Abstract
Homocysteine is associated with several diseases, and a series of dietary factors are known to modulate homocysteine levels. As mice are often used as model organisms to study the effects of dietary hyperhomocysteinemia, we collected data about concentrations of vitamin B12, vitamin B6, folate, methionine, cystine, and choline in mouse diets and the associated plasma/serum homocysteine levels. In addition, we more closely examined the composition of the control diet, the impact of the mouse strain, sex and age, and the duration of the dietary intervention on homocysteine levels. In total, 113 out of 1103 reviewed articles met the inclusion criteria. In the experimental and control diets, homocysteine levels varied from 0.1 to 280 µmol/l. We found negative correlations between dietary vitamin B12 (rho = − 0.125; p < 0.05), vitamin B6 (rho = − 0.191; p < 0.01) and folate (rho = − 0.395; p < 0.001) and circulating levels of homocysteine. In contrast, a positive correlation was observed between dietary methionine and homocysteine (methionine: rho = 0.146; p < 0.05). No significant correlations were found for cystine or choline and homocysteine levels. In addition, there was no correlation between the duration of the experimental diets and homocysteine levels. More importantly, the data showed that homocysteine levels varied widely in mice fed control diets as well. When comparing control diets with similar nutrient concentrations (AIN-based), there were significant differences in homocysteine levels caused by the strain (ANOVA, p < 0.05) and age of the mice at baseline (r = 0.47; p < 0.05). When comparing homocysteine levels and sex, female mice tended to have higher homocysteine levels than male mice (9.3 ± 5.9 µmol/l vs. 5.8 ± 4.5 µmol/l; p = 0.069). To conclude, diets low in vitamin B12, vitamin B6, or folate and rich in methionine are similarly effective in increasing homocysteine levels. AIN recommendations for control diets are adequate with respect to the amounts of homocysteine-modulating dietary parameters. In addition, the mouse strain and the age of mice can affect the homocysteine level.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12986-021-00594-9.
Keywords: Age, Amino acids, Diet composition, Homocysteine, Mice, Sex, Strain, B vitamins
Introduction
Homocysteine is a sulfur-containing essential amino acid. Its accumulation is associated with several diseases, including cardiovascular diseases such as stroke, cancer, Alzheimer’s disease and Parkinson’s disease [1]. Homocysteine is a component of one-carbon metabolism that is involved in the provision of methyl groups for biological methylation reactions. The enzyme S-adenosylmethionine synthetase catalyzes the synthesis of S-adenosylmethionine (SAM) through the reaction of methionine and adenosine triphosphate. SAM, an important methyl donor for methylation reactions, is converted to S-adenosylhomocysteine (SAH) after dispensing the methyl group. The formation of homocysteine from SAH is catalyzed by adenosylhomocysteinase. Homocysteine can be converted to methionine through the vitamin B12-dependent enzyme methionine synthase [2]. The acquired methyl group for remethylation comes from 5-methyltetrahydrofolate or from betaine [3]. Folate is the precursor of tetrahydrofolate [4], which is converted through methyl-tetrahydrofolate reductase to 5-methyl-tetrahydrofolate. Betaine can be formed from its precursor choline [5]. Homocysteine can also be converted to cystathionine via transsulfuration through the vitamin B6-dependent enzyme cystathionine β-synthase [6].
The metabolic steps clearly show that several nutrients are involved in the one-carbon pathway and therefore can modulate homocysteine levels: methionine, vitamin B12, B6, folate and choline (Fig. 1). Thus, any excess in methionine intake or deficiencies in vitamin B12, B6, folate and choline can contribute to an increase in homocysteine levels [7].
Fig. 1.
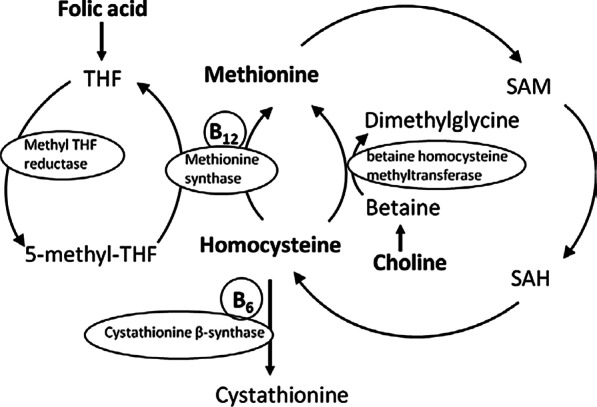
The biochemical pathways of homocysteine involving vitamin B12, vitamin B6, folate, methionine and choline. SAH—S-adenosylhomocysteine, SAM—S-adenosylmethionine, THF—tetrahydrofolate
Mice are often used as models of induced hyperhomocysteinemia and to study the impact of homocysteine on disease development. Thus, the current review evaluates different diets regarding their efficacy in increasing homocysteine levels in mice. We particularly focused on vitamin B12, vitamin B6, folate, the sulfur-containing amino acids methionine and cystine, and choline. In addition to the experimental diets, special focus was also placed on the control diets, which were used as reference. Additionally, we reviewed the impact of mouse strains, sex, age and feeding period on plasma/serum homocysteine levels. This review may be used as a reference for planning future nutrition studies on this topic.
Methods
A systematic literature search was conducted using the database PubMed and the search items (vitamin B12 OR cobalamin OR vitamin B6 OR pyridoxine OR B vitamins OR folic acid OR folate OR folates OR homocysteine OR hyperhomocysteinemia) AND (mice OR mouse OR murine) in the title of publications. Studies were included if they met the following criteria: (I) the study was written in English and published through July 2020, (II) wild-type mice were used as the model organism, and (III) plasma or serum homocysteine levels were measured. Studies were excluded when nutrients were administered via injections, gavage or drinking water or when any kind of surgery was performed. A total of 113 studies with 305 data sets (Additional file 1: Table S1) were eligible to be included in the evaluation of this review.
The following data were extracted from each study: mouse strain, sex, age and/or body weight at baseline, duration of feeding, dietary concentrations of vitamin B12, vitamin B6, folate, the added S-containing amino acids methionine and cystine, choline and plasma or serum homocysteine levels (in the following term "plasma" is used for plasma and serum concentrations). If diet composition was not shown in the publications but was based on commercial diets, we added the manufacturer's information on nutrient contents. If diets were termed AIN-based, we used data on the composition of the AIN-93/G and AIN-93/M diets [8]. Otherwise, corresponding authors were asked for further information (which also included information regarding strain, sex or age of the mice as well as duration of dietary intervention). Correlations between plasma homocysteine levels (means and medians) and dietary compounds, age of the mice and duration of dietary intervention were analyzed using Pearson’s correlation testing since variables are normally distributed and Spearman correlation since variables are not normally distributed. Differences between plasma homocysteine levels and sex variables were analyzed using Student’s t test, and strain differences were analyzed with Levene’s test to assess homogeneity of variances and single-factor analysis of variance (ANOVA) followed by Hochberg’s GT2 post hoc test (SPSS 2020).
Results
Dietary parameters
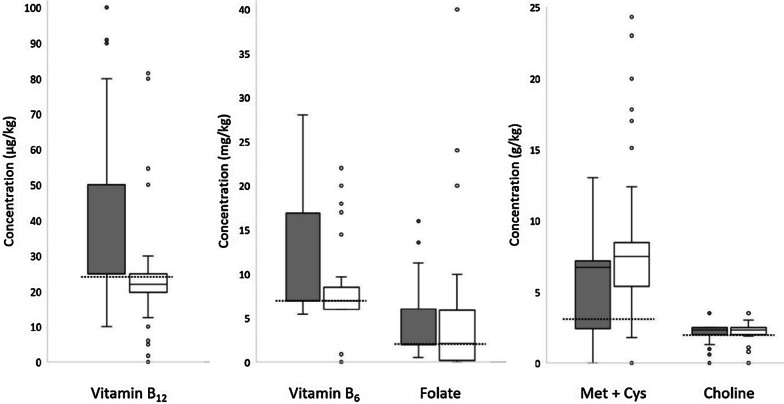
In 56 out of 113 studies, the composition of the experimental diets was described in detail. Experimental diets had vitamin B12 concentrations varying from 0 to 81.6 µg/kg diet, vitamin B6 concentrations varying from 0 to 22 mg/kg diet, folate concentrations varying from 0 to 40 mg/kg diet, methionine + cystine concentrations varying from 0 to 24.3 g/kg diet, and choline concentrations varying from 0 to 3.5 g/kg diet (Fig. 2).
