Abstract
Aseptic loosening of total knee arthroplasty continues to be a challenging clinical problem. The progression of the loosening process, from the initial well-fixed component, is not fully understood. In this work, loss of fixation of cemented hemiarthroplasty was explored using 9-month-old Sprague-Dawley rats with 0, 2, 6, 12, 26 week end points. Morphological and cellular changes of cement-bone fixation were determined for regions directly below the tibial tray (epiphysis) and distal to the tray (metaphysis). Loss of fixation, with a progressive increase in cement-bone gap volume was found in the epiphysis (0.162 mm3/week), but did not progress appreciably in the metaphysis (0.007 mm3/week). In the epiphysis, there was an early and sustained elevation of osteoclasts adjacent to the cement border and development of a fibrous tissue layer between the cement and bone. There was early formation of bone around the cement in the metaphysis, resulting in a condensed bone layer without osteoclastic bone resorption or development of a fibrous tissue layer. Implant positioning was also an important factor in the cement-bone gap formation, with greater gap formation for implants that were placed medially on the tibial articular surface. Loss of fixation in the rat model mimicked patterns found in human arthroplasty where cement-bone gaps initiate under the tibial tray, at the periphery of the implant. This preclinical model could be used to study early biological response to cemented fixation and associated contributions of mechanical instability, component alignment, and periprosthetic inflammation.
Keywords: Preclinical model, knee arthroplasty, cement, fixation, osteolysis, morphology
Graphical Abstract
INTRODUCTION:
Aseptic loosening of cemented knee arthroplasty continues to be a primary mechanism leading to clinical loosening. Patients often present with joint pain due to periprosthetic osteolysis and progressive migration of the implant components. Periprosthetic osteolysis is thought to occur prior to aseptic loosening and is often evident by progressive radiolucency between implant and bone (1). Changes in mechanical stress around the implant, wear particles from the polyethylene insert, and fluid flow acting along the cement-bone interface may contribute to focal osteolysis at the cement-bone interface (2).
Evaluation of postmortem-retrieved knee arthroplasties has shown that the initial interlock achieved at time of cementation, is lost over time due to resorption of trabeculae that initially interlocked with the cement (3). With progression of the loosening process, gaps form between the cement layer and bone, and are later filled with a fibrous tissue layer (4). However, the cellular events that accompany the loss of fixation of interdigitated bone are not fully understood. It is not possible to explore the progression of these events with human postmortem retrievals because of cellular degradation after death. Tissue from revision arthroplasties is not retrieved with intact cement-bone interfaces. Preclinical animal models that recapitulate features of cement-bone interdigitation in the knee can be used to study biological changes at the cement-bone interface and develop a mechanistic understanding of aseptic loosening.
Recently, a cemented hemiarthroplasty model for the rat knee (5) with provision for cement-bone interdigitation was developed. In that model, ovariectomy (OVX) was used in 3-month-old Sprague-Dawley rats to simulate estrogen-deficient osteoporosis that occurs in an elderly human population with knee arthroplasties. The OVX/hemiarthroplasty model resulted in an early and dramatic loss of bone surrounding the cement in the metaphysis, with near complete loss of metaphyseal trabecular bone at 3 months post-op. However, this rapid bone loss did not appropriately simulate the morphologic changes found in the human postmortem retrievals where there was a 50% loss of supporting trabecular bone (6) with aging. In addition, there was an early loss of bone viability in interdigitated cement/bone regions in the 3 month-old rats, and this remained non-viable for the duration of the study. It was not clear if the use of young rats contributed to the early loss of bone viability.
Given concerns that OVX in relatively young rats may not adequately model the effect of aging in humans (in the context of bone loss for the hemiarthroplasty model), we propose here to use older rats (9-month-old, without OVX) with much longer follow up (6 months). Use of older rats may be more relevant as there is a loss of 40% of the metaphyseal bone volume in the proximal tibia of Sprague-Dawley from an age of 3 to 16 months (7), which is similar to the 50% loss found in human arthroplasty with aging (6). We posit that use of older rats that demonstrate bone loss due to natural aging may be more representative of the human knee replacement patient population with respect to progressive changes in cement-bone fixation in our knee replacement model. The goals of this study were to: 1) determine if there is progressive loss of fixation in the cemented hemiarthroplasty model using an aged rat, 2) determine if loss of fixation initiates and progresses under the tibial tray (in the epiphyseal region) as has been documented in human arthroplasty and, 3) to determine the cellular changes associated with cementation and any loss of implant fixation.
METHODS:
Forty female SAS Sprague-Dawley rats were obtained from Charles River Labs (Wilmington, MA) at 7–8 weeks of age and maintained in community housing (2–3 rats/cage, 22°C) on a 12-hour light/dark cycle with pellet chow (Formulab Diet 5008, LabDiet, St. Louis, MO) and with water available ad libitum. Daily welfare observation and biweekly cage and bedding changes were performed in our AAALAC-accredited housing facility (PHS Assurance A3514–01). Joint arthroplasty surgeries were performed at 9 months of age, and animals were assigned (8/end point, except week 6 with 7/end point) to one of 5 end points (0, 2, 6, 12 and 26 weeks) for this longitudinal study. One animal was euthanized prior to surgery due to presence of a progressive soft tissue tumor. Power analysis was conducted a priori, using pilot bivariate data obtained from the previous study with 3-month-old rats, where cement-bone gap volume was estimated to increase by 0.135 mm3/wk (5). Using a more conservative estimate to detect a monotonic increase in cement-bone gap volume with 9-month-old rats (0.1 mm3/wk) would require 35 rats total for this longitudinal study with a power of 95% with a p-value of 0.05. A sample size of 40 was used, because of concern about animal death due to natural causes in older rats. Animal mass was measured biweekly. Euthanasia was performed using carbon dioxide asphyxiation followed by cervical dislocation. All animal procedures were approved by our Institutional Animal Care and Use Committee.
