq and ELISA is described in the supplementary material.
Statistical analysis
IL-6 comparisons were made using t-tests, Wilcoxon rank-sum tests, or Kruskal–Wallis tests, as appropriate. Relationships between IL-6 levels and clinical variables were analysed using linear and logistic regression models adjusted for age and sex. IL-6 levels were right-skewed and log-transformed for analyses. Associations between IL-6 levels and survival were studied in the overall cohort and in prespecified disease subtypes using Kaplan–Meier analysis, in which subjects were dichotomised based on the median IL-6 level, and using Cox proportional hazard models adjusted for potential confounders of the relationship between IL-6 and survival. The proportional hazards assumption was examined for all covariates on the basis of Schoenfeld residuals. 33 subjects for whom survival data were not available were not included in time-to-event analyses. A p-value <0.05 was considered statistically significant. Bonferroni correction for multiple testing was performed for variable associations with IL-6 in table 2 (n=12), yielding a threshold for significance of 0.0042. All analyses were performed using Stata (version 15.1; StataCorp, College Station, TX, USA).
TABLE 2.
Interleukin-6 associations with clinical variables
| Regression coefficient (95% CI) | OR (95% CI) | p-value | |
|---|---|---|---|
| RAP mmHg | 0.50 (0.33–0.67) | <0.001 | |
| mPAP mmHg | −0.04 (−0.46–0.37) | ns | |
| PAWP mmHg | 0.17 (0.05–0.30) | 0.007 | |
| PVR Wood units | −0.24 (−0.42 – −0.06) | 0.010 | |
| Cardiac output L·min−1 | 0.08 (0.03–0.13) | 0.004 | |
| Cardiac index L·min−1·m−2 | 0.01 (−0.03–0.05) | ns | |
| Stroke volume mL | 0.00 (0.00–0.00) | ns | |
| PA compliance mL·mmHg−1 | 0.00 (−0.04–0.03) | ns | |
| Heart rate beats·min−1 | 1.17 (0.59–1.75) | <0.001 | |
| 6MWD m | −15.99 (−21.74 – −10.25) | <0.001 | |
| RV stroke work index | −0.41 (−0.81 – −0.01) | 0.042 | |
| RV power | 4.84 (1.28–8.40) | 0.008 | |
| CTD-PAH | 1.22 (1.13–1.31) | <0.001 | |
| PoPH-PAH | 1.37 (1.18–1.59) | <0.001 | |
| Dyspnoea at rest | 1.17 (1.07–1.28) | 0.001 | |
| Intravenous/subcutaneous prostacyclin | 1.08 (1.01–1.15) | 0.019 | |
All regression coefficients and odds ratios were adjusted for age and sex. Associations with p<0.0042 are significant after Bonferroni correction for multiple testing. RAP: right atrial pressure; mPAP: mean pulmonary arterial pressure; PAWP: pulmonary artery wedge pressure; PVR: pulmonary vascular resistance; PA: pulmonary arterial; 6MWD: 6-min walk distance; RV: right ventricle; CTD-PAH: connective tissue disease-associated pulmonary arterial hypertension; PoPH-PAH: portopulmonary hypertension-associated pulmonary arterial hypertension; ns: nonsignificant.
Results
Patient demographics
2017 subjects were included in the PAH cohort. The cohort was 80% female and 82% white with a mean age at enrolment of 55 years and median 6-min walk distance (6MWD) of 348 m (table 1). Subjects had moderate to severe PAH, with average mean pulmonary arterial pressure (mPAP) of 50 mmHg, pulmonary vascular resistance (PVR) of 10 Wood units and cardiac output of 4.7 L·min−1. The median time from right heart catheterisation to enrolment was 48 months (interquartile range (IQR) 14–92 months). Most subjects received treatment with phosphodiesterase-5 inhibitors and endothelin receptor antagonists (ERAs). The majority of subjects had IPAH (43%) or connective tissue disease-associated PAH (CTD-PAH) (31%). Other subtypes included portal hypertension-associated PAH (5%), familial PAH (4%) and congenital heart disease associated PAH (2%), among others. Overall, 324 out of 1984 subjects with survival data died (16.3% mortality). Subjects were followed for a median of 41 months (IQR 28–55 months) from the time of enrolment to the time of death or censor. Demographic data for the control cohort, PHBI cell line donors and the 33 subjects for whom survival data were not available are provided in supplementary tables S1–S3.
TABLE 1.