Fig. 2.
Boxplots show medians, interquartile ranges and 1.5 × interquartile ranges of concentrations of vitamin B12 (µg/kg), vitamin B6 (mg/kg), folate (mg/kg), methionine + cystine (g/kg) and choline (g/kg) in control (gray box, n = 118 data sets out of 85 studies) and experimental (white box, n = 137 data sets out of 56 studies) diets, which were used to increase plasma levels of homocysteine. AIN-93-based nutrient recommendations [8] for control diets are depicted as dashed lines for each nutrient. For vitamin B12, vitamin B6 and folate the medians coincide with the 25th percentile
In addition to the experimental diets, we also evaluated the control diets that were used as a reference, especially with regard to their potential to minimize homocysteine levels. In 85 out of 113 studies, the composition of the control diets was described in detail. Studies have shown high variations in homocysteine-relevant nutrients in control diets. The concentration of vitamin B12 varied from 10 to 100 µg/kg diet, that of vitamin B6 from 5.4 to 28 mg/kg diet, that of folate from 0.5 to 16 mg/kg diet, that of methionine and cysteine from 0 to 13 g/kg diet, and that of choline from 0 to 3.5 g/kg diet (Fig. 2). Compared to nutrient recommendations for mice [8], concentrations of vitamin B12, vitamin B6, folate and S-containing amino acids in the control diets used were often markedly higher (Fig. 2). However, AIN-based control diets were administered in only 14 out of 113 studies (Table 1 and Additional file 1: Table S1).
Table 1.
Plasma homocysteine levels in mice fed AIN-93-based control diets
| Mouse strain | Sex | Age at baseline | Duration (weeks) | Plasma Hcy (µmol/l) | n | References |
|---|---|---|---|---|---|---|
| 129/Sv | f + m | 3 wks | 6 | 0.1# | 15 | [9] |
| 129/Sv | f + m | 3 wks | 9 | 0.1# | 15 | [9] |
| 129/Sv | f + m | 3 wks | 9–13 | 2.0 ± 0.6b | nda | [10] |
| CD-1 | f + m | Adult | 9 | 2.5# | 13 | [11] |
| C57BL/6 | m | 6 wks | 8 | 2.5 ± 0.7b | nda | [12] |
| C57BL/6 | m | 6 wks | 4 | 2.6 ± 0.8b | nda | [12] |
| 129/Sv | f + m | 3 wks | 9 | 2.8 ± 0.3nda | nda | [13] |
| Swiss | m | 3 wks | 27 | 3.0 ± 0.4b | 6–8 | [14] |
| C57BL/6 | m | 3 wks | 5 | 3.0 ± 2.2c | nda | [15] |
| C57BL/6 | m | 6 wks | 8 | 3.3 ± 0.8b | 6 | [16] |
| C57BL/6 | f | 8 wks | 9 | 3.6 ± 0.7b | 15 | [17] |
| SAMP8 | m | 13 wks | 4 | 4.0# | nda | [18] |
| BALB/c | f | 17 wks | 2 | 5.2 ± 0.2b | 23 | [19] |
| Swiss | m | 3 wks | 10 | 5.2 ± 0.6b | 6–8 | [14] |
| C57BL/6 | f | 3 wks | 5 | 5.4 ± 1.7c | nda | [15] |
| C57BL/6 | f | 6–8 wks | 7 | 5.5 ± 5.4c | 10 | [15] |
| SAMP8 | m | 17 wks | 26 | 6.5# | 15 | [18] |
| SAMR1 | m | 17 wks | 26 | 6.5# | nda | [18] |
| Swiss | f | 3 wks | 10 | 7.1 ± 0.7b | 6–8 | [14] |
| Swiss | f | 3 wks | 27 | 8.1 ± 0.8b | 6–8 | [14] |
| Swiss | m | 3 wks | 1 | 8.7 ± 0.9b | 6–8 | [14] |
| Swiss | f | Adult | 3 | 9.2# | 6–8 | [14] |
| Swiss | f | 3 wks | 1 | 9.7 ± 0.6b | 6–8 | [14] |
| C57BL/6 | f | 7 wks | 2 | 16.7 ± 1.5a | 20 | [20] |
| BALB/c | m | 17 wks | 52 | 18# | nda | [19] |
| BALB/c | f | 17 wks | 52 | 22.5# | nda | [19] |
f, female; m, male; n, number of included mice; nda, no data available; wks, weeks
aMean ± standard deviation
bMean ± standard error
cMedian ± interquartile range
#Read from diagrams of data from mice that received AIN-93-based control diets containing 25 µg vitamin B12, 7 mg vitamin B6, 2 mg folate, 3 g methionine + cystine and 2.5 g choline per kg diet; only studies with complete data sets about mouse strain, sex, age at baseline, and the duration of feeding (in weeks) were included
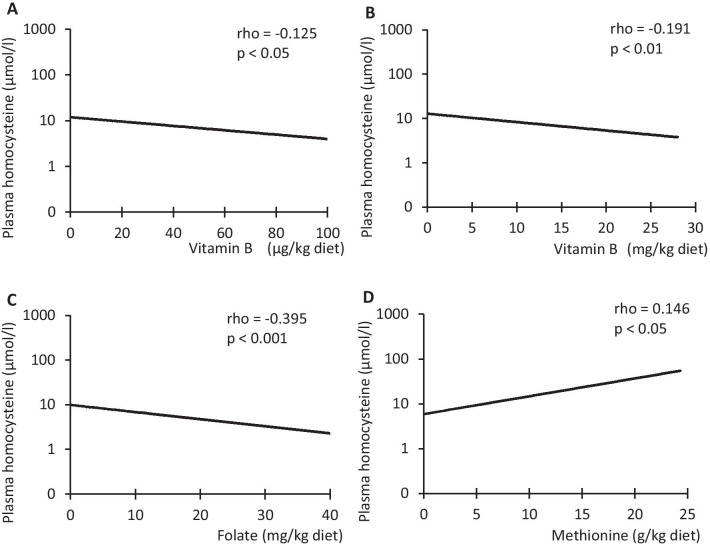
In mice fed the experimental or control diets, circulating homocysteine levels varied from 0.1 to 280 µmol/l. Analysis of the associations between components of the experimental and control diets and plasma homocysteine levels revealed negative correlations for vitamin B12 (rho = − 0.125; p < 0.05), vitamin B6 (rho = − 0.191; p < 0.01) and folate (rho = − 0.395; p < 0.001; Fig. 3). A positive correlation was observed between dietary methionine and plasma homocysteine levels (methionine: rho = 0.146; p < 0.05; Fig. 3). No significant correlations were found for homocysteine levels and dietary cystine (rho = − 0.076; p > 0.05) or choline (rho = 0.044; p > 0.05). The duration of the analyzed feeding experiments varied between 3 and 17 weeks. However, there was no correlation between feeding duration and plasma homocysteine level (r = − 0.05; p > 0.5).
Fig. 3.