Surgical Procedure
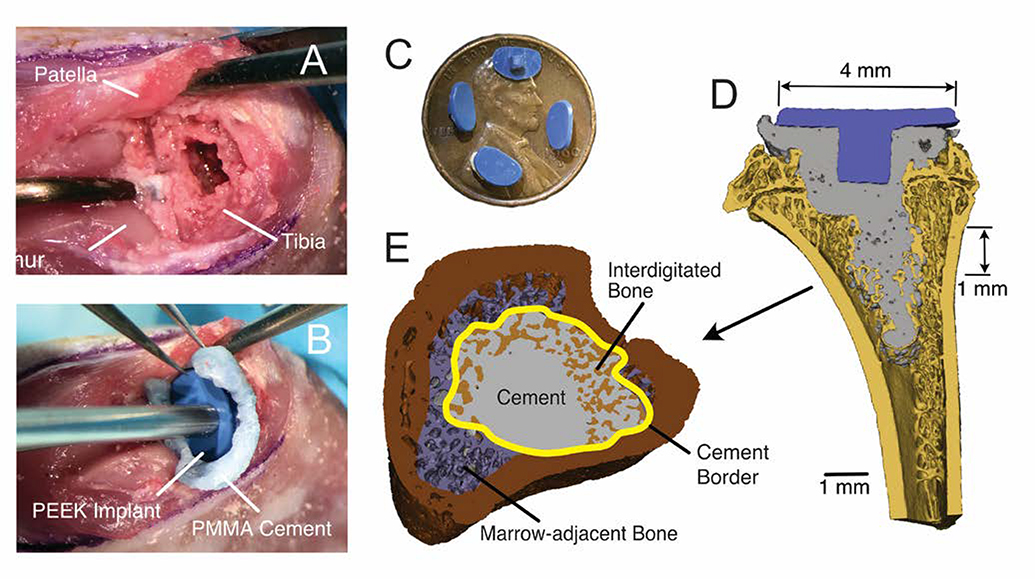
Unilateral (left) tibial knee hemiarthroplasties were performed using a previously described procedure (5). Under isoflurane anesthesia, a medial parapatellar arthrotomy was performed and the patella was dislocated laterally exposing the articulating tibial surface. A 1 mm surgical burr was used to remove epiphyseal cartilage and subchondral bone. A cavity in the epiphysis was created to accept the implant keel, and a central drill hole was made into the metaphysis to 4 mm depth (Fig 1A). Brush lavage and saline irrigation were used to prepare the bone surface for cementing. PMMA cement (Simplex, Stryker, Mahwah, NJ) was mixed by hand, applied to the bone surface in a doughy state and pressurized using a stainless-steel rod. A custom polyether ether ketone (PEEK) implant (6.0 mm width) was applied (Fig 1B). The patella was returned to the trochlear groove, and the incision was closed using interrupted absorbable sutures (5–0 Vicryl, Ethicon Inc., Cornelia, GA). Postoperatively, buprenorphine was administered at 12-hour intervals for three days. Fluorochrome labels were administered via subcutaneous injection 14-days (calcein green, 15 mg/kg) and 4-days (alizarin complexone, 30 mg/kg, Sigma-Aldrich, St. Louis, MO) prior to euthanasia for dynamic histomorphometry.
Figure 1.
Surgical procedure included lateral dislocation of the patella, followed by use of a burr to create a surface at the tibial epiphysis (A) to accept a PEEK implant that was cemented into place (B). The PEEK implant had a major diameter of 6 mm and minor diameter of 4 mm and a small keel to provide rotational stability (C). A section through the mid-plane of the implant in a sagittal view (lateral left) (D) illustrates cement fill extended into metaphysis with the goal of achieving regions of interdigitated bone. Morphological analysis of various regions of bone was performed on a 1mm thick section in the metaphysis (E).
Rationale for use of a PEEK Hemiarthroplasty Model
Small laboratory animals have been used extensively to study the biologic response of bone to PMMA cement. Many of these preclinical models have use preformed cement plugs placed in the medullary canal to investigate the biological response of bone to the poly(methylmethacrylate) (PMMA) cement biomaterial (8–10). This approach has been particularly useful to explore the effect of polyethylene debris in the vicinity of the cement. However, in contrast to human knee implantation, there is no joint load applied through the cement plug and there is no mechanical interlock between cement and trabecular bone. To explore biomaterial interfaces with joint loading, tibial hemiarthroplasty models have been developed for the mouse (11; 12), rat (13), and rabbit (14). The goal of these studies was not to assess functional total joint replacement per se, but rather to develop a model that could address specific questions with regards to bony ingrowth, fibrous tissue formation, or influence of debris or infection on the bone-implant interface. The current study uses an analogous approach.
A hemiarthroplasty model of the proximal tibia was developed using the rat because, unlike mice, rats have ample trabecular bone for interdigitation with the cement. This was an important criterion, as the main goal was to explore changes in interdigitated cement-bone fixation across the joint in an ambulatory animal. PEEK was chosen as the implant material because it is a very low wear biomaterial with a favorable biocompatibility profile, and due to material radiolucency would not product artifacts in micro-CT imaging. A flat and polished implant articulating surface was thought to be a reasonable compromise to minimize wear and allow for a reasonable contact patch with the distal femur. A relatively short keel was used in this design to provide stability between the PEEK and cement. Due to the physical constraint in the surgical field, the implant placement was achieved by tucking the edge of the tray under the patellar tendon/patella, and then pressing into position (Figure 1B). A reciprocating saw was not used due to concern over damage to the collateral ligaments—instead, a 1 mm burr was used to remove cartilage and subchondral bone in the epiphysis. The cement filled in any irregularities on the bone surface. The plunge distally (1 mm diameter) into the metaphysis was done to allow for more distal cement penetration in the metaphysis—because the goal was to obtain regions of cement-bone interdigitation for biologic study.
