Abstract
Background
Several inflammatory cytokines are upregulated in severe coronavirus disease 2019 (COVID-19). We compared cytokines in COVID-19 versus influenza to define differentiating features of the inflammatory response to these pathogens and their association with severe disease. Because elevated body mass index (BMI) is a known risk factor for severe COVID-19, we examined the relationship of BMI to cytokines associated with severe disease.
Methods
Thirty-seven cytokines and chemokines were measured in plasma from 135 patients with COVID-19, 57 patients with influenza, and 30 healthy controls. Controlling for BMI, age, and sex, differences in cytokines between groups were determined by linear regression and random forest prediction was used to determine the cytokines most important in distinguishing severe COVID-19 and influenza. Mediation analysis was used to identify cytokines that mediate the effect of BMI and age on disease severity.
Results
Interleukin-18 (IL-18), IL-1β, IL-6, and tumor necrosis factor-α (TNF-α) were significantly increased in COVID-19 versus influenza patients, whereas granulocyte macrophage colony-stimulating factor, interferon-γ (IFN-γ), IFN-λ1, IL-10, IL-15, and monocyte chemoattractant protein 2 were significantly elevated in the influenza group. In subgroup analysis based on disease severity, IL-18, IL-6, and TNF-α were elevated in severe COVID-19, but not in severe influenza. Random forest analysis identified high IL-6 and low IFN-λ1 levels as the most distinct between severe COVID-19 and severe influenza. Finally, IL-1RA was identified as a potential mediator of the effects of BMI on COVID-19 severity.
Conclusions
These findings point to activation of fundamentally different innate immune pathways in severe acute respiratory syndrome coronavirus 2 and influenza infection, and emphasize drivers of severe COVID-19 to focus both mechanistic and therapeutic investigations.
Keywords: COVID-19, Influenza, Cytokines, SARS-CoV-2, Obesity
Severe COVID-19 is marked by dysregulated inflammation and is associated with elevated BMI. By comparing cytokines and chemokines in patients with either COVID-19 or influenza, we identified distinct inflammatory pathways and a cytokine mediator of the effect of BMI.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) led to more than 2 million deaths worldwide in 2020 [1]. Coronavirus disease 2019 (COVID-19), the disease caused by SARS-CoV-2, spans mild disease to multiorgan failure and death [2, 3]. One hallmark of severe disease is immune dysregulation characterized by elevated proinflammatory markers and cytokines [4–9] including interleukin (IL)-6, IL-10, interferon-γ-induced protein 10 (IP-10), IL-1RA, and monocyte chemoattractant protein 1 (MCP-1 [8, 10–15]. Studies have challenged the uniqueness of the inflammatory cytokine profile of COVID-19 by highlighting similarities to sepsis or acute respiratory distress syndrome from other causes [16–18].
Influenza is another respiratory viral cause of severe pneumonia and pandemics [19]. The case fatality rate for influenza is lower than that of COVID-19, but many of the cytokines upregulated in COVID-19 are also increased in severe influenza infection [15, 20, 21]. Thus, it is unclear what unique cytokine upregulation in SARS-CoV-2 infection leads to more severe disease than influenza [22]. Several clinical factors correlate with severe COVID-19, including advanced age and elevated body mass index (BMI) [23, 24]. Obese patients are at increased risk for hospitalization and death, particularly at younger ages [25, 26]. Obesity leads to chronic inflammation, and elevated BMI is associated with increases in IL-10, IL-6, tumor necrosis factor-α (TNF-α), and IL-1RA [27–29]. Yet, few studies examining these cytokines in COVID-19 have incorporated this in their analyses.
To determine how cytokines produced during COVID-19 and influenza differ and to understand the increased pathogenicity of SARS-CoV-2, we measured 37 cytokines and chemokines in patients hospitalized with either influenza or COVID-19 and compared cytokine levels based on disease severity. We also performed mediation analysis to identify cytokines that mediate the effect of BMI and age on disease severity. We found that severe COVID-19 induces a macrophage proinflammatory cytokine profile, whereas severe influenza leads to interferon induction. We found that although multiple cytokines mediate the effects of advanced age, IL-1RA is the primary mediator underlying the relationship between obesity and severe COVID-19. These findings highlight that disparate immune pathways are activated in these potentially life-threatening respiratory viral infections.
METHODS
Study Participants and Samples
All studies were approved by the Johns Hopkins institutional review board (IRB). Hospitalized patients diagnosed with COVID-19 by positive SARS-CoV-2 RNA testing in the Johns Hopkins Healthcare System were enrolled in a prospective consented protocol to investigate research questions specific to the clinical course of COVID-19 (IRB 00245545). Demographic information, clinical laboratory test results, International Classification of Diseases, 10th revision, coded diagnoses (comorbidities), BMI, and other clinical parameters were linked to data for COVID-19 patients in the study. Those who received tocilizumab before cytokine measurement were excluded. Participants were categorized by maximum COVID-19 disease severity score based on the World Health Organization severity scale [30]. Those with a score <4 were categorized as having mild/moderate disease and those with a score ≥5 were considered severe. Blood was obtained as close to admission as feasible and centrifuged to separate cells from plasma in Basics of Biosafety Level 2+ laboratory conditions.
