Abstract
Heart rate monitoring in free-ranging cetaceans to understand their behavioural ecology and diving physiology is challenging. Here, we developed a simple, non-invasive method to monitor the heart rate of cetaceans in the field using an electrocardiogram-measuring device and a single suction cup equipped with an electrode. The unipolar suction cup was placed on the left lateral body surface behind the pectoral fin of Risso's dolphins (Grampus griseus) and a false killer whale (Pseudorca crassidens) in captivity; their heart rate was successfully monitored. We observed large heart rate oscillations corresponding to respiration in the motionless whales during surfacing (a false killer whale, mean 47 bpm, range 20–75 bpm; Risso's dolphins, mean ± s.d. 61 ± 15 bpm, range 28–120 bpm, n = 4 individuals), which was consistent with the sinus arrhythmia pattern (eupneic tachycardia and apneic bradycardia) observed in other cetaceans. Immediately after respiration, the heart rate rapidly increased to approximately twice that observed prior to the breath. Heart rate then gradually decreased at around 20–50 s and remained relatively constant until the next breath. Furthermore, we successfully monitored the heart rate of a free-swimming Risso's dolphin. The all-in-one suction cup device is feasible for field use without restraining animals and is helpful in further understanding the diving physiology of free-ranging cetaceans.
This article is part of the theme issue ‘Measuring physiology in free-living animals (Part II)’.
Keywords: biologging, marine mammal, cetaceans, electrocardiogram, diving physiology, heart rate
1. Introduction
For breath-hold divers that feed at depth and replenish their oxygen stores at the surface, it is crucial to adopt strategies that minimize oxygen consumption and, thus, maximize foraging time at depth. In particular, diving responses such as a reduction in the heart rate and peripheral vasoconstriction, which are associated with minimizing the oxygen consumption rate, are remarkable in marine mammals [1,2]. To determine how cetaceans accomplish deep-sea pursuits, it is essential to simultaneously monitor the heart rate using electrocardiograms (ECG) and the activity of the animal (i.e. swim speed and fluke stroke). To date, adaptive changes in the heart rate with different levels of underwater activities have been investigated mainly using captive animals. For example, captive common bottlenose dolphins (Tursiops truncatus) were trained to wear a neoprene vest that carried an ECG and a behavioural recorder [3–5]. The heart rates of dolphins were related to their activity and were positively correlated with stroke frequency and the corresponding swim speed. In addition, the degree of bradycardia was strongly correlated with dive depth [5].
The effect of exercise on the degree of bradycardia has been hard to examine in free-ranging cetaceans because of difficulties associated with tagging. In studies under captive conditions, two-suction cup electrodes were attached to dolphins [3–8]: one cup was placed on the sternum along the ventral midline, directly below the pectoral fin insertions, and the other was placed above the right scapula. Multiple electrodes have to be attached to specific places to detect the heartbeat, which is nearly impossible to achieve without prior capture or restraint of the animals. Only one study of a single blue whale (Balaenoptera musculus) successfully monitored the heart rate of a free‐ranging cetacean without its prior capture or restraint: the multiple suction cup tag revealed bradycardia and tachycardia of the blue whale during lunge feeding dives [9].
Here, we developed a non-invasive heart rate monitoring system that is applicable to free-ranging cetaceans. We successfully monitored the heart rate of two captive delphinid species (i.e. four Risso's dolphins Grampus griseus and a false killer whale Pseudorca crassidens) using a single suction cup tag.
2. Material and methods
(a) . Electrocardiogram-logger with a single suction cup tag
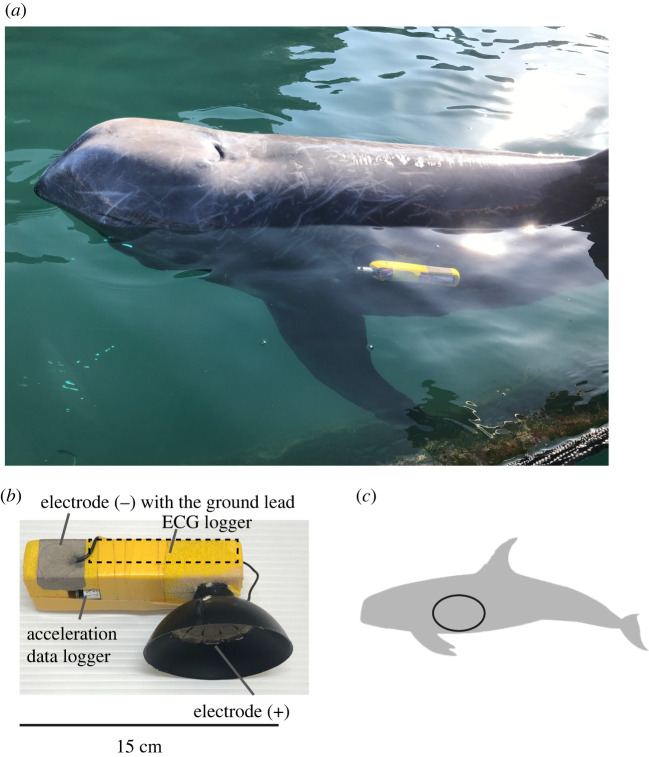
We modified the suction cup tags used in acceleration and speed data loggers [10] to prepare single suction cup tags (float size; width: 4.5 cm, length: 12–20 cm, height: 1.5–3.0 cm) that included a W400-ECG logger (diameter: 21 mm, length: 109 mm, mass: 60 g air; Little Leonardo Co., Tokyo, Japan) (figure 1). The ECGs were recorded at 200 Hz. The maximum recording duration was approximately 3 days at 200 Hz. The voltage span of the input signal was ±5.8 mV. Silicon or rubber suction cups (diameter: 60 mm and 80 mm, respectively) were prepared by Extas Co. (Shizuoka, Japan). Adhesive tape made of electro-conductive fabric (Shield fabric tape KNZ-ST50; Kyowa Harmonet Ltd., Kyoto, Japan) was used as electrodes. The tape was cut to fit the inner side of the suction cup (tape size; diameter: 30 mm for silicon cups, 50 mm for rubber cups) and attached inside the suction cup (+). Another tape electrode (tape size; length: 40 mm, width: 30 mm) was attached outside the tag float (−) to detect differences in the electrical potential between seawater and an attachment located close to the heart of the animal. The ground lead was combined with a tape electrode (−). An acceleration data logger ORI400-D3GT (diameter: 12 mm, length: 45 mm, mass: 9 g air; Little Leonardo Co.) was added to the suction cup tag during swimming trials (see below). The logger recorded longitudinal acceleration at 50 Hz, water temperature and depth at 1 Hz.
