Abstract
Background
Collaborative practice in healthcare has been recommended to improve the quality of antimicrobial stewardship interventions, a behavioral change in antimicrobial use. Insufficient knowledge regarding antibiotic resistance, the fear of complications from infections, and how providers perceive antibiotic use and resistance are likely to influence prescribing behavior. This study's objective was to identify the knowledge and belief healthcare professionals' differences about antibiotic stewardship.
Methods
This cross-sectional survey study of three hospitals in the East Java province, Indonesia utilized a 43-item questionnaire to assess antimicrobial stewardship knowledge and belief. There were 12 knowledge questions (total possible score: 12) and 31 belief questions (total possible score: 155). The Kuder Richardson 20 (KR-20) and Cronbach alpha values of the questionnaire were 0.54 and 0.92, respectively.
Results
Out of the 257 respondents, 19% (48/257) had a low scores of knowledge, and 39% (101/257) had low scores on belief about antibiotic stewardship (101/257). Most midwives had a low scores on knowledge (25/61) and low scores on belief (46/61). Respondents with high scores on belief were 17% (10/59) physicians, 15% (4/27) pharmacists, 8% (5/65) nurses, and 3% (2/61) midwives.
Conclusion
Among healthcare professionals, knowledge and belief differences concerning antibiotic stewardship vary widely. These differences will affect their capability, behavior, and contribution to the healthcare team collaboration and performance. Further studies are needed to evaluate the correlation between the level of inter-professional collaboration and the quality of the antibiotic stewardship implementation.
Keywords: Antibiotic stewardship, Knowledge, Belief
Antibiotic stewardship; Knowledge; Belief.
1. Introduction
Collaborative practices in healthcare optimize antimicrobial use. Antimicrobial use in hospitals remains high [1,2]. A study conducted by the Vermont Oxford Network reported a 34% relative risk reduction of the median antibiotic use rate with an improved collaborative practice (leadership, accountability, drug expertise, actions, tracking, reporting, education) [3]. A decreasing the number of subtherapeutic first troughs (the risk of development of antimicrobial resistance) and increasing the number of therapeutic troughs (the increases of treatment effectiveness) [4] was reported as a result of collaborative practice pharmacist and physician in determining the initial vancomycin dose for adult patients in the intensive care unit (ICU). Vancomycin typically takes 36–48 h to reach a steady state. It was reported that involving a pharmacist, being a medicine expert, in a physician's rounds in an intensive care unit reduced prescribing orders by 66% [5].
It is recommended that, to achieve the antimicrobial stewardship (AMS) goals effectively, an AMS team minimally includes contributions of either an infectious disease (ID) specialist physician, a microbiologist (if available) and a pharmacist. An ID physician supervises the overall function of the ASP and makes recommendations to the ASP team [6]. Pharmacists evaluate antibiotic consumption, participate in Drug and Therapeutics Committee [7] and AMS committee meetings [7,8]. Furthermore, the nurses’ role as a patient caregiver [9], patient advocate [10], and the one who provides education to patient [11] is essential [12]. Along with physicians and pharmacists, nurses are the most consistent patient care givers in reviewing medication charts to administer medications, monitoring duration and indication for antimicrobial treatment, monitoring the possibility of drug allergies and side effects incidence, ensuring timely administration of antimicrobials, and following up on missed doses [9].
According the World Health Organization (WHO): “Collaborative practice happens when multiple health workers from different professional backgrounds work together with patients, families, care givers and communities to deliver the highest quality of care. It allows health workers to engage any individual whose skills can help achieve local health goals.” [13]. There are some theories that support the importance of collaborative practice in antibiotic stewardship. One is the behavior change advocated by Michie et. al (2011), which includes the COM-B model of behavior that identifies three factors for any behavior to occur: capability (C), opportunity (O), and motivation (M) [14]. The COM-B model can be applied to understand behavior and to effect behavioral changes in antibiotic use, whereas the Health Belief Model (HBM) theory explains and predicts person's behavior. Based on the HBM theory by Rosenstock, at an individual level, a person's actions are determined by their beliefs and cues to action [15]. Belief itself consists of perceived threats (possibility of facing the disease), perceived benefits (of understanding the benefits of adapting a new behavior), perceived self-efficacy (one's ability to successfully perform the recommended behavior), and perceived barriers (cost and obstacles which prevent us from doing a behavior) [16,17]. Our beliefs are affected by factors as age, gender, type of profession, length of work, and knowledge. Antibiotics stewardship programs are used worldwide to control antibiotic resistance through improving antibiotic use. One of the strategies of the antibiotic stewardship program is to increase the stakeholder's knowledge about antibiotic stewardship in using antibiotics judiciously. More than half of healthcare practitioners in Fitche town, Ethiopia, were found to have good knowledge (the respondents agree on >70% of the 5-Likert scale statement of practice) in terminology and effectiveness of antibiotic stewardship [18]. However, very few health practitioners (16%) adhered consistently to the management of the antibiotic stewardship program [18]. Moreover, a study at a Riyadh hospital in Saudi Arabia reported that among 212 physicians; 119 (56%) physicians believed that antibiotic resistance causes problems in the community and economic losses for the country; 101 (48%) physicians believed injudicious empiric antibiotics therapy was the main factor in the occurrence of antibiotic resistance; and 95 (45%) physicians were unsure about their knowledge about the appropriate use of antibiotics [19]. A qualitative study in Indonesia explored the education and awareness of healthcare professionals. The participants said that in the university, the lecturer should teach students more about the impact of antibiotic resistance; and the professional organization should provide education for practicing healthcare to keep them up to date with the latest developments [20]. Besides knowledge, the predictor of an effective team performance was trust level, years of previous experience, and the number of team members [21]. The objective of this study was to identify the knowledge’ and belief' (perceived threat, perceived self-efficacy, perceived benefit, and perceived barrier) of healthcare professionals regarding antibiotic stewardship to be able to design training to meet their needs.
