Abstract
Introduction
Malnutrition is a serious problem that causes high morbidity and mortality among cancer patients. There was no sufficient empirical evidence on the prevalence of malnutrition and associated factors among adult cancer hospitalized patients in Ethiopia.
Objective
This study aimed to assess the prevalence and risk factors of malnutrition among adult cancer patients receiving chemotherapy in cancer center, Ethiopia 2019.
Methods
A cross-sectional study was conducted among a sample of 281 patients selected using systematic sampling among cancer patients receiving chemotherapy. Data were collected by patient interviews and chart reviews using a structured questioner adapted from a subjective global assessment tool. Data were analyzed by SPSS version 24.0. Descriptive statistics such as frequency distribution, mean, median, and standard deviation were used to describe characteristics. Bivariate and multivariable analyses using logistic regression models were used.
Result
Of the 281 participating patients, 58.2% had malnutrition, which was higher among females (51.6%) than males (48.4%). The mean body mass index was 20.24 ± 3.6. Of all, 41.9% had moderate weight loss, 21.1% had severe weight loss and 52.3% had weight loss in the past two weeks. Stage four cancer (AOR = 7.2, 95% CI: 1.3–38.5), loss of appetite (AOR = 4.5, 95% CI; 1.5–17.2) and diarrhea (AOR = 7.8, 95% CI: 2.95–20.5) were significantly associated with malnutrition.
Conclusion
The prevalence of malnutrition among cancer patients who receive chemotherapy was high. Stage of cancer, appetite loss, and presence of diarrhea was found to be significant factors for malnutrition.
Keywords: Cancer, Malnutrition, Prevalence, Risk factors, Tikur Anbessa Specialized Hospital, Ethiopia
Cancer; Malnutrition; Prevalence; Risk factors; Tikur Anbessa Specialized Hospital; Ethiopia.
1. Introduction
Globally, cancer is the second leading cause of mortality worldwide, with approximately 17.0 million new cancer cases and 9.6 million deaths from cancer in 2018. Approximately 70% of cancer-related deaths occur in low- and middle-income countries due to the growing incidence of cancer in both developed and developing countries. It is also estimated that around 20% of cancer deaths in cancer patients occurred due to malnutrition and its complications [1]. Malnutrition is a serious problem in cancer patients, and its incidence ranges from 40-80%, which affects approximately 15–20% of cancer patients at diagnosis and up to 80–90% of patients with advanced-stage disease. Hence, low rates of response to treatment have been shown among those patients who are malnourished [2]. Evidence in Latin America showed that the burden of malnutrition among cancer patients ranges from 50% to 80% [3], whereas in Portugal burden of malnutrition ranges from 30 to 60% [4]. The burden of malnutrition is mainly related to tumor stage and site [5].
Chemotherapy is associated with several side effects that play an important role in decreased food intake, nutritional loss, energy expenditure changes, and weight loss, mainly lean body mass [6, 7].
Malnutrition usually occurs as a result of the interaction between the host and the disease, which results in decreased nutrient intake, loss of appetite, changes in taste, and food aversion, fear, depression, and anxiety [8]. Malnutrition in cancer patients has been observed to negatively impact patients’ response to therapy, increase the incidence of treatment-related side effects, interrupt serial treatment regimens, extend hospital stay, impair performance status, immune function, and quality of life, and ultimately affects survival status. Moreover, cancer-related malnutrition was also associated with significant health-care-related costs [9].
Although people with cancer are at high risk of malnutrition, therapy (surgery, chemotherapy, and radiotherapy) is started in Ethiopia without knowing their nutritional status. Since malnutrition in individuals with cancer affects the treatment responses and survival of patients, early assessment of nutritional status is important for nutritional therapy and thereby increases the survival of patients. However, there was no sufficient empirical evidence on the prevalence of malnutrition and associated factors among adult cancer hospitalized patients in Ethiopia. Thus, this study aimed to assess the prevalence and risk factors of malnutrition among adult cancer patients receiving chemotherapy in a cancer center, Ethiopia.
2. Methods and materials
2.1. Study design, study area and period
An institution-based cross-sectional study was conducted at the Tikur Anbessa Specialized Hospital oncology unit in Addis Ababa Ethiopia. The Tikur Anbessa Specialized Hospital (TASH) is a tertiary teaching hospital with 700 beds and provides service for approximately 370,000 to 400,000 patients per year. The oncology center at the hospital is the only referral center in the country. The oncology unit is giving service for more than 60,000 cancer patients annually and has an outpatient, inpatient (33 beds), radiotherapy, and chemotherapy and surgery care service. The study was conducted from February to March at the oncology unit of TASH, College of Health Sciences, and Addis Ababa University, Ethiopia [10].