Figure 1.
(a) A suction cup tag attached to a Risso's dolphin. (b) A suction cup tag equipped with an ECG logger. (c) The region at which the heartbeats were measured: from just behind the posterior edge of the scapula under the thoracic vertebrae. (Online version in colour.)
(b) . Animals
The objective of this study was to understand the diving behaviour and physiology of delphinids using individuals under human care. All procedures, animal husbandry and management were performed under the careful supervision of veterinarians and veterinary nurses in the Whale Museum and Aquarium (Wakayama, Japan). ECG recordings were collected from three out of four Risso's dolphins (ID: gg_mf, gg_nf and gg_rm) and one false killer whale in March 2019, and from the four Risso's dolphins and the false killer whale in October and November 2019 (table 1). One individual (ID: gg_rm) was born in the aquarium and all other individuals have been under professional care in the aquarium for at least 12 years (table 1). Adult females (ID: gg_mf, gg_nf) were considered non-reproductive during the experiment. They were housed in a large natural semi-enclosed area where natural furniture and shelter exist (50 × 210 × 2–6 m, length × width × depth). They are housed temporarily in net pens (12 × 12 × 3–4 m, length × width × depth) for healthcare and/or experiments. A shady environment is created by the natural hills and forests surrounding the enclosure.
Table 1.
Biological information of individuals. Individuals under human care for over 12 years or born in the aquarium were used in this study. Body length and girth were measured at the end of October in 2019. Body mass was estimated from the body length and girth based on Tobayama & Kirihata [11]. All individuals were adults except for ID gg_rm (juvenile).
| species | ID | sex | body length (cm) | girth (cm) | body mass (kg) | estimated age (years) | display periods (years) |
|---|---|---|---|---|---|---|---|
| false killer whale | pc_km | M | 426 | 196 | 599 | 15 | 13 |
| Risso's dolphin | gg_sm | M | 302 | 157 | 334 | 24 | 19 |
| gg_mf | F | 298 | 152 | 312 | 24 | 15 | |
| gg_nf | F | 286 | 167 | 342 | 16 | 12 | |
| gg_rm | M | 270 | 142 | 246 | 5a | 5 |
aThe age was known.
The animals were fed a mixed diet (e.g. mackerel and squid) supplemented with vitamins multiple times daily. The animal ID, body length, body mass, sex and approximate age (known or estimated) of each study animal in the aquarium are summarized in table 1.
(c) . Experiments
The experiments were performed by operant conditioning. A suction cup tag was attached behind the left side of the pectoral fin (just behind the posterior edge of the scapula under the thoracic vertebrae) just before each trial (figure 1). The inner side of the suction cup was covered with conducting cream (Kenz ECG cream; Suzuken Co., Nagoya, Japan).
(i) . Stationary position
The experiments were conducted in the morning (after more than 12 h of fasting) and in the evening (after all performances in the aquarium) from October to November, 2019. The experiments were conducted between aquarium performances during March in 2019. Each trial consisted of an animal floating stationarily at the surface with its blow-hole out of the water to enable spontaneous breathing for up to 15 min. Trainers determined the end of each experiment, and for one Risso's dolphin (ID: gg_nf), the trials ended earlier because the individual started to move its body. The animals were not restrained and could end the trial at any point without any negative consequence. There were no negative responses to the other trials. The timing of each breath was recorded using video cameras. Before deployment, the times of the ECG logger and video cameras were synchronized to the Japan Standard Time. In addition, the time synchronization was verified using the attachment or detachment timing of the suction cup tags recorded by video cameras. Prior to the study, the dolphins were desensitized to the fitting of a single suction cup tag for at least 6 months except for one Risso's dolphin (ID: gg_sm), which was desensitized for one month.
(ii) . Swimming
To check if the heartbeats were detectable during active swimming, a Risso's dolphin (ID: gg_mf) was trained to swim with a suction cup tag in the pen. Each trial involved the dolphin following a trainer who ran around the pen. Another trainer standing on one corner of the pen threw dead prey (whole squids, n = 20 times per trial) when the dolphin passed the opposite corner of the pen, after which the dolphin approached and captured the prey. The experiments were performed by operant conditioning in March 2019. Before deployment, the times of the ECG and acceleration data logger were synchronized to the Japan Standard Time.
(d) . Data analyses
Data were analysed using IGOR Pro software (Wavemetrics Inc., Lake Oswego, OR, USA). We used a custom program ‘ECGtoHR’ (see Sakamoto et al. [12] for more details), in Igor Pro, to automatically detect the time between the R–R peaks using the typical frequency of QRS waves (15 Hz) and maximum heart rates (200 bpm). The detected R peaks were then visually verified, and the instantaneous heart rate (fH) was determined from the time between the R–R peaks. We omitted ‘noisy sections’ where the tag was briefly out of the water. Because the ground electrode was placed in the water, the electrical potential was not detectable when the logger was out of the water.
We observed sinus arrhythmia patterns (eupneic tachycardia and apneic bradycardia) from motionless whales during surfacing, similar to other cetaceans [13–22]. A rapid increase to reach the maximum heart rate that corresponded to respiration was determined as pronounced sinus arrhythmia patterns in this study. We investigated the percentage of synchronicity of respiration with heart rate variability during each trial. Maximum and minimum fH were assessed during inter-breath intervals (i.e. apneic periods, from the end of respiration to the next respiration) for each trial in the experiments during October and November of 2019.
