Abstract
Sepsis, defined as the dysregulated immune response to an infection leading to organ dysfunction, is one of the leading causes of mortality around the globe. Despite the significant progress in delineating the underlying mechanisms of sepsis pathogenesis, there are currently no effective treatments or specific diagnostic biomarkers in the clinical setting. The perturbation of cell signaling mechanisms, inadequate inflammation resolution, and energy imbalance, all of which are altered during sepsis, are also known to lead to defective lipid metabolism. The use of lipids as biomarkers with high specificity and sensitivity may aid in early diagnosis and guide clinical decision making. In addition, identifying the link between specific lipid signatures and their role in sepsis pathology may lead to novel therapeutics. In this review, we discuss the recent evidence on dysregulated lipid metabolism both in experimental and human sepsis focused on bioactive lipids, fatty acids, and cholesterol as well as the enzymes regulating their levels during sepsis. We highlight not only their potential roles in sepsis pathogenesis but also the possibility of using these respective lipid compounds as diagnostic and prognostic biomarkers of sepsis.
Supplementary key words: sepsis pathogenesis, lipid metabolism, biomarkers, inflammation, resolution, energy imbalance, bioactive lipids, fatty acids, cholesterol, prognostics
Abbreviations: AA, arachidonic acid; AEA, N-arachidonoylethanolamine; 2-AG, 2-Arachidonoylglycerol; ARDS, acute respiratory distress syndrome; ATX, autotaxin; CEPT, cholesteryl ester transfer; Cer, ceramide; 2-ClFA, 2-chlorofatty acid; 2-ClFALD, 2-chlorofatty aldehyde; CLP, cecal ligation puncture; 2-ClPA, 2- chloropalmitic acid; 2-ClSA, 2-chlorostearic acid; COX, cyclooxygenase; cPLA2, cytosolic PLA2; CS, cecal slurry; CYP450, cytochrome P450; cys-LTs, cysteinyl leukotrienes; DHET, dihydroxyeicosatrienoic acid; DPA, docosapentaenoic acid; EET, epoxy-eicosatrienoic acid; HOCl, hypochlorous acid; LOX, lipoxygenase; LPA, lysophosphatidic acid; LPC, lysophosphatidylcholine; LPCAT, lysophosphatidylcholine acyltransferase; LPS, lipopolysaccharide; LTA, lipotechoic acid; LTB4, leukotriene B4; MPO, myeloperoxidase; PAF, platelet-activating factor; PC, phosphatidylcholine; PCSK9, proprotein convertase subtilisin/kexin type 9; PLA2, phospholipase A2; rHDL, recombinant HDL; Rv, resolvin; RvD1-RvD6, D series resolvins; RvE1-RvE3, E series resolvins; RvT1-RvT4, T series resolvins; 10S,17S- diHDHA, 10(S),17(S) dihydroxy docosahexaenoic acid; sEH, soluble epoxide hydrolase; SIRS, systemic inflammatory response syndrome; S1P, sphingosine-1-phosphate; sPLA2, secretory phospholipase A2; Sphk, sphingosine kinase; SPMs, specialized pro-resolving mediators; SOFA, sequential organ failure assessment; TC, total cholesterol; TG, triglycerides; TLR, Toll-like receptors; TPPU, N-[1-(1-oxopropyl)-4-piperidinyl]-N′-[4-(trifluoromethoxy)phenyl]-urea; TXB2, thromboxane B2
Graphical abstract
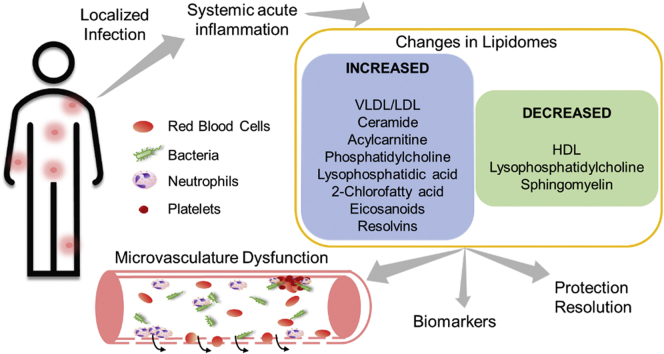
Sepsis is a systemic inflammatory disease with high morbidity and mortality caused by a dysregulated host immune response to an infection. Sepsis strikes more than 1.5 million patients in the United States per year, resulting in 250,000 deaths annually. Globally, sepsis has been estimated to account for 20% of all deaths (1). In the United States, the most common sources of sepsis are bacterial pneumonia, urinary tract infections, and abdominal infections (2). The dysregulated and systemic immune response during sepsis can lead to host tissue injury, organ failure, and death. Central to sepsis-elicited organ injury and mortality are complex interactions between blood cells, microbes, and endothelium, resulting in multiorgan microcirculatory failure. In sepsis, activated endothelial cells lead to an increased propensity for leukocyte and platelet adhesion and increased permeability barrier dysfunction (3, 4). In addition, the coagulation cascade is systemically activated, and endogenous anticoagulant factors are unable to sufficiently match the increased demand to appropriately control clotting (5). Platelets contribute to microthrombi formation, in addition to propagating proinflammatory signaling (6). Collectively, these changes to the endothelium and microvascular environment in sepsis lead to decreased oxygenated blood flow to target organs, contributing to organ failure. In addition to hypoxic injury, tissues can be damaged directly by the release of toxic leukocyte-derived proinflammatory mediators (7, 8). Dysfunctional neutrophils in sepsis are hyperinflammatory (9), have significant defects in localization and trafficking (10), and are resistant to regulatory apoptotic signaling pathways (11). These dysfunctional alterations further drive tissue damage and organ injury through the release of reactive oxygen species and proteases. Treatments for sepsis are limited to antibiotics targeting the infectious source and supportive care for organ dysfunction. Delineating the mechanisms underlying sepsis-induced microcirculatory collapse and the dysregulated immune response may lead to both improved therapeutic interventions and new biomarkers indicative of early sepsis and organ-specific risk.
Alterations in lipid metabolism and the activation of lipid signaling pathways are components of the complex milieu underlying the pathophysiological sequelae of sepsis. Lipid mediators play an important role in the proinflammatory and counterregulatory anti-inflammatory changes in the microvasculature in sepsis. Some of these lipids display significant inherent bioactivity, whereas others may be by-products and reflect important metabolic alterations in a septic patient. A better understanding of the various lipid species, their production and metabolism, and their bioactivity has the potential to lead to the development of early biomarkers and therapeutic targets in sepsis. This review focuses on the role of lipid alterations during sepsis and the potential of lipids to provide both new biomarkers and therapeutic targets to improve outcomes in sepsis.
Eicosanoids and specialized proresolving mediators
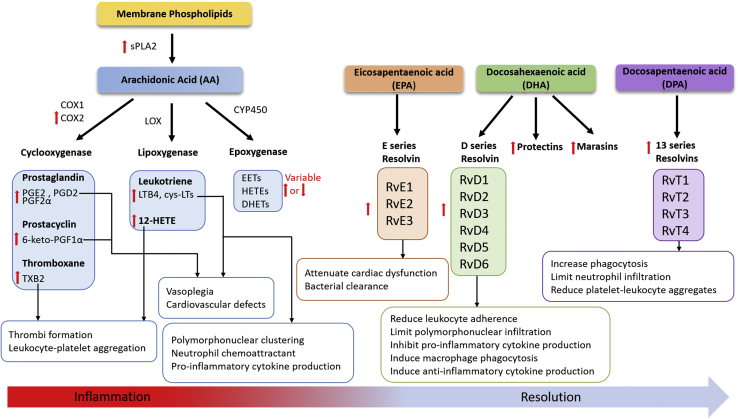
Leukocytes are recruited to sites of infection and inflammation, contributing to a multifactorial proinflammatory response, a component of which is the release of proinflammatory cytokines and eicosanoid mediators. This initial proinflammatory cascade activates antimicrobial systems intended to kill pathogens (12, 13). However, this response requires self-limitation to prevent exaggerated immune reactions from damaging host tissue. Thus, the proinflammatory response is balanced against an anti-inflammatory response mediated by anti-inflammatory cytokines and a class of lipids known as specialized proresolving mediators (SPMs). The proinflammatory eicosanoids are a family of bioactive lipids derived from arachidonic acid (AA). AA is released from membrane phospholipids by the phospholipase A2 (PLA2) enzymes (14, 15, 16). Liberated AA is then oxidized into eicosanoids by a variety of enzymes, including the cyclooxygenase (COX), lipoxygenase (LOX), and cytochrome P450 (CYP450) families. In an analogous manner, omega-3 fatty acids, including DHA, docosapentaenoic acid (n-3DPA), and EPA, exist in esterified pools in membrane phospholipids and are also released by PLA2. These liberated omega-3-fatty acids are themselves subsequently metabolized to yield the SPMs (Fig. 1).
Fig. 1.
Eicosanoids and SPMs in sepsis. Sepsis leads to either increased or decreased (red arrows) eicosanoid and SPM production. Secretory phospholipase A2 (sPLA2) activity is increased to liberate arachidonic (AA) from plasma phospholipids, and then inducible cyclooxygenase 2 (COX2) activity results in elevated production of cyclooxygenase-derived eicosanoids. Eicosanoids are mediators of proinflammatory mechanisms during sepsis. Proinflammatory mechanisms are counter-regulated by SPMs, which aid in the recovery of sepsis. EPA, DHA, and DPA derived SPMs are increased leading to reduction in inflammatory damage. COX1/2, cyclooxygenase 1/2; CYP450, cytochrome P450; cys-LT, cysteinyl leukotriene; DHET, dihydroxyeicosatrienoic acid; EET, epoxyeicosatrienoic acid; LOX, lipoxygenase; LTB4, leukotriene B4; PGD2, prostaglandin D2; PGE2, prostaglandin E2; PGF2α, prostaglandin F2α; RvD1-6, resolvin D 1–6; RvE1-3, resolvin E 1–3; RvT 1–4, resolvin T 1–4; sPLA2, secretory phospholipase A2; TXB2, thromboxane B2.
The roles of eicosanoids and SPMs have been investigated in sepsis and inflammatory processes (17, 18, 19, 20, 21). Mice subjected to the cecal ligation puncture (CLP) model of sepsis accumulate thromboxane B2 (derived from biologically active thromboxane A2) and 12-HETE, major platelet-derived eicosanoids that promote thrombus formation by inducing platelet activation, platelet aggregation, and vasoconstriction (22). The accumulation of these prothrombotic lipids in CLP sepsis is subsequently accompanied by increased thrombi formation in lung capillaries and enhanced monocyte, neutrophil, and platelet aggregates. Thrombosis and thrombocytopenia are common complications in human patients with sepsis owing to overconsumption of platelets for aggregation or adhesion to the endothelium and leukocytes (23, 24). Other studies also demonstrated similar elevations in eicosanoid lipid mediators during sepsis across species. Serum levels of the prostaglandins PGE2 and PGD2 are almost doubled in human subjects with sepsis. This elevated AA-derived eicosanoid production in human sepsis is accompanied by increased secretory PLA2 group IIA and COX-2 activity (25). Similarly, both CLP-septic and lipopolysaccharide (LPS)-treated mice have increased plasma PGE2 and PGE2 levels (26). Prostacyclin levels are also increased in sepsis, indicated by increased stable metabolite levels of 6-keto-PGF1α in plasma and perivascular adipose tissues of septic rats (27, 28). These studies also suggested that prostacyclin has a role in sepsis-induced vasoplegia and cardiovascular failure.
LPS administration and CLP sepsis in mice results in variable responses in plasma levels of various hydroxy-FA, dihydroxy-FA, and epoxy-FA eicosanoid species (26). The dihydroxy-FA species 14,15-DiHETE and 14,15-DiHETrE are elevated in plasma, suggesting targeting of EPA and AA acid, respectively, by CYP450 and then epoxygenase. Epoxy-eicosatrienoic acids (EETs) are metabolized to dihydroxyeicosatrienoic acids (DHETs) by soluble epoxide hydrolase (sEH) (29), and targeting this pathway may be important in sepsis owing to the anti-inflammatory properties of EETs. In CLP mice, sEH protein expression is increased in the brain tissues, mainly in endothelial cells, and is associated with cognitive deficits of sepsis-associated encephalopathy. Pharmacological inhibition of sEH with N-[1-(1-oxopropyl)-4-piperidinyl]-N′-[4-(trifluoromethoxy)phenyl]-urea (TPPU) in septic mice has improved the blood-brain barrier function and cognitive functions (30). TPPU has been shown to be a potent inhibitor of sEH resulting in increased EET levels and reduced inflammation (31). Recently, TPPU treatment of CLP mice was shown to increase EET levels, reduce multiorgan dysfunction, and reduce inflammation (32). The use of TPPU or other sEH inhibitors has yet to go forward in clinical trials, but this approach appears to be a promising potential treatment to be further explored.
Leukotriene B4 (LTB4) is a potent neutrophil chemoattractant and enhances phagocytosis (33). In mice subjected to fungal sepsis, complement activation induces LTB4-elicited intravascular neutrophil clustering (34). Both elevated LTB4 and cysteinyl leukotrienes are associated with pulmonary hemorrhage, hypoxemia, neutrophil infiltration, lung damage, and poor clinical outcomes in septic mice (35). Genetic ablations targeting either the LTB4 receptor or 5-lipoxygenase enzyme have been used to reduce leukotriene biological activity in sepsis models (34, 35). These manipulations attenuate inflammation and neutrophil recruitment in the lungs and the associated lung injury in septic mice. Furthermore, pharmacological inhibition of the LTB4 receptor increases anti-inflammatory cytokine IL-10 production in lungs and plasma of septic mice (35).
Significant alterations in SPM metabolism are evident in sepsis, suggesting defects in the resolution phase of acute inflammation. Resolvins (Rvs) are a class of SPMs and can be categorized into several subclasses: D series resolvins (RvD1-RvD6) derived from DHA, E series resolvins (RvE1-RvE3) derived from EPA, and T series resolvins (RvT1-RvT4) derived from n-3 DPA (Fig. 1). Resolvin concentrations are dramatically altered in human sepsis nonsurvivors and may potentially have prognostic value (21). Plasma RvD1-RvD6 and Maresin-1 are elevated in patients with sepsis, and significantly elevated concentrations of RvD5 are associated with sepsis nonsurvival (19, 36). Plasma RvT1-RvT4 and RvE1-RvE3 are also significantly elevated in patients with sepsis (36). Riché et al. (37) investigated temporal changes in resolvins in patients with sepsis. At the onset of sepsis, plasma RvD1 and RvD5 are at lower concentrations and are accompanied by an upregulated proinflammatory state. Following intensive care treatment and during long-term recovery, resolvin levels are elevated, potentially reflecting an attempt to counterbalance ongoing inflammation (37). Moreover, upregulated plasma protectin D1 isomer, 10(S),17(S) dihydroxy docosahexaenoic acid (10S,17S-diHDHA) levels correlate with acute respiratory distress syndrome (ARDS) development in patients with sepsis (19). In addition to a potential prognostic/diagnostic role, resolvins may also, in part, mediate sepsis pathophysiology, as RvD2 supplementation in CLP septic mice enhances clearance of bacteria, prevents sepsis-induced lethality, reduces neutrophil infiltration into the peritoneum, and inhibits proinflammatory cytokine production (38, 39). Intraperitoneal administration of RvT in Escherichia coli-infected mice protects mice from hypothermia, decreases neutrophil recruitment to sites of inflammation, and increases bacterial phagocytosis (36).