Morphological Analysis
Following euthanasia, tibias were harvested with surrounding soft tissue and fixed for 48 hours with 4% formaldehyde in Dulbecco’s PBS. Micro-CT scans (55 kV, 145 mA, 200 ms integration time, μCT 40, Scanco, Brüttisellen, Switzerland) were obtained of the proximal tibia at 8 μm voxel resolution to document morphology (Fig 1C). Morphological analysis of interdigitated cement-bone regions (MIMICS, Materialise, Leuven, Belgium) was performed on a 1 mm thick axial section in the metaphysis, just distal of the growth plate. The bone mask used a lower threshold of 800 mg/cc hydroxyapatite (HA) equivalent density (Fig 1D). A cement mask was identified, and the intersection between cement and bone represented the interdigitated bone. Marrow-adjacent (non-interdigitated) trabecular bone was also identified. Bone volume fraction (BV/TV) was determined for interdigitated, marrow-adjacent, and trabecular bone from the contralateral tibiae using the BoneJ plugin (15) for Fiji (16). Tissue mineral density (TMD) was determined from the average HA equivalent density for each mask region. Trabecular bone parameters were not assessed in the epiphyseal regions, as there was limited and inconsistent cement-bone interdigitation.
Discernable lytic gaps were found at the cement-bone border, and the volume of these gaps was quantified for metaphyseal and epiphyseal regions. Using sagittal micro-CT images, gaps between the cement and bone were identified, and area masks were created (Photoshop, Adobe Inc., San Joe, CA) at 0.16 mm intervals through the scan set. Gap volume (GV) was calculated using numerical integration of the area masks via the trapezoid rule. The relative medial-lateral position of the placed implant on the tibial surface was documented using micro-CT scans. The perpendicular distance from the center of the keel to a line bisecting the tibia in the frontal and sagittal planes was used to define the medial and anterior offset of the implant on the tibia, respectively.
Tissue processing and Imaging
The medial half of each tibia was decalcified in 10% EDTA, paraffin embedded, and stained with hematoxylin and eosin (H&E) or for tartrate-resistant acid phosphatase (TRAP) expression with ethyl green counterstain (Acid Phosphatase, Leukocyte Kit, Sigma-Aldrich, St. Louis, MO). The lateral half was methyl-methacrylate embedded, sectioned in 0.15 mm intervals, and ground to 0.015 mm thickness for dynamic histomorphometry. Transmitted white light and fluorescent imaging was performed with image stitching to capture full sagittal sections. Digital masks of non-viable or viable bone (presence of vacant or nucleated osteocyte lacunae) in cement-bone interdigitated and marrow-adjacent regions were created using the H&E images. Osteoclast activity was documented by the fraction of bone surface with appositional multinucleated TRAP positive cells (Oc.S/BS). Bone mineralization was assessed using the two fluorochrome labels to quantify length fraction of active bone surface (L.Pm), mineral apposition rate (MAR), and bone formation rate (BFR) (17). Osteoclast activity and bone mineralization were analyzed for interdigitated bone/cement border, marrow-adjacent, and contralateral regions. Finally, the progression of fibroblast/fibrous tissue in the epiphyseal region was determined using a component fraction analysis where space is occupied by bone, marrow, or fibrous tissue.
Statistics
The primary outcome variables were changes in bone morphology with time including bone volume fraction (BV/TV), tissue mineral density (TMD), and gap volume (GV). Linear regression (JMP, SAS, Cary, NC) was used to determine if the dependent variables (BV/TV, TMD, GV) were related to time, as the primary goal was to explore bony changes over time. Linear regression was applied based on region (interdigitated, marrow-adjacent, and contralateral bone). Analysis of covariance (ANCOVA) was used to determine if the change in GV was greater for the epiphysis compared to metaphysis. To explore the relationship between implant placement and GV, a multiple regression model was used. Descriptive statistics were determined for each of the histological measures at each end point and treatment group. When appropriate, grouped cellular data (all end points) were compared between regions (Interdigitated/marrow-adjacent/contralateral, or epiphyseal/metaphyseal) using ANOVA and paired t-tests.
RESULTS:
Body mass and ambulation
Rat body weight decreased an average of 8% one week after surgery. Thereafter, body weight increased in all animals (Fig S-1). Animals exhibited antalgic gait the evening after surgery and all but one animal exhibited full weight bearing and function by one-week post-implantation. One animal from the two-week end point that did not bear weight had inflammation about the knee and extensive osteolysis of the proximal tibia, likely due to an infection of unknown origin; this case was excluded from epiphyseal morphology analysis.
Metaphyseal Bone Morphology and Mineralization
The apparent bone volume fraction (BV/TV) of interdigitated bone in the metaphysis increased with time (Fig 2) (r2=0.41, p<0.0001), but there was not an increase in BV/TV with time for either the marrow-adjacent (r2=0.05, p=0.166) or contralateral (r2=0.07, p=0.314) trabecular bone. Tissue mineral density (TMD) also increased with time in the interdigitated region (r2=0.54, p<0.0001), but there was a transient decrease in mineralization (2 weeks). The marrow-adjacent regions also exhibited a transient decrease in mineralization, and overall there was an increase in TMD with time (r2=0.18, p=0.0078). TMD did not increase with time in the contralateral metaphyseal trabecular regions (r2=0.07, p=0.315). Overall, these results show that the interdigitated bone hypermineralized with time and there was an apparent increase in interdigitated bone volume fraction. Marrow-adjacent regions maintained bone density and volume consistent with the contralateral (non-implanted) tibia. Additional morphological metrics are included in Table S-1.
Figure 2.
Metaphyseal trabecular bone volume fraction (BV/TV) and tissue mineral density (TMD) for interdigitated, marrow-adjacent, and contralateral (not cemented) regions from a 1 mm thick section of the cemented tibia. N=7–8/end point. Mean and standard error bars are shown.