To compare the measured heart rate with the heart rate expected from the body mass of terrestrial mammals (predicted heart rate = 241 × [body mass (kg)]−0.25, [23]), we averaged fH of motionless whales (while surfacing) when pronounced sinus arrhythmia patterns were observed. Data are presented as mean ± s.d.
3. Results
(a) . Heartbeats recorded by the electrocardiogram tag with a single suction cup
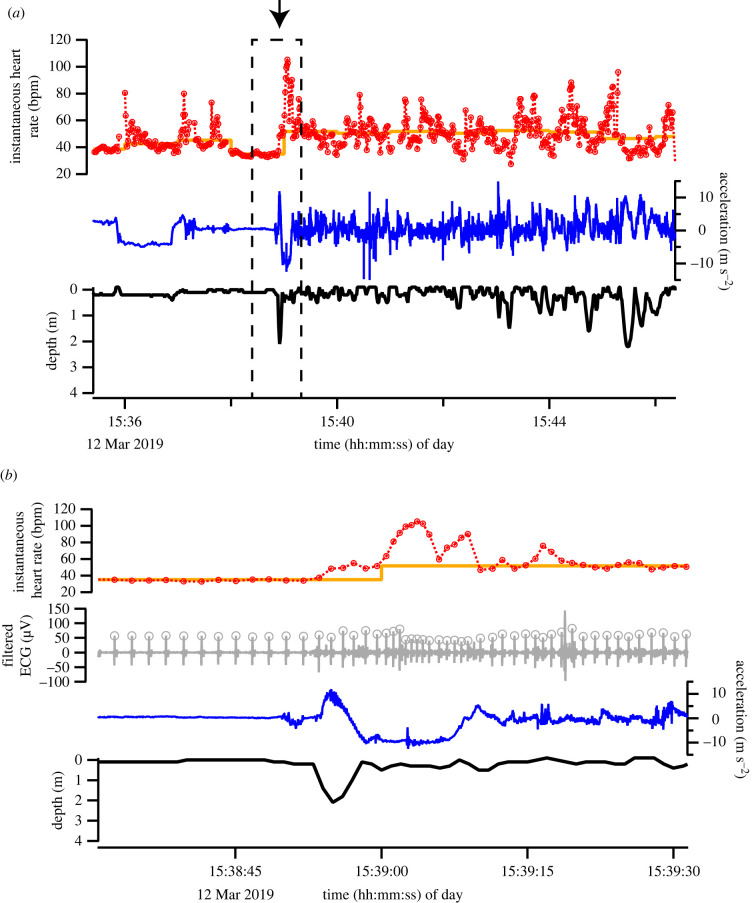
Clear heartbeat signals were obtained from all individuals when the tag was deployed behind the flipper of motionless whales at the surface (figure 2). The shapes of the QRS waves differed among the individuals (figure 2), possibly caused by the differences in the attachment positions of the suction cup tags. To confirm that heartbeats were detectable during active swimming, we monitored a Risso's dolphin (ID: gg_mf) equipped with a suction cup tag when it swam and captured dead prey in the pen by operant conditioning (n = 3 trials, duration 13 ± 0.7 min). The filtered heartbeat signals were clearly observed during swimming (figure 3). The average fH was more varied during the swimming trial (59 ± 6 bpm, range 28–105 bpm) than during the inactive period before the trial (46 ± 3 bpm, range 30–81 bpm) (electronic supplementary material, table S1).
Figure 2.
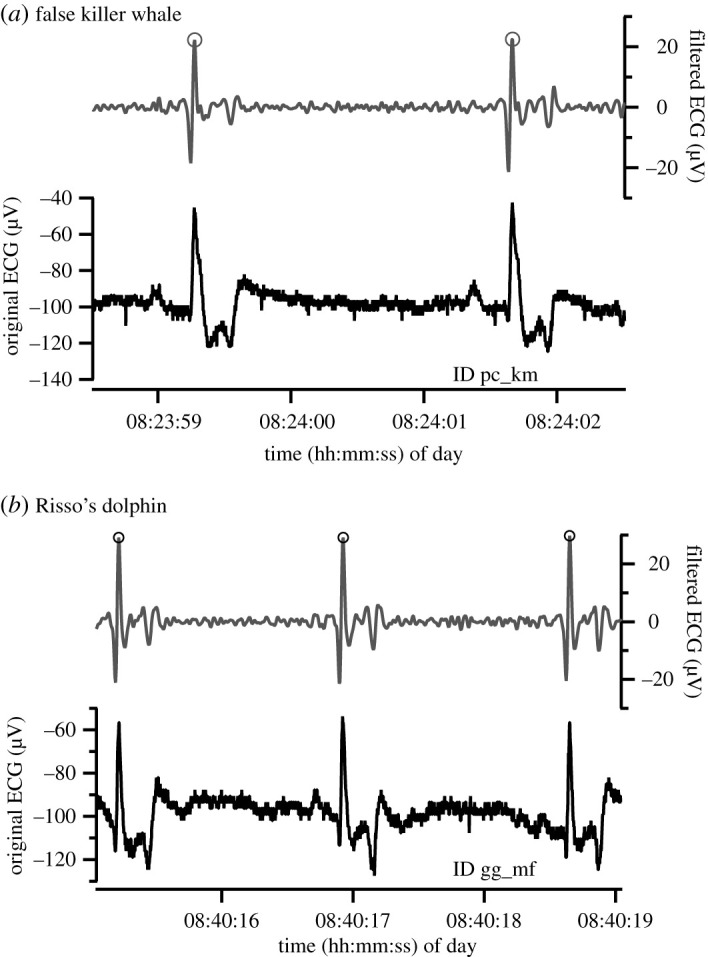
Examples of ECG data and filtered ECG data of a false killer whale and a Risso's dolphin that were motionless at the surface. Grey circles indicate each heartbeat.
Figure 3.