Taken together, it is well established that oxidation products derived from n-3 and n-6 fatty acids are produced in human and animal models of sepsis. These oxidation products have both proinflammatory and anti-inflammatory roles, and they have major roles in the complex biochemistry that drives sepsis pathophysiology. The complex nature of inflammatory balance during sepsis, in which the early-onset proinflammatory condition is followed by a compensatory immunosuppressive response, makes the role of these oxidized lipids crucial targets of further investigation, since they likely have a key mechanistic role in this balance. These anti- and proinflammatory roles as they relate to sepsis inflammation and resolution are summarized in Fig. 1.
Lysophosphatidylcholine
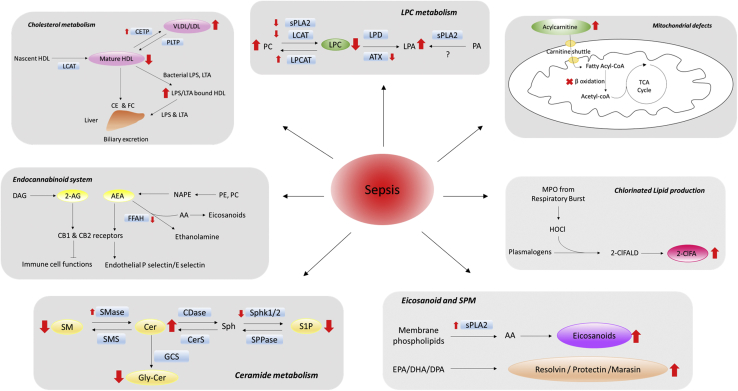
Products of PLA2 activity are fatty acids and lysophospholipids. Phosphatidylcholine (PC) hydrolysis by PLA2 yields fatty acid and lysophosphatidylcholine (LPC). LPC levels are tightly regulated by PLA2 activity and several isozymes of lysophosphatidylcholine acyltransferase (LPCAT), which are responsible for the reacylation of LPC (40). Alternatively, LPC can be further hydrolyzed by phospholipase D or autotaxin (ATX) to produce lysophosphatidic acid (LPA) (Fig. 2). LPC contributes to inflammation by increasing chemokine production and activating endothelium, neutrophils, monocytes, macrophages, and lymphocytes. Total serum LPC levels are significantly lower in human sepsis (21, 41), and other studies demonstrated that the major LPC molecular species (16:0 LPC, 18:0 LPC, 18:1 LPC, and 18:2 LPC) are decreased 50% in serum and plasma of patients with sepsis (42, 43). Less common species including 15:0 LPC, 18:3 LPC, 20:3 LPC, 20:4 LPC, and 20:5 LPC are also decreased in plasma and erythrocytes isolated from patients with sepsis (44).
Fig. 2.
Lipids altered in sepsis. Sepsis induces alterations in lipid signaling mechanisms (gray boxes). Red arrows indicated up- or downregulated lipids (big red arrows) and enzymes (small red arrows) relevant to respective lipid biosynthesis that are evident in sepsis. 2-AG, 2-arachidonoylglycerol; 2-ClFA, 2-chlorofatty acid; 2-ClFALD, 2-chlorofatty aldehyde; AA, arachidonic acid; AEA, N-arachidonoylethanolamine; ATX, autotaxin; CB1/2, cannabinoid receptor 1/2; CDase, ceramidase; CE, cholesterol ester; Cer, ceramide; CerS, ceramide synthase; CETP, cholesteryl ester transfer protein; DAG, diacylglycerol; FC, free cholesterol; FFAH, fatty acid amide hydrolase; GCS, glucosylceramide synthase; Gly-Cer, glycosylated ceramide; HOCl, hypochlorous acid; LPA, lysophosphatidic acid; LPC, lysophosphatidylcholine; LPCAT, lysophosphatidylcholine acyltransferase; LPD, lysophospholipase D; LPS, lipopolysaccharide; LTA, lipoteichoic acid; MPO, myeloperoxidase; NAPE, N-acyl phosphatidylethanolamine; PA, phosphatidic acid; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PLTP, phospholipid transfer protein; S1P, sphingosine-1-phosphate; SMase, sphingomyelinase; SMS, sphingomyelin synthase; Sph, sphingosine; Sphk1/2, sphingosine kinase 1/2; sPLA2, secretory phospholipases A2; SPPase, S1P-phosphatase.
Serum LPC concentrations may have utility in predicting sepsis severity. In human patients with sepsis, serum and plasma LPC concentrations increase over time in survivors but not in nonsurvivors, while persistently lower plasma LPC levels associate with 28- and 90-day mortality (45, 46). Of interest, lower plasma 24:0 LPC at day 7 is a strong predictor of 90-day mortality in patients with sepsis (45, 46). In addition, the molar ratio of LPC/PC is markedly decreased both in plasma and erythrocytes of patients with sepsis compared with heathy subjects (42, 44). Moreover, plasma LPC/PC is significantly decreased in sepsis nonsurvivors compared with survivors, and this ratio can predict 30-day mortality (42). Serum LPC may predict the source of infection in sepsis, potentially guiding appropriate antimicrobial therapy. For instance, elevated 26:1 LPC discriminates patients sick due to community-acquired pneumonia from those sick from other sources. In addition, LPC is lower in bacteremic compared with nonbacteremic sepsis (41, 47).
Ahn et al. (48) investigated mechanisms responsible for this decrease in plasma LPC levels in sepsis by assessing precursors, metabolites, and relevant enzymes in mice subjected to CLP. LPC was the only examined lipid that was decreased, whereas PC and LPA levels were increased in CLP mice. The activities of enzymes responsible for LPC production in plasma, lecithin-cholesterol acyltransferase and sPLA2, were greatly attenuated in these mice. Upregulated plasma LPCAT1-3 activity, accompanied with higher PC concentration, suggests that sepsis triggers LPC conversion to PC. Of interest, plasma ATX levels were decreased, yet plasma LPA levels were increased in septic mice, suggesting that mechanisms responsible for the reacylation of LPA were not activated, unlike those for LPC reacylation (48).
Exogenous LPC enhances neutrophil bacterial killing and augments monocyte chemotaxis in vitro (49, 50). Exogenous LPC acts through the G protein–coupled receptor, G2A, to inhibit the production of the proinflammatory cytokines IL-1β and TNF-α (51). In vivo, exogenous LPC increases bacterial clearance, reduces organ damage, and improves survival rates in septic mice and rats when administered alone or in combination with an antimicrobial agent (51, 52, 53, 54).
The role of LPC in sepsis needs to be further explored, as plasma and serum levels may provide improved prognostic biomarkers in human sepsis. Although animal studies suggest that increasing plasma LPC with exogenous administration is protective in sepsis, this would need to be carefully examined further in animal models to determine critical levels that are therapeutically advantageous. Alternately, pathways downstream of LPC could be identified to replicate the potentially beneficial role of LPC in sepsis, as LPC also has many deleterious consequences, including proinflammatory, atherogenic, and arrhythmogenic effects (55, 56, 57).
Phospholipase A2
Both secretory phospholipase A2 (sPLA2) and cytosolic PLA2 (cPLA2) have roles in regulating arachidonic acid release and subsequent oxylipid production as well as lysophospholipid production and degradation. Several studies have shown an upregulation of sPLA2 and cPLA2 in serum and plasma of patients with sepsis (58, 59, 60). cPLA2 activity in peripheral neutrophils isolated from patients with sepsis is increased 2-fold compared with activity in control neutrophils (61). This increased neutrophil cPLA2 activity returns to normal levels as sepsis resolves (4). Using a genetic knockout approach in mice, cPLA2-α knockout mice subjected to CLP have been shown to have an attenuated inflammatory response to sepsis. These mice had lower PGE2, LTB4, and 6-keto-PGF1α levels in peritoneal lavage fluid compared with control CLP mice. However, genetic ablation of cPLA2-α did not increase survival rates in these sepsis studies (62). It should also be appreciated that cPLA2-α is known to have lysophospholipase activity, which can modulate LPC levels and may contribute to deleterious effects in sepsis (63).
Of the two groups of sPLA2, subtype A (of the ten subtypes) of class II (i.e., sPLA2-IIA) has been shown to be associated with sepsis (64). Serum sPLA2-IIA activity has a positive correlation with early sepsis diagnosis in adult patients as well as with bacterial etiology (64, 65). Similarly, in neonatal sepsis plasma, sPLA2 activity was elevated, whereas infants with respiratory distress syndrome had higher sPLA2 activity (66). Furthermore, sPLA2-IIA has been shown to promote antibacterial activity (67, 68, 69). In other studies, reducing sPLA2 and cPLA2 activity with oligonucleotides against sPLA2-IIA and cPLA2-α led to increased survival in septic rats (70). Although sPLA2-IIA inhibitors have some beneficial effects in animal models of sepsis, clinical trials of the sPLA2-IIA inhibitor, LY315920NA/S-5920, failed to improve outcomes in human sepsis (71).
Platelet-activating factor
Platelet-activating factor (PAF) also likely contributes to sepsis pathophysiology. PAF is synthesized either by a remodeling pathway or by de novo biosynthesis (72, 73, 74, 75, 76). Proinflammatory signals such as LPS and thrombin induce PAF production by neutrophils and endothelial cells (77, 78, 79). Many cells and tissues express the PAF receptor, including monocytes, neutrophils, B-cells, platelets, endothelial cells, and lung tissue (80, 81). PAF can bind to, activate, and induce the aggregation of leukocytes and platelets (82, 83, 84) as well as reactive oxygen species production, NETosis, and platelet adherence (85, 86, 87, 88, 89). Considering its effects on cells in the microvasculature environment, it is unsurprising that PAF and enzymes responsible for PAF metabolism are implicated in sepsis (90).
In endotoxemia models, PAF levels associate with poor clinical outcomes. Overexpression of the PAF receptor increases LPS-induced mortality, as does PAF administration directly after LPS injections (91, 92). Conversely, enhancement of PAF metabolism leads to improved outcomes. PAF is metabolized by PAF-acetylhydrolase, which is expressed by many tissues and also associates with plasma lipoproteins (93, 94, 95, 96). Recombinant PAF-acetylhydrolase improves bacterial clearance in CLP models and survival after both CLP and endotoxemia (97, 98). In addition, PAF receptor blockade improves survival, leukocyte migration, and pathogen clearance after CLP (99).
Data from human patients with sepsis reinforces the association between PAF and mortality. Patients with sepsis have increased platelet-associated PAF compared with other patients (100). One study demonstrated that PAF in sepsis nonsurvivors has an increased half-life in plasma compared with sepsis survivors or healthy subjects. The same study also demonstrated that patients with sepsis have reduced plasma PAF-acetylhydrolase activity, increased plasma PLA2 activity, and decreased plasma lyso-PAF concentrations (101). Decreased PAF-acetylhydrolase activity is associated with multiorgan failure (102). Conversely, increased activity of PAF-acetylhydrolase 7 days following the diagnosis of ARDS is associated with survival (103). Unfortunately, however, recombinant PAF-acetylhydrolase did not improve sepsis outcomes in a phase III clinical trial (104).
Sphingolipids
Sphingolipids have profound functions in cell signaling and regulating inflammatory responses (105). Cellular processes regulated by sphingolipids include lymphocyte trafficking, mediating pro- and anti-inflammatory effects, and maintaining endothelial barrier integrity (105, 106). Investigations focusing on the role of sphingolipids in sepsis have predominantly focused on SM, ceramide (Cer) and sphingosine-1-phosphate (S1P) as biomarkers of sepsis outcomes. Several studies have shown decreased plasma levels of SM in patients with sepsis and decreased plasma levels of SM in patients with sepsis with acute lung injury (44, 107). A statistical model using combined serum 22:3 SM and 24:0 LPC concentrations discriminates sepsis from systemic inflammatory response syndrome (SIRS), a nonspecific state of systemic inflammation that does not require an infectious source (47). Other studies demonstrate that nonsurvivors within 28 days of sepsis diagnosis had a marked decline in plasma 20:2 SM compared with survivors (46). However, CLP rodents do not have decreased levels of plasma SM. Rather, 24 h after CLP, rodents have increased SM levels (108, 109). This is just one of many examples of the differences between human and rodent sepsis, which have made dissection of biochemical mechanisms in rodents difficult to translate into humans and have generated controversy regarding the applicability of rodent models of sepsis (110, 111).
Others have assessed the sphingomyelinase product, Cer, in sepsis. Both total Cer content and individual molecular species of Cer, including 16:0, 18:0, 20:0, 22:1, and 24:1, are elevated in plasma from patients with sepsis (42, 112). Furthermore, increased levels of Cer in peripheral blood mononuclear cells isolated from patients with sepsis are associated with the incidence of multiple organ dysfunction (113). The product/precursor ratio of Cer/SM has also been shown to progressively increase from day 1 to day 11 in human sepsis nonsurvivors (42).
S1P is a lipid mediator that regulates many physiological and pathological processes (114). Serum or plasma S1P levels in patients with septic are approximately 50% lower compared with healthy controls (115, 116, 117, 118), and S1P concentrations inversely correlate with Cer concentration and sequential organ failure assessment (SOFA) score, an assessment of the severity of critically ill patients (112). In blood, S1P is bound to apo-M on HDL particles and albumin (119). A marked loss of HDL and apo-M is observed in both septic patients and animal models of sepsis, which may account for decreased plasma S1P levels (116, 117). Diminished S1P levels likely have a role in lung injury during sepsis as treatments with S1P as well as its analog, FTY720, have been shown to improve barrier function (120, 121).
Plasma acid sphingomyelinase and neutral sphingomyelinase activity in platelets are increased in septic patients, suggesting an increase in SM metabolism in sepsis (112, 122). Treating endothelial cells with serum from septic patients resulted in endothelial cell SM loss accompanied by a sharp and rapid production of Cer, which then is further metabolized to glycosylated Cers (122). However, these results were not observed by Goeritzer et al. (123), who observed decreases in brain endothelial cell levels of Cer and an associated loss of barrier function after treatment with septic patient serum. Decreased S1P levels in human sepsis is likely the result of reduced sphingosine kinase (Sphk1 and Sphk2) activity (112). Sepsis-induced cardiac dysfunction, lung edema, and declined anti-inflammatory responses can be reversed by genetic ablation or pharmacological inhibition of sphingomyelin phosphodiesterase (SMPD1) and Sphk in septic rodents (118, 124, 125, 126). Overall, these investigations indicate that dysregulated sphingomyelin and Cer homeostasis have a significant role in sepsis pathogenesis.
Plasma lipoproteins, cholesterol, and triglycerides
HDL can bind to LPS and modulate infection and inflammation. LPS has greater affinity for HDL than it has for VLDL and LDL (127). It has been suggested that phosphates and the diglucosamine backbone, the functional groups of LPS, interact with HDL particles (128). Henning et al. (129) demonstrated that LPS binds to apo-A1, the major protein found in HDL particles. HDL can also bind to lipoteichoic acid (LTA), a component of gram-positive bacterial cell walls (130). The binding of HDL to either LPS or LTA interferes with its ability to bind to Toll-like receptors (TLRs) in macrophages (131). HDL also impairs TLR signaling by interfering with lipid rafts and by inducing ATF3, a key transcriptional modulator of innate immune response (131, 132). ATF3 activation by HDL acts as a negative-feedback loop to downregulate TLR-driven inflammatory responses (131). In addition to its endotoxin detoxification function, HDL modulates immune cell responses. HDL inhibits integrin CD11b and cytokines in monocyte and neutrophils (133, 134). It also suppresses neutrophil adhesion, spreading, and migration (134). HDL protects endothelium by modulating eNOS-dependent vascular tone, decreasing leukocyte adhesion to the endothelium, and promoting COX2 and prostacyclin to inhibit thrombosis (135, 136, 137). Nofer et al. demonstrated that HDL binds to S1P and interacts with S1P receptors on endothelium to activate eNOS in an Akt-dependent manner, whereas Yuhanna et al. demonstrated that HDL binds to scavenger receptor B1 to stimulate eNOS activity, resulting in vasorelaxation (135, 138). A recent study has shown that HDL-S1P levels are negatively correlated with endothelial dysfunction in septic patients and animal models (139). HDL-S1P administration also reduces LPS-induced acute lung injury in rats (139). Collectively, the protective actions of HDL on endothelium and sequestration of endotoxin suggest that HDL is protective during sepsis.