Progression of Cement-Bone Gaps
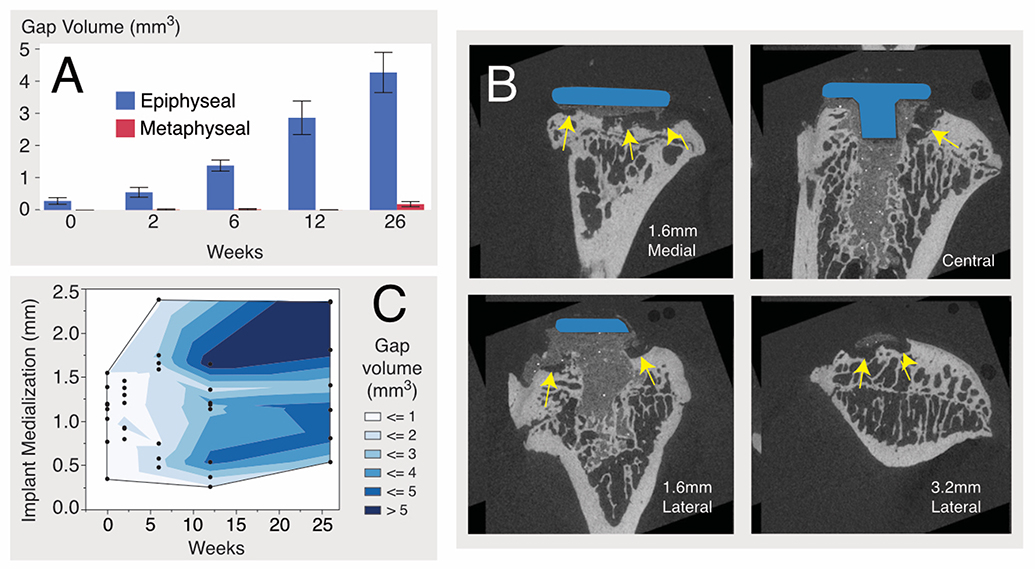
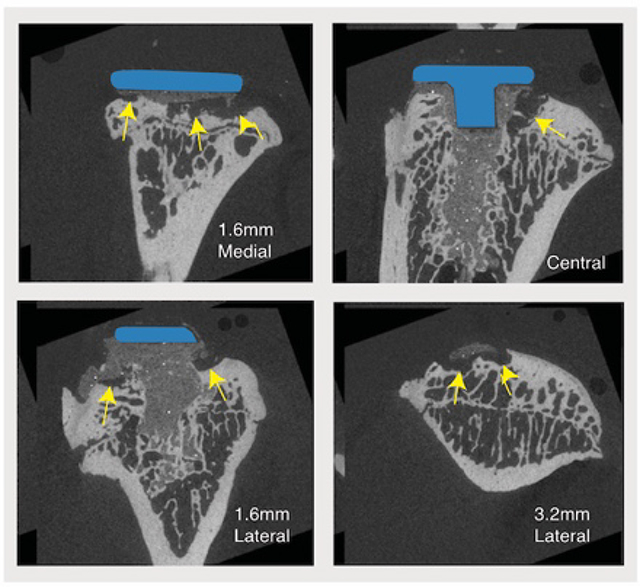
The gap volume (GV) between the cement and bone was greater for the epiphyseal region compared to the metaphyseal region (least squares mean: epiphysis=1.98 mm3, metaphysis=0.05 mm3, p<0.0001), increased monotonically with time (p<0.0001), and increased at a much greater rate (p<0.0001) for the epiphysis (0.162 mm3/wk) compared to metaphysis (0.007 mm3/wk), tested using ANCOVA (Fig 3A). Cement-bone gaps formed along the periphery of the implant in the epiphysis, while the central drilled and cemented region, that extended into the metaphysis, remained well fixed without gaps (Fig 3B). There were several instances of bone cyst formation with an ellipsoidal or spherical appearance in the epiphyseal region.
Figure 3.
Cement-bone gap volume (GV) as a function of time (A) for epiphyseal and metaphyseal regions. N=7–8/time point with mean and standard error bars are shown. Representative epiphyseal gaps (yellow arrows) are shown for sagittal sections from a 26-week implantation, illustrating osteolytic lesions between cement and bone (B). Contour plot relating implantation time, medial position of the implant, and gap volume (C).
The tibial implants were, on average, placed medially (1.16±0.54 mm) and slightly anteriorly (0.17±0.41 mm) from the centerline of the tibial surface. GV increased with both time (p<0.0001) and implant medialization (p=0.0093) (Fig 3C), using a multiple regression model. This suggests that variations in mechanical loading, perhaps due to uneven loading of the implant surface, may contribute to formation of epiphyseal gaps.
Bone Viability
At implantation, 98–100% of osteocyte lacunae were nucleated (Fig 4). However, the area fraction of viable bone in the interdigitated metaphysis was reduced substantially at two weeks (mean±SD viable bone: 28.0±2.0%), and remained suppressed through 26 weeks. The marrow-adjacent bone in the metaphysis had a smaller decrease in viability (82.1 ± 10.7% for 2–26 weeks); regions of non-viable bone in marrow-adjacent regions were generally located near the cement border.
Figure 4.
Viable bone fraction was quantified from masks created for interdigitated and marrow-adjacent regions. Image illustrate regions of non-viable bone at 2 weeks for interdigitated regions of the metaphysis. N=3/time point with mean and standard error bars shown. Some end points did not have measurable interdigitated bone in the epiphysis.
There was a substantial decrease in viable bone in interdigitated regions of the epiphysis at two weeks (21.5%±4.9 %). This increased with time, although the quantity of interdigitation was small and not all epiphyseal sections had interdigitated cement-bone regions. The marrow-adjacent regions of the epiphysis had a much lower fraction of viable bone at 2 weeks (47.7±8.1%) compared to the metaphysis, indicating more early damage to the bone in the epiphysis. Areas of non-viable bone were located near the cement border of marrow-adjacent bone, suggesting the proximity to the cement border was associated with loss of viability. By 26 weeks, viable bone fractions in the epiphysis (83.3±20.2%) and metaphysis (82.7±12.7%) of marrow-adjacent regions were similar.