Correlations between the diet ingredients vitamin B12 (A), vitamin B6 (B), folate (C) as well as methionine (D) and the plasma homocysteine level (logarithmic scale) of mice fed experimental or control diets; Spearman correlation (rho) was performed, since variables are not normally distributed; n = 255 data sets
Strain, sex, and age of mice
When comparing the circulating homocysteine levels in mice resulting from all analyzed control diets (including AIN-based control diets), we found varying homocysteine levels ranging from 0.1 to 24.1 µmol/l (Additional file 1: Table S1). Surprisingly, homocysteine levels in mice consuming strictly AIN-based control diets also varied in a wide range (from 0.1 to 22.5 µmol/l; Table 1), indicating that parameters other than nutrients influenced homocysteine levels.
In mice that received AIN-93-based control diets (Table 1), there were differences in homocysteine levels related to the strain (p < 0.05; Fig. 4) and age of the mice at baseline (r = 0.474; p < 0.05). When comparing homocysteine levels and sex, female mice tended to have higher homocysteine levels than male mice (9.3 ± 5.9 µmol/l vs. 5.8 ± 4.5 µmol/l; p = 0.069, Table 1).
Fig. 4.
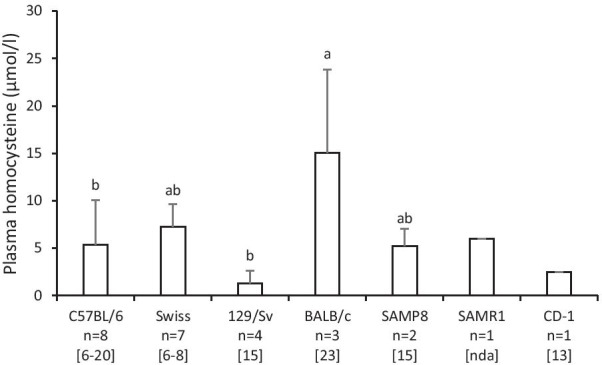
Circulating plasma homocysteine levels (µmol/l) in mice of different strains that received AIN-93-based control diets containing 25 µg vitamin B12, 7 mg vitamin B6, 2 mg folate, 3 g methionine + cystine and 2.5 g choline per kg diet; mean + standard deviation, when more than one data set was available; different letters indicate a statistically significant difference (p < 0.05, ANOVA followed by Hochberg’s GT2 post hoc test); n, number of included data sets (Table 1); number of integrated animals per study (ranges) are stated in square brackets; nda, no data available
Discussion
The current review shows that hyperhomocysteinemia can be induced by numerous different dietary interventions, such as a reduction in vitamin B6, vitamin B12 or folate concentration and an increase in methionine concentration. Study data showed that dietary cystine and choline had no effects on plasma homocysteine levels in mice. In addition, there was no correlation between the duration of feeding the experimental diets and plasma homocysteine levels. When diets were fed over varying periods, in most cases, there was no difference between homocysteine levels at different time points (Additional file 1: Table S1). One study found differences after 2 weeks of feeding the experimental diets, but no differences between 2 and up to 10 weeks. Thus, dietary interventions to increase homocysteine levels appear to be rapidly effective.
The type of control diet used in these studies showed great variations (Additional file 1: Table S1). The intake of AIN-93-based diets resulted in homocysteine levels similar to those of the other control diets. Hence, higher doses of vitamin B12, vitamin B6 and folate than recommended in the AIN-93 diet [8] do not seem to further decrease homocysteine levels. However, it should be mentioned that AIN-based control diets were only used in 14 out of 113 studies (Additional file 1: Table S1).
Plasma levels of homocysteine depend on the mouse strain because the growth rate of mice and thus the nutrient requirements depend on the genetic background [21]. Older mice have higher homocysteine levels than younger mice, which is in line with homocysteine data in humans [22, 23]. An age-related reduction in renal function is attributable to this effect [24]. In addition, females tend to have higher homocysteine levels than males. It is assumed that the renal activity of cystathionine β-synthase, which catalyzes an important step in the formation of cysteine from homocysteine, is regulated by testosterone [25] and thus is commonly higher in males than in females [26].
In addition, it must be kept in mind that the different methods used for quantification of homocysteine such as chromatography, immunoassays or capillary electrophoresis could have influenced the results [27]. In our review, the high-performance liquid chromatography (HPLC) was the most frequently used method to quantify plasma homocysteine (in 72 out of 113 studies, Additional file 1: Table S1).
To conclude, vitamin B12, vitamin B6, folate, and methionine are similarly effective in reducing homocysteine levels. AIN recommendations for control diets are adequate with respect to the amounts of homocysteine-modulating dietary parameters. In addition to dietary parameters, the mouse strain and the age of mice can affect homocysteine levels.
Supplementary Information
Additional file 1. Supplemental Table: Diet composition and mouse data.
Acknowledgements
We thank Annette Schuhmacher (Sniff), Barbara Mickelson (Envigo), Helena Sprafke (Altromin Spezialfutter GmbH & Co. KG), Lisa Wagner (Dyets, Inc.) and Melanie Hoar (LabDiet) for their support regarding diet compositions.
Abbreviations
- ANOVA
Analysis of variance
- SAH
S-adenosylhomocysteine
- SAM
S-adenosylmethionine
Authors’ contributions
ChB collected, analyzed and interpreted the data and was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Availability of data and materials
All data analysed during this study are included in this published article and its supplementary information file.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kumar A, Palfrey HA, Pathak R, Kadowitz PJ, Gettys TW, Murthy SN. The metabolism and significance of homocysteine in nutrition and health. Nutr Metab. 2017;14(1):1–12. doi: 10.1186/s12986-017-0233-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoffer LJ. Homocysteine remethylation and trans-sulfuration. Metabolism. 2004;53(11):1480–1483. doi: 10.1016/j.metabol.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Selhub J. Homocysteine metabolism. Annu Rev Nutr. 1999;19(1):217–246. doi: 10.1146/annurev.nutr.19.1.217. [DOI] [PubMed] [Google Scholar]
- 4.Scott JM. Folate and vitamin B 12. Proc Nutr Soc. 1999;58(2):441–448. doi: 10.1017/S0029665199000580. [DOI] [PubMed] [Google Scholar]
- 5.Olthof MR, Verhoef P. Effects of betaine intake on plasma homocysteine concentrations and consequences for health. Curr Drug Metab. 2005;6(1):15–22. doi: 10.2174/1389200052997366. [DOI] [PubMed] [Google Scholar]
- 6.Jhee KH, Kruger WD. The role of cystathionine β-synthase in homocysteine metabolism. Antioxid Redox Sign. 2005;7(5–6):813–822. doi: 10.1089/ars.2005.7.813. [DOI] [PubMed] [Google Scholar]
- 7.Langman LJ, Cole DEC. Homocysteine. Crit Rev Clin Lab Sci. 1999;36(4):365–406. doi: 10.1080/10408369991239231. [DOI] [PubMed] [Google Scholar]
- 8.Reeves PG. Components of the AIN-93 diets as improvements in the AIN-76A diet. J Nutr. 1997;127(5):838–841. doi: 10.1093/jn/127.5.838S. [DOI] [PubMed] [Google Scholar]