However, plasma HDL levels are decreased in human sepsis. An approximate 30% drop of HDL occurs in patients with sepsis on the day of hospital admission (usually defined as day 0) compared with healthy controls (140, 141). Decreased HDL levels are associated with increased sepsis mortality and can predict multiorgan dysfunction (142, 143, 144, 145). Furthermore, longitudinal studies reveal that serum HDL continually declines in sepsis nonsurvivors at day 0, day 3, and day 10 compared with survivors (144). Septic patients who have HDL concentrations lower than 25.1 mg/dl on hospital admission are highly susceptible to adverse outcomes, such as requirement of intensive care unit care, development of multiple- or single-organ dysfunctions (respiratory, circulatory, hepatic, or renal), and mortality (143). Low HDL has been associated with the development of sepsis-associated acute kidney injury and long-term declines in estimated glomerular filtration rate, suggesting that HDL may serve as biomarker of renal dysfunction in sepsis (146). HDL levels in septic patients are inversely correlated with proinflammatory cytokines such as TNF-α and IL-6 (144, 147). This decrease in HDL levels appears to be specific for systemic inflammation due to infection, as no decreases are observed in trauma, local infection, or SIRS (148, 149). Even after clinical recovery from sepsis, HDL remains at lower concentrations with marked reduction of anti-inflammatory properties, leaving the patients more vulnerable to secondary infection (141).
Small HDL particles have anti-inflammatory and antioxidant properties, and HDL particle size is altered in sepsis. Following low-dose endotoxemia in humans, the number of small and medium-sized HDL particles are decreased (150, 151). Patients with sepsis have shifts to large HDL particle sizes from intermediate and small HDL particles (140). This shift is due to impaired HDL maturation and increased catabolism of the small and medium-sized HDL particles. During endotoxemia, HDL particle remodeling is associated with increased endothelial lipases and sPLA2 and decreased plasma cholesteryl ester transfer protein (CETP) and lecithin-cholesterol acyltransferase activity (150). In addition, carriers of a rare variant in CETP have greater CETP activity and decreased HDL levels along with increased sepsis mortality (152).
Several studies have also examined the amounts of LDL-C, total cholesterol (TC), triglycerides (TGs), and apoproteins during sepsis (144, 145, 153). LDL-C, TC, apoA-1, and apoB were significantly decreased in sepsis nonsurvivors and decreased apoA-1 predicted sepsis-related 30-day mortality (142). TC, VLDL, and apoA-1 concentrations are significantly increased from day 1 to day 7 in pediatric patients with sepsis (153). Moreover, TC is negatively correlated with C-reactive protein, a well-known inflammatory biomarker of sepsis (153). Another study demonstrated an association of decreased TG levels with sepsis nonsurvival (145). Furthermore, adding TG levels to SOFA score improves the predictive accuracy of 28-day mortality (145). However, the utility of plasma TG as a prognostic marker is controversial, as Sharma et al. (154) found that TG concentrations do not change between sepsis survivors, sepsis nonsurvivors, and healthy controls.
Both LDL and HDL are important scavengers of toxins and bioactive lipids that can be taken up by the liver to provide protection. LDL uptake by the liver is dependent on membrane surface LDL receptor, which is reduced in the presence of proprotein convertase subtilisin/kexin type 9 (PCSK9). Walley and coworkers have shown human PCSK9 loss of function subjects in septic shock have improved survival and decreased inflammatory cytokine production (155). Furthermore, in mouse sepsis models, PCSK9 inhibition improved survival and reduced inflammation, which was not observed in similar experiments performed with LDL receptor–deficient mice. HDL has also been tested as a sepsis therapeutic in animal models. Recombinant high density lipoprotein (rHDL) reconstituted with apoA-1 Milano administered to endotoxin-challenged rats attenuates hepatic and renal dysfunction and enhances the anti-inflammatory and antioxidant capacity (156). Tanaka et al. (157) demonstrated that HDL improved survival rates, reduced inflammation, and enhanced bacterial clearance in animal models. In addition, the apoA-1 mimetic peptide 4F improves survival rates and cardiac performance in septic rats (158, 159). rHDL administered to experimental endotoxemic humans shows remarkable HDL elevation in plasma (160). Although these studies showing HDL protective actions in sepsis are promising, to date there have been no human sepsis studies conducted with either rHDL or mimetic peptides. However, there is a related ongoing phase I/II trial designed to stabilize cholesterol levels in sepsis and septic shock patients using a lipid emulsion containing fish oil (161, 162).
Chlorinated lipids
Neutrophils are early mediators of the host immune response during sepsis. The oxidants produced during neutrophil activation are important for bacterial killing, and they also have a role in organ damage through the release of reactive oxygen species (8, 163, 164, 165, 166). These reactive oxygen species target host tissue lipids. The chlorinated lipidome represents an important group of lipids produced by neutrophil-derived reactive oxygen species. Neutrophil-derived myeloperoxidase (MPO) produces hypochlorous acid (HOCl), a strong halogenating and oxidizing agent that reacts with both microbe and host molecules. The Ford group discovered that MPO-derived HOCl targets the vinyl ether bond at the sn-1 position of plasmalogen lipids (167). Plasmalogen vinyl ether oxidation by HOCl liberates 2-chlorofatty aldehyde (2-ClFALD) (168, 169), which is the precursor of other members of the chlorinated lipidome including 2-chlorofatty acid (2-ClFA) and 2-chlorofatty alcohol (170, 171).
Chlorinated lipids have been examined in both animal and human sepsis. Pike et al. (172) used a rat sepsis cecal slurry (CS) model that mimics early stages of human sepsis with antibiotic treatment and fluid resuscitation. Following CS treatment, rat sepsis nonsurvivors have higher plasma free 2-ClFA levels compared with survivors. Both free and esterified 2-ClFA are also increased in kidney, liver, lung, spleen, colon, and ileum of CS-treated rats. Moreover, exogenous 2-ClFA administration to rats results in loss of renal barrier function (172). Plasma 2-ClFA levels are also elevated in rat endotoxemia (173). In addition, the urinary metabolic clearance product of 2-ClFA, 2-chloroadipic acid, resulting from ω-oxidation and subsequent β-oxidation of 2-ClFA, is elevated in rats administered LPS (173). Brain and heart levels of 2-ClFALD have also been shown to be elevated in mouse endotoxemia (174, 175).
2-ClFA has also been investigated as a biomarker of human sepsis (176). Plasma levels of both free and esterified 2-chloropalmitic acid (2-ClPA) and 2-chlorostearic acid (2-ClSA) were assessed in the Molecular Epidemiology of Sepsis in the ICU cohort of human sepsis. Free and esterified forms of 2-ClSA and 2-ClPA plasma levels are significantly elevated in both sepsis survivors and sepsis nonsurvivors compared with healthy controls. Moreover, plasma 2-ClFA levels are elevated in patients who develop ARDS and 2-ClFA levels associate with 30-day mortality. When the plasma free 2-ClSA level is combined with the acute physiology and chronic health evaluation (APACHE) III score, ARDS prediction is significantly improved. Of interest, in septic neutropenic patients, plasma 2-ClFA levels are decreased, providing evidence that chlorinated lipid production is MPO dependent.
The potential roles of 2-ClFALD and 2-ClFA in sepsis and proinflammatory actions in the circulatory system have been investigated. Early studies demonstrated 2-ClFALD is a neutrophil chemoattractant and elicits endothelial P-selectin surface expression (168, 177). Subsequently, 2-ClFALD was shown to inhibit eNOS activity (178). 2-ClFA associates with endothelial Weibel-Palade bodies and mobilizes these granules, resulting in P-selectin surface expression, von Willebrand factor release, and angiopoietin 2 release. The mobilization of these granule contents results in increased neutrophil and platelet adherence, as well as loss of endothelial barrier function (179). 2-ClFA also induces neutrophil extracellular trap formation (180). Human monocytes treated with 2-ClFA undergo ER stress and produce reactive oxygen species (181). 2-ClFALD has also been shown to elicit brain endothelial barrier dysfunction (174) . However, the effects of 2-ClFALD on endothelial dysfunction and adhesion molecule surface expression is heterogeneous and varies with the tissue of origin of the endothelial vascular bed (182). 2-ClFA also elicits COX-2 expression in endothelial cells (183) and induces ER stress and mitochondrial dysfunction in brain endothelial cells (184). In vivo effects of 2-ClFA coupled with pharmacological manipulation have also been observed in the mesenteric microcirculation utilizing intravital microscopy (185, 186). Both 2-ClFALD and 2-ClFA elicit leukocyte rolling and adherence during superfusion in the mesenteric circulation (185). Furthermore, MPO inhibition with the pharmacologic inhibitor KYC reduces 2-ClFA levels, leukocyte rolling and adherence, and acute lung injury in CLP septic rats (186).
The role of chlorinated lipids in sepsis is a relatively new area of investigation compared with other lipids reviewed herein. 2-ClFA appears to be a good biomarker of sepsis outcomes that requires further multicenter evaluation and longitudinal studies. Furthermore, in addition to chlorinated lipids being biomarkers, they have profound effects at the level of the blood-vascular interface, which needs to be mechanistically explored as a therapeutic target to prevent microcirculatory collapse, organ failure, and mortality.
Endocannabinoids
Although endocannabinoids have a long-established role in central nervous system function, significant roles have been identified in inflammation, cardiovascular function, and gastrointestinal function (187). Among nine endocannabinoid species identified so far, the two most extensively studied are anandamide (N-arachidonoylethanolamine: AEA) and 2-arachidonoylglycerol (2-AG) (188). AEA and 2-AG are found mainly in brain tissue; however, 2-AG can also be found in the gut (189, 190) . Both AEA and 2-AG bind to cannabinoid receptors CB1 and CB2, which are G protein–coupled receptors (191). CB1 receptors are enriched in the brain and spinal cord, whereas they are expressed at a lower degree in liver and pancreatic islet cells. CB2 receptors are found in immune cells such as macrophages and lymphocytes, with a reduced expression in neuronal cells (192).
The endocannabinoids have biological activity directed at the blood-vascular interface, which is critical in sepsis. 2-AG induces E-selectin and P-selectin on endothelial cells, resulting in leukocyte adhesion to endothelial cells, as well as promoting eosinophil chemotaxis and reducing endothelial cell viability (193, 194, 195). Endocannabinoids also have profound effects on immune cells (196); both 2-AG and AEA have immunosuppressive functions. AEA suppresses IL-12 and IL-6 production in monocytes (197), and endocannabinoids inhibit TNF-α release in LPS-treated microglia cells (198). Mestre et al. (199) demonstrated that AEA suppresses VCAM-1 and leukocyte migration through CB1 activation following viral infection in endothelial cells. Endocannabinoid metabolites also mediate inflammation, as AEA is hydrolyzed by fatty acid amide hydrolase into ethanolamine and AA, and the liberated AA serves as a substrate for COX-2, LOX, and P450, as discussed above (200). Fatty acid amide hydrolase mRNA levels in the whole blood of patients with sepsis is significantly reduced compared with healthy controls and remains low in sepsis mortality (201).
Endocannabinoid receptors have been examined as potential targets for sepsis therapeutics. Tschöp et al. (202) and Gui et al. (203) demonstrated that deletion of CB2 receptors results in increased mortality following CLP-induced sepsis or LPS-induced endotoxemia. Twenty-four hours following CLP sepsis induction, CB2-deficient mice have increased lung damage, neutrophil activation, and leukocyte recruitment in lungs (202). However, Kapellos et al. (204) did not observe a significant difference in neutrophil recruitment to the lungs following 2 and 8 h of LPS challenge in CB2 knockout mice. In these mice, splenic chemokines were increased following 2 h of LPS challenge, which was accompanied by transient neutrophil recruitment to the spleen (204). The conflicting data on neutrophil recruitment to the lungs with CB2 knockdown may be attributed to both the differences in the duration of the treatment and differences in bacterial sepsis and LPS endotoxemia (202, 204). On the other hand, pharmacological activation of CB2 receptors reduces sepsis-induced leukocyte adherence in the microcirculation, neutrophil recruitment to lungs, and lung damage in rodents (202, 205, 206). Deleting the CB2 receptor in LPS-treated or CLP-septic mice results in increased serum IL-6 and TNF-α levels, and CB2 agonism prevents the increases in proinflammatory cytokine levels in septic animals (202, 203, 206). Also, the CB2 agonist, HU308, decreases plasma levels of ICAM and VCAM in septic mice (206).
CB2 agonism may have a beneficial role in sepsis, whereas antagonism of the CB1 receptor seems to have a protective role (207). The CB1 antagonist, rimonabant, administered 4 h following the induction of CLP-sepsis in rats provides a significant increase in survival compared with vehicle-treated rats. CB1 blocking does not change sepsis-induced leukopenia, neutrophil migration, or plasma IL-6 levels. However, CB1 blockage in late-stage sepsis in CLP rats significantly increases plasma arginine vasopressin, a mediator associated with septic shock, multiple-organ failure, and death (208, 209). Thus, blocking CB1 may have a beneficial role in restoring patient blood pressure in late phase sepsis.
Acylcarnitine and fatty acid metabolism
During sepsis, lipids are mobilized from adipose tissue and liver (210, 211). Accordingly, investigations have evaluated fatty acid intermediates as biomarkers of sepsis. Chung et al. (212) and Schmerler et al. (213) demonstrated short- and medium-chain acylcarnitines (C2–C10) are elevated in the plasma of patients with sepsis. The elevated plasma acylcarnitines are associated with SOFA score, hepatobiliary and renal dysfunction, thrombocytopenia, and hyperlactatemia (212). Plasma acetylcarnitine has a positive correlation with plasma IL-6, IL-8, and IL-10 as well as sepsis severity in patients (212). Plasma acylcarnitine levels may also provide a prognostic tool in human sepsis. Twenty-eight-day sepsis nonsurvivors have a 2-fold increase in plasma concentrations of cis-4-decenoylcarnitine (C10:1), hexanoylcarnitine (C6), butyroylcarnitine (C4) compared with sepsis survivors (214). Furthermore, increased plasma concentrations of medium-chain acylcarnitines differentiate sepsis from noninfectious SIRS (213). Elevated acylcarnitine levels detected during neonatal blood spot screening associate strongly with development of neonatal sepsis (215).
Mitochondrial function and fatty acid β-oxidation is compromised in human sepsis (216, 217, 218). In addition to elevated levels of acylcarnitines, elevated concentrations of tricarboxylic acid cycle metabolites such as lactic acid, pyruvate, and citric acid further confirm dysfunctional energy production due to mitochondrial defects (21, 214). Several investigators have studied the underlying molecular mechanisms of dysregulated lipid oxidation observed in sepsis (219, 220, 221, 222). Experimental animal sepsis identified decreased expression of fatty acid transport protein-2, fatty acyl-CoA synthase, carnitine palmitoyltransferase-1, medium-chain acyl-CoA dehydrogenase, acyl-CoA oxidase, and PPARs (219, 220, 221, 222). The role of PPAR-α is of particular interest, as leukocyte PPAR-α expression declines with the severity of sepsis in humans (223). In addition, in mouse CLP, liver PPAR-α is decreased, leading to increased plasma free fatty acids and hepatic lipotoxicity (211). The PPAR-α agonist pemafibrate reversed liver lipotoxicity following CLP sepsis (211). PPAR-α agonists also reduce LPS-elicited acute lung injury (223, 224). Septic PPAR-α-deficient mice also have reduced cardiac function and hepatic metabolic compensation compared with wild-type mice (225, 226). Although these studies suggest PPAR-α agonists to be potential human sepsis therapeutics, it is unclear whether altered PPAR-α levels are causal or coincidental (227). No clinical trials have been attempted with PPAR-α agonists in sepsis, which may be due to the cardiac, skeletal muscle, renal, and bone marrow toxicities caused by PPAR-α agonist in clinical trials for patients with atherogenic hypercholesterolemia or dyslipidemia (228).