2. Methods
This research was an observational descriptive study with a cross-sectional design. The research material was drawn from a questionnaire completed by healthcare professionals in a private hospital in Surabaya and two public hospitals in Mojokerto and Pasuruan. The questionnaire was developed and validated with the different respondents at the respective hospital before collecting the data. The Kuder Richardson 20 (KR-20) for the knowledge and Cronbach's alpha values for the belief, of the questionnaire were 0.54 and 0.92, respectively. Participants are healthcare practitioners who are associated with antibiotic prescribing and use. The professions included in this study are doctors, pharmacists, midwives, the AMS team members, nurses. The excluded professions are psychiatrists, radiologists, obstetric and neonatal nurses, and hemodialysis nurses. The minimum sample size (n) required is 24 respondent; based on the formula below, where n is the minimum sample size, the Z value for p <0.05 is 1.96, the unknown population size (P) is 0.5, and to obtain 0.2 effect difference (d) [22].
The research questionnaire was delivered face-to-face or collected by the chief nursing officer in the hospital. The respondents provided a written consent, after they understood the research objective and agree to participate; and answered the research questionnaire. The questionnaire consisted of 43 questions (Appendix 1). Of these, 12 questions were used to assess knowledge, and 31 questions to assess belief. The 31 belief questions consisted of 10 questions to assess perceived threats, 11 questions to assess perceived self-efficacy, 8 questions to assess perceived benefits, and 2 questions to assess perceived barriers. The responses to the knowledge questions were measured using the Guttman scale, which specifies a Yes or No answer. The maximum score of the knowledge questionnaire was 12, whereas the belief questionnaire was measured using a 5-point Likert scale. The belief score was a composite score (31 item questions; maximum score 155) which included the perceived threat score (10 item questions; maximum score 50), perceived self-efficacy score (11 item questions; maximum score 55), perceived benefit score (8 item questions; maximum score 40), and perceived barrier score (2 item questions; maximum score 10). There were three categories: high, moderate, and low. The number of respondents in the high category was the number of respondents who had scores higher than the mean value ± one standard deviation (SD). The number of the respondents in the moderate knowledge or belief category was the number of respondents who had scored in the range of mean value ± one SD. Any respondent with a score lower than the moderate category's score (mean value ± one SD) was counted in the low category. We performed a simple random data collection (probability sampling) for nurses' groups (65 nurses from 406 nurses) and complete data collection (nonprobability-sampling) for other groups of professions to get a proportionate number among groups. This study is a descriptive analysis to statistically describe the knowledge and belief score. The healthcare professional's knowledge and belief differences were analyzed with the Kruskal–Wallis test because the data are not normally distributed.
2.1. Ethical considerations
All procedures performed involving human participants were done in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Written consent to participate was obtained from each study participant. The study was approved by the respective hospital managements (Surabaya city: No. 1299/RSHU/Dir./X/2017; Mojokerto district: No. 423.4/4882/416–207/2017; and Pasuruan district: No: 455.1/2752/424.202/2017) and was conducted in accordance with the Indonesian Law for the Protection of Personal Data. The study was ethically cleared by the Health Research Ethics Committee of Politeknik Kesehatan Kemenkes Surabaya, Kementerian Kesehatan No.025/S/KEPK/V/2017.
3. Results
The study population consisted of representatives from five professions: nurses, midwives, pharmacists, pharmacy technicians, and physicians. Table 1 shows the baseline characteristics of the respondents. At a private hospital in Surabaya city, the response rate was 83% (245/296), and 179 met the inclusion and exclusion criteria; at a public hospital in Mojokerto district, the response rate was 77% (153/200), and 153 met the inclusion and exclusion criteria; and at a public hospital in Pasuruan district, the response rate was 87% (307/352), and 257 met the inclusion and exclusion criteria. There were 65 nurses, 61 midwives, 27 pharmacists, 45 pharmacy technicians, and 59 physicians included in the analysis.
Table 1.
Baseline characteristics of healthcare practitioners.
| Variable | Professions (N = 257) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nurses (n = 65) |
Midwives (n = 61) |
Pharmacists (n = 27) |
Pharmacy technicians (n = 45) |
Physicians (n = 59) |
|||||||
| n | % | n | % | n | % | n | % | n | % | ||
| Gender | Female | 48 | 74 | 61 | 100 | 25 | 93 | 43 | 96 | 30 | 51 |
| Male | 17 | 26 | - | - | 2 | 7 | 2 | 4 | 29 | 49 | |
| Work experience | ≤3 years | 10 | 15 | 7 | 11 | 16 | 59 | 7 | 16 | 15 | 25 |
| >3 years | 55 | 85 | 54 | 89 | 11 | 41 | 38 | 84 | 44 | 75 | |
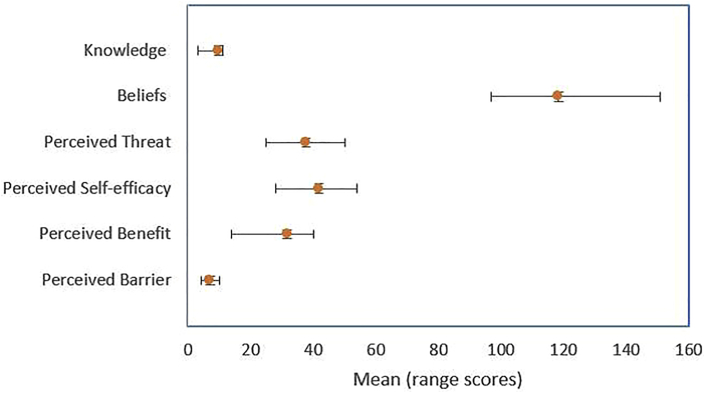
Nineteen percent (48/257) of the respondents had low scores (below mean value ± one SD) knowledge about antimicrobial stewardship (Table 2). The mean of antibiotic stewardship knowledge of total respondents was 9.9 (1.5) (Table 3, Figure 1). The mean of antibiotic stewardship knowledge of the pharmacist and physicians was higher than that of the pharmacy technicians, nurses, and midwives.