Demographics and clinical characteristics of the overall cohort and connective tissue disease-associated pulmonary arterial hypertension (CTD-PAH) and idiopathic pulmonary arterial hypertension (IPAH) subgroups
| Overall | CTD-PAH | IPAH | p-value# | |
|---|---|---|---|---|
| Demographics | ||||
| Subjects | 2017 | 623 | 870 | |
| Age years | 55±15 | 59±14 | 55±15 | <0.01 |
| Female | 1611 (80) | 565 (91) | 698 (80) | <0.01 |
| Race white | 1662 (82) | 564 (91) | 780 (90) | ns |
| NYHA FC I/II/III/IV (III/IV) | 90/451/789/118 (45) | 24/140/266/34 (48) | 38/188/340/56 (45) | ns |
| 6MWD m | 347±141 | 327±160 | 351±136 | <0.01 |
| BMI kg·m−2 | 30±10 | 29±12 | 31±9 | <0.01 |
| Deaths | 338 (17) | 138 (22) | 110 (13) | <0.01 |
| Aetiology | ||||
| CTD-PAH | 623 | |||
| IPAH | 870 | |||
| FPAH | 81 | |||
| PVOD-PAH | 8 | |||
| PoPH-PAH | 111 | |||
| Congenital | 171 | |||
| Drug-PAH | 93 | |||
| HIV-PAH | 42 | |||
| Other | 18 | |||
| IL-6 pg·mL−1 | 1.82 (0.86–3.34) | 2.24 (1.09–4.33) | 1.62 (0.72–2.94) | <0.01 |
| Haemodynamics | ||||
| RAP mmHg | 9±5 | 9±5 | 9±6 | <0.01 |
| mPAP mmHg | 50±15 | 44±11 | 51±14 | <0.01 |
| PAWP mmHg | 10±4 | 10±4 | 10±4 | ns |
| PVR Wood units | 10±6 | 8±5 | 10±6 | <0.01 |
| Cardiac output L·min−1 | 4.7±1.7 | 4.7±1.6 | 4.6±1.6 | 0.01 |
| Cardiac index L·min−1·m−2 | 2.7±1.2 | 2.8±0.9 | 2.6±1.1 | <0.01 |
| Therapies | ||||
| PDE-5 inhibitor | 1546 (77) | 470 (75) | 641 (74) | ns |
| ERA | 1205 (60) | 370 (59) | 515 (59) | ns |
| Intravenous/subcutaneous prostacyclin | 699 (35) | 161 (26) | 355 (41) | <0.01 |
| CCB | 199 (10) | 51 (8) | 99 (11) | 0.05 |
Data are presented as n, mean±sd, n (%) or median (interquartile range), unless otherwise stated. NYHA FC: New York Heart Association functional class; 6MWD: 6-min walk distance; BMI: body mass index; FPAH: familial PAH; PVOD-PAH: pulmonary veno-occlusive disease-associated PAH; PoPH-PAH: portopulmonary hypertension-associated PAH; IL: interleukin; RAP: right atrial pressure; mPAP: mean pulmonary arterial pressure; PAWP: pulmonary artery wedge pressure; PVR: pulmonary vascular resistance; PDE: phosphodiesterase; ERA: endothelin receptor antagonist; CCB: calcium channel blocker; ns: nonsignificant.
p-values reflect comparisons between CTD-PAH and IPAH.
IL-6 in PAH subtypes and associations with clinical phenotypes
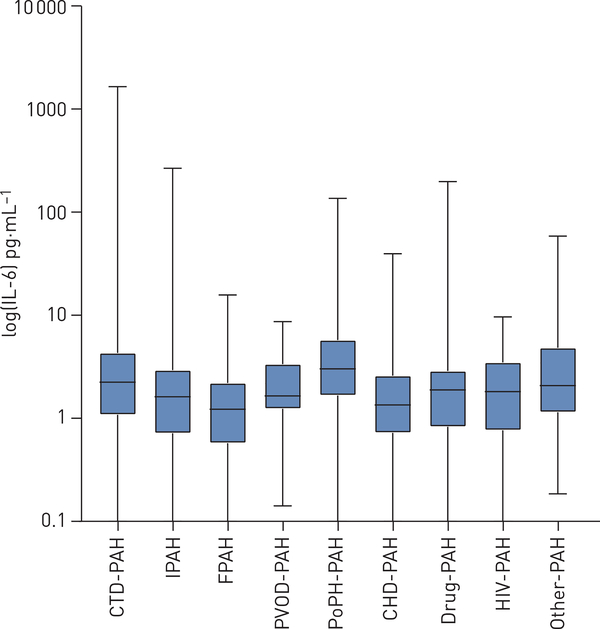
The median (IQR) IL-6 level in the overall PAH cohort was 1.82 (0.86–3.34) pg·mL−1 compared with 0 (0–2.72) pg·mL−1 in controls (p<0.001) (supplementary table S1). Subjects with portal hypertension-associated PAH (3.02, 1.68–5.78 pg·mL−1) and CTD-PAH (2.25, 1.09–4.33 pg·mL−1) had significantly higher serum IL-6 levels than those with IPAH (1.62, 0.72–2.94 pg·mL−1) (p<0.001) (figure 1). Among subjects with CTD-PAH, subjects with rheumatoid arthritis (3.38, 1.19–9.96 pg·mL−1) had higher IL-6 levels than subjects with systemic sclerosis (2.34, 1.19–4.19 pg·mL−1) or systemic lupus erythematosus (1.53, 0.77–4.01 pg·mL−1). Each log-unit higher IL-6 concentration was associated with 22% greater odds of having CTD-PAH (OR 1.22, 95% CI 1.13–1.31; p<0.001) and 37% greater odds of having PAH associated with portopulmonary hypertension (OR 1.37, 95% CI 1.18–1.59; p<0.001) (table 2).