Healthy control (HC) plasma was obtained from human immunodeficiency virus/hepatitis C virus-antibody seronegative participants enrolled before 2020 in the Baltimore Before and After Acute Study of Hepatitis study (IRB NA_00046368), an ongoing prospective, community-recruited, observational cohort study of people who inject drugs, as previously described [31].
Plasma from hospitalized patients infected with influenza between 2017 and 2019 was obtained as previously described for comparison to hospitalized COVID-19 patients in this study [31, 32] (IRB 00091667). Patients hospitalized with influenza requiring no more than nasal cannula and those that required higher levels of oxygen support were classified as having mild/moderate disease or severe disease, respectively, which approximates the World Health Organization COVID-19 severity score [30].
All plasma samples were frozen at -80°C until thawed for cytokine measurement as described in the following section.
Cytokine Measurement
Plasma cytokines and chemokines (interferon-α2a [IFN-α2a], IFN-β, IL-18, IL-1RA, IL-23, IFN-λ1, IL-2Ra, MCP-2, granulocyte macrophage colony-stimulating factor [GM-CSF], IL-23p40, IL-15, IL-16, IL-17A, IL-1α, IL-5, IL-7, TNF-β, vascular endothelial growth factor [VEGF], Eotaxin, Eotaxin-3, induced protein-10 [IP-10], MCP-1, MCP-4, macrophage derived chemokine (MDC), macrophage inflammatory protein-1α [MIP-1α], MIP-1β, thymus- and activation-regulated chemokine (TARC), IFN-γ, IL-10, IL-12p70, IL-13, IL-1β, IL-2, IL-4, IL-6, IL-8, TNF-α) were measured using a custom multiplex kit from Meso Scale Diagnostics (Rockville, MD) according to the manufacturer’s protocol, and data were acquired on a MESO QuickPlex SQ 120. Each sample was measured on first thaw and in duplicate. If an analyte signal was below background, it was set to 0; if detectable, but below the manufacturer’s lower limit of quantification, it was set to the lower limit of detection.
Statistical Analysis
Data were analyzed using the statistical computing software R version 3.6.3 [33]. The cytokine/chemokine (i.e., analyte) signals were first log2 transformed after adding a pseudocount of 1. To compare the analytes between patient groups, a linear regression analysis, which is equivalent to a 2-tailed t test after adjusting for covariates, was applied. For instance, a linear regression model was fitted for each analyte to test the difference between every pair of patient groups (e.g., COVID-19 vs influenza) after adjusting for covariates (age, gender, and BMI). P values of the coefficient for the patient group from the model were obtained and converted to false discovery rates (FDRs) using the Benjamini-Hochberg procedure [34]. An FDR of 0.25 was considered significant. Random forest and mediation analysis were performed as described in the Supplementary Methods [35–37].
RESULTS
Cohort Characteristics
A total of 135 participants with SARS-CoV-2, 57 participants with influenza, and 30 HCs were studied. Based on final infection outcome, we categorized influenza and COVID-19 subgroups as mild/moderate or severe, as described in Materials and Methods. Thirteen of 57 (23%) participants in the influenza cohort and 80 of 135 (59%) patients with COVID-19 had severe disease (Table 1). The influenza and COVID-19 cohorts were not significantly different in gender, non-White race, or BMI. The influenza cohort was younger than the COVID-19 cohort on average (mean age, 48.2 vs 56.2 years). The interval between admission and cytokine measurement was not significantly different between the mild/moderate and severe disease subgroups (Supplementary Figure 1A). Principal component analysis of the 2 cohorts revealed clustering of the severe subgroups together in the principal component space, suggesting a similar level of overall inflammation between the 2 subgroups (Supplementary Figure 1B).
Table 1.