Table 2.
The respondent knowledge classification.
| Classification | Professions (N = 257) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nurses (n = 65) |
Midwives (n = 61) |
Pharmacists (n = 27) |
Pharmacy technicians (n = 45) |
Physicians (n = 59) |
Total (N = 257) |
|||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| High | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Moderate | 54 | 83 | 36 | 59 | 26 | 96 | 42 | 93 | 51 | 86 | 209 | 81 |
| Low | 11 | 17 | 25 | 41 | 1 | 4 | 3 | 7 | 8 | 14 | 48 | 19 |
Table 3.
The descriptive statistics (N = 257).
| Variable | Mean | Standard deviation | Minimum | Maksimum | N of item question (maximum scores) |
|---|---|---|---|---|---|
| Knowledge | 9.85 | 1.532 | 3 | 11 | 12 (12) |
| Belief | 118.41 | 8.933 | 97 | 151 | 31 (155) |
| Threat | 37.77 | 5.418 | 25 | 50 | 10 (50) |
| Self-efficacy | 41.86 | 3.891 | 28 | 54 | 11 (55) |
| Benefit | 31.68 | 2.880 | 14 | 40 | 8 (40) |
| Barrier | 7.10 | 1.217 | 4 | 10 | 2 (10) |
Figure 1.
Mean scores of variables.
The physicians had a high score of perceived threat, perceived self-efficacy, perceived benefit, and an average score of perceived barrier compared with other professions. The pharmacists had a high score for perceived threat and perceived barrier and a low score for perceived self-efficacy and perceived benefit compared with other professions (Table 4). The total score knowledge and belief healthcare professionals differences were statistically significant (p <0.05).
Table 4.
The respondent belief classification.
| Category | Professions (N = 257) |
Total |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nurses (n = 65) |
Midwives (n = 61) |
Pharmacists (n = 27) |
Pharmacy technicians (n = 45) |
Physicians (n = 59) |
||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| Belief | ||||||||||||
| Strong | 5 | 8 | 2 | 3 | 4 | 15 | 1 | 2 | 10 | 17 | 22 | 9 |
| Moderate | 54 | 83 | 13 | 21 | 9 | 33 | 13 | 29 | 45 | 76 | 134 | 52 |
| Weak |
6 |
9 |
46 |
75 |
14 |
52 |
31 |
69 |
4 |
7 |
101 |
39 |
| Perceived Threat | ||||||||||||
| High | 4 | 6 | 0 | 0 | 5 | 19 | 0 | 0 | 18 | 31 | 27 | 11 |
| Moderate | 55 | 85 | 41 | 67 | 22 | 81 | 41 | 91 | 40 | 68 | 199 | 77 |
| Low |
6 |
9 |
20 |
33 |
0 |
0 |
4 |
9 |
1 |
2 |
31 |
12 |
| Perceived Self-efficacy | ||||||||||||
| High | 14 | 22 | 7 | 11 | 3 | 11 | 1 | 2 | 8 | 14 | 33 | 13 |
| Moderate | 50 | 77 | 54 | 89 | 20 | 74 | 44 | 98 | 51 | 86 | 219 | 85 |
| Low |
1 |
2 |
0 |
0 |
4 |
15 |
0 |
0 |
0 |
0 |
5 |
2 |
| Perceived Benefit | ||||||||||||
| High | 9 | 14 | 4 | 7 | 0 | 0 | 0 | 0 | 7 | 12 | 20 | 8 |
| Moderate | 52 | 80 | 54 | 89 | 19 | 70 | 39 | 87 | 50 | 85 | 214 | 83 |
| Low |
4 |
6 |
3 |
5 |
8 |
30 |
6 |
13 |
2 |
3 |
23 |
9 |
| Perceived Barrier | ||||||||||||
| High | 7 | 11 | 9 | 15 | 7 | 26 | 0 | 0 | 8 | 14 | 31 | 12 |
| Moderate | 55 | 85 | 48 | 79 | 20 | 74 | 45 | 100 | 48 | 81 | 216 | 84 |
| Low | 3 | 5 | 4 | 7 | 0 | 0 | 0 | 0 | 3 | 5 | 10 | 4 |
Work experience affected the knowledge (p = 0.07) and belief (p = 0.001) of the pharmacists significantly, but the Kruskal–Wallis test showed that the association between work experience and knowledge or belief of other professions (nurses, midwives, pharmacy technicians, and physicians) was not significant.