Depth, longitudinal acceleration, instantaneous heart rate and filtered ECG data of a Risso's dolphin (ID: gg_mf) during a swimming trial. The thick orange line shows the median instantaneous heart rate per minute. The plus and minus of longitudinal acceleration are downward and upward direction of the logger, respectively. (b) An enlarged section of time series data from a dashed square of figure part (a). The arrow in (a) indicates when the dolphin started swimming. (Online version in colour.)
(b) . Sinus arrhythmia associated with respiration observed from motionless individuals at surface
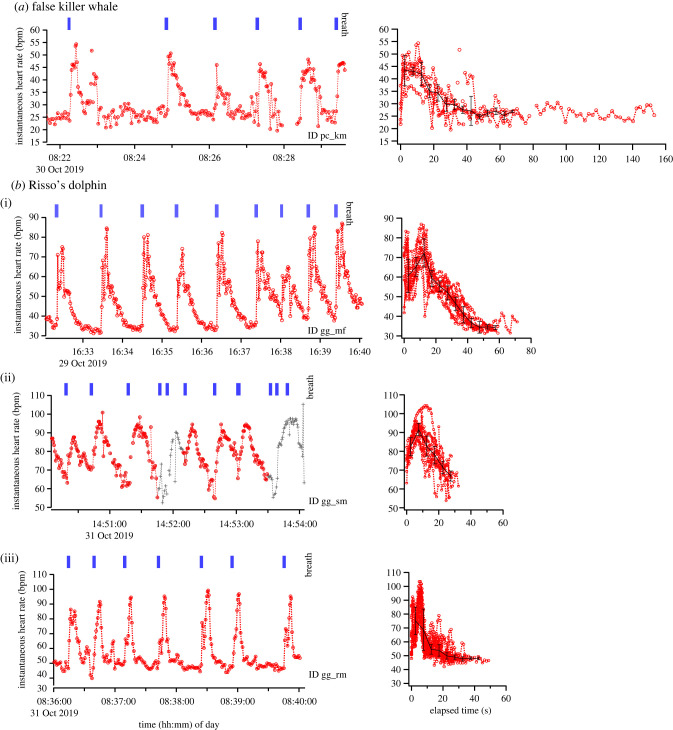
The pronounced sinus arrhythmia patterns associated with respiration were constantly observed in all individuals, but not always seen during trials (tables 2 and 3). The percentage of pronounced sinus arrhythmia patterns during a trial was relatively low in ID gg_sm (24–31%) and more varied in ID gg_nf (0%–100%, tables 2 and 3) than that in the two other Risso's dolphins (ID: gg_mf and gg_rm, 76–100%) and in the false killer whale (ID: pc_km, 58–100%).
Table 2.
Instantaneous heart rates (fH) and respiration rates of motionless delphinids at the surface when pronounced sinus arrhythmia (PSA) patterns were observed. Mean ± s.d. is shown. Measurements were conducted only during non-fasting periods in March 2019.
| species | ID | non-fasting periods during the day |
non-fasting periods in the evening |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| fH (bpm) | range (bpm) | respiration rate (breaths min−1) | % of the PSA | trial duration (min) | fH (bpm) | range (bpm) | respiration rate (breaths min−1) | % of the PSA | trial duration (min) | ||
| false killer whale | pc_km | — | — | — | — | — | 56 ± 18 | 40–75 | 1.4 | 58 | 3.7 |
| Risso's dolphin | gg_nf | 52 ± 11 | 37–83 | 1.3 | 55 | 4.3 | — | — | — | — | — |
| gg_rm | 84 ± 14 | 58–109 | 2.7 | 100 | 3.7 | 87 ± 15 | 55–110 | 2.9 | 100 | 4.5 | |
Table 3.
Instantaneous heart rates (fH) and respiration rates of motionless delphinids at the surface when pronounced sinus arrhythmia (PSA) patterns were observed. Mean ± s.d. is shown. Maximum and minimum fH of apneic periods are shown in electronic supplementary material, table S2. Measurements were conducted from the end of October in 2019 until the beginning of November in 2019.
| species | ID | fasting periods in the morning |
non-fasting periods in the evening |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| fH (bpm) | range (bpm) | respiration rate (breaths min−1) | % of the PSA | trial duration (min) | fH (bpm) | range (bpm) | respiration rate (breaths min−1) | % of the PSA | trial duration (min) | ||
| false killer whale | pc_km | 33 ± 8 | 20–54 | 0.7 | 100 | 8.1 | 51 ± 11 | 24–75 | 1.2 | 84 | 8.3 |
| Risso's dolphin | gg_sm | 67 ± 13 | 46–90 | 3.3 | 31 | 4.9 | 81 ± 11 | 54–108 | 2.2 | 24 | 12.3 |
| gg_mf | 45 ± 11 | 28–81 | 0.9 | 100 | 10.7 | 51 ± 14 | 31–87 | 1.2 | 100 | 9.2 | |
| gg_nf | — | — | — | 0 | 3.9 | 48 ± 14 | 37–120 | 1.5 | 100 | 3.4 | |
| gg_rm | 62 ± 15 | 40–104 | 2.1 | 94 | 10.3 | 69 ± 16 | 46–108 | 1.7 | 76 | 8.7 | |
In all individuals, the instantaneous heart rate (fH) varied considerably with respiration (tables 2, 3; figure 4). Immediately after respiration, fH rapidly increased to approximately twice that observed prior to the breath (false killer whale: maximum fH, 62 ± 11 bpm, n = 13 inter-breath intervals; Risso's dolphin [ID: gg_mf]: maximum fH, 74 ± 8 bpm, n = 20 inter-breath intervals, table S2). The fH then gradually decreased, returning to and stabilizing at pre-breath levels after about 45–50 s (false killer whale: minimum fH, 33 ± 10 bpm, n = 13 inter-breath intervals; Risso's dolphin [ID: gg_mf]: minimum fH, 35 ± 5 bpm, n = 20 inter-breath intervals). When the respiration rate of a Risso's dolphin (ID: gg_mf) increased (after 16:38 h local time), the fH did not stabilize before the next respiration. Indeed, apneic fH of a Risso's dolphin (ID: gg_sm) that showed a relatively high respiration rate compared with ID gg_mf did not stabilize before the next respiration (figure 4, maximum fH, 93 ± 6 bpm, minimum fH, 59 ± 7 bpm, n = 15 inter-breath intervals, table S2). The fH of a juvenile male Risso's dolphin (ID: gg_rm) rapidly rose just after respiration (maximum fH, 94 ± 7 bpm, n = 31 breath intervals) and then decreased, returning to and stabilizing at pre-breath levels after about 20 s (minimum fH, 49 ± 4 bpm, n = 31 inter-breath intervals, table S2).