Characteristics of Study Participants
| Subject Group | COVID-19 | Influenza | Healthy Controls | |
|---|---|---|---|---|
| Demographics | ||||
| Male, N (%) | 70 (52) | 26 (46) | P = .53 | 19 (63) |
| Female, N (%) | 65 (48) | 31 (54) | … | 11 (37) |
| Mean age (range), y | 56.2 (20–90) | 48.2 (19–89) | P = .006 | 31.2 (20–45) |
| Mean BMI (range) | 32.8 (12–70) | 30.2 (18–60) | P = .06 | NA |
| Race and ethnicity | ||||
| Race | N (%) | … | … | |
| Black | 62 (46) | 40 (70) | … | 11 (37) |
| White | 26 (19) | 10 (18) | … | 19 (63) |
| Othera | 38 (28) | 5 (9) | … | 0 (0) |
| Asian | 9 (7) | 2 (3) | … | 0 (0) |
| White vs non-White | P = .84 | |||
| Ethnicity | ||||
| Hispanic/Latinx | N (%) | … | … | |
| Yes | 33 (24) | NA | … | 0 (0) |
| No | 102 (76) | NA | … | 30 (100) |
| Maximum disease severity b, N (%) | ||||
| Mild/moderate | 55 (41) | 44 (77) | … | NA |
| Severe | 80 (59) | 13 (23) | … | NA |
| Comorbidities | N (%) | |||
| Diabetes mellitus | 56 (41) | NA | … | 0 (0) |
| HIV infection | 4 (3) | 7 (12) | … | 0 (0) |
Abbreviations: BMI, body mass index; COVID-19, coronavirus disease 2019; HIV, human immunodeficiency virus; NA, data not available.
aMost self-identified as Hispanic/Latino.
bMaximum disease severity indicates the most severe COVID-19 disease class for the patient when under observation: Mild/moderate = no or low flow oxygen required, or high flow oxygen or noninvasive positive-pressure ventilation required; severe = patient required intubation or patient died (ventilated or not). P values were calculated as described in the Supplementary Methods.
Cytokine Elevations in COVID-19 and Influenza Compared With Healthy Controls
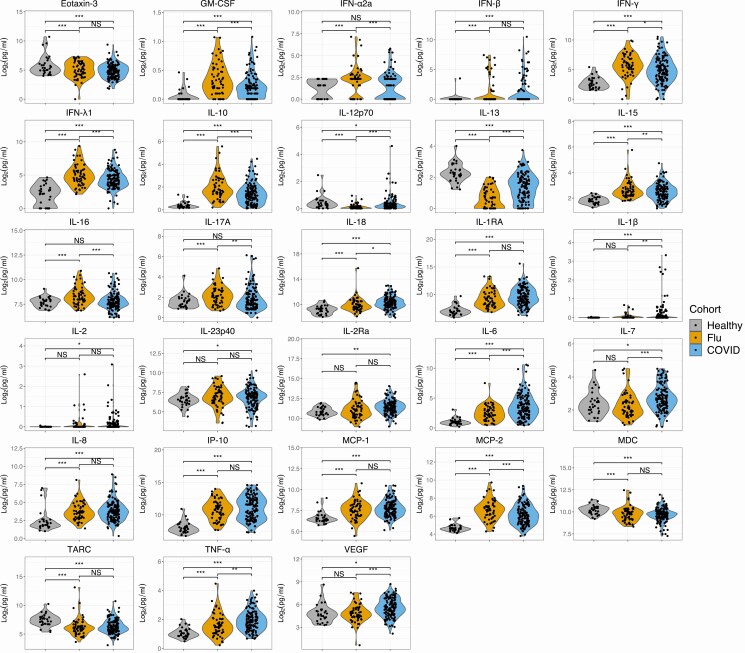
To determine which cytokines and chemokines are upregulated in influenza and COVID-19, we compared cytokines/chemokines in HCs to those with influenza and COVID-19. We selected potentially important markers of disease severity for our custom panel based on prior publications in SARS-CoV-1, SARS-CoV-2, and influenza [8, 15, 38–40]. We found that GM-CSF, IFN-β, IFN-γ, IFN-λ1, IL-10, IL-15, IL-18, IL-1RA, IL-6, IL-8, IP-10, MCP-1, MCP-2, and TNF-α were significantly increased in both influenza and COVID-19 compared with HCs (Figure 1). In contrast, IL-12p70, IL-13, eotaxin-3, MDC, and TARC were significantly decreased in COVID-19 and influenza compared with HCs. Seven analytes were not significantly elevated in either virus group: IL-1α, IL-23, IL-4, IL-5, MCP-4, MIP-1α, and MIP-1β (Supplementary Figure 1C). IL-1β, IL-2, IL-23p40, IL-2Ra, IL-7, and VEGF were elevated in COVID-19 exclusively, not influenza, compared with HCs. Conversely, IFN-α2a, IL-16, and IL-17A were significantly elevated solely in the influenza cohort, with no difference between COVID-19 participants and HCs.
Figure 1.
Cytokines and chemokines in influenza and COVID-19 compared with healthy controls. Differences between the COVID-19 cohort (blue) or influenza cohort (orange) and healthy controls (gray) were determined by 2-tailed t test after adjusting for sex and age. Differences between the COVID-19 cohort and influenza cohort for each analyte were determined by 2-tailed t test after adjusting for sex, age, and BMI. FDR was obtained using the Benjamini-Hochberg procedure. Statistical significance is indicated by NS, *, **, or *** above the brackets indicating FDR >0.25, <0.25, <0.1, or <0.05, respectively. Abbreviations: BMI, body mass index; COVID-19, coronavirus disease 2019; FDR, false discovery rate; NS, not significant.