Concluding Remarks
Sepsis is the most common cause of death in hospitalized patients. The diverse sources of infection, the heterogeneity of immune and vascular responses, as well as multiorgan failure make sepsis extremely difficult to biochemically dissect to improve therapeutics. There is also a desperate need to improve early sepsis detection since mortality from sepsis increases 4% for every 1-h delay in sepsis diagnosis (229). Understanding the role of lipid biology during sepsis has the potential to provide new insights into the pathophysiology of sepsis and provide new therapeutic targets. This review has focused on lipid signaling molecules; fatty acid and cholesterol metabolism and transit via their macromolecular complexes; and new classes of lipids produced as a result of interactions with reactive oxygen species. As we move forward with future investigations focusing on the lipid biology of sepsis, it will be important to consider the limitations of animal models and consider studies that include human cells or organoids. There are considerable interspecies differences in genomes and innate and adaptive immunity responses, and within the human population itself, there is significant heterogeneity and variable comorbidities (230). Although animal models may not completely recapitulate human sepsis, they have been a key tool in understanding the pathogenicity of sepsis. Variability between different animal models (e.g., CLP, LPS administration, pneumonia models), the infection dosing strategy, and any clinical interventions such as antibiotics or fluid resuscitation may affect the identification of lipid-mediated pathology and lipid biomarkers. In addition, the diversity of sepsis sources, immune responses, and outcomes requires large sample sizes and multicenter studies in order to move forward with translational studies involving lipids. Finally, the integration of lipidomics with other omic platforms and big data sets from patient studies, in conjunction with artificial intelligence and computational modeling, should provide new insights in the lipid biology in sepsis.
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Acknowledgments
Author contributions
K. A. and D. P. P. writing-original draft; K. A., D. P. P., and D. A. F. writing-review and editing; K.A. visualization.
Funding and additional information
This study was supported by research funding from the National Institutes of Health R01GM115553 and R01GM129508 to D. A. F. and F30HL142193 to D. P. P. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Rudd K.E., Johnson S.C., Agesa K.M., Shackelford K.A., Tsoi D., Kievlan D.R., Colombara D.V., Ikuta K.S., Kissoon N., Finfer S., Fleischmann-Struzek C., Machado F.R., Reinhart K.K., Rowan K., Seymour C.W. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the gobal burden of disease study. Lancet. 2020;395:200–211. doi: 10.1016/S0140-6736(19)32989-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mayr F.B., Yende S., Angus D.C. Epidemiology of severe sepsis. Virulence. 2014;5:4–11. doi: 10.4161/viru.27372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Opal S.M., van der Poll T. Endothelial barrier dysfunction in septic shock. J. Int. Med. 2015;277:277–293. doi: 10.1111/joim.12331. [DOI] [PubMed] [Google Scholar]
- 4.Colbert J.F., Schmidt E.P. Endothelial and microcirculatory function and dysfunction in sepsis. Clin. Chest Med. 2016;37:263–275. doi: 10.1016/j.ccm.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levi M., van der Poll T. Inflammation and coagulation. Crit. Care Med. 2010;38:S26–34. doi: 10.1097/CCM.0b013e3181c98d21. [DOI] [PubMed] [Google Scholar]
- 6.Katz J.N., Kolappa K.P., Becker R.C. Beyond thrombosis: the versatile platelet in critical illness. Chest. 2011;139:658–668. doi: 10.1378/chest.10-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delano M.J., Ward P.A. The immune system's role in sepsis progression, resolution, and long-term outcome. Immunol. Rev. 2016;274:330–353. doi: 10.1111/imr.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen X.F., Cao K., Jiang J.P., Guan W.X., Du J.F. Neutrophil dysregulation during sepsis: an overview and update. J. Cell Mol. Med. 2017;21:1687–1697. doi: 10.1111/jcmm.13112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drifte G., Dunn-Siegrist I., Tissières P., Pugin J. Innate immune functions of immature neutrophils in patients with sepsis and severe systemic inflammatory response syndrome. Crit. Care Med. 2013;41:820–832. doi: 10.1097/CCM.0b013e318274647d. [DOI] [PubMed] [Google Scholar]
- 10.Lerman Y.V., Kim M. Neutrophil migration under normal and sepsis conditions. Cardiovasc. Hematol. Disord. Drug Targets. 2015;15:19–28. doi: 10.2174/1871529x15666150108113236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hotchkiss R.S., Monneret G., Payen D. Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat. Rev. Immunol. 2013;13:862–874. doi: 10.1038/nri3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cicchese J.M., Evans S., Hult C., Joslyn L.R., Wessler T., Millar J.A., Marino S., Cilfone N.A., Mattila J.T., Linderman J.J., Kirschner D.E. Dynamic balance of pro- and anti-inflammatory signals controls disease and limits pathology. Immunol. Rev. 2018;285:147–167. doi: 10.1111/imr.12671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rastogi D., Ratner A.J., Prince A. Host-bacterial interactions in the initiation of inflammation. Paediatr. Respir. Rev. 2001;2:245–252. doi: 10.1053/prrv.2001.0147. [DOI] [PubMed] [Google Scholar]
- 14.Dennis E.A., Cao J., Hsu Y.-H., Magrioti V., Kokotos G. Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 2011;111:6130–6185. doi: 10.1021/cr200085w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balsinde J., Balboa M.A., Insel P.A., Dennis E.A. Regulation and inhibition of phospholipase A2. Annu. Rev. Pharmacol. Toxicol. 1999;39:175–189. doi: 10.1146/annurev.pharmtox.39.1.175. [DOI] [PubMed] [Google Scholar]
- 16.Lambeau G., Gelb M.H. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu. Rev. Biochem. 2008;77:495–520. doi: 10.1146/annurev.biochem.76.062405.154007. [DOI] [PubMed] [Google Scholar]
- 17.Tam V.C. Lipidomic profiling of bioactive lipids by mass spectrometry during microbial infections. Semin. Immunol. 2013;25:240–248. doi: 10.1016/j.smim.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serhan C.N., Levy B.D. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J. Clin. Invest. 2018;128:2657–2669. doi: 10.1172/JCI97943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dalli J., Colas R.A., Quintana C., Barragan-Bradford D., Hurwitz S., Levy B.D., Choi A.M., Serhan C.N., Baron R.M. Human sepsis eicosanoid and proresolving lipid mediator temporal profiles: correlations with survival and clinical outcomes. Crit. Care Med. 2017;45:58–68. doi: 10.1097/CCM.0000000000002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruegel M., Ludwig U., Kleinhempel A., Petros S., Kortz L., Ceglarek U., Holdt L.M., Thiery J., Fiedler G.M. Sepsis-associated changes of the arachidonic acid metabolism and their diagnostic potential in septic patients. Crit. Care Med. 2012;40:1478–1486. doi: 10.1097/CCM.0b013e3182416f05. [DOI] [PubMed] [Google Scholar]
- 21.Wang J., Sun Y., Teng S., Li K. Prediction of sepsis mortality using metabolite biomarkers in the blood: a meta-analysis of death-related pathways and prospective validation. BMC Med. 2020;18:83. doi: 10.1186/s12916-020-01546-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vardon Bounes F., Mémier V., Marcaud M., Jacquemin A., Hamzeh-Cognasse H., Garcia C., Series J., Sié P., Minville V., Gratacap M.-P., Payrastre B. Platelet activation and prothrombotic properties in a mouse model of peritoneal sepsis. Sci. Rep. 2018;8:13536. doi: 10.1038/s41598-018-31910-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Claushuis T.A.M., van Vught L.A., Scicluna B.P., Wiewel M.A., Klein Klouwenberg P.M.C., Hoogendijk A.J., Ong D.S.Y., Cremer O.L., Horn J., Franitza M., Toliat M.R., Nürnberg P., Zwinderman A.H., Bonten M.J., Schultz M.J. Thrombocytopenia is associated with a dysregulated host response in critically ill sepsis patients. Blood. 2016;127:3062–3072. doi: 10.1182/blood-2015-11-680744. [DOI] [PubMed] [Google Scholar]
- 24.Larkin C.M., Santos-Martinez M.-J., Ryan T., Radomski M.W. Sepsis-associated thrombocytopenia. Thromb. Res. 2016;141:11–16. doi: 10.1016/j.thromres.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 25.Ahmad N.S., Tan T.L., Arifin K.T., Ngah W.Z.W., Yusof Y.A.M. High sPLA2-IIA level is associated with eicosanoid metabolism in patients with bacterial sepsis syndrome. PLoS One. 2020;15 doi: 10.1371/journal.pone.0230285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willenberg I., Rund K., Rong S., Shushakova N., Gueler F., Schebb N.H. Characterization of changes in plasma and tissue oxylipin levels in LPS and CLP induced murine sepsis. Inflamm. Res. 2016;65:133–142. doi: 10.1007/s00011-015-0897-7. [DOI] [PubMed] [Google Scholar]
- 27.Awata W.M.C., Gonzaga N.A., Borges V.F., Silva C.B.P., Tanus-Santos J.E., Cunha F.Q., Tirapelli C.R. Perivascular adipose tissue contributes to lethal sepsis-induced vasoplegia in rats. Eur. J. Pharmacol. 2019;863:172706. doi: 10.1016/j.ejphar.2019.172706. [DOI] [PubMed] [Google Scholar]
- 28.Höcherl K., Schmidt C., Kurt B., Bucher M. Activation of the PGI2/IP system contributes to the development of circulatory failure in a rat model of endotoxic shock. Hypertension. 2008;52:330–335. doi: 10.1161/HYPERTENSIONAHA.108.112029. [DOI] [PubMed] [Google Scholar]
- 29.Spector A.A. Arachidonic acid cytochrome P450 epoxygenase pathway. J. Lipid Res. 2009;50 Suppl:S52–56. doi: 10.1194/jlr.R800038-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang P., Wang W., Hu Y., Li Y. Prolonged soluble epoxide hydrolase reactivity in brain endothelial cells is associated with long cognitive deficits in sepsis. Mol. Neurobiol. 2020;57:2846–2855. doi: 10.1007/s12035-020-01925-2. [DOI] [PubMed] [Google Scholar]
- 31.Schmelzer K.R., Kubala L., Newman J.W., Kim I.H., Eiserich J.P., Hammock B.D. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc. Natl. Acad. Sci. U.S.A. 2005;102:9772–9777. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Z., Tang Y., Yu J., Dong R., Yang Y., Fu M., Luo J., Hu S., Wang D.W., Tu L., Xu X. sEH inhibitor Tppu ameliorates cecal ligation and puncture-induced sepsis by regulating macrophage functions. Shock. 2020;53:761–771. doi: 10.1097/SHK.0000000000001408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brandt S.L., Serezani C.H. Too much of a good thing: How modulating LTB(4) actions restore host defense in homeostasis or disease. Semin. Immunol. 2017;33:37–43. doi: 10.1016/j.smim.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee E.K.S., Gillrie M.R., Li L., Arnason J.W., Kim J.H., Babes L., Lou Y., Sanati-Nezhad A., Kyei S.K., Kelly M.M., Mody C.H., Ho M., Yipp B.G. Leukotriene B4-mediated neutrophil recruitment causes pulmonary capillaritis during lethal fungal sepsis. Cell Host Microbe. 2018;23:121–133.e4. doi: 10.1016/j.chom.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 35.Monteiro A.P.T., Soledade E., Pinheiro C.S., Dellatorre-Teixeira L., Oliveira G.P., Oliveira M.G., Peters-Golden M., Rocco P.R.M., Benjamim C.F., Canetti C. Pivotal role of the 5-lipoxygenase pathway in lung injury after experimental sepsis. Am. J. Respir. Cell Mol. Biol. 2014;50:87–95. doi: 10.1165/rcmb.2012-0525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dalli J., Chiang N., Serhan C.N. Elucidation of novel 13-series resolvins that increase with atorvastatin and clear infections. Nat. Med. 2015;21:1071–1075. doi: 10.1038/nm.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riché F., Chousterman B.G., Valleur P., Mebazaa A., Launay J.-M., Gayat E. Protracted immune disorders at one year after ICU discharge in patients with septic shock. Crit. Care. 2018;22:42. doi: 10.1186/s13054-017-1934-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spite M., Norling L.V., Summers L., Yang R., Cooper D., Petasis N.A., Flower R.J., Perretti M., Serhan C.N. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461:1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiang N., de la Rosa X., Libreros S., Serhan C.N. Novel resolvin D2 receptor axis in infectious inflammation. J. Immunol. 2017;198:842–851. doi: 10.4049/jimmunol.1601650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang B., Tontonoz P. Phospholipid remodeling in physiology and disease. Ann. Rev. Physiol. 2019;81:165–188. doi: 10.1146/annurev-physiol-020518-114444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cho W.H., Park T., Park Y.Y., Huh J.W., Lim C.M., Koh Y., Song D.K., Hong S.B. Clinical significance of enzymatic lysophosphatidylcholine (LPC) assay data in patients with sepsis. Eur. J. Clin. Microbiol. Infect. Dis. 2012;31:1805–1810. doi: 10.1007/s10096-011-1505-6. [DOI] [PubMed] [Google Scholar]
- 42.Drobnik W., Liebisch G., Audebert F.-X., Fröhlich D., Glück T., Vogel P., Rothe G., Schmitz G. Plasma ceramide and lysophosphatidylcholine inversely correlate with mortality in sepsis patients. J. Lipid Res. 2003;44:754–761. doi: 10.1194/jlr.M200401-JLR200. [DOI] [PubMed] [Google Scholar]
- 43.Park J.-M., Noh J.-Y., Kim M.-J., Yun T.G., Lee S.-G., Chung K.S., Lee E.H., Shin M.H., Ku N.S., Yoon S., Kang M.-J., Park M.S., Pyun J.-C. MALDI-TOF mass spectrometry based on parylene-matrix chip for the analysis of lysophosphatidylcholine in sepsis patient sera. Anal. Chem. 2019;91:14719–14727. doi: 10.1021/acs.analchem.9b04019. [DOI] [PubMed] [Google Scholar]