Figure 4.
Examples of variations in the instantaneous heart rates of two delphinid species with respiration (a) and (b). The red dashed lines and circles show pronounced sinus arrhythmia patterns associated with respiration. The grey dashed lines and crosses show less synchronized sinus arrhythmia patterns with respiration. The panels on the right show the changes in heart rate from the end of each respiration to the next respiration when pronounced sinus arrhythmia patterns were observed. The black lines and the error bars show the median and the quantile deviance at intervals of 5 s. We did not calculate the median and the quantile deviance after 70 s for ID pc_km, 60 s for ID gg_mf and 45 s for ID gg_rm because of few data points. (Online version in colour.)
We compared the heart rate of the motionless whale, when pronounced sinus arrhythmia patterns were observed, with the resting heart rate expected from the body mass of terrestrial mammals: HRpredicted = 241 × [body mass (kg)]−0.25 [23]. The mean fH of the false killer whale was 68% and 105–119% of that expected from the body mass, when fasting in the morning and when non-fasting during the day or evening, respectively. The mean fH of Risso's dolphins was 79–119% and 85–145% of that expected from the body mass, when fasting in the morning and when non-fasting during the day or evening, respectively.
4. Discussion
(a) . Toward non-invasive heart rate monitoring in free-ranging cetaceans
We successfully observed clear heart beats in all individuals using the newly developed single suction cup heart rate monitoring system. So far, ECG recording using a unipolar electrode in cetaceans has been successful in just one study; Meijler et al. [24] recorded the ECG of an entrapped humpback whale (Megaptera novaengliae) with a suction cup electrode, placed in a similar position as in this study, and long coaxial cables connected to a portable ECG recorder and Holter monitor. However, ECG recording of a beluga whales (Delphinapterus leucas) was not successful using a unipolar harpoon electrode possibly due to the reduced salt content of a mixture of seawater and river water [25]. More recent studies used the two-suction cup systems to monitor the heart rate in free-ranging common bottlenose dolphins and harbour porpoises (Phocoena phocoena) under captive condition [3–8,19]. But the system requires the deployment of two electrodes at specific locations (i.e. two electrodes placed on either side of the heart); therefore, it is technically difficult to apply to free-ranging cetaceans without their prior capture or restraint. By contrast, we only had to attach one suction cup tag to a larger target area around the heart (figure 1).
To apply our single suction cup tags to free-ranging cetaceans under natural conditions, a VHF transmitter (e.g. MM100 series; Advanced telemetry systems, Inc.) and/or a satellite transmitter (e.g. Spot tags; Wildlife Computer, Inc.) are required to recover the tags, and this makes the tags larger than those used in this study. Larger tags might easily slide during deployment due to a relatively large drag. If the tag slides and moves out of the target range, the heartbeats cannot be detected. However, we expect several hours of heart rate measurements based on our past experience using similar-sized single suction cup tags. So far, the behavioural single suction cup tags that include multiple sensor loggers (but not ECG sensor), a VHF transmitter and/or satellite transmitter have been applied to free-ranging sperm whales (Physeter macrocephalus), long-finned pilot whales (Globicephala melas), killer whales (Orcinus orca), a northern bottlenose whale (Hyperoodon ampullatus) and humpback whales (electronic supplementary material, figure S1, e.g. [10,26–30], K.A. 2010, unpublished data). The size of the behavioural tags applied to these species was similar to or larger than those used in this study (float size, length: 19 cm, width: 4 cm, height: 4.5 cm, total mass approx. 310 g for pilot whales and killer whales; float size, length: 21 cm, width: 4 cm, height: 5 cm, total mass approx. 370–410 g for sperm whales). Based on our experience using these tags, the average deployment durations were approximately 10 h (13 ± 10 h, n = 16 trials, for sperm whales; 12 ± 13 h, n = 38 trials, for humpback whales; 11 ± 8 h, n = 3 trials, for pilot whales; overall range, 1.3–58 h). We visually confirmed that the tags were attached to the same place for several hours at least in these species. Further modification might be required depending on the target species (e.g. logger size, float size and suction cup materials). Especially for smaller species, smaller sized loggers and suction cups might be preferable. We note that the diameter of the suction cup in relation to the diameter of the electrode, fitted inside of the suction cup, was likely to be important for better attachment of the tag on whales (30 mm electrode versus 60 mm for silicon suction cup or 50 mm electrode versus 80 mm for rubber suction cup used in this study). Although tagging suction cup tags to specific locations using a launcher system [31] and short or long poles is still challenging, our all-in-one single suction cup device shows a high possibility of detecting the heart rate of free-ranging cetaceans without its prior capture or restraint.
(b) . Sinus arrhythmia associated with respiration at rest
We observed heart rate variability that was synchronous with respiration in all individuals. The instantaneous heart rate (fH) rapidly increased, corresponding to breathing; then, it gradually decreased and remained relatively constant during apnea until the next breath (figure 4). Such a variation in the heart rate associated with breathing has been reported in inactive cetaceans such as common bottlenose dolphins [13,14,20,21,32], harbour porpoises [22,33], common dolphin (Delphinus delphis) [32], short-finned pilot whales (Globicephala macrorhynchus), killer whales [18,20], beluga whales [16,20] and California grey whale (Eschrichtius robustus) [15]. For example, Ridgway [16] reported that the heart rate of common bottlenose dolphins increased just after inspiration to 70–100 bpm. The heart rate then dropped to 30–40 bpm and remained at this rate until the next breath, regardless of whether the breath-hold lasted for 20 s or 4 min. Ponganis & Kooyman [15] reported similar sinus arrhythmia patterns (apneic heart rate of 15–25 bpm and eupneic heart rate of 34–40 bpm) in young grey whale during resting.