- 9.Fuso A, Nicolia V, Cavallaro RA, Ricceri L, D'Anselmi F, Coluccia P, et al. B-vitamin deprivation induces hyperhomocysteinemia and brain S-adenosylhomocysteine, depletes brain S-adenosylmethionine, and enhances PS1 and BACE expression and amyloid-β deposition in mice. Mol Cell Neurosci. 2008;37(4):731–746. doi: 10.1016/j.mcn.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 10.Fuso A, Nicolia V, Ricceri L, Cavallaro RA, Isopi E, Mangia F, et al. S-adenosylmethionine reduces the progress of the Alzheimer-like features induced by B-vitamin deficiency in mice. Neurobiol Aging. 2012;33(7):1482–e1. doi: 10.1016/j.neurobiolaging.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Holstein JH, Herrmann M, Schmalenbach J, Obeid R, Ölkü I, Klein M, et al. Deficiencies of folate and vitamin B12 do not affect fracture healing in mice. Bone. 2010;47(1):151–5. doi: 10.1016/j.bone.2010.04.592. [DOI] [PubMed] [Google Scholar]
- 12.Yao L, Wang C, Zhang X, Peng L, Liu W, Zhang X, et al. Hyperhomocysteinemia activates the aryl hydrocarbon receptor/CD36 pathway to promote hepatic steatosis in mice. Hepatology. 2016;64(1):92–105. doi: 10.1002/hep.28518. [DOI] [PubMed] [Google Scholar]
- 13.Persichilli S, Gervasoni J, Di Napoli A, Fuso A, Nicolia V, Giardina B, et al. Plasma thiols levels in alzheimer's disease mice under diet-induced hyperhomocysteinemia: effect of s-adenosylmethionine and superoxide-dismutase supplementation. J Alzheimer's Dis. 2015;44(4):1323–31. doi: 10.3233/JAD-142391. [DOI] [PubMed] [Google Scholar]
- 14.da Silva VC, Fernandes L, Haseyama EJ, Agamme ALDA, Shinohara EMG, Muniz MTC, et al. Effect of vitamin B deprivation during pregnancy and lactation on homocysteine metabolism and related metabolites in brain and plasma of mice offspring. PLoS ONE. 2014;9(4):e92683. doi: 10.1371/journal.pone.0092683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castaño-Moreno E, Castillo V, Peñailillo R, Llanos MN, Valenzuela R, Ronco AM. Fatty acid and lipid metabolism in liver of pregnant mice and their offspring is influenced by unbalanced folates/vitamin B12 diets. Prostaglandins Leukot Essent Fatty Acids. 2020;154:102057. doi: 10.1016/j.plefa.2020.102057. [DOI] [PubMed] [Google Scholar]
- 16.Yao L, Cao B, Cheng Q, Cai W, Ye C, Liang J, et al. Inhibition of soluble epoxide hydrolase ameliorates hyperhomocysteinemia-induced hepatic steatosis by enhancing β-oxidation of fatty acid in mice. Am J Physiol-Gastrointest Liver Physiol. 2019;316(4):G527–38. doi: 10.1152/ajpgi.00148.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martínez-Vega R, Partearroyo T, Vallecillo N, Varela-Moreiras G, Pajares MA, Varela-Nieto I. Long-term omega-3 fatty acid supplementation prevents expression changes in cochlear homocysteine metabolism and ameliorates progressive hearing loss in C57BL/6J mice. J Nutr Biochem. 2015;26(12):1424–33. doi: 10.1016/j.jnutbio.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 18.Lv X, Wang X, Wang Y, Zhou D, Li W, Wilson JX, et al. Folic acid delays age-related cognitive decline in senescence-accelerated mouse prone 8: alleviating telomere attrition as a potential mechanism. Aging (Albany NY) 2019;11(22):10356. doi: 10.18632/aging.102461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwahn BC, Wang XL, Mikael LG, Wu Q, Cohn J, Jiang H, et al. Betaine supplementation improves the atherogenic risk factor profile in a transgenic mouse model of hyperhomocysteinemia. Atherosclerosis. 2007;195(2):e100–7. doi: 10.1016/j.atherosclerosis.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 20.Huang Y, He Y, Sun X, He Y, Li Y, Sun C. Maternal high folic acid supplement promotes glucose intolerance and insulin resistance in male mouse offspring fed a high-fat diet. Int J Mol Sci. 2014;15(4):6298–313. doi: 10.3390/ijms15046298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacoby RO, Fox JG, Davisson M. Biology and diseases of mice. Lab Anim Med. 2002 doi: 10.1016/B978-012263951-7/50006-5. [DOI] [Google Scholar]
- 22.Frick B, Schroecksnadel K, Neurauter G, Leblhuber F, Fuchs D. Increasing production of homocysteine and neopterin and degradation of tryptophan with older age. Clin Biochem. 2004;37(8):684–7. doi: 10.1016/j.clinbiochem.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Powers RW, Majors AK, Lykins DL, Sims CJ, Lain KY, Roberts JM. Plasma homocysteine and malondialdehyde are correlated in an age-and gender-specific manner. Metab Clin Exp. 2002;51(11):1433–8. doi: 10.1053/meta.2002.35587. [DOI] [PubMed] [Google Scholar]
- 24.Norlund L, Grubb A, Fex G, Leksell H, Nilsson JE, Schenck H, et al. The increase of plasma homocysteine concentrations with age is partly due to the deterioration of renal function as determined by plasma cystatin C. Clin Chem Lab Med. 1998;36(3):175–8. doi: 10.1515/CCLM.1998.032. [DOI] [PubMed] [Google Scholar]
- 25.Vitvitsky V, Prudova A, Stabler S, Dayal S, Lentz SR, Banerjee R. Testosterone regulation of renal cystathionine β-synthase: implications for sex-dependent differences in plasma homocysteine levels. Am J Physiol Renal Physiol. 2007;293(2):F594–600. doi: 10.1152/ajprenal.00171.2007. [DOI] [PubMed] [Google Scholar]
- 26.Vitvitsky V, Dayal S, Stabler S, Zhou Y, Wang H, Lentz SR, et al. Perturbations in homocysteine-linked redox homeostasis in a murine model for hyperhomocysteinemia. A J Physiol Regul Integr Comp Physiol. 2004;287(1):R39–46. doi: 10.1152/ajpregu.00036.2004. [DOI] [PubMed] [Google Scholar]
- 27.Alam SF, Kumar S, Ganguly P. Measurement of homocysteine: a historical perspective. J Clin Biochem Nutr. 2019;65(3):171–177. doi: 10.3164/jcbn.19-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pickell L, Li D, Brown K, Mikael LG, Wang XL, Wu Q, et al. Methylenetetrahydrofolate reductase deficiency and low dietary folate increase embryonic delay and placental abnormalities in mice. Birth Defects Res A Clin Mol Teratol. 2009;85(6):531–41. doi: 10.1002/bdra.20575. [DOI] [PubMed] [Google Scholar]
- 29.Pickell L, Brown K, Li D, Wang XL, Deng L, Wu Q, et al. High intake of folic acid disrupts embryonic development in mice. Birth Defects Res A Clin Mol Teratol. 2011;91(1):8–19. doi: 10.1002/bdra.20754. [DOI] [PubMed] [Google Scholar]
- 30.Chen Z, Karaplis AC, Ackerman SL, Pogribny IP, Melnyk S, Lussier-Cacan S, et al. Mice deficient in methylenetetrahydrofolate reductase exhibit hyperhomocysteinemia and decreased methylation capacity, with neuropathology and aortic lipid deposition. Hum Mol Genet. 2001;10(5):433–44. doi: 10.1093/hmg/10.5.433. [DOI] [PubMed] [Google Scholar]
- 31.Devlin AM, Arning E, Bottiglieri T, Faraci FM, Rozen R, Lentz SR. Effect of Mthfr genotype on diet-induced hyperhomocysteinemia and vascular function in mice. Blood. 2004;103(7):2624–9. doi: 10.1182/blood-2003-09-3078. [DOI] [PubMed] [Google Scholar]