Differences in Cytokines and Chemokines Between Influenza and COVID-19 Reveal a Proinflammatory Macrophage Signature in COVID-19
Focusing on analytes elevated in 1 or both viral cohorts, we found that IL-18, IL-1β, IL-6, IL-7, TNF-α, and VEGF were higher in COVID-19, whereas GM-CSF, IFN-α2a, IFN-γ, IFN-λ1, IL-10, IL-15, IL-16, IL-17A, and MCP-2 were higher in influenza (Figure 1) (Supplementary Table 1 and 2). The cytokines most elevated in the COVID-19 group are produced primarily by macrophages and characterize macrophage activation syndrome [41, 42]. Elevated levels of IL-18 and IL-1β suggest prominent inflammasome activation in COVID-19 relative to influenza [43]. Macrophages are major sources of inflammasome cytokines in other viral infections [44–46]. Conversely, IFN-λ1 was nearly 2-fold higher in influenza compared with COVID-19, consistent with a small study demonstrating lower interferon production in COVID-19 versus influenza [47] and an in vitro study demonstrating limited induction of IFN-λ1, IFN-α2a, and IFN-β by SARS-CoV-2 [39]. Though IL-10 was implicated in COVID-19 pathogenesis [15, 48, 49], IL-10 levels were actually higher in moderate influenza compared with COVID-19.
Plotting the correlation of each analyte with every other analyte in correlation matrices by disease revealed that many of the cytokines/chemokines elevated in influenza relative to COVID-19 strongly correlate, including IL-10, IFN-λ1, MCP-2, and IFN-γ. Similarly, those increased in COVID-19 relative to influenza positively correlate including IL-18, TNF-α, and IL-6 (Supplementary Figure 2A and 2B). A similar pattern emerged after generating a heatmap grouped by disease subgroup (Supplementary Figure 2C). These findings suggest that distinct inflammatory pathways are activated in these respiratory viral infections.
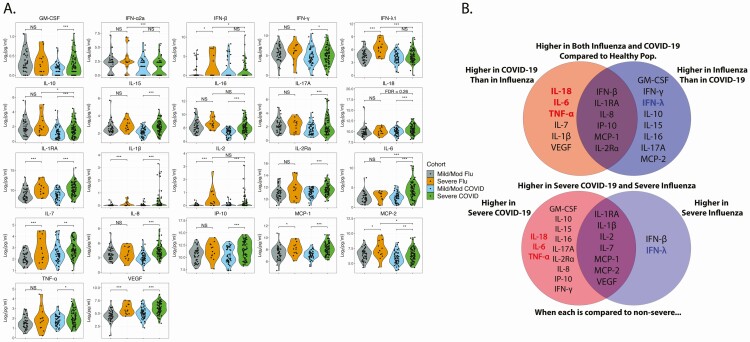
When we compared elevated analytes statistically by severity subgroups, we found minimal overlap in the cytokines/chemokines that distinguished severe from mild/moderate influenza and those that distinguished severe from mild/moderate COVID-19 (Figure 2A and 2B). IL-1RA, IL-1β, IL-2, IL-7, MCP-1, MCP-2, and VEGF were elevated in both severe diseases compared with their mild/moderate counterpart. Only IFN-β and IFN-λ1 were elevated in severe influenza, but not in severe COVID-19 (Figure 2 and Supplementary Table 1), consistent with low interferon responses in COVID-19. Cytokines elevated in severe COVID-19, but not severe influenza, include GM-CSF, IL-10, IL-15, IL-16, IL-17A, IL-18, IL-2Ra, IL-6, IL-8, and IP-10. Zero influenza and 22 COVID-19 participants received steroids before cytokine measurement. When excluding these, IFN-γ and TNF-α were also significantly elevated in severe COVID-19 (Figure 2A and 2B, Supplementary Figure 3, and Supplementary Table 3). Of the cytokines elevated in severe COVID-19, but not severe influenza, only IL-18, IL-6, and TNF-α were also elevated in the whole COVID-19 cohort compared with the whole influenza cohort (Figures 1 and 2). These 3 cytokines are highly associated with proinflammatory macrophages [50].
Figure 2.