FIGURE 1.
Comparison of interleukin (IL)-6 levels by pulmonary arterial hypertension (PAH) subtype: connective tissue disease-associated PAH (CTD-PAH), idiopathic PAH (IPAH), familial PAH (FPAH), pulmonary veno-occlusive disease-associated PAH (PVOD-PAH), portopulmonary hypertension-associated PAH (PoPH-PAH), congenital heart disease-associated PAH (CHD-PAH), drug-associated PAH (Drug-PAH), HIV-associated PAH (HIV-PAH) and other unspecified disease-associated forms of PAH (Other-PAH).
As shown in table 2, each log-unit higher IL-6 was associated with higher right atrial pressure (RAP), pulmonary artery wedge pressure and cardiac output, and with lower PVR. In addition, each log-unit higher IL-6 was associated with lower right ventricular (RV) stroke work index and higher RV power output. Functionally, each log-unit higher IL-6 was associated with 17% greater odds of having dyspnoea at rest (OR 1.17, 95% CI 1.07–1.28; p=0.001), 8% greater odds of requiring treatment with prostacyclin analogues (OR 1.08, 95% CI 1.01–1.15; p=0.019), and a 16.0 m shorter 6MWD (95% CI 10.2–21.7; p<0.001). Overall, higher IL-6 was associated with a more severe New York Heart Association functional class (NYHA FC) (p<0.001) (supplementary figure S1a) and a higher REVEAL (Registry to Evaluate Early and Long-Term Disease Management in PAH) risk score (p<0.001) (supplementary figure S1b), a multivariable score that predicts 1-year survival based on a combination of patient demographics, aetiological factors and physical exam and laboratory results [19–21].
IL-6 associations with survival in the overall cohort and in PAH subgroups
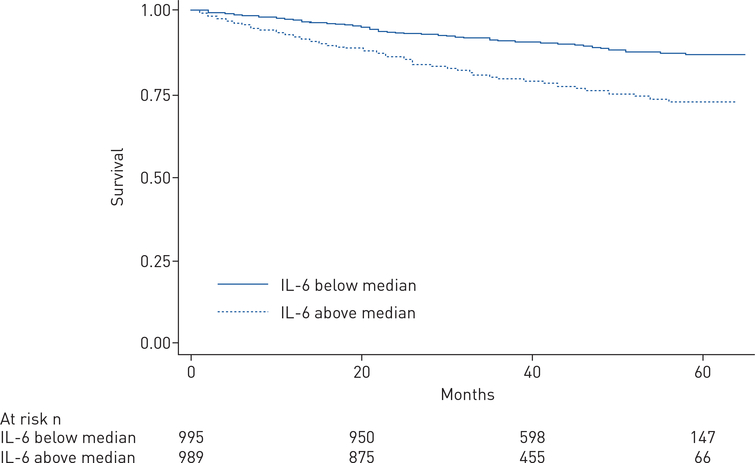
As shown in the Kaplan–Meier plot in figure 2, 5-year survival was shorter among subjects with IL-6 levels above the cohort median 1.82 pg·mL−1 (log-rank p<0.0001). In Cox proportional hazard modelling, each log-unit higher IL-6 was associated with a 35% greater risk of death, with an unadjusted hazard ratio (HR) of 1.35 (95% CI 1.25–1.46; p<0.01). This relationship remained significant when adjusted for age, sex, PAH subtype, PAH-specific therapy drug class, NYHA FC, 6MWD, body mass index and haemodynamic variables (RAP, mPAP, PVR, cardiac index) (HR 1.22, 95% CI 1.08–1.38; p=0.002).
FIGURE 2.
Kaplan–Meier survival analysis among pulmonary arterial hypertension subjects with available survival data with interleukin (IL)-6 levels above versus below the median (log rank p<0.0001).