- 44.Mecatti G.C., Fernandes Messias M.C., Sant’Anna Paiola R.M., Figueiredo Angolini C.F., da Silva Cunha I.B., Eberlin M.N., de Oliveira Carvalho P. Lipidomic profiling of plasma and erythrocytes from septic patients reveals potential biomarker candidates. Biomark Insights. 2018;13 doi: 10.1177/1177271918765137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park D.W., Kwak D.S., Park Y.Y., Chang Y., Huh J.W., Lim C.-M., Koh Y., Song D.-K., Hong S.-B. Impact of serial measurements of lysophosphatidylcholine on 28-day mortality prediction in patients admitted to the intensive care unit with severe sepsis or septic shock. J. Crit. Care. 2014;29:882.e5–882.e11. doi: 10.1016/j.jcrc.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 46.Ferrario M., Cambiaghi A., Brunelli L., Giordano S., Caironi P., Guatteri L., Raimondi F., Gattinoni L., Latini R., Masson S., Ristagno G., Pastorelli R. Mortality prediction in patients with severe septic shock: a pilot study using a target metabolomics approach. Sci. Rep. 2016;6:20391. doi: 10.1038/srep20391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neugebauer S., Giamarellos-Bourboulis E.J., Pelekanou A., Marioli A., Baziaka F., Tsangaris I., Bauer M., Kiehntopf M. Metabolite profiles in sepsis: developing prognostic tools based on the type of infection. Crit. Care Med. 2016;44:1649–1662. doi: 10.1097/CCM.0000000000001740. [DOI] [PubMed] [Google Scholar]
- 48.Ahn W.-G., Jung J.-S., Kwon H.Y., Song D.-K. Alteration of lysophosphatidylcholine-related metabolic parameters in the plasma of mice with experimental sepsis. Inflammation. 2017;40:537–545. doi: 10.1007/s10753-016-0500-6. [DOI] [PubMed] [Google Scholar]
- 49.Hong C.-W., Kim T.-K., Ham H.-Y., Nam J.-S., Kim Y.H., Zheng H., Pang B., Min T.-K., Jung J.-S., Lee S.-N., Cho H.-J., Kim E.-J., Hong I.-H., Kang T.-C., Lee J. Lysophosphatidylcholine increases neutrophil bactericidal activity by enhancement of azurophil granule-phagosome fusion via glycine·GlyRα2/TRPM2/p38 MAPK signaling. J. Immunol. 2010;184:4401–4413. doi: 10.4049/jimmunol.0902814. [DOI] [PubMed] [Google Scholar]
- 50.Rolin J., Vego H., Maghazachi A.A. Oxidized lipids and lysophosphatidylcholine induce the chemotaxis, up-regulate the expression of CCR9 and CXCR4 and abrogate the release of IL-6 in human monocytes. Toxins. 2014;6:2840–2856. doi: 10.3390/toxins6092840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yan J.-J., Jung J.-S., Lee J.-E., Lee J., Huh S.-O., Kim H.-S., Jung K.C., Cho J.-Y., Nam J.-S., Suh H.-W., Kim Y.-H., Song D.-K. Therapeutic effects of lysophosphatidylcholine in experimental sepsis. Nat. Med. 2004;10:161–167. doi: 10.1038/nm989. [DOI] [PubMed] [Google Scholar]
- 52.Smani Y., Domínguez-Herrera J., Ibáñez-Martínez J., Pachón J. Therapeutic efficacy of lysophosphatidylcholine in severe infections caused by Acinetobacter baumannii. Antimicrob. Agents Chemother. 2015;59:3920–3924. doi: 10.1128/AAC.04986-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Murch O., Collin M., Sepodes B., Foster S.J., Mota-Filipe H., Thiemermann C. Lysophosphatidylcholine reduces the organ injury and dysfunction in rodent models of gram-negative and gram-positive shock. Br. J. Pharmacol. 2006;148:769–777. doi: 10.1038/sj.bjp.0706788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Parra Millán R., Jiménez Mejías M.E., Sánchez Encinales V., Ayerbe Algaba R., Gutiérrez Valencia A., Pachón Ibáñez M.E., Díaz C., Pérez Del Palacio J., López Cortés L.F., Pachón J., Smani Y. Efficacy of lysophosphatidylcholine in combination with antimicrobial agents against Acinetobacter baumannii in experimental murine peritoneal sepsis and pneumonia models. Antimicrob. Agents Chemother. 2016;60:4464–4470. doi: 10.1128/AAC.02708-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schmitz G., Ruebsaamen K. Metabolism and atherogenic disease association of lysophosphatidylcholine. Atherosclerosis. 2010;208:10–18. doi: 10.1016/j.atherosclerosis.2009.05.029. [DOI] [PubMed] [Google Scholar]
- 56.Han J.H., Cao C., Kim S.M., Piao F.L., Kim S.H. Attenuation of lysophosphatidylcholine-induced suppression of ANP release from hypertrophied atria. Hypertension. 2004;43:243–248. doi: 10.1161/01.HYP.0000107779.92645.89. [DOI] [PubMed] [Google Scholar]
- 57.Cedars A., Jenkins C.M., Mancuso D.J., Gross R.W. Calcium-independent phospholipases in the heart: mediators of cellular signaling, bioenergetics, and ischemia-induced electrophysiologic dysfunction. J. Cardiovasc. Pharmacol. 2009;53:277–289. [PMC free article] [PubMed] [Google Scholar]
- 58.Endo S., Inada K., Nakae H., Takakuwa T., Yamada Y., Suzuki T., Taniguchi S., Yoshida M., Ogawa M., Teraoka H. Plasma levels of type II phospholipase A2 and cytokines in patients with sepsis. Res. Comm. Mol. Path. Pharmacol. 1995;90:413–421. [PubMed] [Google Scholar]
- 59.Vadas P., Scott K., Smith G., Rajkovic I., Stefanski E., Schouten B.D., Singh R., Pruzanski W. Serum phospholipase A2 enzyme activity and immunoreactivity in a prospective analysis of patients with septic shock. Life Sci. 1992;50:807–811. doi: 10.1016/0024-3205(92)90186-s. [DOI] [PubMed] [Google Scholar]
- 60.Green J.A., Smith G.M., Buchta R., Lee R., Ho K.Y., Rajkovic I.A., Scott K.F. Circulating phospholipase A2 activity associated with sepsis and septic shock is indistinguishable from that associated with rheumatoid arthritis. Inflammation. 1991;15:355–367. doi: 10.1007/BF00917352. [DOI] [PubMed] [Google Scholar]
- 61.Levy R., Dana R., Hazan I., Levy I., Weber G., Smoliakov R., Pesach I., Riesenberg K., Schlaeffer F. Elevated cytosolic phospholipase A(2) expression and activity in human neutrophils during sepsis. Blood. 2000;95:660–665. [PubMed] [Google Scholar]
- 62.Uozumi N., Kita Y., Shimizu T. Modulation of lipid and protein mediators of inflammation by cytosolic phospholipase A2 alpha during experimental sepsis. J. Immunol. 2008;181:3558–3566. doi: 10.4049/jimmunol.181.5.3558. [DOI] [PubMed] [Google Scholar]
- 63.Yamashita A., Tanaka K., Kamata R., Kumazawa T., Suzuki N., Koga H., Waku K., Sugiura T. Subcellular localization and lysophospholipase/transacylation activities of human group IVC phospholipase A2 (cPLA2gamma) Biochim. Biophys. Acta. 2009;1791:1011–1022. doi: 10.1016/j.bbalip.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 64.Tan T.L., Ahmad N.S., Nasuruddin D.N., Ithnin A., Tajul Arifin K., Zaini I.Z., Wan Ngah W.Z. CD64 and group II secretory phospholipase A2 (sPLA2-IIA) as biomarkers for distinguishing adult sepsis and bacterial infections in the emergency department. PLoS One. 2016;11 doi: 10.1371/journal.pone.0152065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tan T.L., Goh Y.Y. The role of group IIA secretory phospholipase A2 (sPLA2-IIA) as a biomarker for the diagnosis of sepsis and bacterial infection in adults-A systematic review. PLoS One. 2017;12 doi: 10.1371/journal.pone.0180554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schrama A.J., de Beaufort A.J., Poorthuis B.J., Berger H.M., Walther F.J. Secretory phospholipase A2 in newborn infants with sepsis. J. Perinatology. 2008;28:291–296. doi: 10.1038/sj.jp.7211929. [DOI] [PubMed] [Google Scholar]
- 67.Weinrauch Y., Abad C., Liang N.S., Lowry S.F., Weiss J. Mobilization of potent plasma bactericidal activity during systemic bacterial challenge. Role of group IIA phospholipase A2. J. Clin. Invest. 1998;102:633–638. doi: 10.1172/JCI3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nevalainen T.J., Graham G.G., Scott K.F. Antibacterial actions of secreted phospholipases A2. Biochim. Biophys. Acta. 2008;1781:1–9. doi: 10.1016/j.bbalip.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 69.Movert E., Wu Y., Lambeau G., Kahn F., Touqui L., Areschoug T. Secreted group IIA phospholipase A2 protects humans against the group B streptococcus: experimental and clinical evidence. J. Infect. Dis. 2013;208:2025–2035. doi: 10.1093/infdis/jit359. [DOI] [PubMed] [Google Scholar]
- 70.Liu M.S., Liu C.H., Wu G., Zhou Y. Antisense inhibition of secretory and cytosolic phospholipase A2 reduces the mortality in rats with sepsis. Crit. Care Med. 2012;40:2132–2140. doi: 10.1097/CCM.0b013e31824e1e20. [DOI] [PubMed] [Google Scholar]
- 71.Zeiher B.G., Steingrub J., Laterre P.F., Dmitrienko A., Fukiishi Y., Abraham E., EZZI Study Group LY315920NA/S-5920, a selective inhibitor of group IIA secretory phospholipase A2, fails to improve clinical outcome for patients with severe sepsis. Crit. Care Med. 2005;33:1741–1748. doi: 10.1097/01.ccm.0000171540.54520.69. [DOI] [PubMed] [Google Scholar]
- 72.Lordan R., Tsoupras A., Zabetakis I., Demopoulos C.A. Forty years since the structural elucidation of platelet-activating factor (PAF): historical, current, and future research perspectives. Molecules. 2019;24:4414. doi: 10.3390/molecules24234414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Prescott S.M., Zimmerman G.A., Stafforini D.M., McIntyre T.M. Platelet-activating factor and r elated lipid mediators. Annu. Rev. Biochem. 2000;69:419–445. doi: 10.1146/annurev.biochem.69.1.419. [DOI] [PubMed] [Google Scholar]
- 74.Wykle R.L., Malone B., Snyder F. Enzymatic synthesis of 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine, a hypotensive and platelet-aggregating lipid. J. Biol. Chem. 1980;255:10256–10260. [PubMed] [Google Scholar]
- 75.Holland M.R., Venable M.E., Whatley R.E., Zimmerman G.A., McIntyre T.M., Prescott S.M. Activation of the acetyl-coenzyme A:lysoplatelet-activating factor acetyltransferase regulates platelet-activating factor synthesis in human endothelial cells. J. Biol. Chem. 1992;267:22883–22890. [PubMed] [Google Scholar]
- 76.Lee T.C., Malone B., Snyder F. A new de novo pathway for the formation of 1-alkyl-2-acetyl-sn-glycerols, precursors of platelet activating factor. Biochemical characterization of 1-alkyl-2-lyso-sn-glycero-3-P:acetyl-CoA acetyltransferase in rat spleen. J. Biol. Chem. 1986;261:5373–5377. [PubMed] [Google Scholar]
- 77.Triggiani M., Schleimer R.P., Warner J.A., Chilton F.H. Differential synthesis of 1-acyl-2-acetyl-sn-glycero-3-phosphocholine and platelet-activating factor by human inflammatory cells. J. Immunol. 1991;147:660–666. [PubMed] [Google Scholar]
- 78.Watanabe J., Marathe G.K., Neilsen P.O., Weyrich A.S., Harrison K.A., Murphy R.C., Zimmerman G.A., McIntyre T.M. Endotoxins stimulate neutrophil adhesion followed by synthesis and release of platelet-activating factor in microparticles. J. Biol. Chem. 2003;278:33161–33168. doi: 10.1074/jbc.M305321200. [DOI] [PubMed] [Google Scholar]
- 79.Prescott S.M., Zimmerman G.A., McIntyre T.M. Human endothelial cells in culture produce platelet-activating factor (1-alkyl-2-acetyl-sn-glycero-3-phosphocholine) when stimulated with thrombin. Proc. Natl. Acad. Sci. U.S.A. 1984;81:3534–3538. doi: 10.1073/pnas.81.11.3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Muller E., Dagenais P., Alami N., Rola-Pleszczynski M. Identification and functional characterization of platelet-activating factor receptors in human leukocyte populations using polyclonal anti-peptide antibody. Proc. Natl. Acad. Sci. U.S.A. 1993;90:5818–5822. doi: 10.1073/pnas.90.12.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Honda Z., Nakamura M., Miki I., Minami M., Watanabe T., Seyama Y., Okado H., Toh H., Ito K., Miyamoto T., Shimizu T. Cloning by functional expression of platelet-activating factor receptor from guinea-pig lung. Nature. 1991;349:342–346. doi: 10.1038/349342a0. [DOI] [PubMed] [Google Scholar]
- 82.Zimmerman G.A., McIntyre T.M., Prescott S.M. Thrombin stimulates the adherence of neutrophils to human endothelial cells in vitro. J. Clin. Invest. 1985;76:2235–2246. doi: 10.1172/JCI112232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lorant D.E., Patel K.D., McIntyre T.M., McEver R.P., Prescott S.M., Zimmerman G.A. Coexpression of GMP-140 and PAF by endothelium stimulated by histamine or thrombin: a juxtacrine system for adhesion and activation of neutrophils. J. Cell Biol. 1991;115:223–234. doi: 10.1083/jcb.115.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Weyrich A.S., McIntyre T.M., McEver R.P., Prescott S.M., Zimmerman G.A. Monocyte tethering by P-selectin regulates monocyte chemotactic protein-1 and tumor necrosis factor-alpha secretion. Signal integration and NF-kappa B translocation. J. Clin. Invest. 1995;95:2297–2303. doi: 10.1172/JCI117921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rouis M., Nigon F., Chapman M.J. Platelet activating factor is a potent stimulant of the production of active oxygen species by human monocyte-derived macrophages. Biochem. Biophys. Res. Commun. 1988;156:1293–1301. doi: 10.1016/s0006-291x(88)80773-3. [DOI] [PubMed] [Google Scholar]
- 86.Takahashi S., Yoshikawa T., Naito Y., Tanigawa T., Yoshida N., Kondo M. Role of platelet-activating factor (PAF) in superoxide production by human polymorphonuclear leukocytes. Lipids. 1991;26:1227–1230. doi: 10.1007/BF02536537. [DOI] [PubMed] [Google Scholar]
- 87.Yost C.C., Cody M.J., Harris E.S., Thornton N.L., McInturff A.M., Martinez M.L., Chandler N.B., Rodesch C.K., Albertine K.H., Petti C.A., Weyrich A.S., Zimmerman G.A. Impaired neutrophil extracellular trap (NET) formation: a novel innate immune deficiency of human neonates. Blood. 2009;113:6419–6427. doi: 10.1182/blood-2008-07-171629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhou W., Javors M.A., Olson M.S. Platelet-activating factor as an intercellular signal in neutrophil-dependent platelet activation. J. Immunol. 1992;149:1763–1769. [PubMed] [Google Scholar]
- 89.Ninio E., Leyravaud S., Bidault J., Jurgens P., Benveniste J. Cell adhesion by membrane-bound paf-acether. Int. Immunol. 1991;3:1157–1163. doi: 10.1093/intimm/3.11.1157. [DOI] [PubMed] [Google Scholar]
- 90.Yost C.C., Weyrich A.S., Zimmerman G.A. The platelet activating factor (PAF) signaling cascade in systemic inflammatory responses. Biochimie. 2010;92:692–697. doi: 10.1016/j.biochi.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ishii S., Nagase T., Tashiro F., Ikuta K., Sato S., Waga I., Kume K., Miyazaki J., Shimizu T. Bronchial hyperreactivity, increased endotoxin lethality and melanocytic tumorigenesis in transgenic mice overexpressing platelet-activating factor receptor. EMBO J. 1997;16:133–142. doi: 10.1093/emboj/16.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jeong Y.I., Jung I.D., Lee C.M., Chang J.H., Chun S.H., Noh K.T., Jeong S.K., Shin Y.K., Lee W.S., Kang M.S., Lee S.Y., Lee J.D., Park Y.M. The novel role of platelet-activating factor in protecting mice against lipopolysaccharide-induced endotoxic shock. PLoS One. 2009;4 doi: 10.1371/journal.pone.0006503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stafforini D.M., McIntyre T.M., Zimmerman G.A., Prescott S.M. Platelet-activating factor acetylhydrolases. J. Biol. Chem. 1997;272:17895–17898. doi: 10.1074/jbc.272.29.17895. [DOI] [PubMed] [Google Scholar]