Absence of Osteoclasts in Interdigitated Regions, Elevated Presence in Marrow-Adjacent Regions
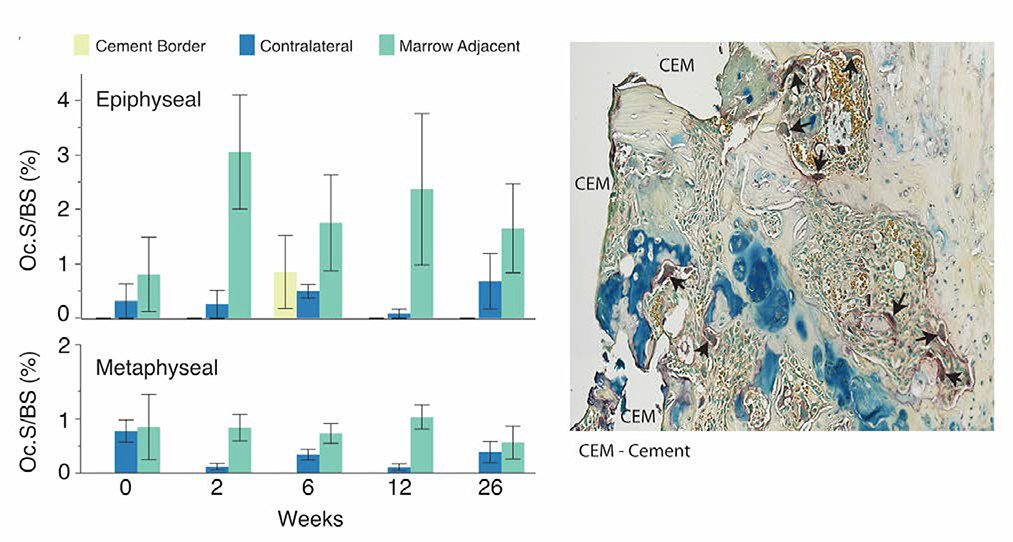
No TRAP-positive multinucleated cells (osteoclasts) were found in interdigitated cement-bone regions at any end point. Similarly, very few osteoclasts were located at the cement border (Figure 5). In contrast, osteoclast fraction was larger (p<0.0001) for the marrow-adjacent regions of the metaphysis (Oc.S/BS, for 2–26 weeks: 0.79±0.30%) compared to the contralateral tibia (0.24±0.22%). Oc.S/BS for marrow-adjacent regions in the epiphysis was even greater (2.2±1.7%) compared to the metaphysis (p<0.01), or compared to the contralateral epiphysis (0.375±0.49%) (p<0.0001).
Figure 5.
The fraction of osteoclast surface to bone surface (Oc.S/BS) was determined from TRAP stained sections for interdigitated, cement border, marrow-adjacent, and contralateral regions of the epiphysis and metaphysis. Values for interdigitated bone were not included because all were 0.0 for Oc.S/BS. Osteoclasts are noted by black arrows in this example epiphyseal section at 2 weeks indicating substantial osteoclast surface. N=3/end point with mean and standard error bars shown.
Dynamic Histomorphometry
The active mineralizing bone surface (L.Pm) (was diminished (p<0.0052) in the interdigitated/cement border regions of the metaphysis (8.6±5.7%) compared to the contralateral bone (21.0±7.1%) Fig 6A). In the marrow-adjacent regions of the metaphysis, there was a transient increase in mineralizing bone surface at 2 weeks (36.8±19.8%), and this was associated with new bone formation adjacent to the cement border (Fig 6B). At other end points, the mineralizing bone surface was similar for marrow-adjacent and contralateral regions of the metaphysis.
Figure 6.
Calcein green and alizarin complexone were administered 14 and 4 days prior to euthanasia, respectively (A). Figure illustrates extensive focal bone formation at 2 weeks post operatively, adjacent to the cement border in the marrow adjacent region of the metaphysis. Double labels are not evident in the interdigitated bone. Active mineralizing bone surface (L.Pm) (B), mineral apposition rate (MAR) (C), and bone formation rate (BFR) (D). N=3/end point with mean and standard error bars shown.
In the epiphysis, L.Pm for interdigitated/cement border regions (11.6±8.8%) was diminished compared to the contralateral bone (18.7±9.7%), but this difference was not significant (p=0.17). The epiphyseal marrow-adjacent bone had a greater L.Pm (25.9±9.7%) compared the interdigitated/cement border (p=0.002).
The mineral apposition rate (MAR) and bone formation rate (BFR) for the interdigitated/cement border region were not calculated because there were not discernable double labels in this region. MAR (Fig 6C) and BFR (Fig 6D) for the marrow-adjacent and contralateral regions followed the same pattern. The BFR for the marrow-adjacent region of the metaphysis had an early transient increase at 2 weeks (0.81±0.42 μm3/μm2/d), followed by a decrease in BFR to levels slightly higher (0.30±0.11 μm3/μm2/d) than contralateral tibia (0.21±0.07 μm3/μm2/d) over 6–26 weeks. In the epiphysis, marrow-adjacent regions (0.42±0.31 μm3/μm2/d) had a BFR that was elevated and sustained compared to the contralateral tibia (0.18±0.08 μm3/μm2/d) over 2–26 weeks. Together these results suggest that the interdigitated bone was not modeling (forming new bone) after initial cementing of the tibia, there was a transient increase in new bone formation in the marrow-adjacent regions of the metaphysis, and elevated and sustained bone formation in the marrow-adjacent regions of the epiphysis.