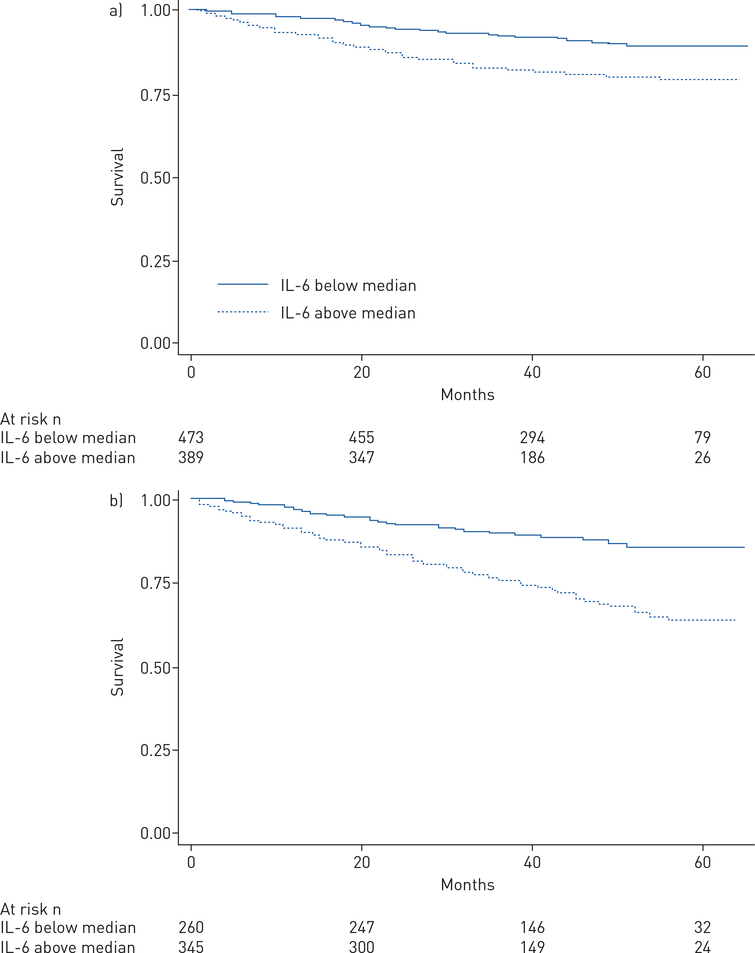
Kaplan–Meier analysis conducted within the two largest disease subtypes demonstrated shorter survival in both IPAH and CTD-PAH among subjects with IL-6 levels above the median (each log-rank p<0.001) (figure 3a and b). Cox multivariable analysis of the IPAH subgroup demonstrated that each log-unit higher IL-6 was associated with a 31% greater risk of death (HR 1.31, 95% CI 1.01–1.71; p=0.039). However, the significance of the relationship between IL-6 and survival was attenuated in multivariable analysis of the CTD-PAH subgroup (HR 1.18, 95% CI 0.98–1.42; p=0.074).
FIGURE 3.
Kaplan–Meier survival analyses among subjects with a) idiopathic pulmonary arterial hypertension and b) connective tissue disease-associated pulmonary arterial hypertension with interleukin (IL)-6 levels above versus below the median of the respective subgroup (log rank p<0.0001 for each).
In Kaplan–Meier analysis, IL-6 above the median was associated with worse survival in subjects in REVEAL risk categories 1 (n=123, log-rank p<0.01), 2 (n=73, log-rank p<0.001) and 3 (n=62, log-rank p<0.01). IL-6 was not significantly associated with survival in REVEAL risk categories 4 (n=57, log-rank p=0.06) or 5 (n=9, log-rank p=0.31), although sample sizes were smaller in higher risk categories. Univariable associations between log-transformed IL-6 levels and survival for each REVEAL risk category (table 3) align with the results of Kaplan–Meier analysis, with significant relationships demonstrated in lower risk categories and significance of associations lost in higher risk categories.
TABLE 3.
Interleukin-6 associations with survival in REVEAL (Registry to Evaluate Early and Long-Term Disease Management in PAH) risk categories
| Univariable hazard ratio (95% CI) | p-value | |
|---|---|---|
| Category 1 | 1.29 (1.13–1.48) | <0.001 |
| Category 2 | 1.31 (1.10–1.57) | 0.003 |
| Category 3 | 1.21 (1.02–1.44) | 0.02 |
| Category 4 | 1.12 (0.93–1.35) | 0.22 |
| Category 5 | 1.12 (0.36–3.46) | 0.85 |
As shown in table 4, higher IL-6 was associated with worse survival in subgroups of subjects with low-risk clinical features as defined by European Society of Cardiology (ESC) and European Respiratory Society (ERS) guidelines [22], including N-terminal pro-brain natriuretic peptide (NT-proBNP) <300 pg·mL−1 (n=623; HR 1.41, 95% CI 1.08–1.83; p=0.011), 6MWD >440 m (n=1192; HR 1.43, 95% CI 1.29–1.58; p<0.001), RAP <8 mmHg (n=867; HR 1.43, 95% CI 1.26–1.62; p<0.001) and cardiac index >2.5 L·min−1·m−2 (n=1053; HR 1.36, 95% CI 1.23–1.52; p<0.001).