4. Discussion
Knowledge about antibiotic stewardship varies among different groups of healthcare practitioners. The lack of knowledge may cause inappropriate use of antibiotics. However, antibiotic stewardship knowledge can be increased with education and training. A study in Dire Dawa, Ethiopia with a total of 218 paramedical staffs (41 health officers, 96 nurses, 31 pharmacists, 21 midwives, and 29 medical laboratory technologists) showed that the level of knowledge about the causes of antimicrobial resistance for the pharmacists (77.4%), health officers (75.6%), and nurses (63.5%) was higher than that of lab technologists (44.8%) and midwives (38.1%). That study also showed that 90.4% (197/218) had not attended any training on antimicrobial resistance; that 96.8% (211/218) of the participants of the study had not used antimicrobial sensitivity test results for treating the patients, and that only 15.7% of them reported referring to the guidelines whenever caring for a patient [23]. Along with education and training, capabilities of healthcare practitioners will also increase. A study on long-term educational effects of antibiotic prescribing in 171 doctors showed that antibiotic prescribing in the intervention group was reported as having a more significant decrease compared with that in the control group. There were two intervention subgroups. First, a 2-day seminar about evidence-based medicine for respiratory infections (evidence-based medicine subgroup). Second, an additional 1-day seminar focused on problem-solving strategies (evidence-based medicine plus problem-solving strategies subgroup) [24] The studies indicated that the education about the benefits of limiting antibiotic use is urgently needed for healthcare professionals [25,26]. These findings will be used by hospital management to design better educational material for the next antibiotic stewardship training for healthcare professional that associated with antibiotic use.
There are differences in knowledge and belief among healthcare professionals. There is a positive correlation between knowledge and behavior. The knowledge about antibiotic stewardship will affect antibiotic use behavior. Furthermore, there is a positive correlation between self-efficacy and perceived benefit, and a negative correlation between self-efficacy and perceived barrier [16]. Nair et al. reported that there was a statistically significant difference in average scores of knowledge, attitude, and practice questions among allopathic doctors, nurses, pharmacy shopkeepers, and informal health providers in Paschim Bardhaman District, India [27]. A study of 135 nurses from two hospitals in Mashhad, Iran showed that education intervention in the intervention group increased the participants’ knowledge, and that there was a significant relationship between knowledge and perceived threat or perceived benefit [17]. A study of the HBM as an explanatory framework in communication research recommended further research to evaluate the causal effect of the behavior variable and which variable was a strong predictor of behavior change [15].
Education is effective in reducing antibiotic prescribing, but education combined with direct intervention in supporting the implementation of rational antibiotic use is more effective than education alone [28,29]. Some interventions identified were public awareness campaigns [26], antimicrobial guidelines [30], professional regulation [31], restricted reimbursement [32], pay for performance, and prescription requirements [32,33]. Collaborative practice and recommendation acceptance by the clinical provider is also important for an antibiotic stewardship program [34] and for collaborative approaches to appropriate antimicrobial use [35]. Logan's study in 28 hospitals showed that collaborative practice in antibiotic stewardship increased days of therapy (DOT) per 1,000 patient-days of broad-spectrum antibiotics reduction from 1%–2.5% to 5%–10% [36].
In this study, the association of work experience and the knowledge or belief were inconsistent. Among the pharmacists' subgroup, the work experience was associated with different score knowledge or belief, but among other professions (nurses, midwives, pharmacy technicians, and physicians) subgroup, the work experience was not associated with the knowledge or belief score. These results are comparable to the findings reported by by Tegagn et al. from a study in Fitche Hospital, Ethiopia, were profession and years of experience were not significant predictors of healthcare professionals’ knowledge, attitude, and practices towards antimicrobial stewardship [18].
Our study has a number of limitations. First, it is not fully representative for the sample population because not all healthcare practitioners filled in the questionnaire. This is the nonresponse error, reflecting the results of the individuals who did not respond to the survey. Second, there is measurement error, occurring when survey responses are not accurate reflections of the true value because of social desirability bias [37].
5. Conclusion
Among healthcare professionals, knowledge and belief differences of antibiotic stewardship vary widely. Antibiotic knowledge is associated with positive belief and behavior that contribute to adherence to a judicious use of antibiotics and reduce antibiotic utilization. Knowledge about antibiotics and resistance relates to the understanding of the antibiotic misuse concept and awareness of antimicrobial resistance. The Health Belief Model (HBM) theory presume several constructs (perceived severity, perceived benefit, self-efficacy, cues of action) to predict behavior. Perception about the severity and consequences of the disease and the benefits of antibiotic treatment for infectious disease are determinants of antibiotic use and prescribing behavior [15]. Further studies are needed to evaluate the correlation between the level of interprofessional collaboration and the quality of the antibiotic stewardship implementation.
Declarations
Author contribution statement
Fauna Herawati and Diantha Soemantri: Conceived and designed the experiments; Wrote the paper.
Abdul Kadir Jaelani and Heru Wijono: Performed the experiments.
Setiasih: Analyzed and interpreted the data.
Rika Yulia: Analyzed and interpreted the data; Wrote the paper.
Abdul Rahem: Contributed reagents, materials, analysis tools or data.
Retnosari Andrajati: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
This research was presented at the 5th International Conference and Exhibition on Indonesian Medical Education and Research Institute (5th ICE on IMERI), Faculty of Medicine, Universitas Indonesia, Jakarta. We thank the 5th ICE on IMERI Committee for supporting the peer review and manuscript preparation before submission. We are grateful to the management and the hospital staff for allowing us to collect data and for their contributions to the evaluation process. We would like to thank Erik Christopher, Klinik Bahasa, Faculty Kedokteran, Universitas Gadjah Mada (Yogyakarta) for translating and editing the manuscript.