- 94.Stafforini D.M., McIntyre T.M., Carter M.E., Prescott S.M. Human plasma platelet-activating factor acetylhydrolase. Association with lipoprotein particles and role in the degradation of platelet-activating factor. J. Biol. Chem. 1987;262:4215–4222. [PubMed] [Google Scholar]
- 95.Blank M.L., Lee T., Fitzgerald V., Snyder F. A specific acetylhydrolase for 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine (a hypotensive and platelet-activating lipid) J. Biol. Chem. 1981;256:175–178. [PubMed] [Google Scholar]
- 96.Farr R.S., Wardlow M.L., Cox C.P., Meng K.E., Greene D.E. Human serum acid-labile factor is an acylhydrolase that inactivates platelet-activating factor. Fed. Proc. 1983;42:3120–3122. [PubMed] [Google Scholar]
- 97.Teixeira-da-Cunha M.G., Gomes R.N., Roehrs N., Bozza F.A., Prescott S.M., Stafforini D., Zimmerman G.A., Bozza P.T., Castro-Faria-Neto H.C. Bacterial clearance is improved in septic mice by platelet-activating factor-acetylhydrolase (PAF-AH) administration. PLoS One. 2013;8 doi: 10.1371/journal.pone.0074567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gomes R.N., Bozza F.A., Amancio R.T., Japiassu A.M., Vianna R.C., Larangeira A.P., Gouvea J.M., Bastos M.S., Zimmerman G.A., Stafforini D.M., Prescott S.M., Bozza P.T., Castro-Faria-Neto H.C. Exogenous platelet-activating factor acetylhydrolase reduces mortality in mice with systemic inflammatory response syndrome and sepsis. Shock. 2006;26:41–49. doi: 10.1097/01.shk.0000209562.00070.1a. [DOI] [PubMed] [Google Scholar]
- 99.Moreno S.E., Alves-Filho J.C., Rios-Santos F., Silva J.S., Ferreira S.H., Cunha F.Q., Teixeira M.M. Signaling via platelet-activating factor receptors accounts for the impairment of neutrophil migration in polymicrobial sepsis. J. Immunol. 2006;177:1264–1271. doi: 10.4049/jimmunol.177.2.1264. [DOI] [PubMed] [Google Scholar]
- 100.Lopez Diez F., Nieto M.L., Fernandez-Gallardo S., Gijon M.A., Sanchez Crespo M. Occupancy of platelet receptors for platelet-activating factor in patients with septicemia. J. Clin. Invest. 1989;83:1733–1740. doi: 10.1172/JCI114074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Graham R.M., Stephens C.J., Silvester W., Leong L.L., Sturm M.J., Taylor R.R. Plasma degradation of platelet-activating factor in severely ill patients with clinical sepsis. Crit. Care Med. 1994;22:204–212. doi: 10.1097/00003246-199402000-00009. [DOI] [PubMed] [Google Scholar]
- 102.Partrick D.A., Moore E.E., Moore F.A., Biffl W.L., Barnett C.C. Reduced PAF-acetylhydrolase activity is associated with postinjury multiple organ failure. Shock. 1997;7:170–174. doi: 10.1097/00024382-199703000-00003. [DOI] [PubMed] [Google Scholar]
- 103.Li S., Stuart L., Zhang Y., Meduri G.U., Umberger R., Yates C.R. Inter-individual variability of plasma PAF-acetylhydrolase activity in ARDS patients and PAFAH genotype. J. Clin. Pharm. Ther. 2009;34:447–455. doi: 10.1111/j.1365-2710.2008.01014.x. [DOI] [PubMed] [Google Scholar]
- 104.Opal S., Laterre P.F., Abraham E., Francois B., Wittebole X., Lowry S., Dhainaut J.F., Warren B., Dugernier T., Lopez A., Sanchez M., Demeyer I., Jauregui L., Lorente J.A., McGee W. Recombinant human platelet-activating factor acetylhydrolase for treatment of severe sepsis: results of a phase III, multicenter, randomized, double-blind, placebo-controlled, clinical trial. Crit. Care Med. 2004;32:332–341. doi: 10.1097/01.CCM.0000108867.87890.6D. [DOI] [PubMed] [Google Scholar]
- 105.Maceyka M., Spiegel S. Sphingolipid metabolites in inflammatory disease. Nature. 2014;510:58–67. doi: 10.1038/nature13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hannun Y.A., Obeid L.M. Sphingolipids and their metabolism in physiology and disease. Nat. Rev. Mol. Cell Biol. 2018;19:175–191. doi: 10.1038/nrm.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stringer K.A., Serkova N.J., Karnovsky A., Guire K., Robert Paine I., Standiford T.J. Metabolic consequences of sepsis-induced acute lung injury revealed by plasma 1H-nuclear magnetic resonance quantitative metabolomics and computational analysis. Am. J. Physiol. Lung Cell Mol. Physiol. 2011;300:L4–L11. doi: 10.1152/ajplung.00231.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ahn W.-G., Jung J.-S., Song D.-K. Lipidomic analysis of plasma lipids composition changes in septic mice. Korean J. Physiol. Pharmacol. 2018;22:399–408. doi: 10.4196/kjpp.2018.22.4.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shi X., Y F., Zheng Y.-N., Zhang H., Wang X.-X., Shao G.-J., Lai X.-L. Metabolomic approach for the identification of therapeutic targets of erythropoietin against sepsis in rat models. Eur. Rev. Med. Pharmacol. Sci. 2016;20:537–546. [PubMed] [Google Scholar]
- 110.Takao K., Miyakawa T. Genomic responses in mouse models greatly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. U. S. A. 2015;112:1167–1172. doi: 10.1073/pnas.1401965111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Seok J., Warren H.S., Cuenca A.G., Mindrinos M.N., Baker H.V., Xu W., Richards D.R., McDonald-Smith G.P., Gao H., Hennessy L., Finnerty C.C., López C.M., Honari S., Moore E.E., Minei J.P. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. U. S. A. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wu X., Hou J., Li H., Xie G., Zhang X., Zheng J., Wang J., Gao F., Yao Y., Liu H., Fang X. Inverse correlation between plasma sphingosine-1-phosphate and ceramide concentrations in septic patients and their utility in predicting mortality. Shock. 2019;51:718–724. doi: 10.1097/SHK.0000000000001229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Delogu G., Famularo G., Amati F., Signore L., Antonucci A., Trinchieri V., Di Marzio L., Cifone M.G. Ceramide concentrations in septic patients: a possible marker of multiple organ dysfunction syndrome. Crit. Care Med. 1999;27:2413–2417. doi: 10.1097/00003246-199911000-00015. [DOI] [PubMed] [Google Scholar]
- 114.Cartier A., Hla T. Sphingosine 1-phosphate: lipid signaling in pathology and therapy. Science. 2019;366:6463. doi: 10.1126/science.aar5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Winkler M.S., Nierhaus A., Holzmann M., Mudersbach E., Bauer A., Robbe L., Zahrte C., Geffken M., Peine S., Schwedhelm E., Daum G., Kluge S., Zoellner C. Decreased serum concentrations of sphingosine-1-phosphate in sepsis. Crit. Care. 2015;19:372. doi: 10.1186/s13054-015-1089-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Frej C., Linder A., Happonen K.E., Taylor F.B., Lupu F., Dahlbäck B. Sphingosine 1-phosphate and its carrier apolipoprotein M in human sepsis and in Escherichia coli sepsis in baboons. J. Cell Mol. Med. 2016;20:1170–1181. doi: 10.1111/jcmm.12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Winkler M.S., Märtz K.B., Nierhaus A., Daum G., Schwedhelm E., Kluge S., Gräler M.H. Loss of sphingosine 1-phosphate (S1P) in septic shock is predominantly caused by decreased levels of high-density lipoproteins (HDL) J. Intensive Care. 2019;7:23. doi: 10.1186/s40560-019-0376-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Coldewey S.M., Benetti E., Collino M., Pfeilschifter J., Sponholz C., Bauer M., Huwiler A., Thiemermann C. Elevation of serum sphingosine-1-phosphate attenuates impaired cardiac function in experimental sepsis. Sci. Rep. 2016;6:27594. doi: 10.1038/srep27594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Książek M., Chacińska M., Chabowski A., Baranowski M. Sources, metabolism, and regulation of circulating sphingosine-1-phosphate. J. Lipid Res. 2015;56:1271–1281. doi: 10.1194/jlr.R059543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Garcia J.G., Liu F., Verin A.D., Birukova A., Dechert M.A., Gerthoffer W.T., Bamberg J.R., English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J. Clin. Invest. 2001;108:689–701. doi: 10.1172/JCI12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang L., Sammani S., Moreno-Vinasco L., Letsiou E., Wang T., Camp S.M., Bittman R., Garcia J.G., Dudek S.M. FTY720 (s)-phosphonate preserves sphingosine 1-phosphate receptor 1 expression and exhibits superior barrier protection to FTY720 in acute lung injury. Crit. Care Med. 2014;42:e189–199. doi: 10.1097/CCM.0000000000000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chung H.-Y., Hupe D.C., Otto G.P., Sprenger M., Bunck A.C., Dorer M.J., Bockmeyer C.L., Deigner H.-P., Gräler M.H., Claus R.A. Acid sphingomyelinase promotes endothelial stress response in systemic inflammation and sepsis. Mol. Med. 2016;22:412–423. doi: 10.2119/molmed.2016.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Goeritzer M., Bernhart E., Plastira I., Reicher H., Leopold C., Eichmann T.O., Rechberger G., Madreiter-Sokolowski C.T., Prasch J., Eller P., Graier W.F., Kratky D., Malle E., Sattler W. Myeloperoxidase and septic conditions disrupt sphingolipid homeostasis in murine brain capillaries in vivo and immortalized human brain endothelial cells in vitro. Int. J. Mol. Sci. 2020;21:1143. doi: 10.3390/ijms21031143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chung H.Y., Kollmey A.S., Schrepper A., Kohl M., Bläss M.F., Stehr S.N., Lupp A., Gräler M.H., Claus R.A. Adjustment of dysregulated ceramide metabolism in a murine model of sepsis-Induced cardiac dysfunction. Int. J. Mol. Sci. 2017;18:839. doi: 10.3390/ijms18040839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chung H.Y., Witt C.J., Jbeily N., Hurtado-Oliveros J., Giszas B., Lupp A., Gräler M.H., Bruns T., Stallmach A., Gonnert F.A., Claus R.A. Acid sphingomyelinase inhibition prevents development of sepsis sequelae in the murine liver. Sci. Rep. 2017;7:12348. doi: 10.1038/s41598-017-11837-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Peng H., Li C., Kadow S., Henry B.D., Steinmann J., Becker K.A., Riehle A., Beckmann N., Wilker B., Li P.L., Pritts T., Edwards M.J., Zhang Y., Gulbins E., Grassmé H. Acid sphingomyelinase inhibition protects mice from lung edema and lethal Staphylococcus aureus sepsis. J. Mol. Med. 2015;93:675–689. doi: 10.1007/s00109-014-1246-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Levels J.H.M., Abraham P.R., van den Ende A., van Deventer S.J.H. Distribution and kinetics of lipoprotein-bound endotoxin. Infect. Immun. 2001;69:2821–2828. doi: 10.1128/IAI.69.5.2821-2828.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Brandenburg K., Jürgens G., Andrä J., Lindner B., Koch M.H., Blume A., Garidel P. Biophysical characterization of the interaction of high-density lipoprotein (HDL) with endotoxins. Eur. L. Biochem. 2002;269:5972–5981. doi: 10.1046/j.1432-1033.2002.03333.x. [DOI] [PubMed] [Google Scholar]
- 129.Henning M.F., Herlax V., Bakás L. Contribution of the C-terminal end of apolipoprotein AI to neutralization of lipopolysaccharide endotoxic effect. Innate Immun. 2010;17:327–337. doi: 10.1177/1753425910370709. [DOI] [PubMed] [Google Scholar]
- 130.Levels J.H., Abraham P.R., van Barreveld E.P., Meijers J.C., van Deventer S.J. Distribution and kinetics of lipoprotein-bound lipoteichoic acid. Infect. Immun. 2003;71:3280–3284. doi: 10.1128/IAI.71.6.3280-3284.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.De Nardo D., Labzin L.I., Kono H., Seki R., Schmidt S.V., Beyer M., Xu D., Zimmer S., Lahrmann C., Schildberg F.A., Vogelhuber J., Kraut M., Ulas T., Kerksiek A., Krebs W. High-density lipoprotein mediates anti-inflammatory reprogramming of macrophages via the transcriptional regulator ATF3. Nat. Immunol. 2014;15:152–160. doi: 10.1038/ni.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yamada H., Umemoto T., Kawano M., Kawakami M., Kakei M., Momomura S.-i., Ishikawa S.-e., Hara K. High-density lipoprotein and apolipoprotein A-I inhibit palmitate-induced translocation of toll-like receptor 4 into lipid rafts and inflammatory cytokines in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2017;484:403–408. doi: 10.1016/j.bbrc.2017.01.138. [DOI] [PubMed] [Google Scholar]
- 133.Murphy A.J., Woollard K.J., Hoang A., Mukhamedova N., Stirzaker R.A., McCormick S.P.A., Remaley A.T., Sviridov D., Chin-Dusting J. High-density lipoprotein reduces the human monocyte inflammatory response. Arterioscler. Thromb. Vasc. Biol. 2008;28:2071–2077. doi: 10.1161/ATVBAHA.108.168690. [DOI] [PubMed] [Google Scholar]
- 134.Murphy A.J., Woollard K.J., Suhartoyo A., Stirzaker R.A., Shaw J., Sviridov D., Chin-Dusting J.P.F. Neutrophil activation is attenuated by high-density lipoprotein and apolipoprotein A-I in in vitro and in vivo models of inflammation. Arterioscler. Thromb. Vasc. Biol. 2011;31:1333–1341. doi: 10.1161/ATVBAHA.111.226258. [DOI] [PubMed] [Google Scholar]
- 135.Yuhanna I.S., Zhu Y., Cox B.E., Hahner L.D., Osborne-Lawrence S., Lu P., Marcel Y.L., Anderson R.G.W., Mendelsohn M.E., Hobbs H.H., Shaul P.W. High-density lipoprotein binding to scavenger receptor-BI activates endothelial nitric oxide synthase. Nat. Med. 2001;7:853–857. doi: 10.1038/89986. [DOI] [PubMed] [Google Scholar]
- 136.Zhang Q.-H., Zu X.-Y., Cao R.-X., Liu J.-H., Mo Z.-C., Zeng Y., Li Y.-B., Xiong S.-L., Liu X., Liao D.-F., Yi G.-H. An involvement of SR-B1 mediated PI3K–Akt–eNOS signaling in HDL-induced cyclooxygenase 2 expression and prostacyclin production in endothelial cells. Biochem. Biophys. Res. Commun. 2012;420:17–23. doi: 10.1016/j.bbrc.2012.02.103. [DOI] [PubMed] [Google Scholar]