TABLE 4.
Interleukin-6 associations with survival in low-risk pulmonary arterial hypertension clinical features
| Univariable hazard ratio (95% CI) | p-value | |
|---|---|---|
| NT-proBNP <300 ng·mL−1 | 1.41 (1.08–1.83) | 0.011 |
| 6MWD >440 m | 1.43 (1.29–1.58) | <0.001 |
| RAP <8 mmHg | 1.43 (1.26–1.62) | <0.001 |
| Cardiac index >2.5 L·min−1·m−2 | 1.36 (1.23–1.52) | <0.001 |
NT-proBNP: N-terminal pro-brain natriuretic peptide; 6MWD: 6-min walk distance; RAP: right atrial pressure.
IL-6 in pulmonary artery cell lines
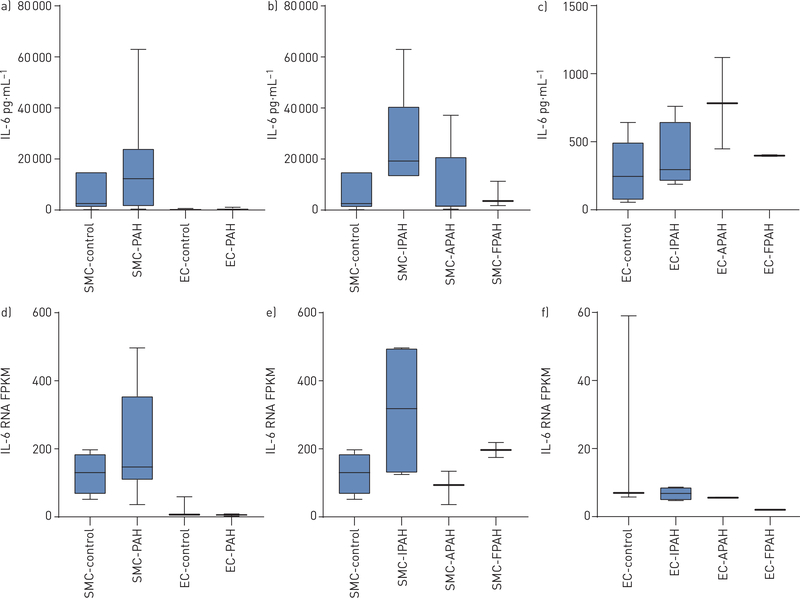
Median (IQR) IL-6 concentrations in conditioned media were significantly higher in PASMCs (12301, 1694–21822 pg·mL−1) than in PAECs (398, 298–525 pg·mL−1) (p<0.0001) in PAH cell lines (figure 4a). No significant difference in IL-6 concentrations existed between PASMCs and PAECs in controls (figure 4a) or between PAH subtypes among either PASMCs (figure 4b) or PAECs (figure 4c). IL-6 concentrations were higher in PASMCs from PAH patients (12301, 1694–21822 pg·mL−1) than in PASMCs from controls (2445, 2253–14799 pg·mL−1), although this difference did not reach statistical significance (figure 4a). RNASeq results aligned with these findings, with significantly higher IL-6 gene expression in PASMCs compared to PAECs in PAH, and a trend toward higher IL-6 gene expression in PASMCs in PAH patients compared to controls (figure 4d). Furthermore, cellular IL-6 gene expression levels by PAH subtype closely mirrored IL-6 protein concentrations in conditioned media (figure 4e and f).
FIGURE 4.
Comparison of interleukin (IL)-6 levels in conditioned media from a) smooth muscle cells (SMCs) and endothelial cells (EC) from transplanted pulmonary arterial hypertension (PAH) subjects and non-transplanted donor controls (p<0.0001 for SMC-PAH versus EC-PAH); b) SMCs in controls and PAH subtypes; and c) ECs in controls and PAH subtypes. Comparison of IL-6 gene expression levels by fragments per kilobase of exon model per million reads mapped (FPKM) between d) SMCs and ECs from PAH subjects and controls (p<0.0001 for SMC-PAH versus EC-PAH); e) SMCs in controls and PAH subtypes; and f) ECs in controls and PAH subtypes. APAH: disease-associated PAH subtypes; IPAH: idiopathic PAH; FPAH: familial PAH.