2.2. Population
The study population was all adults diagnosed with cancer and treated with chemotherapy at Tikur Anbessa Specialized Referral Hospital in the time interval of the study period were eligible. Patients aged 18 years or older, competent subjects who can give written informed consent and all adults inpatients and outpatients taking chemotherapy treatment above 3 cycles were included in the study.
2.3. Sampling procedure and sample size determination
A total of 281 population sample sizes were determined using a single proportion formula. From the oncology unit of TASH, patients who fulfilled the criteria were identified from their charts each day. The sample size was allocated proportionally to the size of inpatient and outpatient cancer treatment attendants using the previous month's rate of flow of the cases. According to the one-year record of adults receiving chemotherapy treatment for cancer, 9000 cases were seen in the oncology unit at Tikur Anbessa Specialized Hospital [10]. Since the duration of the study was four weeks, the flow within the study period was 750 cases that came for treatment during the data collection period. A systematic random sampling technique was used, and every 2 study participants were selected in the study.
The primary outcome of interest was the prevalence of malnutrition, whereas age, sex, residence, marital status, education, occupation and household monthly income, body mass index, type of cancer, stage of cancer, type of treatment, appetite status, mode of feeding and mobility, alcohol consumption, smoking and chat chewing were explanatory variables.
Malnutrition is characterized by the presence of two or more of the following characteristics: insufficient energy intake, weight loss, loss of muscle mass, and loss of subcutaneous fat, localized or generalized fluid accumulation, or decreased functional status. SGA Score of nutritional status according to the sum of points assigned to each item, patients have initially classified in Well-nourished: < 17 points. Malnourished (moderate and severe): ≥17 points.
2.4. Data collection procedures
Data were collected by using a pretested global assessment tool. The tool adopted from similar studies from India [11] was prepared in English, translated to Amharic, and retranslated back to English to check for consistency of meaning. The training was given for two female and two male nurses who were oncology nurses for one day. The training focused mainly on the aim of the study, on each part of questionnaires, about consent, the right to participate or not, the right to draw at any time, confidentiality, and how to approach. Data were collected from two sources. The primary data source was responses of sampled respondents who were eligible for interviews and from their medical records. The SGA tool was adopted from India modifications on gastrointestinal surgical oncology malnutrition screening cancer patients. Pretesting of the questionnaire was undertaken in 5% of the sample size in other sites before the actual data collection took place, and corrections on the instruments were made accordingly. Data were checked daily for completeness by the supervisors and the principal investigator. The overall data collection was supervised by the principal investigator.
2.5. Data quality assurance
To ensure the quality of the data, the following measures were undertaken. Pretesting of the questionnaire was undertaken in 5% of the sample size in amstegna police tabiya cancer center before the actual data collection took place and corrections on the instruments were made accordingly. The training was given to all supervisors and data collectors. Data were checked for completeness, clarity, and consistency by the supervisors and the principal investigator daily. Finally, the data collectors collected the filled questionnaires and supervisors cross-checked the completeness of the questionnaire. The overall data collection process was monitored by the principal investigator.
2.6. Data processing and analysis
The collected data were checked at the end of each data collection day for completeness. Then, the collected data were coded, entered, and cleaned using Epi data version 3.1 software (Jens M. Lauritsen & Michael Bruus, Odense, Denmark). Then, it was exported to SPSS version 24.0 for analysis (Norman H. Nie, C. Hadlai (Tex) Hull and Dale H. Bent, Stanford, England). During the process of analysis, the frequency distribution and percentage of variables were computed to describe and summarize the basic socio-demographic characteristics of the respondents. Bivariate and multivariable analysis using a logistic regression model was used to determine the association between predictor variables and the dependent variable (malnutrition). The first bivariate relationship between each independent variable and outcome variable was investigated using binary logistic regression analysis. The variables that were fitted on bivariate analysis with a p-value of <0.25 were used in multivariate logistic regression. Multivariable logistic regression analyses were used to minimize the effect of confounding variables and to identify the significant factors of malnutrition. Adjusted odds ratios with 95% confidence intervals and p-values<0.05 were used to determine the strength of the association between dependent and independent variables. The result was illustrated in the form of text, tables, pie charts, and graphs to give a glance at the variables.
2.7. Ethics approval and consent to participation
Ethical clearance was obtained from Addis Ababa University, School of Nursing and Midwifery research ethical committee, and ethical approval was performed following the declaration of Helsinki. A letter was written from the School of Nursing and midwifery to TASH. Informed verbal consent was obtained from each respondent after providing sufficient information on the purpose and procedure of the study and the right of the participant. Confidentiality of the information was kept throughout the study, and the data were used only for the proposed study. To maintain confidentiality, all collected data were coded and locked in a separate room before entering the computer.