Cytokines and chemokines elevated in severe disease compared with mild/moderate disease and according to infection. A, Differences between disease severity subgroups were determined by 2-tailed t test after adjusting for sex, age, and BMI. Participants who received steroids before cytokine measurement were excluded. Differences between the severe COVID-19 cohort (green) and severe influenza cohort (orange) for each analyte were determined by 2-tailed t test after adjusting for sex, age, and BMI. False discovery rate (FDR) was obtained using the Benjamini-Hochberg procedure. Statistical significance is indicated by NS, *, **, or *** above the brackets indicating FDR >0.25, <0.25, <0.1, or <0.05 respectively. B, Top: Cytokines/chemokines higher in the COVID-19 cohort compared with influenza (left), influenza compared with COVID-19 (right) both COVID-19 and influenza compared with healthy controls, but not significantly different between COVID-19 and influenza (overlap center). Bottom: Cytokines/chemokines elevated in severe COVID-19 relative to mild/mod COVID-19 (left side), those elevated in severe influenza relative to mild/mod influenza (right side), and those that are elevated in severe forms of both diseases (overlap center). Abbreviations: BMI, body mass index; COVID-19, coronavirus disease 2019; NS, not significant.
When comparing severe COVID-19 to severe influenza directly, only IL-6 was significantly higher in severe COVID-19, whether those who received steroids were included or not (Figure 2 and Supplementary Tables 1 and 3). When excluding steroid recipients, IL-18 narrowly missed our predetermined FDR cutoff for significance of 0.25 (FDR = 0.26) (Supplementary Tables 1 and 2). Elevated analytes higher in severe influenza compared with severe COVID-19 included IFN-λ1, IFN-α2a, IFN-β, IL-10, and MCP-2 (Figure 2 and Supplementary Table 5).
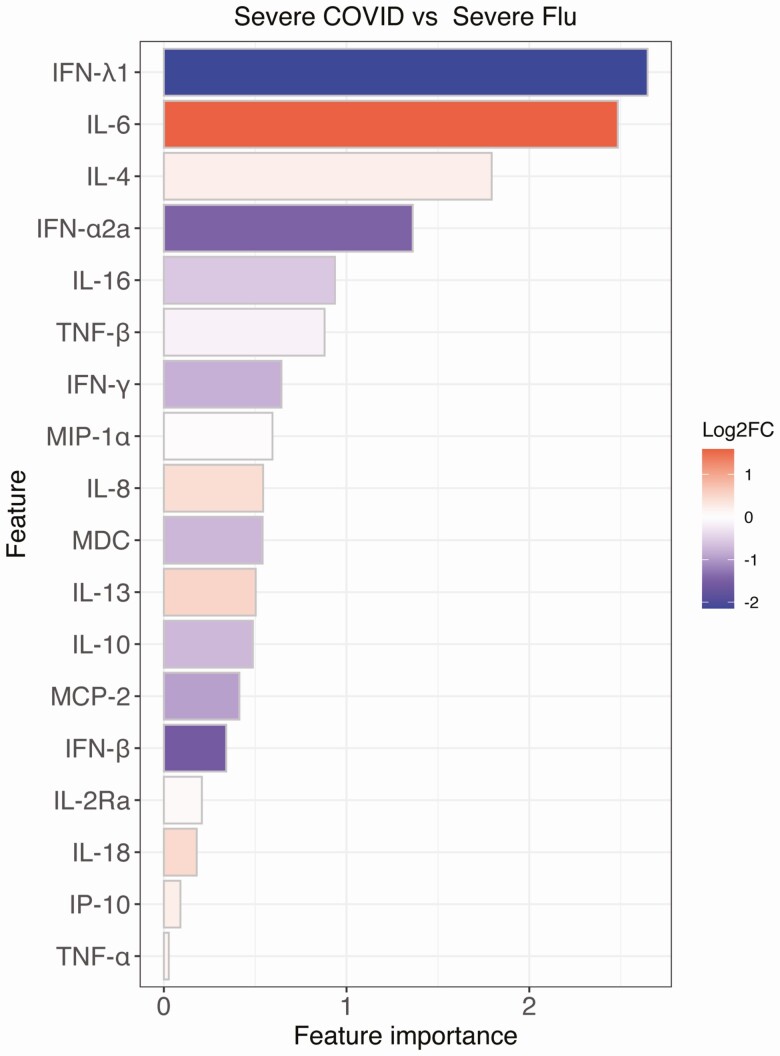
IL-6 and IFN-λ1 Are the Most Important Cytokines in Distinguishing Severe COVID-19 From Severe Influenza
To further characterize differences in the inflammatory pathways activated, we performed a multivariate analysis based on random forest using all the analytes and basic demographic information to compare severe COVID-19 and severe influenza. IL-6 and IFN-λ1 emerged as the most important factors distinguishing these 2 diseases in this analysis (Figure 3), with the highest fold changes between the severe subgroups. The importance of IL-6 and IFN-λ1 were confirmed when removing participants treated with steroids and when using a univariate analysis (Supplementary Figure 4 and Supplementary Methods). These findings underscore differences in the innate immune programs activated by these viruses; inflammatory macrophage activation pathways in COVID-19 and interferon pathways in influenza.
Figure 3.