Appendix.
Appendix 1: Knowledge and belief questionnaire
RESPONDENT PROFILE
Age:
Gender:
Profession:
Profession length:
Work place (unit/division):
| Number | Statement | True | False |
|---|---|---|---|
| 1. |
Penggunaan antibiotik yang tidak sesuai denganindikasi, menyebabkan bakteri resisten terhadap antibiotik. The use of antibiotics outside the indications, causes bacteria to become resistant to antibiotics. |
||
| 2. |
Frekuensi pemberian antibiotikyang tidak tepat dengan waktu minum obat tersebut dapat menyebabkan bakteri resisten terhadap antibiotik. The inappropriate administration of antibiotic can cause bacterial resistant to antibiotics. |
||
| 3. |
Pemilihan antibiotik yang tidak sesuai denganjenis infeksi, menyebabkan bakteri resisten terhadap antibiotik. The selection of antibiotics that are not related to the type of infection, causes bacteria to become resistant to antibiotics. |
||
| 4. |
Pemantauan dan evaluasi penggunaan antibiotik cukup dilakukan oleh perawat saja. Monitoring and evaluation of the use of antibiotics are performed by nurses only. |
||
| 5. |
Penggunaan dosis antibiotik yangkurang dari dosisterapi dapat menyebabkan bakteri resisten terhadap antibiotik. The use of doses of antibiotics lower than the therapeutic dose can cause bacteria to become resistant to antibiotics. |
||
| 6. |
Penggunaan dosis antibiotik yanglebih dari dosis terapidapat meningkatkan efek samping obat. The use of doses of antibiotics that are higher than the therapeutic dose can increase the side effects of the drug. |
||
| 7. |
Pemberian informasi penggunaan antibiotik kepada pasien dapat mencegah bakteri resisten terhadap antibiotik. Providing information on the use of antibiotics to patients can prevent bacteria becoming resistant to antibiotics. |
||
| 8. |
Peta kuman di rumah sakit dapat membantu mengetahui bakteri yang resisten terhadap antibiotik. Antibiotic sensitivity patterns in the hospital can help to identify antibiotic-resistant bacteria. |
||
| 9. |
Lama pemberian antibiotikyang tidak sesuai dengan jenis infeksi, menyebabkan bakteri resisten terhadap antibiotik. The duration of antibiotic administration not following the type of infection, may cause bacteria to become resistant to antibiotics. |
||
| 10. |
Cara pemberian antibiotikyang tidak sesuai dengan aturan dapat menyebabkan bakteri resisten terhadap antibiotik. Give antibiotics without following the rules can cause bacteria to become resistant to antibiotics. |
||
| 11. |
Pelaksanaan Program Pengendalian Resistensi Antibiotik (PPRA) cukup dilakukan oleh dokter saja. The implementation of the Antibiotic Resistance Control Program (PPRA) can only be done by a doctor. |
||
| 12. |
Mencuci tangan sebelum dan sesudah kontak fisik/penyiapan sediaan injeksi kepada pasien dapat mengurangi transmisi/penularan penyakit infeksi. Washing hands before and after physical contact/preparing injections to patients can reduce the transmission of infectious diseases. |
| Number | Statement | Strongly Disagree | Disagree | Neutral | Agree | Strongly Agree |
|---|---|---|---|---|---|---|
| 1. |
Pemberian antibiotik pada bakteri yang resisten terhadap antibiotik tersebut dapatmemperpanjang lama rawat inap. Giving antibiotics to bacteria that are resistant to antibiotics can prolong the length of hospitalization. |
|||||
| 2. |
Bakteri resisten terhadap antibiotik dapatmeningkatkan biayapengobatan pada pasien yang terinfeksi. Antibiotic-resistant bacteria can increase treatment costs in infected patients. |
|||||
| 3. |
Bakteri resisten terhadap antibiotikmeningkatkan frekuensipemeriksaan laboratorium pada pasien yang terinfeksi. Antibiotic-resistant bacteria increase the frequency of laboratory tests in infected patients. |
|||||
| 4. |
Bakteri resisten terhadap antibiotik dapatmenyebabkan terjadinya sepsis. Bacterial resistance to antibiotics can cause sepsis. |
|||||
| 5. |
Bakteri resisten terhadap antibiotik dapatmenyebabkan risiko pasien dirawat di intensive care unit (ICU) atau ruang isolasi. Bacterial resistance to antibiotics can put the patient at risk of being admitted to the intensive care unit (ICU) or isolation room. |
|||||
| 6. |
Bakteri resisten terhadap antibiotik dapatmeningkatkan resiko mortalitaspada pasien yang terinfeksi. Antibiotic-resistant bacteria can increase the risk of mortality in infected patients. |
|||||
| 7. |
Profesi saya beresiko terinfeksi bakteri yang resisten terhadap antibiotik. My profession is at risk of infection with antibiotic-resistant bacteria. |
|||||
| 8. |
Penggunaan kombinasi antibiotik karena bakteri resisten dapat meningkatkan efek samping obat. The use of a combination of antibiotics due to resistant bacteria can increase the side effects of the drug. |
|||||
| 9. |
Bakteri resisten terhadap antibiotik dapat menyebabkan risiko penggunaan ventilator pada pasien yang terinfeksi. Antibiotic-resistant bacteria may pose a risk of ventilator use in infected patients. |
|||||
| 10. |
Bakteri resisten terhadap antibiotik membebani keuangan negara dalam pembiayaan jaminan kesehatan nasional. Antibiotic-resistant bacteria burden the state's finances in financing national health insurance. |
|||||
| 11. |