3. Results
3.1. Socio-demographic characteristics of adult cancer patients
A total of 281 respondents participated in the study with a 96% response rate. Of 281 respondents, 145 (51.6%) were females. The mean age of the participants was 44.51 ± 13.45 Of the total cancer patients, 257 (91.1%) were <65, whereas 25 (8.9%) were > 65 years of age. Of the participants, 69 (24.6%) had no formal education, and 50 (17.8%) attended higher education. Regarding region, 91 (32.4%) were from Oromo, 71 (25.3%) from Amhara, 43 (15.3%) from Tigray, 39 (13.9%) from South Nation and Nationalities people of Representatives (SNNPR) and 23 (8.2%) from Addis Ababa. Approximately 162 (57.7%) of the participants were urban residents. Approximately 167 (59.4%) of the respondents were married (Table 1).
Table 1.
Socio-demographic characteristics among adult cancer patients receiving chemotherapy treatment in the cancer center, Ethiopia (n = 281).
| Characteristics | Category | n (%) |
|---|---|---|
| Age | <65 | 257(91.5%) |
| ≥65 | 24(8.5%) | |
| Mean(±SD) | 44.51 ± 13.45 | |
| Range | 19–83 | |
| Sex | Male | 136(48.4%) |
| Female | 145(51.6%) | |
| Residence | Urban | 162(57.7%) |
| Rural | 119(42.3%) | |
| Region | Oromo | 91(32.4%) |
| Amhara | 71(25.3%) | |
| Tigray | 43(15.3%) | |
| SNNPR | 39(13.9%) | |
| Addis Ababa | 23(8.2%) | |
| Others | 14(4.98.0%) | |
| Religion | Orthodox | 171(60.8%) |
| Muslim | 59(21.0%) | |
| Protestant | 38(13.5%) | |
| Catholic | 10(3.6%) | |
| Others | 3(1.1%) | |
| Marital status | Married | 167(59.4%) |
| Single | 66(23.5%) | |
| Divorced | 28(10.0%) | |
| Widowed | 20(7.1%) | |
| Occupation | Farmer | 67(23.8%) |
| Merchant | 59(20.1%) | |
| Government employee | 57(20.2%) | |
| Self employee | 58(20.6%) | |
| Student | 24(8.5%) | |
| Other | 14(4.9%) | |
| Income | ≤500 birr | 80(28.5%) |
| 500-1600birr | 65(23.1%) | |
| >1600 | 136(48.4%) | |
| Education | No education | 69(24.6%) |
| Primary education | 83(29.5%) | |
| Secondary education | 79(28.1) | |
| higher education | 50(17.8%) |
SNNPR: South Nation and Nationalities people of Representatives; SD: Standard deviation.
3.2. Lifestyle: Alcohol consumption, cigarette smoking, and chat chewing among adult cancer patients
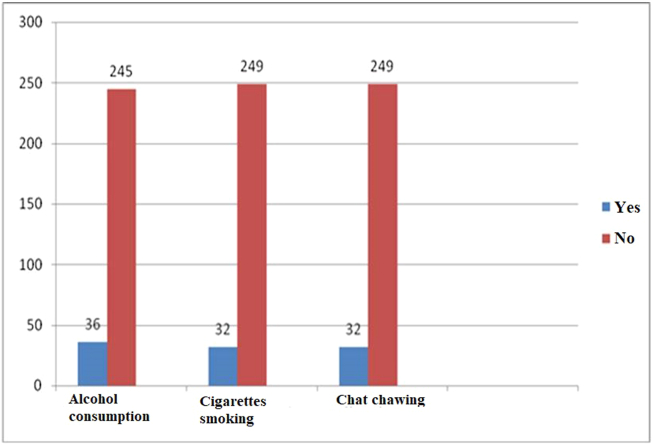
Overall, 36 (12.8%) had a history of alcohol consumption, 32 (11.3%) had a history of smoking, and 32 (11.3%) had a history of chat chewing (Figure 1).
Figure 1.
Alcohol consumption, cigarette smoking and chat chewing among adult cancer patients receiving chemotherapy treatment in Tikur Anbessa Specialized Hospital Addis Ababa, Ethiopia, 2019 (n = 281).
3.3. Disease- and treatment-related factors among adult cancer patients
Among 281 study participants, about 14.5% had pre-existing comorbidities and 14.5% had a family history of cancer. Concerning the type of cancer, 28.8% had breast cancer, 22.8% had colorectal cancer, 15.3% had lung cancer, 14.5% had gastric cancer, 14.0% had nasopharyngeal cancer, and others (4.6%). The proportion of respondents diagnosed with cancer at stage I was 4.6% and stage II was 26.4%; stage III was 34.9%, stage IV was 28.1% and 6.0% was unknown. Concerning the treatment type, 73.0% of the respondents were on chemotherapy, 17.4% chemotherapy and surgery and 9.3% received both chemotherapy and radiotherapy treatment (Table 2).
Table 2.