- 32.Liu WH, Zhao YS, Gao SY, Cao J, Zhang KQ, Zou CG. Hepatocyte proliferation during liver regeneration is impaired in mice with methionine diet-induced hyperhomocysteinemia. Am J Pathol. 2010;177(5):2357–65. doi: 10.2353/ajpath.2010.091131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsu CC, Cheng CH, Hsu CL, Lee WJ, Huang SC, Huang YC. Role of vitamin B6 status on antioxidant defenses, glutathione, and related enzyme activities in mice with homocysteine-induced oxidative stress. Food Nutr Res. 2015;59(1):25702. doi: 10.3402/fnr.v59.25702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mikael LG, Deng L, Paul L, Selhub J, Rozen R. Moderately high intake of folic acid has a negative impact on mouse embryonic development. Birth Defects Res A Clin Mol Teratol. 2013;97(1):47–52. doi: 10.1002/bdra.23092. [DOI] [PubMed] [Google Scholar]
- 35.Christensen KE, Wu Q, Wang X, Deng L, Caudill MA, Rozen R. Steatosis in mice is associated with gender, folate intake, and expression of genes of one-carbon metabolism. J Nutr. 2010;140(10):1736–41. doi: 10.3945/jn.110.124917. [DOI] [PubMed] [Google Scholar]
- 36.Li D, Pickell L, Liu Y, Wu Q, Cohn JS, Rozen R. Maternal methylenetetrahydrofolate reductase deficiency and low dietary folate lead to adverse reproductive outcomes and congenital heart defects in mice. Am J Clin Nutr. 2005;82(1):188–95. doi: 10.1093/ajcn/82.1.188. [DOI] [PubMed] [Google Scholar]
- 37.Christensen KE, Hou W, Bahous RH, Deng L, Malysheva OV, Arning E, et al. Moderate folic acid supplementation and MTHFD1-synthetase deficiency in mice, a model for the R653Q variant, result in embryonic defects and abnormal placental development. Am J Clin Nutr. 2016;104(5):1459–69. doi: 10.3945/ajcn.116.139519. [DOI] [PubMed] [Google Scholar]
- 38.Christensen KE, Bahous RH, Hou W, Deng L, Malysheva OV, Arning E, et al. Low dietary folate interacts with MTHFD1 synthetase deficiency in mice, a model for the R653Q variant, to increase incidence of developmental delays and defects. J Nutr. 2018;148(4):501–9. doi: 10.1093/jn/nxy013. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, Chen S, Li L, Wang Q, Le W. Folic acid protects motor neurons against the increased homocysteine, inflammation and apoptosis in SOD1G93A transgenic mice. Neuropharmacology. 2008;54(7):1112–9. doi: 10.1016/j.neuropharm.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 40.Champier J, Claustrat F, Nazaret N, Montange MF, Claustrat B. Folate depletion changes gene expression of fatty acid metabolism, DNA synthesis, and circadian cycle in male mice. Nutr Res. 2012;32(2):124–32. doi: 10.1016/j.nutres.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 41.Uthus EO, Ross SA. Dietary selenium affects homocysteine metabolism differently in Fisher-344 rats and CD-1 mice. J Nutr. 2007;137(5):1132–6. doi: 10.1093/jn/137.5.1132. [DOI] [PubMed] [Google Scholar]
- 42.Claes L, Schmalenbach J, Herrmann M, Ölkü I, Garcia P, Histing T, et al. Hyperhomocysteinemia is associated with impaired fracture healing in mice. Calcif Tissue Int. 2009;85(1):17–21. doi: 10.1007/s00223-009-9262-6. [DOI] [PubMed] [Google Scholar]
- 43.Neuman JC, Albright KA, Schalinske KL. Exercise prevents hyperhomocysteinemia in a dietary folate-restricted mouse model. Nutr Res. 2013;33(6):487–493. doi: 10.1016/j.nutres.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 44.Lam TY, Seto SW, Au ALS, Poon CCW, Li RWS, Lam HY, et al. Folic Acid Supplementation Modifies β-Adrenoceptor–Mediated In Vitro Lipolysis of Obese/Diabetic (+ db/+ db) Mice. Exp Biol Med. 2009;234(9):1047–55. doi: 10.3181/0902-RM-44. [DOI] [PubMed] [Google Scholar]
- 45.Ghosh S, Sinha JK, Putcha UK, Raghunath M. Severe but not moderate vitamin B12 deficiency impairs lipid profile, induces adiposity, and leads to adverse gestational outcome in female C57BL/6 mice. Front Nutr. 2016;3:1. doi: 10.3389/fnut.2016.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yun KU, Ryu CS, Oh JM, Kim CH, Lee KS, Lee CH, et al. Plasma homocysteine level and hepatic sulfur amino acid metabolism in mice fed a high-fat diet. Eur J Nutr. 2013;52(1):127–34. doi: 10.1007/s00394-011-0294-0. [DOI] [PubMed] [Google Scholar]
- 47.da Costa KA, Gaffney CE, Fischer LM, Zeisel SH. Choline deficiency in mice and humans is associated with increased plasma homocysteine concentration after a methionine load. Am J Clin Nutr. 2005;81(2):440–4. doi: 10.1093/ajcn.81.2.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Troen AM, Shea-Budgell M, Shukitt-Hale B, Smith DE, Selhub J, Rosenberg IH. B-vitamin deficiency causes hyperhomocysteinemia and vascular cognitive impairment in mice. PNAS. 2008;105(34):12474–9. doi: 10.1073/pnas.0805350105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chwatko G, Boers GH, Strauss KA, Shih DM, Jakubowski H. Mutations in methylenetetrahydrofolate reductase or cystathionine β-syntase gene, or a high-methionine diet, increase homocysteine thiolactone levels in humans and mice. FASEB J. 2007;21(8):1707–13. doi: 10.1096/fj.06-7435com. [DOI] [PubMed] [Google Scholar]
- 50.Looft-Wilson RC, Ashley BS, Billig JE, Wolfert MR, Ambrecht LA, Bearden SE. Chronic diet-induced hyperhomocysteinemia impairs eNOS regulation in mouse mesenteric arteries. Am J Physiol-Reg I. 2008;295(1):R59–66. doi: 10.1152/ajpregu.00833.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang H, Ha L, Hui X, Lin Y, He R, Baixiao Z. Effect of moxibustion on hyperhomocysteinemia and oxidative stress induced by high-methionine diet. Evid-Based Compl Alt Med. 2020;2020:1–8. doi: 10.1155/2020/3184785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lentz SR, Erger RA, Dayal S, Maeda N, Malinow MR, Heistad DD, Faraci FM. Folate dependence of hyperhomocysteinemia and vascular dysfunction in cystathionine β-synthase-deficient mice. Am J Physiol Heart Circ Physiol. 2000;279(3):H970–5. doi: 10.1152/ajpheart.2000.279.3.H970. [DOI] [PubMed] [Google Scholar]
- 53.Dayal S, Wilson KM, Leo L, Arning E, Bottiglieri T, Lentz SR. Enhanced susceptibility to arterial thrombosis in a murine model of hyperhomocysteinemia. Blood. 2006;108(7):2237–43. doi: 10.1182/blood-2006-02-005991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dayal S, Chauhan AK, Jensen M, Leo L, Lynch CM, Faraci FM, et al. Paradoxical absence of a prothrombotic phenotype in a mouse model of severe hyperhomocysteinemia. Blood J Am Soc Hematol. 2012;119(13):3176–83. doi: 10.1182/blood-2011-09-380568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dayal S, Blokhin IO, Erger RA, Jensen M, Arning E, Stevens JW, et al. Protective vascular and cardiac effects of inducible nitric oxide synthase in mice with hyperhomocysteinemia. PLoS ONE. 2014;9(9):e107734. doi: 10.1371/journal.pone.0107734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tan H, Jiang X, Yang F, Li Z, Liao D, Trial J, et al. Hyperhomocysteinemia inhibits post-injury reendothelialization in mice. Cardiovasc Res. 2006;69(1):253–262. doi: 10.1016/j.cardiores.2005.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vitvitsky V, Dayal S, Stabler S, Zhou Y, Wang H, Lentz SR, et al. Perturbations in homocysteine-linked redox homeostasis in a murine model for hyperhomocysteinemia. Am J Physiol-Reg I. 2004;287(1):R39–46. doi: 10.1152/ajpregu.00036.2004. [DOI] [PubMed] [Google Scholar]