Laboratorium mikrobiologi di rumah sakit saya dapat mendukung keberhasilan Program Pengendalian Resistensi Antibiotik (PPRA). The microbiology laboratory at my hospital can support the success of the Antibiotic Resistance Control Program (PPRA). |
|||||
| 12. |
Penjelasan saya tentang penggunaan antibiotik dapat dipahami pasien. My explanation about the use of antibiotics is understandable for the patient. |
|||||
| 13. |
Saya berkoordinasi dengan tenaga kesehatan lain ketika pasien mendapatkan antibiotik bersamaan dengan obat lain. I attune with other healthcare practitioners when patients receive antibiotics along with other drugs. |
|||||
| 14. |
Pencampuran (rekonstitusi) sediaan antibiotik dilakukan dengan baik di rumah sakit saya. Mixing (reconstitution) of antibiotic preparations is done well in my hospital. |
|||||
| 15. |
Saya mengkonfirmasi peresepan/cara penggunaan antibiotik sebelum diberikan ke pasien dengan tenaga kesehatan lain. I confirm the prescribing/how to use antibiotics before giving them to patients with other healthcare practitioners. |
|||||
| 16. |
Farmasis di rumah sakit saya menjamin stabilitas hasil pencampuran sediaan antibiotik. The pharmacists in my hospital guarantee the stability of the reconstituted antibiotic preparations. |
|||||
| 17. |
Sayamenggantiantibiotik bila hasil laboratorium menunjukkan bakteri resisten terhadap antibiotik meskipun kondisi klinik membaik. I change antibiotics when the laboratory results show that bacteria are resistant to antibiotics even though the clinical condition is improving. |
|||||
| 18. |
Pedoman Penggunaan Antibiotik (PPAB) di rumah sakit saya telah digunakan sebagai pedoman dalam penggunaan antibiotik. The Antibiotic Use Guideline (PPAB) in my hospital have been used as guidelines in the use of antibiotics. |
|||||
| 19. |
Formularium rumah sakit saya telah memberi batasan (restriksi) yang jelas tentang penggunaan antibiotik. My hospital formulary has clearly defined the use of antibiotics. |
|||||
| 20. |
Farmasis di rumah sakit saya merekomendasikan perubahan antibiotik parenteral ke oral jika kondisi pasien membaik. The pharmacists in my hospital recommends changing parenteral to oral antibiotics if the patient's condition improves. |
|||||
| 21. |
Sayamelanjutkan antibiotikbila kondisi klinikpasien membaiksetelah 48–72 jam. I continue antibiotics if the patient's clinical condition improves after 48–72 h. |
|||||
| 22. |
Penggunaan antibiotik di rumah sakit saya rasional (sesuai indikasi, tepat dosis, frekuensi, rute pemberian dan lama terapi). The use of antibiotics in my hospital is rational (according to indication, dosage, frequency, route of administration, and duration of therapy). |
|||||
| 23. |
Penggunaan antibiotik pada pasien dapat dipantau/dievaluasi dengan baik. The use of antibiotics in patients can be monitored/evaluated properly. |
|||||
| 24. |
Tenaga kesehatan di rumah sakit saya dapat memilih dan merekomendasikan antibiotik dengan baik. The healthcare practitioners in my hospital are well able to choose and recommend antibiotics. |
|||||
| 25. |
Pedoman Penggunaan Antibiotik (PPAB) di rumah sakit saya diperbarui secara berkala. The Antibiotic Use Guideline (PPAB) in my hospital are updated regularly. |
|||||
| 26. |
Pemeriksaan laboratorium untuk pasien yang terinfeksi diperiksa sesuai kebutuhan. Laboratory tests for infected patients are checked as needed. |
|||||
| 27. |
Koordinasi antar tenaga kesehatan (dokter, perawat, apoteker) yang baik mendukung keberhasilan terapi. Good coordination between healthcare practitioners (doctors, nurses, pharmacists) supports the success of therapy. |
|||||
| 28. |
Pembatasan penggunaan antibiotik oleh formularium rumah sakit menurunkan kejadian bakteri resisten terhadap antibiotik. Restrictions on the use of antibiotics by hospital formularies reduce the incidence of antibiotic-resistant bacteria. |
|||||
| 29. |
Penggantian antibiotik dari spektrum luas ke spektrum lebih sempit sesuai penyebab infeksi menurunkan kejadian bakteri resisten terhadap antibiotik. Switching from broad-spectrum to narrower-spectrum antibiotics according to the cause of infection reduces the incidence of antibiotic-resistant bacteria. |
|||||
| 30. |
Pengetahuan tentang bakteri yang resisten dapat membantu keberhasilan terapi. Knowledge of resistant bacteria can help in successful therapy. |
|||||
| 31. |
Waktu pemeriksaan yang lama di laboratorium mikrobiologitidak berpengaruhpada keberhasilan terapi pada pasien yang terinfeksi. Long examination time in microbiology laboratory has no effect on the success of therapy in infected patients. |
References
- 1.Zanichelli V., Monnier A.A., Gyssens I.C., Adriaenssens N., Versporten A., Pulcini C., Le Maréchal M., Tebano G., Vlahovic-Palcevski V., Benic M.S., Milanic R., Harbarth S., Hulscher M.E., Huttner B. Variation in antibiotic use among and within different settings: a systematic review. J. Antimicrob. Chemother. 2018;73(suppl_6):vi17–vi29. doi: 10.1093/jac/dky115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pouwels K.B., Dolk F.C.K., Smith D.R.M., Smieszek T., Robotham J.V. Explaining variation in antibiotic prescribing between general practices in the UK. J. Antimicrob. Chemother. 2018;73(suppl_2):ii27–ii35. doi: 10.1093/jac/dkx501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dukhovny D., Buus-Frank M.E., Edwards E.M., Ho T., Morrow K.A., Srinivasan A., Pollock D.A., Zupancic J.A.F., Pursley D.M., Goldmann D., Puopolo K.M., Soll R.F., Horbar J.D. A collaborative multicenter QI initiative to improve antibiotic stewardship in newborns. Pediatrics Dec. 2019;144(6) doi: 10.1542/peds.2019-0589. [DOI] [PubMed] [Google Scholar]