In terrestrial mammals, variations in the heart rate associated with breathing are called as respiratory sinus arrhythmia (RSA) [34,35]. The RSA results in shortened R–R interval during inspiration and prolonged R–R interval during expiration [36]. The RSA has been shown to vary with both breathing frequency and tidal volume in humans [37,38]. Terrestrial mammals show continuous breathing, and heart rate oscillation with respiration is minimal [39]. For example, a breath causes changes in the heart rate by 5–15% in healthy young humans [37,40]. By contrast, cetaceans show apnea (breath-hold in which the blow-hole closes after inspiration) between respirations even if they are motionless and the blow-hole is out of water. Here, Risso's dolphins and the false killer whale showed a rapid increase in fH just after respirations to about twice that observed prior to the breath (figure 4). The fH then gradually decreased, returning to and stabilizing at pre-breath levels after about 20–50 s. Therefore, patterns of oscillation of heart rate in relation to respiration seemed to differ between these individuals and terrestrial mammals. Strictly speaking, it may not be physiologically equivalent to RSA in terrestrial mammals, although it is consistent that heart rates of both cetaceans and terrestrial mammals vary with respirations.
Cetaceans appear to ventilate their lungs within around 1 s [1,41–43], and they are capable of sustaining high expiratory and inspiratory flow rates (e.g. common bottlenose dolphins, more than 130 l s−1 and more than 30 l s−1 [44]) over a large percentage of their lung volume, owing to the reinforcement of the airways by cartilage and/or muscle that extends to the alveolar sac [1]. These structural features of cetacean lungs enable whales to exchange almost entire their lung gas (e.g. 88% of total lung capacity, pilot whales [42,43]) rapidly during brief periods when they surface through the air–water interface. Because it is known that the amplitude of oscillation of heart rate with respiration is related to inspired tidal volume [21], the quick large oscillation of the heart rate, observed in this study, might be associated with an exchange of a large gas volume in short periods. Further research is required to understand the mechanism.
(c) . Comparison of the mean heart rate of motionless whales at the surface with the resting heart rate expected from the body mass of terrestrial mammals
The mean fH during pronounced sinus arrhythmia patterns was 68–140% of the resting heart rate expected from the body mass of terrestrial mammals: 68–119% when fasting in the morning and 85%–140% when non-fasting during the day and evening, respectively. The slightly higher fH in the day and evening (tables 2 and 3; electronic supplementary material, figure S2) may have been caused by non-fasting or prior daytime activities, or both. The heart rate can be increased by tension even when the animal is inactive, due to the following: (i) less active parasympathetic nerves, (ii) dominant sympathetic nerves and (iii) humoral regulation (secretion of catecholamines) [45]. Our current data are limited and couldn't separate the effects of non-fasting/fasting from the time of day. Measuring the resting heart rate in more species at different times and conditions, and/or simultaneously measuring ventilation and oxygen consumption rates, will be useful to understand the resting heart rate of delphinids.
(d) . Conclusion
The majority of heart rate data in free-ranging cetaceans have been obtained only from captive common bottlenose dolphins and harbour porpoises [3–5,7,8]. A few recent studies have monitored the heart rate of cetaceans under natural conditions: lunge feeding of free-ranging single blue whale [9] and escape response of narwhals (Monodon monoceros) from net entanglement or stranding [46]. To the best of our knowledge, this is the first study to record the heart rate of Risso's dolphins when motionless at the surface and swimming. Our study shows that the all-in-one single suction cup device is feasible for field use with free-ranging cetaceans. Measuring heart rates will further our understanding of the diving physiology of free-ranging cetaceans under natural conditions.
Acknowledgements
We thank all staff members of the Taiji Whale Museum and Aquarium for their invaluable assistance in handling the animals and ECG and behavioural data recording. M. Sakai and F. Miyamoto also helped with ECG data collection. M. Kikuchi and K. Sato provided data loggers. T. Narazaki, K. Sato and R.W. Davis offered helpful suggestions.
Ethics
All ECG data were obtained from unrestrained dolphins using routine husbandry training. All procedures, animal husbandry and management were performed under the careful veterinary supervision at the aquarium. The experimental procedures were approved by the Animal Ethics Committee of the Atmosphere and Ocean Research Institute, University of Tokyo (P19-1) and complied with the recommendations of the Life Science Research Ethics and Safety of University of Tokyo for Experiments on Animals.
Data accessibility
The datasets supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
K.A. participated in the preparation of fieldwork, collected the field data, analysed the tag data, designed the study and drafted the manuscript; Y.W., D.I. and N.F. collected the field data and coordinated the study; K.Q.S participated in the preparation of the fieldwork, collected the field data and designed the study. All authors edited the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This study was supported by the education program of the Center for Ocean Literacy and Education in the University of Tokyo, in cooperation with the Japan Science Society and Grant-in-Aids from the Ministry of Education, Culture, Sports, Science, and Technology, Japan [grant nos. 17K12813 and 20K21362].