Multivariable analysis based on random forest revealed the most important variables in distinguishing severe COVID-19 and severe influenza. Feature importance was obtained from the random forest model for predicting severe COVID-19 versus severe influenza. The color indicates the log2 fold change of the analyte signal between severe COVID-19 and severe influenza. Red color indicates a higher value in COVID-19 and blue color indicates a higher value in influenza. Abbreviation: COVID-19, coronavirus disease 2019.
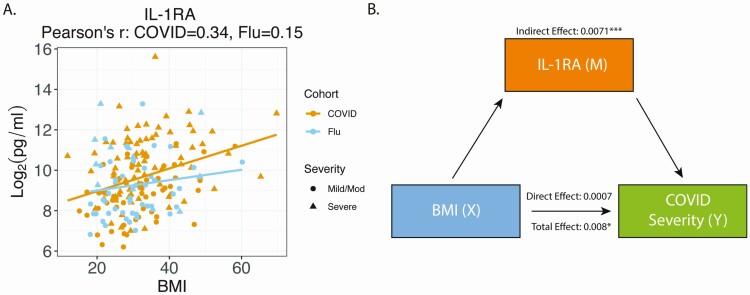
IL-1RA Is a Potential Mediator of the Effect of BMI on COVID-19 Severity
Previous studies demonstrated an association between BMI and elevation of multiple cytokines increased in severe COVID-19, including IL-6, IL-1β, and IL-1RA [27, 29, 51, 52]. Plotting BMI versus cytokine concentration demonstrated a positive association between IL-1RA, IL-23p40, MDC, IL-17A, and MCP-2 (Figure 4A and Supplementary Figure 5). With mediation analysis and after adjusting for multiple testing, diabetes, and heart disease, only IL-1RA had a significant mediation effect. Although the total effect of BMI on COVID-19 severity was 0.0078 (FDR = 0.13), most of the effect was indirectly through IL-1RA. Indeed, the direct effect of BMI on COVID-19 severity was minimal (0.0007, FDR = 0.88) compared with the indirect effect of BMI on severity through IL-1RA (0.0071, FDR <0.05). This suggests that the effect of increased BMI on the likelihood of severe COVID-19 may be mediated by IL-1RA (Figure 4B, Supplementary Table 6). Cardiovascular disease and diabetes are associated with elevated BMI, but we compared the mild/moderate and severe COVID-19 subgroups while adjusting for these conditions and found no differences in the cytokines that were significant between severe and mild/moderate COVID-19 (Supplementary Table 4). Analysis of the influenza cohort did not reveal any cytokines/chemokines mediating BMI and disease severity (Supplementary Table 7).
Figure 4.
Mediation analysis of BMI and IL-1RA on COVID-19 severity. A, BMI (X axis) was plotted versus IL-1RA level (Y axis). The yellow line is a linear regression line for the COVID-19 cohort and the blue line is a linear regression line for the influenza cohort. The Pearson’s correlation coefficient between IL-1RA and BMI is 0.34 for the COVID-19 cohort and 0.15 for the influenza cohort. B, Diagram of the mediation analysis of BMI and IL-1RA on COVID-19 severity while controlling for diabetes and heart disease. The indirect, direct, and total effects were showed in the diagram. Statistical significance is indicated by *, **, or *** representing FDR <0.25, <0.1, or <0.05, respectively. Abbreviations: BMI, body mass index; COVID-19, coronavirus disease 2019; FDR, false discovery rate; IL, interleukin.
Given that advanced age is a risk factor for severe COVID-19, we also performed mediation analysis using age as the independent variable. Unlike BMI, numerous cytokines are potential mediators of the effect of age on severity including IL-10, IL-1RA, IL-2Ra, and MCP-1 (Supplementary Figure 6 and Supplementary Table 8).
DISCUSSION
Our analysis of cytokines and chemokines elevated in COVID-19 compared with influenza reveals distinct cytokine profiles of these respiratory diseases. We found that several cytokines previously reported to be elevated in COVID-19 were not different from or were higher in influenza than in COVID-19, including IFN-γ, IL-10, and IL-15 [15]. Some cytokines (GM-CSF, IL-10, IL-15, IL-16, and IL-17A) that were higher in influenza than COVID-19 overall and distinguished severe COVID-19 from moderate COVID-19 did not differ between severe and moderate influenza. Influenza infection generally induces high levels of these cytokines and severe influenza is not marked by additional elevations.
Another study also found that many cytokines were either not different or were significantly less elevated in COVID-19 versus influenza [53]. Consistent with their results, we found that IFN-γ and IL-17A were elevated in influenza. They found significant increases in IL-1RA, IL-2, and MIP-1α in their influenza cohort relative to COVID-19, whereas we did not. These differences may be due to our adjustment for BMI, our larger cohort size, different platforms used to measure cytokines, or that a higher percentage of their influenza patients had severe disease. They observed a trend toward increased IL-6 in the COVID-19 group compared with influenza and we found high IL-6 to be one of the most distinguishing cytokines in COVID-19. They did not measure IL-18 or IFN-λ1, important in distinguishing severe COVID-19 and severe influenza in our analysis. Although the “cytokine storm” hypothesis was proposed to explain the pathology observed in COVID-19, our study and others demonstrate lower overall cytokine levels in COVID-19 than those observed in other inflammatory diseases [17, 18, 53, 54].