- 137.Xiong S.-L., Liu X., Yi G.-H. High-density lipoprotein induces cyclooxygenase-2 expression and prostaglandin I-2 release in endothelial cells through sphingosine kinase-2. Mol. Cell Biochem. 2014;389:197–207. doi: 10.1007/s11010-013-1941-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Nofer J.-R., van der Giet M., Tölle M., Wolinska I., von Wnuck Lipinski K., Baba H.A., Tietge U.J., Gödecke A., Ishii I., Kleuser B., Schäfers M., Fobker M., Zidek W., Assmann G., Chun J. HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J. Clin. Invest. 2004;113:569–581. doi: 10.1172/JCI18004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fan Y., Chen J., Liu D., Li W., Wang H., Huang Y., Gao C. HDL-S1P protects endothelial function and reduces lung injury during sepsis in vivo and in vitro. Int. J. Biochem. Cell Biol. 2020;126:105819. doi: 10.1016/j.biocel.2020.105819. [DOI] [PubMed] [Google Scholar]
- 140.Tanaka S., Diallo D., Delbosc S., Genève C., Zappella N., Yong-Sang J., Patche J., Harrois A., Hamada S., Denamur E., Montravers P., Duranteau J., Meilhac O. High-density lipoprotein (HDL) particle size and concentration changes in septic shock patients. Ann. Intensive Care. 2019;9:68. doi: 10.1186/s13613-019-0541-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Vavrova L., Rychlikova J., Mrackova M., Novakova O., Zak A., Novak F. Increased inflammatory markers with altered antioxidant status persist after clinical recovery from severe sepsis: a correlation with low HDL cholesterol and albumin. Clin. Exp. Med. 2016;16:557–569. doi: 10.1007/s10238-015-0390-1. [DOI] [PubMed] [Google Scholar]
- 142.Barlage S., Gnewuch C., Liebisch G., Wolf Z., Audebert F.-X., Glück T., Fröhlich D., Krämer B.K., Rothe G., Schmitz G. Changes in HDL-associated apolipoproteins relate to mortality in human sepsis and correlate to monocyte and platelet activation. Intensive Care Med. 2009;35:1877–1885. doi: 10.1007/s00134-009-1609-y. [DOI] [PubMed] [Google Scholar]
- 143.Cirstea M., Walley K.R., Russell J.A., Brunham L.R., Genga K.R., Boyd J.H. Decreased high-density lipoprotein cholesterol level is an early prognostic marker for organ dysfunction and death in patients with suspected sepsis. J. Crit. Care. 2017;38:289–294. doi: 10.1016/j.jcrc.2016.11.041. [DOI] [PubMed] [Google Scholar]
- 144.Lekkou A., Mouzaki A., Siagris D., Ravani I., Gogos C.A. Serum lipid profile, cytokine production, and clinical outcome in patients with severe sepsis. J. Crit. Care. 2014;29:723–727. doi: 10.1016/j.jcrc.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 145.Lee S.H., Park M.S., Park B.H., Jung W.J., Lee I.S., Kim S.Y., Kim E.Y., Jung J.Y., Kang Y.A., Kim Y.S., Kim S.K., Chang J., Chung K.S. Prognostic implications of serum lipid metabolism over time during sepsis. Biomed. Res. Intl. 2015;2015:789298. doi: 10.1155/2015/789298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Roveran Genga K., Lo C., Cirstea M., Zhou G., Walley K.R., Russell J.A., Levin A., Boyd J.H. Two-year follow-up of patients with septic shock presenting with low HDL: the effect upon acute kidney injury, death and estimated glomerular filtration rate. J. Int. Med. 2017;281:518–529. doi: 10.1111/joim.12601. [DOI] [PubMed] [Google Scholar]
- 147.Chien J.-Y., Jerng J.-S., Yu C.-J., Yang P.-C. Low serum level of high-density lipoprotein cholesterol is a poor prognostic factor for severe sepsis. Crit. Care Med. 2005;33:1688–1693. doi: 10.1097/01.ccm.0000171183.79525.6b. [DOI] [PubMed] [Google Scholar]
- 148.Tanaka S., Labreuche J., Drumez E., Harrois A., Hamada S., Vigué B., Couret D., Duranteau J., Meilhac O. Low HDL levels in sepsis versus trauma patients in intensive care unit. Ann. Intensive Care. 2017;7:60. doi: 10.1186/s13613-017-0284-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zou G., He J., Ren B., Xu F., Xu G., Zhang W. The delta high-density lipoprotein cholesterol ratio: a novel parameter for gram-negative sepsis. SpringerPlus. 2016;5:1044. doi: 10.1186/s40064-016-2685-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.de la Llera Moya M., McGillicuddy F.C., Hinkle C.C., Byrne M., Joshi M.R., Nguyen V., Tabita-Martinez J., Wolfe M.L., Badellino K., Pruscino L., Mehta N.N., Asztalos B.F., Reilly M.P. Inflammation modulates human HDL composition and function in vivo. Atherosclerosis. 2012;222:390–394. doi: 10.1016/j.atherosclerosis.2012.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Camont L., Chapman M.J., Kontush A. Biological activities of HDL subpopulations and their relevance to cardiovascular disease. Trends Mol. Med. 2011;17:594–603. doi: 10.1016/j.molmed.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 152.Trinder M., Genga K.R., Kong H.J., Blauw L.L., Lo C., Li X., Cirstea M., Wang Y., Rensen P.C.N., Russell J.A., Walley K.R., Boyd J.H., Brunham L.R. Cholesteryl ester transfer protein influences high-density lipoprotein levels and survival in sepsis. Am. J. Respir. Crit. Care Med. 2019;199:854–862. doi: 10.1164/rccm.201806-1157OC. [DOI] [PubMed] [Google Scholar]
- 153.Bermudes A.C.G., de Carvalho W.B., Zamberlan P., Muramoto G., Maranhão R.C., Delgado A.F. Changes in lipid metabolism in pediatric patients with severe sepsis and septic shock. Nutrition. 2018;47:104–109. doi: 10.1016/j.nut.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 154.Sharma N.K., Ferreira B.L., Tashima A.K., Brunialti M.K.C., Torquato R.J.S., Bafi A., Assuncao M., Azevedo L.C.P., Salomao R. Lipid metabolism impairment in patients with sepsis secondary to hospital acquired pneumonia, a proteomic analysis. Clin. Proteomics. 2019;16:29. doi: 10.1186/s12014-019-9252-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Walley K.R., Thain K.R., Russell J.A., Reilly M.P., Meyer N.J., Ferguson J.F., Christie J.D., Nakada T.A., Fjell C.D., Thair S.A., Cirstea M.S., Boyd J.H. PCSK9 is a critical regulator of the innate immune response and septic shock outcome. Sci. Transl. Med. 2014;6:258ra143. doi: 10.1126/scitranslmed.3008782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang X., Wang L., Chen B. Recombinant HDL (Milano) protects endotoxin-challenged rats from multiple organ injury and dysfunction. Biol. Chem. 2015;396:53–60. doi: 10.1515/hsz-2014-0188. [DOI] [PubMed] [Google Scholar]
- 157.Tanaka S., Genève C., Zappella N., Yong-Sang J., Planesse C., Louedec L., Viranaïcken W., Bringart M., Montravers P., Denamur E., Duranteau J., Couret D., Meilhac O. Reconstituted high-density lipoprotein therapy improves survival in mouse models of sepsis. Anesthesiology. 2020;132:825–838. doi: 10.1097/ALN.0000000000003155. [DOI] [PubMed] [Google Scholar]
- 158.Zhang Z., Datta G., Zhang Y., Miller A.P., Mochon P., Chen Y.-F., Chatham J., Anantharamaiah G.M., White C.R. Apolipoprotein A-I mimetic peptide treatment inhibits inflammatory responses and improves survival in septic rats. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H866–H873. doi: 10.1152/ajpheart.01232.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Datta G., Gupta H., Zhang Z., Mayakonda P., Anantharamaiah G.M., White C.R. HDL mimetic peptide administration improves left ventricular filling and cardiac output in lipopolysaccharide-treated rats. J. Clin. Exp. Cardiol. 2011;2:172. doi: 10.4172/2155-9880.1000172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Levels J.H.M., Pajkrt D., Schultz M., Hoek F.J., van Tol A., Meijers J.C.M., van Deventer S.J.H. Alterations in lipoprotein homeostasis during human experimental endotoxemia and clinical sepsis. Biochim. Biophys. Acta. 2007;1771:1429–1438. doi: 10.1016/j.bbalip.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 161.Guirgis F.W., Black L.P., Rosenthal M.D., Henson M., Ferreira J., Leeuwenburgh C., Kalynych C., Moldawer L.L., Miller T., Jones L., Crandall M., Reddy S.T., Wu S.S., Moore F.A. Lipid intensive drug therapy for sepsis pilot (LIPIDS-P): phase I/II clinical trial protocol of lipid emulsion therapy for stabilising cholesterol levels in sepsis and septic shock. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-029348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Guirgis F.W., Black L.P., DeVos E., Henson M., Ferreira J., Miller T., Rosenthal M., Leeuwenburgh C., Kalynych C., Moldawer L., Jones L., Crandall M., Reddy S.T., Gao H., Wu S. Lipid intensive drug therapy for sepsis pilot: a bayesian phase I clinical trial. J. Am. Coll. Emerg. Physicians Open. 2020;1:1332–1340. doi: 10.1002/emp2.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Thijs A., Thijs L.G. Pathogenesis of renal failure in sepsis. Kidney Int. Suppl. 1998;66:S34–37. [PubMed] [Google Scholar]
- 164.Windsor A.C., Mullen P.G., Fowler A.A., Sugerman H.J. Role of the neutrophil in adult respiratory distress syndrome. Br. J. Surg. 1993;80:10–17. doi: 10.1002/bjs.1800800106. [DOI] [PubMed] [Google Scholar]
- 165.Brown K.A., Brain S.D., Pearson J.D., Edgeworth J.D., Lewis S.M., Treacher D.F. Neutrophils in development of multiple organ failure in sepsis. Lancet. 2006;368:157–169. doi: 10.1016/S0140-6736(06)69005-3. [DOI] [PubMed] [Google Scholar]
- 166.Sônego F., Castanheira F.V., Ferreira R.G., Kanashiro A., Leite C.A., Nascimento D.C., Colón D.F., Borges Vde F., Alves-Filho J.C., Cunha F.Q. Paradoxical roles of the neutrophil in sepsis: protective and deleterious. Front. Immunol. 2016;7:155. doi: 10.3389/fimmu.2016.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Albert C.J., Crowley J.R., Hsu F.-F., Thukkani A.K., Ford D.A. Reactive chlorinating species produced by myeloperoxidase target the vinyl ether bond of plasmalogens: identification of 2-chlorohexadecanal. J. Biol. Chem. 2001;276:23733–23741. doi: 10.1074/jbc.M101447200. [DOI] [PubMed] [Google Scholar]
- 168.Thukkani A.K., Hsu F.-F., Crowley J.R., Wysolmerski R.B., Albert C.J., Ford D.A. Reactive chlorinating species produced during neutrophil activation target tissue plasmalogens: production of the chemoattractant, 2-chlorohexadecanal. J. Biol. Chem. 2002;277:3842–3849. doi: 10.1074/jbc.M109489200. [DOI] [PubMed] [Google Scholar]
- 169.Thukkani A.K., Albert C.J., Wildsmith K.R., Messner M.C., Martinson B.D., Hsu F.F., Ford D.A. Myeloperoxidase-derived reactive chlorinating species from human monocytes target plasmalogens in low density lipoprotein. J. Biol. Chem. 2003;278:36365–36372. doi: 10.1074/jbc.M305449200. [DOI] [PubMed] [Google Scholar]
- 170.Wildsmith K.R., Albert C.J., Anbukumar D.S., Ford D.A. Metabolism of myeloperoxidase-derived 2-chlorohexadecanal. J. Biol. Chem. 2006;281:16849–16860. doi: 10.1074/jbc.M602505200. [DOI] [PubMed] [Google Scholar]
- 171.Palladino E.N.D., Hartman C.L., Albert C.J., Ford D.A. The chlorinated lipidome originating from myeloperoxidase-derived HOCl targeting plasmalogens: metabolism, clearance, and biological properties. Arch. Biochem. Biophys. 2018;641:31–38. doi: 10.1016/j.abb.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pike D.P., Vogel M.J., McHowat J., Mikuzis P.A., Schulte K.A., Ford D.A. 2-Chlorofatty acids are biomarkers of sepsis mortality and mediators of barrier dysfunction in rats. J. Lipid Res. 2020;61:1115–1127. doi: 10.1194/jlr.RA120000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Brahmbhatt V.V., Albert C.J., Anbukumar D.S., Cunningham B.A., Neumann W.L., Ford D.A. {Omega}-oxidation of {alpha}-chlorinated fatty acids: identification of {alpha}-chlorinated dicarboxylic acids. J. Biol. Chem. 2010;285:41255–41269. doi: 10.1074/jbc.M110.147157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ullen A., Singewald E., Konya V., Fauler G., Reicher H., Nusshold C., Hammer A., Kratky D., Heinemann A., Holzer P., Malle E., Sattler W. Myeloperoxidase-derived oxidants induce blood-brain barrier dysfunction in vitro and in vivo. PLoS One. 2013;8 doi: 10.1371/journal.pone.0064034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Prasch J., Bernhart E., Reicher H., Kollroser M., Rechberger G.N., Koyani C.N., Trummer C., Rech L., Rainer P.P., Hammer A., Malle E., Sattler W. Myeloperoxidase-derived 2-chlorohexadecanal is generated in mouse heart during endotoxemia and induces modification of distinct cardiomyocyte protein subsets in vitro. Int. J. Mol. Sci. 2020;21:9235. doi: 10.3390/ijms21239235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Meyer N.J., Reilly J.P., Feng R., Christie J.D., Hazen S.L., Albert C.J., Franke J.D., Hartman C.L., McHowat J., Ford D.A. Myeloperoxidase-derived 2-chlorofatty acids contribute to human sepsis mortality via acute respiratory distress syndrome. JCI Insight. 2017;2 doi: 10.1172/jci.insight.96432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Thukkani A.K., McHowat J., Hsu F.F., Brennan M.L., Hazen S.L., Ford D.A. Identification of alpha-chloro fatty aldehydes and unsaturated lysophosphatidylcholine molecular species in human atherosclerotic lesions. Circulation. 2003;108:3128–3133. doi: 10.1161/01.CIR.0000104564.01539.6A. [DOI] [PubMed] [Google Scholar]
- 178.Marsche G., Heller R., Fauler G., Kovacevic A., Nuszkowski A., Graier W., Sattler W., Malle E. 2-chlorohexadecanal derived from hypochlorite-modified high-density lipoprotein-associated plasmalogen is a natural inhibitor of endothelial nitric oxide biosynthesis. Arterioscler. Thromb. Vasc. Biol. 2004;24:2302–2306. doi: 10.1161/01.ATV.0000148703.43429.25. [DOI] [PubMed] [Google Scholar]