Disease- and treatment-related factors among adult cancer patients receiving chemotherapy treatment in the cancer center, Ethiopia (n = 281).
| Characteristics | Category | n (%) |
|---|---|---|
| Family history of cancer | Yes | 41 (14.5%) |
| No | 240 (85.1%) | |
| Comorbidity | Yes | 41 (14.5%) |
| No | 240 (85.1%) | |
| Types of cancer | Breast cancer | 81 (28.8%) |
| Colorectal | 64 (22.8%) | |
| Lung | 43 (15.3%) | |
| Gastric | 41 (14.5%) | |
| Nasopharyngeal | 39 (14.0%) | |
| Others | 13 (4.6%) | |
| Stages of cancer | Stage one | 13 (4.6%) |
| Stage two | 74 (26.4%) | |
| Stage three | 98 (34.9%) | |
| Stage four | 79 (28.1%) | |
| Unknown | 17 (6.0%) | |
| Type of treatment | Chemotherapy | 206 (73%) |
| Chemotherapy surgery | 49 (17.4%) | |
| chemo radiation | 26 (9.3%) | |
| Cycle | Third | 72 (25.6%) |
| Fourth | 81 (28.8%) | |
| Fifth | 60 (21.4%) | |
| Sixth | 68 (24.2%) |
3.4. Feeding habit and nutritional status among adult cancer patients
Regarding feeding habits, more than two-thirds (73.3%) of respondents consumed semisolid food, 13.5% consumed a liquid diet, 11.1% consumed a solid diet and 2.1% were on tube feeding. Nearly half of the respondents (49.5%) took food regularly. A total of 16.4% of respondents had problems during chewing/eating, 21.7% had pain during swallowing, 79.7% had a loss of appetite, 75.1% felt nausea, 59.8% had vomiting and 27.1% had diarrhea. The nutritional status of respondents shows that the mean BMI was 20.24 ± 3.6 SD, and 38.1% were underweight (Supple 1).
The subjective global nutritional assessment findings showed that 28.9% of respondents had decreased food intake, 12.8% were unable to eat, 41.9% had moderate weight loss, 36.0% had severe weight loss and 52.3% had weight loss in the past two weeks. Of all, 42.7% had mild to moderate stress, and 41.6% had severe stress. With regard to functional status, 41.6% had mild to moderate loss of strength, and 41.0% had severe loss of function and strength. In addition, 47.3% of respondents had mild to moderate loss of subcutaneous fat in all areas, and 23.9% had severe loss of subcutaneous fat in most areas. Furthermore, 40.6% of respondents had mild to moderate edema, 3.2% severe edema, 21.7% mild to moderate ascites and 2.5% had severe ascites (supple 2).
3.5. Prevalence of malnutrition among adult cancer patients receiving chemotherapy
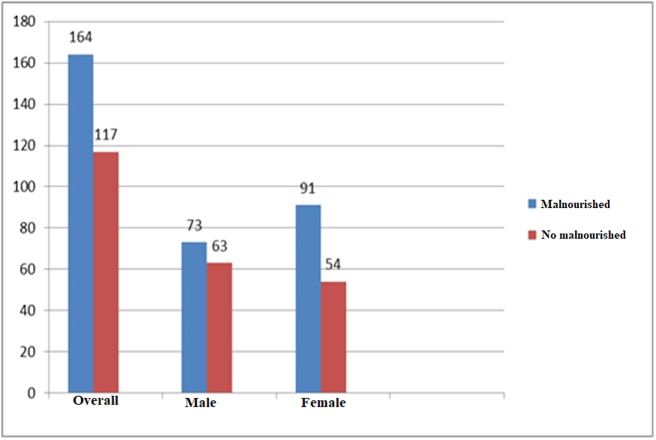
The prevalence of malnutrition was 58.4%. The prevalence of malnutrition was higher among female patients (62.8%) than among males (53.7%) (Figure 2).
Figure 2.
Prevalence of malnutrition among adult cancer patients receiving chemotherapy treatment in Tikur Anbessa Specialized Hospital Addis Ababa, Ethiopia, 2019 (n = 281).
3.6. Disease- and treatment-related factors and nutritional status among adult cancer patients
Of all types of cancer, malnutrition was higher among lung cancer patients (64.4%), followed by breast cancer (56.8%), colorectal cancer (56.2%), gastric cancer (46.3%) and Nasal Pharyngeal Cancer (NPC) (46.3%). From the stage of cancer, malnutrition was higher among stage four (74.7%) and stage three (58.2%) patients. Regarding the type of treatment, malnutrition was higher among patients on chemoradiation (73.1%), followed by chemosurgery (59.2%) and then chemotherapy treatment (56.3%). Malnutrition was higher in patients in the fifth (71.7%) and fourth (66.7%) cycles of treatment (Table 3).
Table 3.