Progression of Epiphyseal Fibrous Tissue Formation
Fibroblasts were found near the epiphyseal cement border at early end points (Fig 7), and these progressed to create regions of extensive organized fibrous tissue in the epiphysis. The fibrous tissue was associated with the cement-bone gaps described above. The area fraction of fibrous tissue increased with time, while the normal marrow fraction decreased with time. The fraction of nonviable bone was highest at two weeks, and this diminished at later end points. Overall, there was very little change in the overall bone area fraction (sum of viable and nonviable bone) with time. The finding of elevated osteoclastic and bone formation measures described above in the epiphysis, combined with the diminished nonviable bone suggests that non-viable bone was being resorbed, but there were also substantial changes in the spatial distribution of epiphyseal bone. This resulted in epiphyseal bone with progressively larger fibrous filled gaps at the cement border, and condensation of bone away from the cement border.
Figure 7.
The area fraction of viable bone, nonviable bone, marrow, and fibroblast/fibrous tissue in the epiphyseal region of the cemented tibia. There is progression of fibrotic tissue near the cement border with time and loss of normal marrow space. There is an early increase in nonviable bone, that then is replaced with viable bone at late end points. N=3 time/point with mean and standard error bars shown.
DISCUSSION:
We found a progressive loss of fixation between cement and bone using a knee hemiarthroplasty model in an aged rat. The loss of fixation initiated and progressed under the tibial tray resulting in discernable gaps between the cement and bone filled with fibrous tissue, that did not extend to the metaphysis. Distally, the construct remained well-fixed with cement interdigitated with metaphyseal bone. The cellular response to implantation over time in the epiphysis and metaphysis were also markedly different. In the epiphysis, there was an early and sustained elevation of osteoclasts adjacent to the cement border, with active bone formation more distant from the cement border, and development of a fibrous tissue layer between the cement border and bone. The fibrous tissue layer is thought to be the result of the loosening process rather than the cause (14; 18). In the metaphysis, the interdigitated trabecular bone was not viable, but also did not resorb with time. There was early formation of bone around the cement border in the metaphysis, resulting in a condensed bone layer without osteoclastic bone resorption or development of a fibrous tissue layer.
The hyper-mineralization of interdigitated trabecular bone found in the current study with 9-month-old rats was also found in the earlier study using 3-month-old rats (5) (Table S-2). In both cases, there was extensive osteocyte death and low bone turnover, similar to what is found in hyper-mineralized auditory ossicles (19; 20). The hyper-mineralization of the trabeculae could also explain, in part, the apparent increase in bone volume fraction of interdigitated bone, as more ‘bone’ at the edges would be captured with higher mineralization. While there was evidence of active mineralization on the interdigitated bone surface, the mineral apposition rate was not discernable between fluorescent labels in this study. Whether hyper-mineralization occurs in interdigitated bone of human arthroplasties is not known.
In general, loss of fixation in the rat hemiarthroplasty model mimics the pattern found in human arthroplasty where progressive radiolucencies (1; 21) initiate at the periphery of the implant under the tibial tray (Fig 8). However, there was limited interdigitation between cement and bone in the epiphyseal bone in the rats, which is different from a well-cemented human knee arthroplasty. The epiphyseal gaps found in the present study are similar to cavitary defects found in canine total knee replacement for cases where there was not initial interdigitation between cement and the tibial surface cut (22). Future work could focus on achieving more epiphyseal interdigitation by focusing cement application to only the epiphysis to the level of the growth plate, which would more closely mimic human implants.
Figure 8.
Sagittal section of proximal tibia from 15 month-old rat hemiarthroplasty following 26 weeks in vivo service (top) and post-mortem retrieved proximal tibia from a 61 year-old male total knee arthroplasty following 5 years in vivo service (bottom). Both exhibit fibrous tissue (FT) at the periphery of the implant between the cement (C) and bone (B). Note that the human arthroplasty exhibits cement bone interdigitated (Int), while the rat does not.
A number of factors are known to contribute to periprosthetic osteolysis including bone necrosis from the surgical procedure and application of bone cement (23), mechanical factors including stress shielding (24) and fluid flow-induced osteolysis (25), and over the longer term, the inflammatory response to wear particles generated from the joint arthroplasty (2; 26). In the current rat model, non-viable bone was found early (at 2 weeks) in regions adjacent to the cement in the epiphysis. It is likely that local cytotoxic effects of the monomer and disruption of the vasculature from surgery (27) contribute to the necrosis in regions adjacent to the cement border. Osteocyte apoptosis has been shown to result in an increase in receptor activator of NFκB ligand (RANKL), which is essential for osteoclast differentiation, function, and survival (28). Osteocyte apoptosis has also been shown to result in a large transient increase in bone resorption (29). However, using a model of instability-induced loosening, osteoclast differentiation was found to be independent of osteocyte apoptosis (18). In the current study, there was a large increase in osteoclast number in the epiphysis at the earliest end point, which may be associated with osteocyte death. Further work is needed to relate the early stage apoptotic or necrotic fate of the osteocytes adjacent to the cement and any subsequent peri-implant osteolysis.
In contrast to the epiphysis, metaphyseal interdigitated bone was largely non-viable, but this bone did not resorb or remodel. Resorption of interdigitated bone has been shown to initiate at the cement border in post-mortem-retrieved human total knee arthroplasties (6). In the human case, there is access to marrow derived osteoclastic cells and precursors at the cement border, and as the resorption of bone progresses, osteoclasts would have access to bone deeper in the cement layer. With the rat arthroplasty, it is possible that there is less access to osteoclasts due the early condensation of bone at the metaphyseal cement border. Other factors such as greater local implant stability or reduced access to inflammatory cytokines associated with the synovium (and not part of the “effective joint space”(30)) may also contribute to the limited gap formation in the metaphysis.