References
- 1.Davis RW. 2019. Marine mammals: adaptations for an aquatic life. Cham, Switzerland: Springer. [Google Scholar]
- 2.Ponganis PJ. 2015. Diving physiology of marine mammals and seabirds. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 3.Noren SR, Kendall T, Cuccurullo V, Williams TM. 2012. The dive response redefined: underwater behavior influences cardiac variability in freely diving dolphins. J. Exp. Biol. 215, 2735-2741. ( 10.1242/jeb.069583) [DOI] [PubMed] [Google Scholar]
- 4.Williams TM, Friedl WA, Haun JE. 1993. The physiology of bottlenose dolphins (Tursiops truncatus): heart rate, metabolic rate and plasma lactate concentration during exercise. J. Exp. Biol. 179, 31-46. ( 10.1242/jeb.179.1.31) [DOI] [PubMed] [Google Scholar]
- 5.Williams TM, et al. 2015. Exercise at depth alters bradycardia and incidence of cardiac anomalies in deep-diving marine mammals. Nat. Commun. 6, 1-9. ( 10.1038/ncomms7055) [DOI] [PubMed] [Google Scholar]
- 6.Noren SR, Cuccurullo V, Williams TM. 2004. The development of diving bradycardia in bottlenose dolphins (Tursiops truncatus). J. Comp. Physiol. B 174, 139-147. ( 10.1007/s00360-003-0398-9) [DOI] [PubMed] [Google Scholar]
- 7.Elmegaard SL, McDonald BI, Madsen PT. 2019. Drivers of the dive response in trained harbour porpoises (Phocoena phocoena). J. Exp. Biol. 222, jeb208637. ( 10.1242/jeb.208637) [DOI] [PubMed] [Google Scholar]
- 8.Elmegaard SL, Johnson M, Madsen PT, McDonald BI. 2016. Cognitive control of heart rate in diving harbor porpoises. Curr. Biol. 26, R1175-R1176. ( 10.1016/j.cub.2016.10.020) [DOI] [PubMed] [Google Scholar]
- 9.Goldbogen JA, et al. 2019. Extreme bradycardia and tachycardia in the world's largest animal. Proc. Natl Acad. Sci. USA 116, 25 329-25 332. ( 10.1073/pnas.1914273116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aoki K, Amano M, Mori K, Kourogi A, Kubodera T, Miyazaki N. 2012. Active hunting by deep-diving sperm whales: 3D dive profiles and manoeuvres during bursts of speed. Mar. Ecol. Prog. Ser. 444, 289-301. ( 10.3354/meps09371) [DOI] [Google Scholar]
- 11.Tobayama T, Kirihata T. 1999. An Attempt to estimate body weight of small cetaceans. IBI Rep. 9, 135-147. [Google Scholar]
- 12.Sakamoto KQ, Miyayama M, Kinoshita C, Fukuoka T, Ishihara T, Sato K. 2021. A non-invasive system to measure heart rate in hard-shelled sea turtles: potential for field applications. Phil. Trans. R. Soc. B 376, 20200222. ( 10.1098/rstb.2020.0222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blawas AM, Nowacek DP, Allen AS, Rocho-Levine J, Fahlman A. 2021. Respiratory sinus arrhythmia and submersion bradycardia in bottlenose dolphins (Tursiops truncatus). J. Exp. Biol. 224, jeb234096. ( 10.1242/jeb.234096) [DOI] [PubMed] [Google Scholar]
- 14.Irving L, Scholander PF, Grinnell SW. 1941. The respiration of the porpoise, Tursiops truncatus. J. Cell. Comp. Physiol. 17, 145-168. ( 10.1002/jcp.1030170203) [DOI] [Google Scholar]
- 15.Ponganis PJ, Kooyman GL. 1999. Heart rate and electrocardiogram characteristics of a young California gray whale (Eschrictius robustus). Mar. Mam. Sci. 15, 1198-1207. ( 10.1111/j.1748-7692.1999.tb00885.x) [DOI] [Google Scholar]
- 16.Ridgway SH. 1972. Mammals of the sea—biology and medicine. Springfield, IL: Charles C Thomas. [Google Scholar]
- 17.Kanwisher JW, Sundnes G. 1965. Physiology of a small cetacean. Hvalradets Skrifter 48, 45-53. [Google Scholar]
- 18.Spencer MP, Gornall TA, Poulter TC. 1967. Respiratory and cardiac activity of killer whales. J. Appl. Physiol. 22, 974-981. ( 10.1152/jappl.1967.22.5.974) [DOI] [PubMed] [Google Scholar]
- 19.Bickett NJ, Tift MS, Leger JS, Ponganis PJ.. 2019. Heart rates, heart rate profiles, and electrocardiograms in three killer whales, a beluga, and a pilot whale: an exploratory investigation . Mar. Mamm. Sci. 35, 1112.–. ( 10.1111/mms.12578) [DOI] [Google Scholar]
- 20.Fahlman A, Miedler S, Marti-Bonmati L, Ferrero Fernandez D, Muñoz Caballero P, Arenarez J, Rocho-Levine J, Robeck T, Blawas A. 2020. Cardiorespiratory coupling in cetaceans; a physiological strategy to improve gas exchange? J. Exp. Biol. 223, jeb.226365. ( 10.1242/jeb.226365) [DOI] [PubMed] [Google Scholar]
- 21.Cauture F, Sterba-Boatwright B, Rocho-Levine J, Harms C, Miedler S, Fahlman A. 2019. Using respiratory sinus arrhythmia to estimate inspired tidal volume in the bottlenose dolphin (Tursiops truncatus). Front. Physiol. 10, 128. ( 10.3389/fphys.2019.00128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kastelein RA, Meijler FL.. 1989. Respiratory arrhythmia in the hearts of harbour porpoises (Phocoena phocoena). Aquat. Mamm. 15, 57-63. ( 10.1578/am.39.4.2013.389) [DOI] [Google Scholar]
- 23.Stahl WR. 1967. Scaling of respiratory variables in mammals. J. Appl. Physiol. 22, 453-460. ( 10.1152/jappl.1967.22.3.453) [DOI] [PubMed] [Google Scholar]
- 24.Meijler FL, Wittkampf FH, Brennen MKR, Baker V, Wassenar C, Baken EE. 1992. Electrocardiogram of the humpback whale (Megaptera novaeangliae), with specific reference to atrioventricular transmission and ventricular excitation. J. Am. Coll. Cardiol. 20, 475-479. ( 10.1016/0735-1097(92)90120-C) [DOI] [PubMed] [Google Scholar]