Discussion
IL-6 is a pro-inflammatory cytokine shown in animal models to mediate the pulmonary vascular remodelling and progressive occlusion of the pulmonary vessels that characterises PAH in humans [1, 6–8]. Our study is the largest to date to investigate clinical associations with circulating IL-6 levels across diverse PAH subtypes. Our results confirm that IL-6 is higher in PAH compared to controls and demonstrate that IL-6 levels are highest in PAH associated with portal hypertension and connective tissue diseases.
Importantly, we demonstrate significant associations between IL-6 and mortality in multivariable models in both the overall PAH cohort and in the IPAH subgroup. This is in contrast to previous studies that have demonstrated inconsistent associations with mortality. Soon et al. [13] demonstrated unadjusted IL-6 associations with mortality in 57 subjects with severe disease; however, the significance of the relationship was lost with adjustment for important covariates. Cracowski et al. [15] examined a panel of pro-inflammatory cytokines, including IL-6, in 74 PAH patients, although the significance of the association between IL-6 and mortality was borderline (p=0.06).
We found significant relationships between serum IL-6 levels and phenotypic variables across disease subtypes, including higher NYHA FC, shorter 6MWD and the presence of dyspnoea at rest. In alignment with previous studies [13, 14], we found null or unexpected associations between serum IL-6 levels and haemodynamic variables indicative of PAH, such as mPAP and PVR. We did find associations between IL-6 and decreased RV stroke work index and increased RV power, two metrics of RV function [23–26]. One potential explanation for this is that IL-6 may be a poor marker of haemodynamic impairment and instead a better marker of RV dysfunction. Prins et al. [14] found no difference in haemodynamics in PAH patients with high versus low IL-6, but did find significant associations between IL-6 and measures of RV dysfunction and impaired RV–pulmonary arterial coupling. RV dysfunction is the major determinant of mortality in PAH, thus these associations align with the strong relationships between IL-6 levels and mortality observed in our cohort. Unfortunately, we do not have echocardiographic or imaging data available for our cohort to recapitulate Prins et al.’s specific findings.
Our study re-demonstrates the prognostic utility of IL-6 among PAH patients with mild disease, including among patients with low-risk features designated by current ESC/ERS guidelines, such as lower RAP, longer 6MWD and low/normal NT-proBNP levels [22], and among patients in low REVEAL risk categories. These clinical results, together with prior animal studies, suggest that IL-6 is a mechanistic marker of pulmonary vascular disease, rather than a nonspecific marker of critical illness in severe PAH. Perivascular inflammation precedes pulmonary vascular remodelling in experimental models of pulmonary hypertension [27], and it is provocative to speculate that IL-6 may be a biomarker of upstream pathobiological events in PAH, in contrast to NT-proBNP, which reflects cardiomyocyte stretch that occurs once pathological pulmonary vascular remodelling has evolved significantly [28–30]. NT-proBNP levels were only weakly correlated with IL-6 levels in our cohort (Spearman correlation coefficient 0.25, p<0.01), implying that IL-6 reflects different pathobiological mechanisms to NT-proBNP, and therefore may provide additional, multidimensional prognostic information. Establishing markers of mild disease is particularly relevant in light of the recent re-definition of PAH at the 6th World Symposium on Pulmonary Hypertension (with revision of the mPAP threshold from 25 mmHg to 20 mmHg) [31].
Our study shows that, in addition to known production of IL-6 by pulmonary macrophages and other inflammatory cells of the lung, PAMSCs release μg·mL−1 quantities of IL-6 in PAH, which may contribute to circulating IL-6 levels measured in the serum (typically measured in pg·mL−1) or have local effects. Preclinical studies have demonstrated ectopic upregulation of the IL-6 receptor in PASMCs in experimental pulmonary hypertension. Moreover, deletion of the IL-6 receptor in the smooth muscle layer of animal PASMCs prevents development of hypoxia-induced pulmonary hypertension [12].
Collectively, our results corroborate an important role for IL-6 in PAH pathobiology. These findings support the dual potential of IL-6 as a biomarker of a dysfunctional pulmonary circulation and as a possible therapeutic target. Importantly, a pharmacological IL-6 inhibitor, tocilizumab, is currently under investigation for efficacy in PAH (www.clinicaltrials.gov NCT02676947). Notably, the designated co-primary end-points for this phase 2 trial are change in PVR and incidence of adverse events [32]. In light of the clinical associations demonstrated in our study, special attention should be paid to the trial’s secondary outcome measures, especially changes in 6MWD, functional class and quality of life when interpreting the results. Future studies of anti-IL-6 therapies should consider incorporation of end-points such as changes in RV function, time to clinical worsening or changes in IL-6 levels with therapy, and should be powered to analyse results within distinct prespecified disease subtypes. In addition, selectively enriching study populations by preferentially enrolling subjects with high IL-6 pre-intervention could be considered in designing future efficacy trials.