- 58.Kamath AF, Chauhan AK, Kisucka J, Dole VS, Loscalzo J, Handy DE, et al. Elevated levels of homocysteine compromise blood-brain barrier integrity in mice. Blood. 2006;107(2):591–3. doi: 10.1182/blood-2005-06-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Devlin AM, Singh R, Bottiglieri T, Innis SM, Green TJ. Hepatic acyl-coenzyme a: cholesterol acyltransferase-2 expression is decreased in mice with hyperhomocysteinemia. J Nutr. 2010;140(2):231–237. doi: 10.3945/jn.109.112920. [DOI] [PubMed] [Google Scholar]
- 60.Li W, Tang R, Ma F, Ouyang S, Liu Z, Wu J. Folic acid supplementation alters the DNA methylation profile and improves insulin resistance in high-fat-diet-fed mice. J Nutr Biochem. 2018;59:76–83. doi: 10.1016/j.jnutbio.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 61.Qipshidze N, Metreveli N, Lominadze D, Tyagi SC. Folic acid improves acetylcholine-induced vasoconstriction of coronary vessels isolated from hyperhomocysteinemic mice: an implication to coronary vasospasm. J Cell Physiol. 2011;226(10):2712–20. doi: 10.1002/jcp.22621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keil KP, Abler LL, Altmann HM, Wang Z, Wang P, Ricke WA, et al. Impact of a folic acid-enriched diet on urinary tract function in mice treated with testosterone and estradiol. Am J Physiol Renal Physiol. 2015;308(12):F1431–43. doi: 10.1152/ajprenal.00674.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Teng YW, Cerdena I, Zeisel SH. Homocysteinemia in mice with genetic betaine homocysteine S-methyltransferase deficiency is independent of dietary folate intake. J Nutr. 2012;142(11):1964–7. doi: 10.3945/jn.112.166835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bernard DJ, Pangilinan FJ, Cheng J, Molloy AM, Brody LC. Mice lacking the transcobalamin-vitamin B12 receptor, CD320, suffer from anemia and reproductive deficits when fed vitamin B12-deficient diet. Hum Mol Genet. 2018;27(20):3627–40. doi: 10.1093/hmg/ddy267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou J, Werstuck GH, Lhoták Š, Shi YY, Tedesco V, Trigatti B, et al. Hyperhomocysteinemia induced by methionine supplementation does not independently cause atherosclerosis in C57BL/6J mice. FASEB J. 2008;22(7):2569–78. doi: 10.1096/fj.07-105353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hamelet J, Noll C, Ripoll C, Paul JL, Janel N, Delabar JM. Effect of hyperhomocysteinemia on the protein kinase DYRK1A in liver of mice. Biochem Biophys Res Commun. 2009;378(3):673–677. doi: 10.1016/j.bbrc.2008.11.126. [DOI] [PubMed] [Google Scholar]
- 67.Aléssio AC, Santos CX, Debbas V, Oliveira LC, Haddad R, Annichino-Bizzacchi JM. Evaluation of mild hyperhomocysteinemia during the development of atherosclerosis in apolipoprotein E-deficient and normal mice. Exp Mol Pathol. 2011;90(1):45–50. doi: 10.1016/j.yexmp.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 68.Roohi J, Kang B, Bernard D, Bedja D, Dietz HC, Brody LC. Moderately elevated homocysteine does not contribute to thoracic aortic aneurysm in mice. J Nutr. 2017;147(7):1290–5. doi: 10.3945/jn.117.251173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Minami S, Miura K, Ishioka M, Morimoto N, Isoda N, Yamamoto H, et al. Homocysteine supplementation ameliorates steatohepatitis induced by a choline-deficient diet in mice. Hepatol Res. 2019;49(2):189–200. doi: 10.1111/hepr.13234. [DOI] [PubMed] [Google Scholar]
- 70.Wu L, Zhou X, Li T, He J, Huang L, Ouyang Z, et al. Improved Sp1 and betaine homocysteine-S-methyltransferase expression and homocysteine clearance are involved in the effects of zinc on oxidative stress in high-fat-diet-pretreated mice. Biol Trace Elem Res. 2018;184(2):436–441. doi: 10.1007/s12011-017-1214-9. [DOI] [PubMed] [Google Scholar]
- 71.Prieur EA, Pjetri E, Zeisel SH, Jadavji NM. Reduced brain volume and impaired memory in betaine homocysteine S-methyltransferase knockout mice. Appl Physiol Nutr Metab. 2017;42(11):1228–31. doi: 10.1139/apnm-2017-0182. [DOI] [PubMed] [Google Scholar]
- 72.Ai Y, Sun Z, Peng C, Liu L, Xiao X, Li J. Homocysteine induces hepatic steatosis involving ER stress response in high methionine diet-fed mice. Nutrients. 2017;9(4):346. doi: 10.3390/nu9040346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sim WC, Yin HQ, Choi HS, Choi YJ, Kwak HC, Kim SK, et al. L-serine supplementation attenuates alcoholic fatty liver by enhancing homocysteine metabolism in mice and rats. J Nutr. 2015;145(2):260–7. doi: 10.3945/jn.114.199711. [DOI] [PubMed] [Google Scholar]
- 74.Borowczyk K, Tisończyk J, Jakubowski H. Metabolism and neurotoxicity of homocysteine thiolactone in mice: protective role of bleomycin hydrolase. Amino Acids. 2012;43(3):1339–48. doi: 10.1007/s00726-011-1207-5. [DOI] [PubMed] [Google Scholar]
- 75.Berge RK, Bjørndal B, Strand E, Bohov P, Lindquist C, Nordrehaug JE, et al. Tetradecylthiopropionic acid induces hepatic mitochondrial dysfunction and steatosis, accompanied by increased plasma homocysteine in mice. Lipids Health Dis. 2016;15(1):1–16. doi: 10.1186/s12944-016-0192-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jacovina AT, Deora AB, Ling Q, Broekman MJ, Almeida D, Greenberg CB, et al. Homocysteine inhibits neoangiogenesis in mice through blockade of annexin A2–dependent fibrinolysis. J Clin Investig. 2009;119(11):3384–94. doi: 10.1172/JCI39591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zinno P, Motta V, Guantario B, Natella F, Roselli M, Bello C, et al. Supplementation with dairy matrices impacts on homocysteine levels and gut microbiota composition of hyperhomocysteinemic mice. Eur J Nutr. 2020;59(1):345–358. doi: 10.1007/s00394-019-01911-y. [DOI] [PubMed] [Google Scholar]
- 78.Shinohara M, Ji C, Kaplowitz N. Differences in betaine-homocysteine methyltransferase expression, endoplasmic reticulum stress response, and liver injury between alcohol-fed mice and rats. Hepatology. 2010;51(3):796–805. doi: 10.1002/hep.23391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Teng YW, Mehedint MG, Garrow TA, Zeisel SH. Deletion of betaine-homocysteine S-methyltransferase in mice perturbs choline and 1-carbon metabolism, resulting in fatty liver and hepatocellular carcinomas. J Biol Chem. 2011;286(42):36258–67. doi: 10.1074/jbc.M111.265348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jakubowski H, Perła-Kaján J, Finnell RH, Cabrera RM, Wang H, Gupta S, et al. Genetic or nutritional disorders in homocysteine or folate metabolism increase protein N-homocysteinylation in mice. FASEB J. 2009;23(6):1721–7. doi: 10.1096/fj.08-127548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tran P, Hiou-Tim F, Frosst P, Lussier-Cacan S, Bagley P, Selhub J, et al. The curly-tail (ct) mouse, an animal model of neural tube defects, displays altered homocysteine metabolism without folate responsiveness or a defect in Mthfr. Mol Genet Metabol. 2002;76(4):297–304. doi: 10.1016/S1096-7192(02)00108-7. [DOI] [PubMed] [Google Scholar]