Disease- and treatment-related factors and nutritional status among adult cancer patients receiving chemotherapy treatment in the cancer center, Ethiopia (n = 281).
| Variables | Category | Malnourished | Not malnourished |
|---|---|---|---|
| Type of cancer | Breast Colorectal Lung Gastric NPC |
46 (56.8%) 36 (56.2%) 29 (64.4%) 19 (46.3%) 29 (74.4%) |
36 (43.2%) 28 (43.8%) 16 (35.6%) 22 (53.7%) 10 (25.6%) |
| Stage of cancer | Stage one Stage two Stage three Stage four |
5 (38.5%) 35 (47.3%) 57 (58.2%) 59 (74.7%) |
8 (61.5%) 39 (52.7%) 41 (41.8%) 20 (25.3%) |
| Type of treatment | Chemotherapy Chemo radiation Chemo and surgery |
116 (56.3%) 19 (73.1%) 29 (59.2%) |
90 (43.7%) 7 (26.9%) 20 (40.8&) |
| Cycle of treatment | Third cycle Fourth cycle Fifth cycle Sixth cycle |
34 (47.2%) 54 (66.7%) 43 (71.7%) 33 (48.5%) |
38 (52.8%) 27 (33.3%) 17 (28.3%) 35 (51.5%) |
NPC: Nasal Pharyngeal Cancer.
3.7. Bivariate and multivariable analysis of risk factors associated with malnutrition
Bivariate and multivariate logistic regression analyses were performed to analyze factors associated with malnutrition. Age, level of education, income, stage four cancer, loss of appetite, BMI, a problem during chewing, pain during swallowing, diarrhea, vomiting, and nausea were fitted in bivariate analysis.
In the multivariable logistic regression model, variables such as stage of cancer, loss of appetite, and presence of diarrhea were significantly associated at p-value < 0.05. Accordingly, stage four cancer patients are 7.2 times more likely to have malnutrition than those with stage one cancer (AOR = 7.2, 95% CI 1.3–38.51). Also, those with loss of appetite were 4.5 times more likely to have malnutrition than those with no appetite loss (AOR = 4.5, 95% CI 1.5–17.2). Furthermore, adult cancer patients with diarrhea were 7.83 times more likely to have malnutrition than those with no diarrhea (AOR = 7.8, 95% CI 2.95–20.5) (Table 4).
Table 4.
Bivariate and multivariate analysis of patient characteristics with malnutrition among cancer patients in Tikur Anbessa Specialized Hospital cancer center, Ethiopia (n = 281).
| Variable | Malnutrition |
COR (95% CI) | AOR (CI = 95%) | p-value | |
|---|---|---|---|---|---|
| Yes | No | ||||
| Age>65 | 9 (62.5%) | 15 (37.5%) | 2.5 (1.07–6) | 2.5 (.65–9.5) | 0.2 |
| Income (≤500 birr) | 49 (61.3%) | 31 (38.8%) | 2.6 (1.4–4.9) | 1.7 (0.49–6.03) | 0.4 |
| no education | 43 (62.3%) | 26 (37.7%) | 2.9 (1.4–6.3) | 0.9 (0.18–4.6) | 0.92 |
| Primary education | 58 (69.9%) | 25 (30.1%) | 4.1 (1.10–6.8) | 3.6 (0.19–15.95) | 0.06 |
| Secondary education | 45 (57. %) | 34 (43. %) | 2.4 (1.1–4.9) | 1.7 (0.53–5.4) | 0.4 |
| Stage four | 59 (74.7%) | 20 (25.3%) | 3.3 (1.1–9.8) | 7.2 (1.3–38.5) | 0.002 |
| BMI <18.5 kg/m2 | 92 (86. %) | 15 (14%) | 36.8 (4.1–33.6) | 2.5 (0.12–49.8) | 0.55 |
| Problem during chewing (yes) | 38 (8.26%) | 8 (17.4%) | 4.11 (1.9–9.2) | 1.3 (0.35–4.7) | 0.17 |
| Paine during swallowing (yes) | 52 (85.2%) | 9 (14.8%) | 5.6 (2.6–11.6) | 0.79 (0.23–2.8) | 0.75 |
| Loss of appetite (yes) | 156 (69.6%) | 68 (30.4%) | 14.1 (6.3–31.3) | 4.5 (1.5–17.2) | 0.011 |
| Nausea (yes) | 149 (70.6%) | 62 (29.4%) | 8.8 (4.6–16.8) | 1.1 (0.35–3.4) | 0.88 |
| Vomiting (yes) | 131 (78. %) | 37 (22. %) | 8.6 (4.10–14.8) | 1.6 (0.64–3.95) | 0.32 |
| Diarrhea (yes) | 137 (82.5%) | 29 (17.5%) | 2.5 (1.4–4.5) | 7.8 (2.10–20.5) | P < 0.001 |
| Weight loss last 3 months (yes) | 139 (71.3%) | 56 (28.7%) | 6.06 (3.5–10.6) | 1.5 (0.68–3.97) | 0.25 |
| Decrease feeding habit last weeks | 56 (73.7%) | 20 (26.3%) | 15.4 (8.6–27.7) | 0.99 (0.39–2.6) | 0.99 |
| Fluid<3 litters | 61 (74.4%) | 21 (25.6%) | 4.5 (2.2–9.2) | 3.1 (0.91–10.3) | 0.07 |
| Fluid 3-5 litters | 78 (57.8%) | 57 (42.2%) | 2.1 (1.2–3.9) | 1.8 (0.64–4.98) | 0.26 |
AOR: Adjusted Odds Ratio; BMI: Body Mass Index; COR: Crude Odd Ratio.