A major strength of our study is the large overall sample size of subjects with detailed haemodynamic, functional and phenotypic data, enabling a thorough analysis of clinical variables in relation to IL-6 levels. Moreover, this study pairs a large-scale epidemiological investigation of serum IL-6 levels in PAH with cell culture and RNAseq experiments to examine IL-6 production by cells of the pulmonary vasculature. The study was somewhat limited by the composition of the cohort. Some subgroups of interest (for example, high-risk REVEAL categories) had relatively small sample sizes. Furthermore, the majority of subjects were prevalent patients on PAH-specific therapy at the time of enrolment. Therapies may have affected IL-6 measurements in serum, as treatment with ERAs has been shown to reduce circulating IL-6 levels [33]. However, our large overall sample size allowed for adjustment of multiple covariates in Cox proportional hazard models, including adjustment for PAH-specific therapies.
In conclusion, IL-6 is produced by pulmonary vascular cells, is variably upregulated across diverse PAH subtypes and is strongly associated with clinical features of disease, including specific phenotypes and survival times. IL-6 may be a more upstream, mechanistic biomarker of disease development than other biomarkers currently in clinical use, and therefore may aid in efforts toward diagnosis and phenotyping of mild PAH. Serum IL-6 measurements offer insights into disease pathobiology and prognosis. In the future, measurements of mechanistic biomarkers like IL-6 may aid in accurately phenotyping patients, selecting patients most likely to benefit from novel therapies, and monitoring therapeutic effects of tailored therapies.
Supplementary Material
Acknowledgments
Support statement: This study was supported by National Institutes of Health/National Heart, Lung, and Blood Institute awards R01HL135114 (A.D.E., J.Y., R.D., D.V., W.C.N., D.D.I and E.D.A.), R24 HL105333 (W.C.N., D.D.I., E.D.A., M.W.P. and L.J.M.), and T32HL007534 (C.E.S.).
Serum/tissue samples were provided by the Pulmonary Hypertension Breakthrough Initiative (PHBI). Funding for the PHBI is provided under an NHLBI R24 grant, R24HL123767, and by the Cardiovascular Medical Research and Education Fund (CMREF). National Heart, Lung, and Blood Institute; DOI: http://dx.doi.org/10.13039/100000050; Grant: R01HL135114, R24 HL105333, T32HL007534.
Footnotes
Conflict of interest: None declared.
References
- 1.Rafikova O, Al Ghouleh I, Rafikov R. Focus on early events: pathogenesis of pulmonary arterial hypertension development. Antioxid Redox Signal 2019; 31: 933–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vonk Noordegraaf A, Chin KM, Haddad F, et al. Pathophysiology of the right ventricle and of the pulmonary circulation in pulmonary hypertension: an update. Eur Respir J 2019; 53: 1801900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rabinovitch M, Guignabert C, Humbert M, et al. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res 2014; 115: 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jasiewicz M, Knapp M, Waszkiewicz E, et al. Enhanced IL-6 trans-signaling in pulmonary arterial hypertension and its potential role in disease-related systemic damage. Cytokine 2015; 76: 187–192. [DOI] [PubMed] [Google Scholar]
- 5.Humbert M, Monti G, Brenot F, et al. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med 1995; 151: 1628–1631. [DOI] [PubMed] [Google Scholar]
- 6.Steiner K, Syrkina O, Kolliputi N, et al. IL-6 overexpression induces pulmonary hypertension. Circ Res 2009; 104: 236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Savale L, Tu L, Rideau D, et al. Impact of interleukin-6 on hypoxia-induced pulmonary hypertension and lung inflammation in mice. Respir Res 2009; 10: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kylhammar D, Hesselstrand R, Nielsen S, et al. Angiogenic and inflammatory biomarkers for screening and follow-up in patients with pulmonary arterial hypertension. Scand J Rheumatol 2018; 47: 319–324. [DOI] [PubMed] [Google Scholar]
- 9.Sweatt AJ, Hedlin HK, Balasubramanian V, et al. Discovery of distinct immune phenotypes using machine learning in pulmonary arterial hypertension. Circ Res 2019; 124: 904–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 2014; 6: a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhodes CJ, Im H, Cao A, et al. RNA sequencing analysis detection of a novel pathway of endothelial dysfunction in pulmonary arterial hypertension. Am J Respir Crit Care Med 2015; 192: 356–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamura Y, Phan C, Tu L, et al. Ectopic upregulation of membrane-bound IL6R drives vascular remodeling in pulmonary arterial hypertension. J Clin Invest 2018; 128: 1956–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Soon E, Holmes A, Treacy C, et al. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation 2010; 122: 920–927. [DOI] [PubMed] [Google Scholar]