- 82.Maclean KN, Jiang H, Greiner LS, Allen RH, Stabler SP. Long-term betaine therapy in a murine model of cystathionine beta-synthase deficient homocystinuria: decreased efficacy over time reveals a significant threshold effect between elevated homocysteine and thrombotic risk. Mol Genet Metabol. 2012;105(3):395–403. doi: 10.1016/j.ymgme.2011.11.190. [DOI] [PubMed] [Google Scholar]
- 83.Sood HS, Hunt MJ, Tyagi SC. Peroxisome proliferator ameliorates endothelial dysfunction in a murine model of hyperhomocysteinemia. Am J Physiol Lung Cell Mol Physiol. 2003;284(2):L333–41. doi: 10.1152/ajplung.00183.2002. [DOI] [PubMed] [Google Scholar]
- 84.Suszyńska-Zajczyk J, Jakubowski H. Paraoxonase 1 and dietary hyperhomocysteinemia modulate the expression of mouse proteins involved in liver homeostasis. Acta Biochim Pol. 2014;61(4):815–23. doi: 10.18388/abp.2014_1851. [DOI] [PubMed] [Google Scholar]
- 85.Suszyńska-Zajczyk J, Wróblewski J, Utyro O, Łuczak M, Marczak Ł, Jakubowski H. Bleomycin hydrolase and hyperhomocysteinemia modulate the expression of mouse proteins involved in liver homeostasis. Amino Acids. 2014;46(6):1471–80. doi: 10.1007/s00726-014-1712-4. [DOI] [PubMed] [Google Scholar]
- 86.Suszyńska-Zajczyk J, Łuczak M, Marczak Ł, Jakubowski H. Hyperhomocysteinemia and bleomycin hydrolase modulate the expression of mouse brain proteins involved in neurodegeneration. J Alzheimer's Dis. 2014;40(3):713–726. doi: 10.3233/JAD-132033. [DOI] [PubMed] [Google Scholar]
- 87.Suszyńska-Zajczyk J, Utyro O, Jakubowski H. Methionine-induced hyperhomocysteinemia and bleomycin hydrolase deficiency alter the expression of mouse kidney proteins involved in renal disease. Mol Genet Metabol. 2014;112(4):339–46. doi: 10.1016/j.ymgme.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 88.Suszyńska-Zajczyk J, Sikora M, Jakubowski H. Paraoxonase 1 deficiency and hyperhomocysteinemia alter the expression of mouse kidney proteins involved in renal disease. Mol Genet Metabol. 2014;113(3):200–6. doi: 10.1016/j.ymgme.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 89.Ables GP, Ouattara A, Hampton TG, Cooke D, Perodin F, Augie I, et al. Dietary methionine restriction in mice elicits an adaptive cardiovascular response to hyperhomocysteinemia. Sci Rep. 2015;5(1):1–10. doi: 10.1038/srep08886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang JW, Yan R, Tang YS, Guo YZ, Chang Y, Jing L, et al. Hyperhomocysteinemia-induced autophagy and apoptosis with downregulation of hairy enhancer of split 1/5 in cortical neurons in mice. Int J Immunopathol Pharmacol. 2017;30(4):371–382. doi: 10.1177/0394632017740061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xiaoling Y, Li Z, ShuQiang L, Shengchao M, Anning Y, Ning D, et al. Hyperhomocysteinemia in ApoE−/−mice leads to overexpression of enhancer of zeste homolog 2 via miR-92a regulation. PLoS ONE. 2016;11(12):e0167744. doi: 10.1371/journal.pone.0167744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.de Rezende MM, D’Almeida V. Central and systemic responses to methionine-induced hyperhomocysteinemia in mice. PLoS ONE. 2014;9(8):e105704. doi: 10.1371/journal.pone.0105704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mullick AE, Zaid UB, Athanassious CN, Lentz SR, Rutledge JC, Symons JD. Hyperhomocysteinemia increases arterial permeability and stiffness in mice. A J Physiol-Reg I. 2006;291(5):R1349–54. doi: 10.1152/ajpregu.00335.2006. [DOI] [PubMed] [Google Scholar]
- 94.Devlin AM, Bottiglieri T, Domann FE, Lentz SR. Tissue-specific changes in H19 methylation and expression inmice withhyperhomocysteinemia. J Biol Chem. 2005;280(27):25506–11. doi: 10.1074/jbc.M504815200. [DOI] [PubMed] [Google Scholar]
- 95.Behera J, George AK, Voor MJ, Tyagi SC, Tyagi N. Hydrogen sulfide epigenetically mitigates bone loss through OPG/RANKL regulation during hyperhomocysteinemia in mice. Bone. 2018;114:90–108. doi: 10.1016/j.bone.2018.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Devlin AM, Singh R, Wade RE, Innis SM, Bottiglieri T, Lentz SR. Hypermethylation of Fads2 and altered hepatic fatty acid and phospholipid metabolism in mice with hyperhomocysteinemia. J Biol Chem. 2007;282(51):37082–90. doi: 10.1074/jbc.M704256200. [DOI] [PubMed] [Google Scholar]
- 97.Sudduth TL, Weekman EM, Brothers HM, Braun K, Wilcock DM. β-amyloid deposition is shifted to the vasculature and memory impairment is exacerbated when hyperhomocysteinemia is induced in APP/PS1 transgenic mice. Alzheimer's Res Ther. 2014;6(3):1–11. doi: 10.1186/alzrt262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen M, Zhou C, Xu H, Zhang T, Wu B. Chronopharmacological targeting of Rev-erbα by puerarin alleviates hyperhomocysteinemia in mice. Biomed Pharmacother. 2020 doi: 10.1016/j.biopha.2020.109936. [DOI] [PubMed] [Google Scholar]
- 99.Sulistyoningrum DC, Singh R, Devlin AM. Epigenetic regulation of glucocorticoid receptor expression in aorta from mice with hyperhomocysteinemia. Epigenetics. 2012;7(5):514–21. doi: 10.4161/epi.19836. [DOI] [PubMed] [Google Scholar]
- 100.Wang J, Jiang Y, Yang A, Sun W, Ma C, Ma S, et al. Hyperhomocysteinemia-induced monocyte chemoattractant protein-1 promoter DNA methylation by nuclear factor-jB/DNA methyltransferase 1 in apolipoprotein E-deficient mice. Biores Open Access. 2013;2(2):118–27. doi: 10.1089/biores.2012.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Song YS, Rosenfeld ME. Methionine-induced hyperhomocysteinemia promotes superoxide anion generation and NF κ B activation in peritoneal macrophages of C57BL/6 mice. J Med Food. 2004;7(2):229–34. doi: 10.1089/1096620041224021. [DOI] [PubMed] [Google Scholar]
- 102.Xu F, Sudo Y, Sanechika S, Yamashita J, Shimaguchi S, Honda SI, et al. Disturbed biopterin and folate metabolism in the Qdpr-deficient mouse. FEBS Lett. 2014;588(21):3924–31. doi: 10.1016/j.febslet.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 103.Denny KJ, Kelly CF, Kumar V, Witham KL, Cabrera RM, Finnell RH, et al. Autoantibodies against homocysteinylated protein in a mouse model of folate deficiency-induced neural tube defects. Birth Defects Res Part A Clin Mol Teratol. 2016;106(3):201–7. doi: 10.1002/bdra.23483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu Z, Choi SW, Crott JW, Keyes MK, Jang H, Smith DE, et al. Mild depletion of dietary folate combined with other B vitamins alters multiple components of the Wnt pathway in mouse colon. J Nutr. 2007;137(12):2701–8. doi: 10.1093/jn/137.12.2701. [DOI] [PubMed] [Google Scholar]