- 4.Levin D., Glasheen J.J., Kiser T.H. Pharmacist and physician collaborative practice model improves vancomycin dosing in an Intensive Care Unit. Int. J. Clin. Med. 2016;7(10):675–684. doi: 10.4236/ijcm.2016.710073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosen M.A., DiazGranados D., Dietz A.S., Benishek L.E., Thompson D., Pronovost P.J., Weaver S.J. Teamwork in healthcare: key discoveries enabling safer, high-quality care. Am. Psychol. 2018;73(4):433–450. doi: 10.1037/amp0000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Majumder M.A.A., Rahman S., Cohall D., Bharatha A., Singh K., Haque M., Gittens-St Hilaire M. Antimicrobial stewardship: fighting antimicrobial resistance and protecting global public health. Infect. Drug Resist. 2020;13:4713–4738. doi: 10.2147/IDR.S290835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haseeb A., Faidah H.S., Al-Gethamy M., Iqbal M.S., Alhifany A.A., Ali M., Abuhussain S.S.A., Elrggal M.E., Almalki W.H., Alghamdi S., Saleem Z., Verma A.K., Algarni M.A., Ashgar S.S., Qashqari F.S.I., Hassali M.A. Evaluation of antimicrobial stewardship programs (ASPs) and their perceived level of success at makkah region hospitals, Kingdom of Saudi Arabia. Saudi Pharmaceut. J. 2020;28(10):1166–1171. doi: 10.1016/j.jsps.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weier N., Tebano G., Thilly N., Demoré B., Pulcini C., Zaidi S.T.R. Pharmacist participation in antimicrobial stewardship in Australian and French hospitals: a cross-sectional nationwide survey. J. Antimicrob. Chemother. 2018;73(3):804–813. doi: 10.1093/jac/dkx435. [DOI] [PubMed] [Google Scholar]
- 9.Edwards R., Drumright L., Kiernan M., Holmes A. Covering more territory to fight resistance: considering nurses’ role in antimicrobial stewardship. J. Infect. Prev. 2011;12(1):6–10. doi: 10.1177/1757177410389627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carter E.J., Greendyke W.G., Furuya E.Y., Srinivasan A., Shelley A.N., Bothra A., Saiman L., Larson E.L. Exploring the nurses’ role in antibiotic stewardship: a multisite qualitative study of nurses and infection preventionists. Am. J. Infect. Contr. 2018;46(5):492–497. doi: 10.1016/j.ajic.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jayaweerasingham M., Angulmaduwa S., Liyanapathirana V. Knowledge, beliefs and practices on antibiotic use and resistance among a group of trainee nurses in Sri Lanka. BMC Res. Notes. 2019;12(1):601. doi: 10.1186/s13104-019-4640-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Courtenay M., Burnett E., Castro-Sánchez E., Du Toit B., Figueiredo R.M., Gallagher R., Gotterson F., Kennedy H., Manias E., McEwen J., Ness V., Olans R., Padoveze M.C. Preparing nurses for COVID-19 response efforts through involvement in antimicrobial stewardship programmes. J. Hosp. Infect. 2020;106(1):176–178. doi: 10.1016/j.jhin.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization . World Health Organization; Geneva: 2010. Framework for Action on Interprofessional Education and Collaborative Practice. [PubMed] [Google Scholar]
- 14.Michie S., van Stralen M.M., West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement. Sci. 2011;6:42. doi: 10.1186/1748-5908-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones C.L., Jensen J.D., Scherr C.L., Brown N.R., Christy K., Weaver J. The Health Belief Model as an explanatory framework in communication research: exploring parallel, serial, and moderated mediation. Health Commun. 2015;30(6):566–576. doi: 10.1080/10410236.2013.873363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang H.T., Kuo Y.M., Wang S.R., Wang C.F., Tsai C.H. Structural factors affecting health examination behavioral intention. Int. J. Environ. Res. Publ. Health. 2016;13:395. doi: 10.3390/ijerph13040395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeigheimat F., Ebadi A., Rahmati-Najarkolaei F., Ghadamgahi F. An investigation into the effect of health belief model-based education on healthcare behaviors of nursing staff in controlling nosocomial infections. J. Educ. Health Promot. 2016;5:23. doi: 10.4103/2277-9531.184549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tegagn G.T., Yadesa T.M., Ahmed Y. Knowledge, attitudes and practices of healthcare professionals towards antimicrobial stewardship and their predictors in Fitche Hospital. J. Bioanal. Biomed. 2017;9(2):91–97. [Google Scholar]
- 19.Baadani A.M., Baig K., Alfahad W.A., Aldalbahi S., Omrani A.S. Physicians’ knowledge, perceptions, and attitudes toward antimicrobial prescribing in Riyadh, Saudi Arabia. Saudi Med. J. 2015;36(5):613–619. doi: 10.15537/smj.2015.5.11726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mohrs S. Department of Women’s and Children’s Health, IMCH/International Maternal and Child Health, Uppsala University; Uppsala, Sweden: 2015. Factors Influencing the Use of Antibiotics and Knowledge about Antibiotic Resistance in Jakarta: a Qualitative Study on the Perceptions of Stakeholders Involved in Yayasan Orangtua Peduli’s Smart Use of Antibiotics Campaign in Indonesia. [Thesis] [Google Scholar]