Stress shielding has long been identified as a contributor to peri-implant bone loss. In human knee arthroplasty, peri-implant bone density decreases for several years after implantation (31). In the rat hemiarthroplasty, the relative amount of metaphyseal fill with cement is large compared to human tibial components and this could result in stress bypass of the epiphysis, resulting in a compensatory loss of epiphyseal bone. Future work contrasting constructs with metaphyseal fill versus epiphyseal-only fixation could be used to explore the stress bypass phenomenon.
Pressurized synovial fluid flow at the cement border as a result of mechanical instability is another mechanical factor that could contribute to the periprosthetic osteolysis (25; 32). In this scenario, fluid flow in narrow gaps is caused by local micromotion, resulting in supraphysiologic fluid flow and pressure (33). Supraphysiologic flow induces pro-osteoclastic signaling (34) and osteoclast differentiation is induced by mechanical instability (18), resulting in increased focal bone resorption (35). The local flow environment found in human knee arthroplasty appears to be similar to that found in the rat hemiarthroplasty model in terms of magnitude of fluid shear stress, suggesting that any response due to mechanical instability on the local cellular environment would be similar (36).
Debris from the articulating surface of joint arthroplasty has been identified a major factor leading to long term periprosthetic osteolysis (26). There was some observed burnishing on the articulating PEEK surfaces in the rat, but direct measures of PEEK debris were not made. Wear particles produced by PEEK bearings are in the phagocytosable size range (0.1–10 μm) with cytotoxicity similar to ultra-high molecular weight polyethylene (37). A dose-response relationship between amount of debris generation and osteolysis (38) has been reported. Therefore, it is possible that wear debris contributed to the periprosthetic osteolysis.
The ideal age of rat to study age-related bone loss in cemented arthroplasty model would be after reproductive senescence which occurs relatively late in a Sprague Dawley rat (15–20 months). However, the average lifespan is reported to be between 24 and 36 months (39). Long-term longitudinal studies starting at reproductive senescence would be challenging due to the likelihood of losing large fractions of the sample population at later end points. Previously, we used 3-month-old rats with this hemiarthroplasty model, and qualitative changes in bone morphology were similar to the present study using 9-month-old rats (Fig S-2). However, the 3-month-old rats had a much greater rate of hyper-mineralization of trabecular bone interdigitated with cement. The older rats may be a better choice in capturing progressive changes in fixation due to age-related bone loss, as they lack robust anabolic responses.
Despite the technical challenges with this and other small animal models used to investigate implant fixation, this work has several important findings that are relevant to human cemented knee replacement. First, the finding that trabecular bone interdigitated with cement is not viable in this aged rat model suggests that the interdigitated bone in cemented human arthroplasty may also not be viable. Presence of non-viable bone could initiate a resorption process in humans over the long term, resulting in loss of cement-bone fixation. Second, early epiphyseal fixation changes in this model appear to recapitulate the early loosening process in human knee arthroplasty (4), where progressive radiolucencies are seen under the tibial tray. Third, this preclinical rat model could be used to explore pharmacologic interventions as candidates to prevent the early bone loss under the tibial tray. If early interface stability could be maintained, long term progression of peri-implant osteolysis might be prevented. Finally, this model could be used to explore the relationships between early biological response to fixation and the associated contributions of periprosthetic inflammation and mechanical instability.
Supplementary Material
ACKNOWLEDGEMENTS:
This work was funded under NIH/NIAMS award #AR42017. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. TAD is a consultant for Cerament and has received unrelated grant support from Stryker Orthopaedics. KAM is a member of the Journal of Orthopaedic Research Publication Advisory Board.
References
- 1.Guha AR, Debnath UK, Graham NM. 2008. Radiolucent lines below the tibial component of a total knee replacement (TKR)--a comparison between single-and two-stage cementation techniques. Int Orthop 32: 453–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallo J, Goodman SB, Konttinen YT, et al. 2013. Osteolysis around total knee arthroplasty: a review of pathogenetic mechanisms. Acta Biomater 9: 8046–8058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goodheart JR, Miller MA, Mann KA. 2014. In vivo loss of cement-bone interlock reduces fixation strength in total knee arthroplasties. J Orthop Res 32: 1052–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller MA, Goodheart JR, Izant TH, et al. 2014. Loss of Cement-bone Interlock in Retrieved Tibial Components from Total Knee Arthroplasties. Clin Orthop Relat Res 472: 304–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mann KA, Miller MA, Amendola RL, et al. 2019. Early Changes in Cement-Bone Fixation Using a Novel Rat Knee Replacement Model. J Orthop Res 37: 2163–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodheart JR, Miller MA, Oest ME, Mann KA. 2017. Trabecular resorption patterns of cement-bone interlock regions in total knee replacements. J Orthop Res 35: 2773–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li XJ, Jee WSS, Ke HZ, et al. 1991. Age-related Changes of Cancellous and Cortical Bone Histomorphometry in Female Sprague-Dawley Rats. Cells and Materials 25–35. [Google Scholar]
- 8.Millett PJ, Allen MJ, Bostrom MP. 2002. Effects of alendronate on particle-induced osteolysis in a rat model. J Bone Joint Surg Am 84-A: 236–249. [DOI] [PubMed] [Google Scholar]
- 9.Howie DW, Vernon-Roberts B, Oakeshott R, Manthey B. 1988. A rat model of resorption of bone at the cement-bone interface in the presence of polyethylene wear particles. J Bone Joint Surg Am 70: 257–263. [PubMed] [Google Scholar]