- 105.Padmanabhan N, Menelaou K, Gao J, Anderson A, Blake GE, Li T, et al. Abnormal folate metabolism causes age-, sex-and parent-of-origin-specific haematological defects in mice. J Physiol. 2018;596(18):4341–60. doi: 10.1113/JP276419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ernest S, Christensen B, Gilfix BM, Mamer OA, Hosack A, Rodier M, et al. Genetic and molecular control of folate-homocysteine metabolism in mutant mice. Mamm Genome. 2002;13(5):259–67. doi: 10.1007/s00335-001-3054-2. [DOI] [PubMed] [Google Scholar]
- 107.Kopp M, Morisset R, Rychlik M. Characterization and interrelations of one-carbon metabolites in tissues, erythrocytes, and plasma in mice with dietary induced folate deficiency. Nutrients. 2017;9(5):462. doi: 10.3390/nu9050462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jiang H, Hurt KJ, Breen K, Stabler SP, Allen RH, Orlicky DJ, et al. Sex-specific dysregulation of cysteine oxidation and the methionine and folate cycles in female cystathionine gamma-lyase null mice: a serendipitous model of the methylfolate trap. Biol Open. 2015;4(9):1154–62. doi: 10.1242/bio.013433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jadavji NM, Deng L, Malysheva O, Caudill MA, Rozen R. MTHFR deficiency or reduced intake of folate or choline in pregnant mice results in impaired short-term memory and increased apoptosis in the hippocampus of wild-type offspring. Neuroscience. 2015;300:1–9. doi: 10.1016/j.neuroscience.2015.04.067. [DOI] [PubMed] [Google Scholar]
- 110.Schaevitz LR, Picker JD, Rana J, Kolodny NH, Shane B, Berger-Sweeney JE, et al. Glutamate carboxypeptidase II and folate deficiencies result in reciprocal protection against cognitive and social deficits in mice: implications for neurodevelopmental disorders. Dev Neurobiol. 2012;72(6):891–905. doi: 10.1002/dneu.21000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Challet E, Dumont S, Mehdi MK, Allemann C, Bousser T, Gourmelen S, et al. Aging-like circadian disturbances in folate-deficient mice. Neurobiol Aging. 2013;34(6):1589–98. doi: 10.1016/j.neurobiolaging.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 112.Miszewski SG, Trott JF, Berryhill GE, Tat L, Green R, Borowsky AD, et al. Folate Deficiency inhibits development of the mammary gland and its associated lymphatics in fvb mice. J Nutr. 2020;150(8):2120–30. doi: 10.1093/jn/nxaa154. [DOI] [PubMed] [Google Scholar]
- 113.Gospe SM, Jr, Gietzen DW, Summers PJ, Lunetta JM, Miller JW, Selhub J, et al. Behavioral and neurochemical changes in folate-deficient mice. Physiol Behav. 1995;58(5):935–41. doi: 10.1016/0031-9384(95)00156-D. [DOI] [PubMed] [Google Scholar]
- 114.Cavallaro RA, Fuso A, Nicolia V, Scarpa S. S-adenosylmethionine prevents oxidative stress and modulates glutathione metabolism in TgCRND8 mice fed a B-vitamin deficient diet. J Alzheimer's Dis. 2010;20(4):997–1002. doi: 10.3233/JAD-2010-091666. [DOI] [PubMed] [Google Scholar]
- 115.Ernest S, Hosack A, O’Brien WE, Rosenblatt DS, Nadeau JH. Homocysteine levels in A/J and C57BL/6J mice: genetic, diet, gender, and parental effects. Physiol Genomics. 2005;21(3):404–10. doi: 10.1152/physiolgenomics.00199.2004. [DOI] [PubMed] [Google Scholar]
- 116.Zhang J, Handy DE, Wang Y, Bouchard G, Selhub J, Loscalzo J, et al. Hyperhomocysteinemia from trimethylation of hepatic phosphatidylethanolamine during cholesterol cholelithogenesis in inbred mice. Hepatology. 2011;54(2):697–706. doi: 10.1002/hep.24428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pogribny IP, Kutanzi K, Melnyk S, de Conti A, Tryndyak V, Montgomery B, et al. Strain-dependent dysregulation of one-carbon metabolism in male mice is associated with choline-and folate-deficient diet-induced liver injury. FASEB J. 2013;27(6):2233–43. doi: 10.1096/fj.12-227116. [DOI] [PubMed] [Google Scholar]
- 118.Knock E, Deng L, Wu Q, Lawrance AK, Wang XL, Rozen R. Strain differences in mice highlight the role of DNA damage in neoplasia induced by low dietary folate. J Nutr. 2008;138(4):653–658. doi: 10.1093/jn/138.4.653. [DOI] [PubMed] [Google Scholar]
- 119.Jacobs RL, Stead LM, Devlin C, Tabas I, Brosnan ME, Brosnan JT, et al. Physiological regulation of phospholipid methylation alters plasma homocysteine in mice. J Biol Chem. 2005;280(31):28299–305. doi: 10.1074/jbc.M501971200. [DOI] [PubMed] [Google Scholar]
- 120.Choumenkovitch SF, Selhub J, Bagley PJ, Maeda N, Nadeau MR, Smith DE, et al. In the cystathionine β-synthase knockout mouse, elevations in total plasma homocysteine increase tissue S-adenosylhomocysteine, but responses of S-adenosylmethionine and DNA methylation are tissue specific. J Nutr. 2002;132(8):2157–60. doi: 10.1093/jn/132.8.2157. [DOI] [PubMed] [Google Scholar]
- 121.Santiard-Baron D, Aupetit J, Janel N. Plasma homocysteine levels are not increased in murine models of Alzheimer's disease. Neurosci Res. 2005;53(4):447–9. doi: 10.1016/j.neures.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 122.Chassé JF, Santiard-Baron D, Vayssettes C, Chabli A, Aupetit J, Maeda N, et al. Altered gene expression in liver from a murine model of hyperhomocysteinemia. J Biol Chem. 2003;278(34):31504–11. doi: 10.1074/jbc.M213036200. [DOI] [PubMed] [Google Scholar]
- 123.Robert K, Maurin N, Ledru A, Delabar J, Janel N. Hyperkeratosis in cystathionine beta synthase-deficient mice: An animal model of hyperhomocysteinemia. Anat Rec Part A. 2004;280(2):1072–6. doi: 10.1002/ar.a.20082. [DOI] [PubMed] [Google Scholar]
- 124.Robert K, Santiard-Baron D, Chassé JF, Paly E, Aupetit J, Kamoun P, et al. The neuronal SAPK/JNK pathway is altered in a murine model of hyperhomocysteinemia. J Neurochem. 2004;89(1):33–43. doi: 10.1046/j.1471-4159.2003.02297.x. [DOI] [PubMed] [Google Scholar]
- 125.Eberhardt RT, Forgione MA, Cap A, Leopold JA, Rudd MA, Trolliet M, et al. Endothelial dysfunction in a murine model of mild hyperhomocyst (e) inemia. J Clin Investig. 2000;106(4):483–91. doi: 10.1172/JCI8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hamelet J, Demuth K, Paul JL, Delabar JM, Janel N. Hyperhomocysteinemia due to cystathionine beta synthase deficiency induces dysregulation of genes involved in hepatic lipid homeostasis in mice. J Hepatol. 2007;46(1):151–9. doi: 10.1016/j.jhep.2006.07.028. [DOI] [PubMed] [Google Scholar]
- 127.Powers RW, Gandley RE, Lykins DL, Roberts JM. Moderate hyperhomocysteinemia decreases endothelial-dependent vasorelaxation in pregnant but not nonpregnant mice. Hypertension. 2004;44(3):327–33. doi: 10.1161/01.HYP.0000137414.12119.f6. [DOI] [PubMed] [Google Scholar]
- 128.Ernest S, Carter M, Shao H, Hosack A, Lerner N, Colmenares C, et al. Parallel changes in metabolite and expression profiles in crooked-tail mutant and folate-reduced wild-type mice. Hum Mol Genet. 2006;15(23):3387–93. doi: 10.1093/hmg/ddl415. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplemental Table: Diet composition and mouse data.
Data Availability Statement
All data analysed during this study are included in this published article and its supplementary information file.