- 21.Sifaki-Pistolla D., Melidoniotis E., Dey N., Chatzea V.E. How trust affects performance of interprofessional health-care teams. J. Interprof. Care. 2020;34(2):218–224. doi: 10.1080/13561820.2019.1631763. [DOI] [PubMed] [Google Scholar]
- 22.Binu V.S., Mayya S.S., Dhar M. Some basic aspects of statistical methods and sample size determination in health science research. Ayu. 2014;35(2):119–123. doi: 10.4103/0974-8520.146202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tafa B., Endale A., Bekele D. Paramedical staffs knowledge and attitudes towards antimicrobial resistance in Dire Dawa, Ethiopia: a cross sectional study. Ann. Clin. Microbiol. Antimicrob. 2017;16(1):64. doi: 10.1186/s12941-017-0241-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Corvoisier P., Renard V., Roudot-Thoraval F., Cazalens T., Veerabudun K., Canoui-Poitrine F., Montagne O., Attali C. Long-term effects of an educational seminar on antibiotic prescribing by GPs: a randomised controlled trial. Br. J. Gen. Pract. 2013;63(612):e455–e464. doi: 10.3399/bjgp13X669176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pereira N.R., Castro-Sanchez E., Nathwani D. How can multi-professional education support better stewardship? Infect. Dis. Rep. 2017;9(1):6917. doi: 10.4081/idr.2017.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Machowska A., Stålsby Lundborg C. Drivers of irrational use of antibiotics in Europe. Int. J. Environ. Res. Publ. Health. 2018;16(1):27. doi: 10.3390/ijerph16010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nair M., Tripathi S., Mazumdar S., Mahajan R., Harshana A., Pereira A., Jimenez C., Halder D., Burza S. Knowledge, attitudes, and practices related to antibiotic use in Paschim Bardhaman District: a survey of healthcare providers in West Bengal, India. PloS One. 2019;14(5) doi: 10.1371/journal.pone.0217818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van der Velden A.W., Pijpers E.J., Kuyvenhoven M.M., Tonkin-Crine S.K.G., Little P., Verheij T.J.M. Effectiveness of physician-targeted interventions to improve antibiotic use for respiratory tract infections. Br. J. Gen. Pract. 2012;62(605):e801–e807. doi: 10.3399/bjgp12X659268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee C.R., Lee J.H., Kang L.W., Jeong B.C., Lee S.H. Educational effectiveness, target, and content for prudent antibiotic use. BioMed Res. Int. 2015;2015:214021. doi: 10.1155/2015/214021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baraka M.A., Alsultan H., Alsalman T., Alaithan H., Islam M.A., Alasseri A.A. Health care providers’ perceptions regarding antimicrobial stewardship programs (AMS) implementation-facilitators and challenges: a cross-sectional study in the Eastern province of Saudi Arabia. Ann. Clin. Microbiol. Antimicrob. 2019;18(1):26. doi: 10.1186/s12941-019-0325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hamdan S., El-Dahiyat F. Implementation and evaluation of an antimicrobial stewardship program across nine hospitals in the United Arab Emirates: a qualitative study. J. Pharm. Pract. Res. 2020;50(2):124–131. [Google Scholar]
- 32.Birgand G., Castro-Sánchez E., Hansen S., Gastmeier P., Lucet J.C., Ferlie E., Holmes A., Ahmad R. Comparison of governance approaches for the control of antimicrobial resistance: analysis of three European countries. Antimicrob. Resist. Infect. Contr. 2018;7:28. doi: 10.1186/s13756-018-0321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rogers Van Katwyk S., Grimshaw J.M., Nkangu M., Nagi R., Mendelson M., Taljaard M., Hoffman S.J. Government policy interventions to reduce human antimicrobial use: a systematic review and evidence map. PLoS Med. 2019;16(6) doi: 10.1371/journal.pmed.1002819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palavecino E.L., Williamson J.C., Ohl C.A. Collaborative antimicrobial stewardship: working with microbiology. Infect. Dis. Clin. 2020;34(1):51–65. doi: 10.1016/j.idc.2019.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacDougall C., Schwartz B.S., Kim L., Nanamori M., Shekarchian S., Chin-Hong P.V. An interprofessional curriculum on antimicrobial stewardship improves knowledge and attitudes toward appropriate antimicrobial use and collaboration. Open Forum Infect. Dis. 2017;4(1):ofw225. doi: 10.1093/ofid/ofw225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Logan A.Y., Williamson J.E., Reinke E.K., Jarrett S.W., Boger M.S., Davidson L.E. Establishing an antimicrobial stewardship collaborative across a large, diverse health care system. Joint Comm. J. Qual. Patient Saf. 2019;45(9):591–599. doi: 10.1016/j.jcjq.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Smith M.G., Witte M., Rocha S., Basner M. Effectiveness of incentives and follow-up on increasing survey response rates and participation in field studies. BMC Med. Res. Methodol. 2019;19:230. doi: 10.1186/s12874-019-0868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.