- 10.Moran MM, Wilson BM, Ross RD, et al. 2017. Arthrotomy-based preclinical models of particle-induced osteolysis: A systematic review. J Orthop Res 35: 2595–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vertesich K, Sosa BR, Niu Y, et al. 2020. Alendronate enhances osseointegration in a murine implant model. J Orthop Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang X, Ricciardi BF, Dvorzhinskiy A, et al. 2015. Intermittent Parathyroid Hormone Enhances Cancellous Osseointegration of a Novel Murine Tibial Implant. J Bone Joint Surg Am 97: 1074–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pap G, Machner A, Rinnert T, et al. 2001. Development and characteristics of a synovial-like interface membrane around cemented tibial hemiarthroplasties in a novel rat model of aseptic prosthesis loosening. Arthritis Rheum 44: 956–963. [DOI] [PubMed] [Google Scholar]
- 14.Goodman SB, Magee FP, Fornasier VL. 1993. Radiological and histological study of aseptic loosening using a cemented tibial hemiarthroplasty in the rabbit knee. Biomaterials 14: 522–528. [DOI] [PubMed] [Google Scholar]
- 15.Doube M, Klosowski MM, Arganda-Carreras I, et al. 2010. BoneJ: Free and extensible bone image analysis in ImageJ. Bone 47: 1076–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schindelin J, Arganda-Carreras I, Frise E, et al. 2012. Fiji: an open-source platform for biological-image analysis. Nat Methods 9: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dempster DW, Compston JE, Drezner MK, et al. 2013. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 28: 2–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madsen RV, Nam D, Schilcher J, et al. 2020. Mechanical instability induces osteoclast differentiation independent of the presence of a fibrous tissue interface and osteocyte apoptosis in a rat model for aseptic loosening. Acta Orthop 91: 115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rolvien T, Schmidt FN, Milovanovic P, et al. 2018. Early bone tissue aging in human auditory ossicles is accompanied by excessive hypermineralization, osteocyte death and micropetrosis. Sci Rep 8: 1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt FN, Delsmann MM, Mletzko K, et al. 2018. Ultra-high matrix mineralization of sperm whale auditory ossicles facilitates high sould pressure and high-frequency underwater hearing. Proc. R. Soc. B 285: 20181820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ritter MA, Herbst SA, Keating EM, Faris PM. 1994. Radiolucency at the bone-cement interface in total knee replacement. The effects of bone-surface preparation and cement technique. J Bone Joint Surg Am 76: 60–65. [DOI] [PubMed] [Google Scholar]
- 22.Mann KA, Miller MA, Khorasani M, et al. 2012. The dog as a preclinical model to evaluate interface morphology and micro-motion in cemented total knee replacement. Vet Comp Orthop Traumatol 25: 1–10. [DOI] [PubMed] [Google Scholar]
- 23.Mjoberg B, Pettersson H, Rosenqvist R, Rydholm A. 1984. Bone cement, thermal injury and the radiolucent zone. Acta Orthop Scand 55: 597–600. [DOI] [PubMed] [Google Scholar]
- 24.Zhang QH, Cossey A, Tong J. 2016. Stress shielding in periprosthetic bone following a total knee replacement: Effects of implant material, design and alignment. Med Eng Phys 38: 1481–1488. [DOI] [PubMed] [Google Scholar]
- 25.Nam D, Bostrom MP, Fahlgren A. 2013. Emerging ideas: Instability-induced periprosthetic osteolysis is not dependent on the fibrous tissue interface. Clin Orthop Relat Res 471: 1758–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodman SB, Gallo J. 2019. Periprosthetic Osteolysis: Mechanisms, Prevention and Treatment. J Clin Med 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charnley J 1970. The reaction of bone to self-curing acrylic cement. A long-term histological study in man. J Bone Joint Surg Br 52: 340–353. [PubMed] [Google Scholar]
- 28.O’Brien CA, Nakashima T, Takayanagi H. 2013. Osteocyte control of osteoclastogenesis. Bone 54: 258–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tatsumi S, Ishii K, Amizuka N, et al. 2007. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab 5: 464–475. [DOI] [PubMed] [Google Scholar]
- 30.Schmalzried TP, Jasty M, Harris WH. 1992. Periprosthetic bone loss in total hip arthroplasty. Polyethylene wear debris and the concept of the effective joint space. J Bone Joint Surg Am 74: 849–863. [PubMed] [Google Scholar]
- 31.Shi M, Chen L, Wu H, et al. 2018. Effect of bisphosphonates on periprosthetic bone loss after total knee arthroplasty: a meta-analysis of randomized controlled trials. BMC Musculoskelet Disord 19: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johansson L, Edlund U, Fahlgren A, Aspenberg P. 2011. Fluid-induced osteolysis: modelling and experiments. Comput Methods Biomech Biomed Engin 14: 305–318. [DOI] [PubMed] [Google Scholar]
- 33.Mann KA, Miller MA. 2014. Fluid-structure interactions in micro-interlocked regions of the cement-bone interface. Comput Methods Biomech Biomed Engin 17: 1809–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fahlgren A, Bratengeier C, Semeins CM, et al. 2018. Supraphysiological loading induces osteocyte-mediated osteoclastogenesis in a novel in vitro model for bone implant loosening. J Orthop Res 36: 1425–1434. [DOI] [PubMed] [Google Scholar]
- 35.Skripitz R, Aspenberg P. 2000. Pressure-induced periprosthetic osteolysis: a rat model. J Orthop Res 18: 481–484. [DOI] [PubMed] [Google Scholar]
- 36.Mann KA, Miller MA, Tatusko ME, Oest ME. 2020. Similitude of cement-bone micromechanics in cemented rat and human knee replacement. J Orthop Res 38: 1529–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stratton-Powell AA, Pasko KM, Brockett CL, Tipper JL. 2016. The Biologic Response to Polyetheretherketone (PEEK) Wear Particles in Total Joint Replacement: A Systematic Review. Clin Orthop Relat Res 474: 2394–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilkinson JM, Hamer AJ, Stockley I, Eastell R. 2005. Polyethylene wear rate and osteolysis: critical threshold versus continuous dose-response relationship. J Orthop Res 23: 520–525. [DOI] [PubMed] [Google Scholar]
- 39.Sengupta P 2013. The Laboratory Rat: Relating Its Age With Human’s. Int J Prev Med 4: 624–630. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.