4. Discussion
The main purpose of the study was to assess the prevalence of malnutrition and its risk factors among adult cancer patients receiving chemotherapy treatment at Addis Ababa, Tikur Anbessa Specialized Hospital. The overall prevalence of malnutrition among adult cancer patients receiving chemotherapy was 58.4%. This finding was in line with study done in Tehran [51.1%] [12], Korea [61.0%] [13], Malaysia [43.5%], and [61.9%] [14] using the Subjective Global Assessment (SGA) and the Malnutrition Screening Tool (MST) respectively. This is lower than a study done in India [15], Brazil [71.1%] [16], [77.0%] [17]. However, the finding of this study was higher than the study done in Spain [23.7%] [8], French [30.9%] [18], Japan [19.0%] [19], and Kenya [31.0%] [20].
This discrepancy might be due to differences in socio-demographic characteristics, study population, economic status, impaired immune function, and expanded health service provision. The higher prevalence in the current study might due to the presence of side effects related to chemotherapy. Such as oral mucositis, intraoral mucositis, intraoral infection, dry mouth, salivary gland inflammation, mucosal bleeding, and intraoral hemorrhage interfere with the nutritional intake and immune response. Moreover, most of the clients in the current study were gastrointestinal cancer patients; this could potentially interfere with food intake and metabolism. Hence, the site and origin of the tumor determine the nutritional status [21]. The current study was also done among cancer patients who received chemotherapy. This made the clients experience complications and side effects.
From the current finding, the prevalence of malnutrition in males was 73%, which is higher than that in the Korean study (60.5%) for hospitalized male patients [13] and Tehran male patients (54%) [12]. The current finding showed that the prevalence in female patients was 91%, which was higher than that in the Tehran study, which was approximately 46% [12]. This perhaps due to the difference in different treatment interventions to improve the health status of clients and economic constraints to get adequate health care. The exact gender difference is uncertain. But it might be due to immunologic differences, lifestyle in food intake, and sensitivity to chemotherapeutic intervention.
From the current results, the prevalence of malnutrition was higher in the advanced stage of cancer (74.7%), which is higher than that in the Malaysia study (56.9%) [14] and Korea (55.6%) [22]. Moreover, it was consistent with a study done in Kenya that showed that the prevalence of malnutrition was 66.9%. This difference may be due to the economic status of the participants and the quality of treatment. The stage of cancer was significantly associated with malnutrition. Stage four cancer patients were 7.2 times more likely to have malnutrition than those with stage one (AOR = 7.2, 95% CI 1.3–38.51), which was supported by a study performed in Malaysia [18]. This may be due to the poor quality of care cancer-related malnutrition or pathophysiology of cancer cells, which require a high-calorie intake of food in advanced stages.
Besides, those with loss of appetite were 4.5 times more likely to have malnutrition than those with no appetite loss (AOR = 4.5, 95% CI 1.5–17.2). This study is in line with the study conducted by Kumar on Cancer Patients [13]. A study from Italy showed that loss of appetite was the major cause of malnutrition in cancer patients [23]. The current study shows that loss of appetite (79.7%) is a 4.5-fold risk factor for malnutrition in cancer patients compared with good appetite patients, which is supported by an Indian study in which loss of appetite is the major cause of malnutrition in cancer patients (38.60%) [24]. This may be due to cancer therapy affecting nutritional status through alterations in the metabolic system, changes in food tests, and reductions in food intake resulting in damage to normal tissues.
Furthermore, adult cancer patients with the presence of diarrhea were 7.8 times more likely to have malnutrition than those with no diarrhea (AOR = 7.8, 95% CI 2.95–20.5). A study performed in the Republic of Korea showed that the presence of diarrhea in cancer patients was the major cause of malnutrition in cancer patients [7], which is consistent with the current study in which diarrhea was significantly associated with malnutrition in cancer patients. This may be due to the side effects of chemotherapy or the disease itself.