- 25.King RL, Jenks JL, White PD. 1953. The electrocardiogram of a beluga whale. Circulation 8, 387-393. ( 10.1161/01.CIR.8.3.387) [DOI] [PubMed] [Google Scholar]
- 26.Aoki K, Sato K, Isojunno S, Narazaki T, Miller PJO. 2017. High diving metabolic rate indicated by high-speed transit to depth in negatively buoyant long-finned pilot whales. J. Exp. Biol. 220, 3802-3811. ( 10.1242/jeb.158287) [DOI] [PubMed] [Google Scholar]
- 27.Aoki K, Sakai M, Miller PJO, Visser F, Sato K. 2013. Body contact and synchronous diving in long-finned pilot whales. Behav. Proc. 99, 12-20. ( 10.1016/j.beproc.2013.06.002) [DOI] [PubMed] [Google Scholar]
- 28.Miller PJO, Narazaki T, Isojunno S, Aoki K, Smout S, Sato K. 2016. Body density and diving gas volume of the northern bottlenose whale (Hyperoodon ampullatus). J. Exp. Biol. 219, 2458-2468. ( 10.1242/jeb.137349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narazaki T, et al. 2018. Body density of humpback whales (Megaptera novaengliae) in feeding aggregations estimated from hydrodynamic gliding performance. PLoS ONE 13, e0200287. ( 10.1371/journal.pone.0200287) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aoki K, et al. 2021. Aerial photogrammetry and tag-derived tissue density reveal patterns of lipid-store body condition of humpback whales on their feeding grounds. Proc. R Soc. B 288, 20202307. ( 10.1098/rspb.2020.2307) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kleivane L. 1998. A new pneumatic launching device ARTS (aerial rocket transmitter system) especially developed and designed to improve tagging and instrumentation of Baleen whales. Bodø, Norway: Restech A/S. [Google Scholar]
- 32.Kanwisher JW, Ridgway SH. 1983. The physiological ecology of whales and porpoises. Sci. Am. 248, 110-120. ( 10.1038/scientificamerican0683-110) [DOI] [Google Scholar]
- 33.Reed JZ, Chambers C, Hunter CJ, Lockyer C, Kastelein R, Fedak MA, Boutilier RG. 2000. Gas exchange and heart rate in the harbour porpoise, Phocoena phocoena. J. Comp. Physiol. B 170, 1-10. ( 10.1007/s003600050001) [DOI] [PubMed] [Google Scholar]
- 34.Hayano J, Yasuma F. 2003. Hypothesis: respiratory sinus arrhythmia is an intrinsic resting function of cardiopulmonary system. Cardiovasc. Res. 58, 1-9. ( 10.1016/S0008-6363(02)00851-9) [DOI] [PubMed] [Google Scholar]
- 35.Katona PG, Jih F. 1975. Respiratory sinus arrhythmia: noninvasive measure of parasympathetic cardiac control. J. Appl. Physiol. 39, 801-805. ( 10.1152/jappl.1975.39.5.801) [DOI] [PubMed] [Google Scholar]
- 36.Yasuma F, Hayano J. 2004. Respiratory sinus arrhythmia: why does the heartbeat synchronize with respiratory rhythm? Chest 125, 683-690. ( 10.1378/chest.125.2.683) [DOI] [PubMed] [Google Scholar]
- 37.Hirsch JA, Bishop B. 1981. Respiratory sinus arrhythmia in humans: how breathing pattern modulates heart rate. Am. J. Physiol. 241, H620-H629. ( 10.1152/ajpheart.1981.241.4.H620) [DOI] [PubMed] [Google Scholar]
- 38.Guillén-Mandujano A, Carrasco-Sosa S. 2014. Additive effect of simultaneously varying respiratory frequency and tidal volume on respiratory sinus arrhythmia. Auton. Neurosci. 186, 69-76. ( 10.1016/j.autneu.2014.08.003) [DOI] [PubMed] [Google Scholar]
- 39.Piccione G, Giudice E, Giannetto C, Mortola JP. 2019. The magnitude of respiratory sinus arrhythmia of a large mammal (the horse) is like that of humans. Respir. Physiol. Neurobiol. 259, 170-172. ( 10.1016/j.resp.2018.09.006) [DOI] [PubMed] [Google Scholar]
- 40.Sakakibara M, Takeuchi S, Hayano J. 1994. Effect of relaxation training on cardiac parasympathetic tone. Psychophysiology 31, 223-228. ( 10.1111/j.1469-8986.1994.tb02210.x) [DOI] [PubMed] [Google Scholar]
- 41.Kooyman GL, Cornell LH. 1981. Flow properties of expiration and inspiration in a trained bottlenose porpoise. Physiol. Zool. 54, 55-61. ( 10.1086/physzool.54.1.30155804) [DOI] [Google Scholar]
- 42.Olsen CR, Elsner R, Hale FC, Kenney DW. 1969. ‘Blow’ of the pilot whale. Science 163, 953-955. ( 10.1126/science.163.3870.953) [DOI] [PubMed] [Google Scholar]
- 43.Olsen CR, Hale FC, Elsner R. 1969. Mechanics of ventilation in the pilot whale. Respir. Physiol. 7, 137-149. ( 10.1016/0034-5687(69)90001-2) [DOI] [PubMed] [Google Scholar]
- 44.Fahlman A, Loring SH, Levine G, Rocho-Levine J, Austin T, Brodsky M. 2015. Lung mechanics and pulmonary function testing in cetaceans. J. Exp. Biol. 218, 2030-2038. ( 10.1242/jeb.119149) [DOI] [PubMed] [Google Scholar]
- 45.Erickson HH, Detweiler DK. 2004. Regulation of the heart. In Dukes' physiology of domestic animals (ed. Swenson MJ), pp. 261-274, 12th edn. Ithaca, NY: Comstock. [Google Scholar]
- 46.Williams TM, Blackwell SB, Richter B, Sinding MS, Heide-Jørgensen MP. 2017. Paradoxical escape responses by narwhals (Monodon monoceros). Science 358, 1328-1331. ( 10.1126/science.aao2740) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting this article have been uploaded as part of the electronic supplementary material.