- 179.Hartman C.L., Duerr M.A., Albert C.J., Neumann W.L., McHowat J., Ford D.A. 2-Chlorofatty acids induce Weibel-Palade body mobilization. J. Lipid Res. 2018;59:113–122. doi: 10.1194/jlr.M080200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Palladino E.N.D., Katunga L.A., Kolar G.R., Ford D.A. 2-Chlorofatty acids: lipid mediators of neutrophil extracellular trap formation. J. Lipid Res. 2018;59:1424–1432. doi: 10.1194/jlr.M084731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang W.-y., Albert C.J., Ford D.A. α-Chlorofatty acid accumulates in activated monocytes and causes apoptosis through reactive oxygen species production and endoplasmic reticulum stress. Arterioscler. Thromb. Vasc. Biol. 2014;34:526–532. doi: 10.1161/ATVBAHA.113.302544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.McHowat J., Shakya S., Ford D.A. 2-Chlorofatty aldehyde elicits endothelial cell activation. Front. Physiol. 2020;11:460. doi: 10.3389/fphys.2020.00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Messner M.C., Albert C.J., Ford D.A. 2-Chlorohexadecanal and 2-chlorohexadecanoic acid induce COX-2 expression in human coronary artery endothelial cells. Lipids. 2008;43:581–588. doi: 10.1007/s11745-008-3189-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bernhart E., Kogelnik N., Prasch J., Gottschalk B., Goeritzer M., Depaoli M.R., Reicher H., Nusshold C., Plastira I., Hammer A., Fauler G., Malli R., Graier W.F., Malle E., Sattler W. 2-Chlorohexadecanoic acid induces ER stress and mitochondrial dysfunction in brain microvascular endothelial cells. Redox Biol. 2018;15:441–451. doi: 10.1016/j.redox.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Yu H., Wang M., Wang D., Kalogeris T.J., McHowat J., Ford D.A., Korthuis R.J. Chlorinated Lipids Elicit Inflammatory Responses in vitro and in vivo. Shock. 2019;51:114–122. doi: 10.1097/SHK.0000000000001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Yu H., Liu Y., Wang M., Restrepo R.J., Wang D., Kalogeris T.J., Neumann W.L., Ford D.A., Korthuis R.J. Myeloperoxidase instigates proinflammatory responses in a cecal ligation and puncture rat model of sepsis. Am. J. Physiol. Heart Circ. Physiol. 2020;319:H705–H721. doi: 10.1152/ajpheart.00440.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Pacher P., Bátkai S., Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol. Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Witkamp R. Fatty acids, endocannabinoids and inflammation. Eur. J. Pharmacol. 2016;785:96–107. doi: 10.1016/j.ejphar.2015.08.051. [DOI] [PubMed] [Google Scholar]
- 189.Mechoulam R., Parker L.A. The endocannabinoid system and the brain. Ann. Rev. Psychol. 2013;64:21–47. doi: 10.1146/annurev-psych-113011-143739. [DOI] [PubMed] [Google Scholar]
- 190.DiPatrizio N.V., Igarashi M., Narayanaswami V., Murray C., Gancayco J., Russell A., Jung K.M., Piomelli D. Fasting stimulates 2-AG biosynthesis in the small intestine: role of cholinergic pathways. Am. J. Physiol. Reg. Integr. Comp. Physiol. 2015;309:R805–813. doi: 10.1152/ajpregu.00239.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Reggio P.H. Endocannabinoid binding to the cannabinoid receptors: what is known and what remains unknown. Curr. Med. Chem. 2010;17:1468–1486. doi: 10.2174/092986710790980005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Dinu A.R., Rogobete A.F., Bratu T., Popovici S.E., Bedreag O.H., Papurica M., Bratu L.M., Sandesc D. Cannabis sativa revisited-crosstalk between microRNA expression, inflammation, oxidative stress, and endocannabinoid response system in critically ill patients with sepsis. Cells. 2020;9:307. doi: 10.3390/cells9020307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Jehle J., Eich L., Danisch M., Bagheri S., Avraamidou E., Pfeifer P., Tiyerili V., Bindila L., Lutz B., Nickenig G. The endocannabinoid 2-arachidonoylglycerol inhibits endothelial function and repair. Int. J. Cardiol. 2020;323:243–250. doi: 10.1016/j.ijcard.2020.08.042. [DOI] [PubMed] [Google Scholar]
- 194.Gasperi V., Evangelista D., Chiurchiù V., Florenzano F., Savini I., Oddi S., Avigliano L., Catani M.V., Maccarrone M. 2-Arachidonoylglycerol modulates human endothelial cell/leukocyte interactions by controlling selectin expression through CB1 and CB2 receptors. Int. J. Biochem. Cell Biol. 2014;51:79–88. doi: 10.1016/j.biocel.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 195.Larose M.C., Turcotte C., Chouinard F., Ferland C., Martin C., Provost V., Laviolette M., Flamand N. Mechanisms of human eosinophil migration induced by the combination of IL-5 and the endocannabinoid 2-arachidonoyl-glycerol. J. Allergy Clin. Immunol. 2014;133:1480–1482. doi: 10.1016/j.jaci.2013.12.1081. [DOI] [PubMed] [Google Scholar]
- 196.Cabral G.A., Ferreira G.A., Jamerson M.J. Endocannabinoids and the immune system in health and disease. Handbook Exp. Pharmacol. 2015;231:185–211. doi: 10.1007/978-3-319-20825-1_6. [DOI] [PubMed] [Google Scholar]
- 197.Chiurchiù V., Leuti A., Cencioni M.T., Albanese M., De Bardi M., Bisogno T., Centonze D., Battistini L., Maccarrone M. Modulation of monocytes by bioactive lipid anandamide in multiple sclerosis involves distinct Toll-like receptors. Pharmacol. Res. 2016;113:313–319. doi: 10.1016/j.phrs.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 198.Facchinetti F., Del Giudice E., Furegato S., Passarotto M., Leon A. Cannabinoids ablate release of TNFalpha in rat microglial cells stimulated with lypopolysaccharide. Glia. 2003;41:161–168. doi: 10.1002/glia.10177. [DOI] [PubMed] [Google Scholar]
- 199.Mestre L., Iñigo P.M., Mecha M., Correa F.G., Hernangómez-Herrero M., Loría F., Docagne F., Borrell J., Guaza C. Anandamide inhibits Theiler's virus induced VCAM-1 in brain endothelial cells and reduces leukocyte transmigration in a model of blood brain barrier by activation of CB(1) receptors. J. Neuroinflam. 2011;8:102. doi: 10.1186/1742-2094-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Giuffrida A., McMahon L.R. In vivo pharmacology of endocannabinoids and their metabolic inhibitors: therapeutic implications in Parkinson's disease and abuse liability. Prostaglandins Other Lipid Mediat. 2010;91:90–103. doi: 10.1016/j.prostaglandins.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Tanaka M., Yanagihara I., Takahashi H., Hamaguchi M., Nakahira K., Sakata I. The mRNA expression of fatty acid amide hydrolase in human whole blood correlates with sepsis. J. Endotoxin Res. 2007;13:35–38. doi: 10.1177/0968051907078607. [DOI] [PubMed] [Google Scholar]
- 202.Tschöp J., Kasten K.R., Nogueiras R., Goetzman H.S., Cave C.M., England L.G., Dattilo J., Lentsch A.B., Tschöp M.H., Caldwell C.C. The cannabinoid receptor 2 is critical for the host response to sepsis. J. Immunol. 2009;183:499–505. doi: 10.4049/jimmunol.0900203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gui H., Sun Y., Luo Z.-M., Su D.-F., Dai S.-M., Liu X. Cannabinoid receptor 2 protects against acute experimental sepsis in mice. Mediators Inflamm. 2013;2013:741303. doi: 10.1155/2013/741303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Kapellos T.S., Recio C., Greaves D.R., Iqbal A.J. Cannabinoid receptor 2 modulates neutrophil recruitment in a murine model of endotoxemia. Mediators Inflamm. 2017;2017:4315412. doi: 10.1155/2017/4315412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sardinha J., Kelly M.E., Zhou J., Lehmann C. Experimental cannabinoid 2 receptor-mediated immune modulation in sepsis. Mediators Inflamm. 2014;2014:978678. doi: 10.1155/2014/978678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lehmann C., Kianian M., Zhou J., Küster I., Kuschnereit R., Whynot S., Hung O., Shukla R., Johnston B., Cerny V., Pavlovic D., Spassov A., Kelly M.E. Cannabinoid receptor 2 activation reduces intestinal leukocyte recruitment and systemic inflammatory mediator release in acute experimental sepsis. Crit. Care. 2012;16:R47. doi: 10.1186/cc11248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Leite-Avalca M.C., Lomba L.A., Bastos-Pereira A.L., Brito H.O., Fraga D., Zampronio A.R. Involvement of central endothelin ETA and cannabinoid CB1 receptors and arginine vasopressin release in sepsis induced by cecal ligation and puncture in rats. Shock. 2016;46:290–296. doi: 10.1097/SHK.0000000000000598. [DOI] [PubMed] [Google Scholar]
- 208.Landry D.W., Oliver J.A. The pathogenesis of vasodilatory shock. N. Engl. J. Med. 2001;345:588–595. doi: 10.1056/NEJMra002709. [DOI] [PubMed] [Google Scholar]
- 209.Landry D.W., Levin H.R., Gallant E.M., Ashton R.C., Jr., Seo S., D'Alessandro D., Oz M.C., Oliver J.A. Vasopressin deficiency contributes to the vasodilation of septic shock. Circulation. 1997;95:1122–1125. doi: 10.1161/01.cir.95.5.1122. [DOI] [PubMed] [Google Scholar]
- 210.Rittig N., Bach E., Thomsen H.H., Pedersen S.B., Nielsen T.S., Jørgensen J.O., Jessen N., Møller N. Regulation of lipolysis and adipose tissue signaling during acute endotoxin-induced inflammation: a human randomized crossover trial. PLoS One. 2016;11 doi: 10.1371/journal.pone.0162167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Van Wyngene L., Vanderhaeghen T., Timmermans S., Vandewalle J., Van Looveren K., Souffriau J., Wallaeys C., Eggermont M., Ernst S., Van Hamme E., Gonçalves A., Eelen G., Remmerie A., Scott C.L., Rombouts C. Hepatic PPARα function and lipid metabolic pathways are dysregulated in polymicrobial sepsis. EMBO Mol. Med. 2020;12:e11319. doi: 10.15252/emmm.201911319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Chung K.-P., Chen G.-Y., Chuang T.-Y., Huang Y.-T., Chang H.-T., Chen Y.-F., Liu W.-L., Chen Y.-J., Hsu C.-L., Huang M.-T., Kuo C.-H., Yu C.-J. Increased plasma acetylcarnitine in sepsis is associated with multiple organ dysfunction and mortality: a multicenter cohort study. Crit. Care Med. 2019;47:210–218. doi: 10.1097/CCM.0000000000003517. [DOI] [PubMed] [Google Scholar]
- 213.Schmerler D., Neugebauer S., Ludewig K., Bremer-Streck S., Brunkhorst F.M., Kiehntopf M. Targeted metabolomics for discrimination of systemic inflammatory disorders in critically ill patients. J. Lipid Res. 2012;53:1369–1375. doi: 10.1194/jlr.P023309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Langley R.J., Tsalik E.L., Velkinburgh J.C.v., Glickman S.W., Rice B.J., Wang C., Chen B., Carin L., Suarez A., Mohney R.P., Freeman D.H., Wang M., You J., Wulff J., Thompson J.W. An Integrated clinico-metabolomic model improves prediction of death in sepsis. Sci. Transl. Med. 2013;5 doi: 10.1126/scitranslmed.3005893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Fell D.B., Hawken S., Wong C.A., Wilson L.A., Murphy M.S.Q., Chakraborty P., Lacaze-Masmonteil T., Potter B.K., Wilson K. Using newborn screening analytes to identify cases of neonatal sepsis. Sci. Rep. 2017;7:18020. doi: 10.1038/s41598-017-18371-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Brealey D., Brand M., Hargreaves I., Heales S., Land J., Smolenski R., Davies N.A., Cooper C.E., Singer M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360:219–223. doi: 10.1016/S0140-6736(02)09459-X. [DOI] [PubMed] [Google Scholar]
- 217.Quoilin C., Mouithys-Mickalad A., Lécart S., Fontaine-Aupart M.P., Hoebeke M. Evidence of oxidative stress and mitochondrial respiratory chain dysfunction in an in vitro model of sepsis-induced kidney injury. Biochim. Biophys. Acta. 2014;1837:1790–1800. doi: 10.1016/j.bbabio.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 218.Singer M. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence. 2014;5:66–72. doi: 10.4161/viru.26907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Feingold K.R., Wang Y., Moser A., Shigenaga J.K., Grunfeld C. LPS decreases fatty acid oxidation and nuclear hormone receptors in the kidney. J. Lipid Res. 2008;49:2179–2187. doi: 10.1194/jlr.M800233-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Feingold K., Kim M.S., Shigenaga J., Moser A., Grunfeld C. Altered expression of nuclear hormone receptors and coactivators in mouse heart during the acute-phase response. Am. J. Physiol. Endocrinol. Metab. 2004;286:E201–E207. doi: 10.1152/ajpendo.00205.2003. [DOI] [PubMed] [Google Scholar]
- 221.Feingold K.R., Moser A., Patzek S.M., Shigenaga J.K., Grunfeld C. Infection decreases fatty acid oxidation and nuclear hormone receptors in the diaphragm. J. Lipid Res. 2009;50:2055–2063. doi: 10.1194/jlr.M800655-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Maitra U., Chang S., Singh N., Li L. Molecular mechanism underlying the suppression of lipid oxidation during endotoxemia. Mol. Immunol. 2009;47:420–425. doi: 10.1016/j.molimm.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Standage S.W., Caldwell C.C., Zingarelli B., Wong H.R. Reduced peroxisome proliferator-activated receptor α expression is associated with decreased survival and increased tissue bacterial load in sepsis. Shock. 2012;37:164–169. doi: 10.1097/SHK.0b013e31823f1a00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Yoo S.H., Abdelmegeed M.A., Song B.J. Activation of PPARα by Wy-14643 ameliorates systemic lipopolysaccharide-induced acute lung injury. Biochem. Biophys. Res. Commun. 2013;436:366–371. doi: 10.1016/j.bbrc.2013.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Paumelle R., Haas J.T., Hennuyer N., Baugé E., Deleye Y., Mesotten D., Langouche L., Vanhoutte J., Cudejko C., Wouters K., Hannou S.A., Legry V., Lancel S., Lalloyer F., Polizzi A. Hepatic PPARα is critical in the metabolic adaptation to sepsis. J. Hepatol. 2019;70:963–973. doi: 10.1016/j.jhep.2018.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Standage S.W., Bennion B.G., Knowles T.O., Ledee D.R., Portman M.A., McGuire J.K., Liles W.C., Olson A.K. PPARα augments heart function and cardiac fatty acid oxidation in early experimental polymicrobial sepsis. Am. J. Physiol. Heart Circ. Physiol. 2017;312:H239–H249. doi: 10.1152/ajpheart.00457.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Morel J., Singer M. Statins, fibrates, thiazolidinediones and resveratrol as adjunctive therapies in sepsis: could mitochondria be a common target? Intensive Care Med. Exp. 2014;2:9. doi: 10.1186/2197-425X-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Nissen S.E., Nicholls S.J., Wolski K., Howey D.C., McErlean E., Wang M.D., Gomez E.V., Russo J.M. Effects of a potent and selective PPAR-alpha agonist in patients with atherogenic dyslipidemia or hypercholesterolemia: two randomized controlled trials. JAMA. 2007;297:1362–1373. doi: 10.1001/jama.297.12.1362. [DOI] [PubMed] [Google Scholar]
- 229.Seymour C.W., Kahn J.M., Martin-Gill C., Callaway C.W., Yealy D.M., Scales D., Angus D.C. Delays from first medical contact to antibiotic administration for sepsis. Crit. Care Med. 2017;45:759–765. doi: 10.1097/CCM.0000000000002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Stortz J.A., Raymond S.L., Mira J.C., Moldawer L.L., Mohr A.M., Efron P.A. Murine models of sepsis and trauma: can we bridge the gap? ILAR J. 2017;58:90–105. doi: 10.1093/ilar/ilx007. [DOI] [PMC free article] [PubMed] [Google Scholar]