The current study showed that about 35.8% exhibited severe weight loss, moderate weight loss (42.2%) and loss of muscle mass was 61.5%. A similar study done in Brazil showed that cancer clients presented with weight loss and loss of appetite was 40.0%and 41.7% respectively [17]. The co-existence of these conditions could cause not only severe malnutrition but also cause poorer quality of life and worsen physical functioning. Even if the accurate estimates of cachexia were limited. The presence of these conditions weight loss with muscle mass (Cachexia) is indicative of inadequate nutritional intake secondary to the interference of the tumor itself on the body functioning and the side effect of the treatment intervention especially the chemotherapeutic interventions. Besides the current finding revealed 74.8% and 79.4% were presented with nausea and loss of appetite.
4.1. Strength and limitations
The strengths of this study include the presence of a high response rate. The data were collected by interviewing patients, and from their medical records, the study was performed in the only oncology center in Ethiopia where patients are coming to this center from all over Ethiopia; hence, the results of this study can represent the whole population. Limitations include a shortage of literature on the prevalence and risk factors of malnutrition among adult cancer patients receiving chemotherapy. Causal relationships cannot be ascertained because the study used a cross-sectional design.
4.2. Conclusion
The prevalence of malnutrition among adult cancer patients receiving chemotherapy was 58.4%. Malnutrition was higher among female cancer patients than among males. Stage of cancer, appetite loss, and presence of diarrhea was found to be significant factors for malnutrition. This study recommends early identification of malnutrition for proper nutritional intervention during hospitalization. Dealing with medical issues for subjective symptoms, such as appetite loss and diarrhea, as well as there is a need for an integrated support team including a psycho-oncologist who can address and treat the psychological aspects (depression, loss of hope, and anxiety) of cancer patients. In addition to counseling on food preference, lifestyle, energy, and nutrient balance, counseling should address the presence and absence of loss of appetite, diarrhea as nutritional intervention. Increase nutritional assessment and nutritional intervention should be employed. Moreover, training of health professionals about cancer and malnutrition. Establishment of nutritional centers are very important as treatment modalities themselves are one major causes of malnutrition. Intervention on reduction of chemotherapy related side effects like diarrhea and loss of appétit are significant to decrease malnutrition among cancer patients and to give attention to female patients need special attention during cancer treatment due to their high prevalence, and further qualitative and longitudinal studies are needed to explore the state of malnutrition in cancer patients. Abbreviations and data collection tool (supple 3).
Declarations
Author contribution statement
Tuemay Kiros Gebremedhin: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Amsale Cherie and Boka Dugassa Tolera: Conceived and designed the experiments, Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Bantalem Tilaye Atinafu and Tefera Mulugeta Demelew: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Addis Ababa University, School of Nursing and Midwifery.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We would like to acknowledge Addis Ababa University, College of Health Sciences School of nursing, and midwifery for giving us the chance to do this research. Special gratitude and deepest appreciation go to Tikur Anbessa Specialized Hospital Manager, all Oncology Unit staff members, card room officers, and data collectors for their cooperation during data collection.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.WHO. World Health Organization . 2018. Global Cancer burden. [Google Scholar]
- 2.Da Silva T.L., Pretto A.D.B., Gonzalez M.C., Pastore C.A. Association between nutritional subjective global assessment and manual dynamometry in cancer patients of a chemotherapy service in Southern Brazil. Rev. Brasil. Oncol. Clín. 2015;11(40) [Google Scholar]
- 3.De Melo Silva F.R., de Oliveira M.G.O.A., Souza A.S.R., Figueroa J.N., Santos C.S. Factors associated with malnutrition in hospitalized cancer patients: a cross-sectional study. Nutr. J. 2015;14(1):123. doi: 10.1186/s12937-015-0113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pedro Lopes J., de Castro Cardoso Pereira P.M., dos Reis Baltazar Vicente A.F., Bernardo A., de Mesquita M.F. Nutritional status assessment in colorectal cancer patients. Nutr. Hosp. 2013;28(2) doi: 10.3305/nh.2013.28.2.6173. [DOI] [PubMed] [Google Scholar]