Both influenza and SARS-CoV-2 are respiratory RNA viruses, but our study emphasizes that they induce distinct inflammatory pathways. We found that severe COVID-19 leads to upregulation of cytokines associated with a proinflammatory macrophage phenotype [55] characterized by high levels of IL-6, TNF-α, and IL-18, whereas interferons and cytokines involved in T-cell activation (IL-15, IL-16, and IFN-γ) are upregulated in influenza. IL-18 and IL-1β, which is difficult to detect in blood, are released from macrophages upon activation of a component of the innate immune system called the inflammasome [43, 45, 56]. Emerging evidence suggests inflammasome activation is central to the SARS-CoV-2 pathogenesis and marks severe disease [8, 12, 57, 58]. Our study provides additional evidence that the inflammasome is activated in SARS-CoV-2 infection. High IL-6 and low IFN-λ1 were the most distinct features of severe COVID-19 compared with severe influenza, consistent with results of another study of COVID-19 and influenza patients [47]. We do not know if these cytokine disparities mediate pathology differences or are merely correlates of other distinct immune responses. Although steroids have proven beneficial in later stages of the disease in patients with COVID-19 requiring oxygen, early immunosuppression is not beneficial [59]. Given the association we observed with severe COVID-19 and a proinflammatory macrophage phenotype, targeting of the cytokines mediating macrophage activation syndrome, singly or in combination, might provide a more specific approach to immune modulation. Both targeted anti-IL-6 therapy and IL-1 antagonists are associated with benefit in some studies. [60–71].
Another strength of our analysis is the focus on BMI as a contributor to severe disease. Although this association has been widely described, few analyses of inflammatory cytokines have taken this variable into account [72]. We found that IL-1RA is a potential mediator of the effect of BMI on COVID-19 disease severity. This novel observation points to a possible mechanism linking BMI to severe COVID-19. IL-1RA is an acute phase reactant produced by adipocytes, macrophages, and the liver in response to inflammatory cytokines and pathogens through pathways that upregulate IL-6 and TNF-α [73, 74]. This was an unexpected finding given the anti-inflammatory nature of this cytokine and the role of IL-1RA in pathogenesis warrants additional investigation. Inflammation is an important component of aging [75]. In contrast to IL-1RA being the sole potential cytokine mediator of the effect of BMI on disease severity, we identified numerous potential cytokine mediators of the effect of age on disease severity, highlighting the specificity of our association between IL-1RA, BMI, and COVID-19 severity.
Limitations to our study include that outpatients with milder COVID-19 were not included. However, we would predict that differences between those with and without severe disease in our study would be more significant if milder disease were included. In addition, our study participants with influenza also required hospitalization, making them an appropriate comparator. An additional limitation is that we examined a single timepoint, and that the time from admission to sampling was not identical, but principal component analysis revealed similar inflammatory states. It is possible that dynamic changes in these cytokines during the course of hospitalization would make the patterns more or less distinct from influenza. However, as patients remain hospitalized, complications from critical illness arise that could obfuscate this comparison. Finally, although the influenza cohort was, on average, younger than the COVID-19 cohort, we adjusted for age in our analysis.
This study provides insight into pathways activated by SARS-CoV-2 and influenza, demonstrating that some inflammatory cytokines elevated in COVID-19 likely reflect common pathways activated in respiratory tract inflammation, whereas others are more specific to COVID-19 pathogenesis. In summary, this study demonstrates activation of a proinflammatory cytokine macrophage pathway and a role for IL-1RA in the effect of BMI on severe COVID-19, highlighting potential therapeutic targets.
Supplementary Material
Notes
Acknowledgments. We acknowledge the contribution of the Johns Hopkins IVAR team and assistance for clinical data coordination and retrieval from the Core for Clinical Research Data Acquisition. The specimens from COVID-19 patients used for this study were part of the Johns Hopkins Biospecimen Repository, which is based on the contribution of many patients, their families, research teams, and clinicians. We thank members of the Viral Hepatitis Center at Johns Hopkins for advice and discussion and blood processing. We owe additional thanks to Sherry Kelly Gibson, Muhammad Munir, Tingtin Niu, Nikitta Dhillon, Dan Warren, Robin Avery, and Niraj Desai for assistance with the study.