- 14.Prins K, Archer S, Pritzker M, et al. Interleukin-6 is independently associated with right ventricular function in pulmonary arterial hypertension. J Hear Lung Transpl 2018; 37: 376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cracowski JL, Chabot F, Labarère J, et al. Proinflammatory cytokine levels are linked to death in pulmonary arterial hypertension. Eur Respir J 2014; 43: 915–917. [DOI] [PubMed] [Google Scholar]
- 16.Heresi GA, Aytekin M, Hammel JP, et al. Plasma interleukin-6 adds prognostic information in pulmonary arterial hypertension. Eur Respir J 2014; 43: 912–914. [DOI] [PubMed] [Google Scholar]
- 17.Nickel NP, Spiekerkoetter E, Gu M, et al. Elafin reverses pulmonary hypertension via caveolin-1-dependent bone morphogenetic protein signaling. Am J Respir Crit Care Med 2015; 191: 1273–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang J, Nies MK, Fu Z, et al. Hepatoma-derived growth factor predicts disease severity and survival in pulmonary arterial hypertension. Am J Respir Crit Care Med 2016; 194: 1264–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benza R, Miller D, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation 2010; 122: 164–172. [DOI] [PubMed] [Google Scholar]
- 20.Benza R, Gomberg-Maitland M, Miller D, et al. The REVEAL registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest 2012; 141: 354–362. [DOI] [PubMed] [Google Scholar]
- 21.Benza RL, Gomberg-Maitland M, Elliott CG, et al. Predicting survival in patients with pulmonary arterial hypertension: the REVEAL risk score calculator 2.0 and comparison with ESC/ERS-based risk assessment strategies. Chest 2019; 156: 323–337. [DOI] [PubMed] [Google Scholar]
- 22.Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2015; 46: 903–975. [DOI] [PubMed] [Google Scholar]
- 23.Ibe T, Wada H, Sakakura K, et al. Right ventricular stroke work index. Int Heart J 2018; 59: 1047–1051. [DOI] [PubMed] [Google Scholar]
- 24.Brittain E, Pugh M, Wheeler L, et al. Prostanoids but not oral therapies improve right ventricular function in pulmonary arterial hypertension. JACC Heart Fail 2013; 1: 300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xie A, Belcik T, Qi Y, et al. Ultrasound-mediated vascular gene transfection by cavitation of endothelial-targeted cationic microbubbles. JACC Cardiovasc Imaging 2012; 5: 1253–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vonk Noordegraaf A, Westerhof B, Westerhof N. The relationship between the right ventricle and its load in pulmonary hypertension. J Am Coll Cardiol 2017; 69: 236–243. [DOI] [PubMed] [Google Scholar]
- 27.Tamosiuniene R, Tian W, Dhillon G, et al. Regulatory T cells limit vascular endothelial injury and prevent pulmonary hypertension. Circ Res 2011; 109: 867–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pathak V, Aris R, Jensen BC, et al. Effect of 6-min walk test on pro-BNP levels in patients with pulmonary arterial hypertension. Lung 2018; 196: 315–319. [DOI] [PubMed] [Google Scholar]
- 29.Helgeson S, Imam J, Moss J, et al. Comparison of brain natriuretic peptide levels to simultaneously obtained right heart hemodynamics in stable outpatients with pulmonary arterial hypertension. Diseases 2018; 6: E33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frantz R, Farber H, Badesc D, et al. Baseline and serial brain natriuretic peptide level predicts 5-year overall survival in patients with pulmonary arterial hypertension: data from the REVEAL registry. Chest 2018; 143: 126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galiè N, McLaughlin V, Rubin L, et al. An overview of the 6th World Symposium on Pulmonary Hypertension. Eur Respir J 2019; 53: 1802148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hernández-Sánchez J, Harlow L, Church C, et al. Clinical trial protocol for TRANSFORM-UK: a therapeutic open-label study of tocilizumab in the treatment of pulmonary arterial hypertension. Pulm Circ 2018; 8: 2045893217735820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Karavolias GK, Georgiadou P, Gkouziouta A, et al. Short and long term anti-inflammatory effects of bosentan therapy in patients with pulmonary arterial hypertension: relation to clinical and hemodynamic responses. Expert Opin Ther Targets 2010; 14: 1283–1289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.