- 5.Caccialanza R., Pedrazzoli P., Cereda E., Gavazzi C., Pinto C., Paccagnella A. Nutritional support in cancer patients: a position paper from the Italian society of medical oncology (AIOM) and the Italian society of artificial nutrition and metabolism (SINPE) J. Canc. 2016;7(2):131. doi: 10.7150/jca.13818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bincy R., Chacko B. Assessment OF nutritional status OF patients receiving chemotherapy. Nitte Univ. J. Health Sci. 2014;4(3):33. [Google Scholar]
- 7.Kim J.-Y., Wie G.-A., Cho Y.-A., Kim S.-Y., Kim S.-M., Son K.-H. Development and validation of a nutrition screening tool for hospitalized cancer patients. Clin. Nutr. 2011;30(6):724–729. doi: 10.1016/j.clnu.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 8.Álvarez Hernández J., Planas Vilá M., León Sanz M., Garcia de Lorenzo y Mateos A., Celaya Pérez S., García Lorda P. 2012. Prevalence and Costs of Multinutrution in Hospitalized Patients; the PREDyCES Study. [Google Scholar]
- 9.Kumar N.B. Nutritional Management of Cancer Treatment Effects. Springer; 2012. Assessment of malnutrition and nutritional therapy approaches in cancer patients; pp. 7–41. [Google Scholar]
- 10.Tikur T.A.S.H. 2017. Anbessa Specialized Hospital Oncology Unit Unpublished Report from Manager Office. [Google Scholar]
- 11.M Shirodkar K.M.M. Indian Society of Gastroenterology; 2016. Subjective Global Assessment: a Simple and Reliable Screening Tool for Malnutrition Among Indians. [PubMed] [Google Scholar]
- 12.Khoshnevis N., Ahmadizar F., Alizadeh M., Akbari M. Nutritional assessment of cancer patients in Tehran, Iran. Asian Pac. J. Cancer Prev. APJCP. 2012;13(4):1621–1626. doi: 10.7314/apjcp.2012.13.4.1621. [DOI] [PubMed] [Google Scholar]
- 13.Wie G.-A., Cho Y.-A., Kim S.-Y., Kim S.-M., Bae J.-M., Joung H. Prevalence and risk factors of malnutrition among cancer patients according to tumor location and stage in the National Cancer Center in Korea. Nutrition. 2010;26(3):263–268. doi: 10.1016/j.nut.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Norshariza J., Siti Farrah Zaidah M., Aini Zaharah A., Betti Sharina M., Neoh M., Aeininhayatey A. Prevalence of malnutrition among hospitalised adult cancer patients at the national cancer institute, Putrajaya, Malaysia. Malaysian J. Nutr. 2017;23(2) [Google Scholar]
- 15.Parasa1 K., Avvaru2 D.K. Assessment of nutritional status of cancer patients using scored PG-SGA tool. IOSR J. Dent. Med. Sci. 2016;15(Issue 8 Ver):37–40. [Google Scholar]
- 16.Silva FRdM., de Oliveira M.G.O.A., Souza A.S.R., Figueroa J.N., Santos C.S. Factors associated with malnutrition in hospitalized cancer patients: a cross-sectional study. Nutr. J. 2015;14(1):123. doi: 10.1186/s12937-015-0113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferigollo A., Bazzan L.S.T., Ceni G.C., Bohrer C.T. Prevalence of malnutrition and factors associated with the nutritional status of oncological patients. Nutr. Clínica Dietética Hosp. 2018;38(4):137–142. [Internet] [Google Scholar]
- 18.Pressoir M., Desné S., Berchery D., Rossignol G., Poiree B., Meslier M. Prevalence, risk factors and clinical implications of malnutrition in French Comprehensive Cancer Centres. Br. J. Canc. 2010;102(6):966–971. doi: 10.1038/sj.bjc.6605578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fukuda Y., Yamamoto K., Hirao M., Nishikawa K., Maeda S., Haraguchi N. Prevalence of malnutrition among gastric cancer patients undergoing gastrectomy and optimal preoperative nutritional support for preventing surgical site infections. Ann. Surg Oncol. 2015;22(3):778–785. doi: 10.1245/s10434-015-4820-9. [DOI] [PubMed] [Google Scholar]
- 20.Opanga Y., Kaduka L., Bukania Z., Mutisya R., Korir A., Thuita V. Nutritional status of cancer outpatients using scored patient-generated subjective global assessment in two cancer treatment centers, Nairobi, Kenya. BMC Nutr. 2017;3(1):63. doi: 10.1186/s40795-017-0181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bozzetti F., Migliavacca S., Scotti A., Bonalumi M.G., Scarpa D., Baticci F. Impact of cancer, type, site, stage and treatment on the nutritional status of patients. Ann. Surg. 1982;196(2):170–179. doi: 10.1097/00000658-198208000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nho J.-H., Kim S.R., Kwon Y.S. Depression and appetite: predictors of malnutrition in gynecologic cancer. Support. Care Canc. 2014;22(11):3081–3088. doi: 10.1007/s00520-014-2340-y. [DOI] [PubMed] [Google Scholar]
- 23.Muscaritoli M., Lucia S., Farcomeni A., Lorusso V., Saracino V., Barone C. Prevalence of malnutrition in patients at first medical oncology visit: the PreMiO study. Oncotarget. 2017;8(45):79884. doi: 10.18632/oncotarget.20168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma D., Kannan R., Tapkire R., Nath S. Evaluation of nutritional status of cancer patients during treatment by patient-generated subjective global assessment: a hospital-based study. Asian Pac. J. Cancer Prev. 2015;16(18):8173–8176. doi: 10.7314/apjcp.2015.16.18.8173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.