Financial support. This work was supported by the Johns Hopkins COVID-19 Research Response Program, a Johns Hopkins University Provost Research Grant, The Bill and Melinda Gates Foundation (134582), National Cancer Institute (U54CA260491), and National Institutes of Health Centers of Excellence in Influenza Research and Surveillance (HHSN272201400007C). The study was supported in part by a cooperative agreement between Johns Hopkins University and the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, the US Department of Health and Human Services Biomedical Advanced Research and Development Authority (agreement number IDSEP160031-01-00), the National Institute of Allergy and Infectious Diseases U19AI088791 and R01AI108403, and National Institute of Allergy and Infectious Diseases contract HHSN272201400007C awarded to Johns Hopkins Center of Excellence in Influenza Research and Surveillance. H. J. and W. Z. were supported in part by National Institute of Health grant R01HG009518. A. H. K. was supported by the National Institute of Health T32 AI007291-27. A. F. was supported, in part, by grant D18HP29037 from the US Health Resources and Services Administration, Bureau of Health Workforce, Health Careers Opportunity Program. R. E. R. was supported in part by the National Institute of Allergy and Infectious Diseases contract HHSN272201400007C awarded to the Johns Hopkins Center of Excellence in Influenza Research and Surveillance at the Johns Hopkins University and US Department of Health and Human Services Biomedical Advanced Research and Development Authority (agreement number IDSEP160031-01-00).
Potential conflicts of interest. None of the authors has any relevant conflict of interests to disclose.
References
- 1. COVID-19 map. Available at: https://coronavirus.jhu.edu/map.html. Accessed 15 June 2020.
- 2. Argenziano MG, Bruce SL, Slater CL, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ 2020; 369: m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chau NVV, Thanh Lam V, Thanh Dung N, et al. The natural history and transmission potential of asymptomatic SARS-CoV-2 infection. Clin Infect Dis. 2020; 71:2679–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang X, Tan Y, Ling Y, et al. Viral and host factors related to the clinical outcome of COVID-19. Nature 2020; 583:437–40. [DOI] [PubMed] [Google Scholar]
- 5. Giamarellos-Bourboulis EJ, Netea MG, Rovina N, et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host & Microbe 2020; 0. Available at: https://www.cell.com/cell-host-microbe/abstract/S1931-3128(20)30236-5. Accessed 23 April 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet 2020; 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest 2020; 130:2620–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lucas C, Wong P, Klein J, et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 2020; 584:463–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leisman DE, Ronner L, Pinotti R, et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med 2020; 8: 1233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Silvin A, Chapuis N, Dunsmore G, et al. Elevated calprotectin and abnormal myeloid cell subsets discriminate severe from mild COVID-19. Cell 2020; 182:1401–1418.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mandel M, Harari G, Gurevich M, Achiron A. Cytokine prediction of mortality in COVID19 patients. Cytokine 2020; 134:155190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Young BE, Ong SWX, Ng LFP, et al. Viral dynamics and immune correlates of coronavirus disease 2019 (COVID-19) severity. Clin Infect Dis 2021; 73:e2932–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Del Valle DM, Kim-Schulze S, Huang H-H, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med 2020; 26:1636–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Han H, Ma Q, Li C, et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect 2020; 9:1123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhao Y, Qin L, Zhang P, et al. Longitudinal COVID-19 profiling associates IL-1RA and IL-10 with disease severity and RANTES with mild disease. JCI Insight 2020; 5. Available at: 10.1172/jci.insight.139834. Accessed 9 July 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilson JG, Simpson LJ, Ferreira A-M, et al. Cytokine profile in plasma of severe COVID-19 does not differ from ARDS and sepsis. JCI Insight 2020; 5. Available at: 10.1172/jci.insight.140289. Accessed 10 August 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kox M, Waalders NJB, Kooistra EJ, Gerretsen J, Pickkers P. Cytokine levels in critically ill patients with COVID-19 and other conditions. JAMA 2020; 324:1565–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sinha P, Matthay MA, Calfee CS. Is a “cytokine storm” relevant to COVID-19? JAMA Intern Med 2020; 180:1152–4. [DOI] [PubMed] [Google Scholar]
- 19. Krammer F, Smith GJD, Fouchier RAM, et al. Influenza. Nat Rev Dis Primers 2018; 4:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Betakova T, Kostrabova A, Lachova V, Turianova L. Cytokines induced during influenza virus infection. Curr Pharm Des 2017; 23:2616–22. [DOI] [PubMed] [Google Scholar]
- 21. Bradley-Stewart A, Jolly L, Adamson W, et al. Cytokine responses in patients with mild or severe influenza A(H1N1)pdm09. J Clin Virol 2013; 58:100–7. [DOI] [PubMed] [Google Scholar]
- 22. Short KR, Kroeze EJBV, Fouchier RAM, Kuiken T. Pathogenesis of influenza-induced acute respiratory distress syndrome. Lancet Infect Dis 2014; 14:57–69. [DOI] [PubMed] [Google Scholar]
- 23. Garibaldi BT, Fiksel J, Muschelli J, et al. Patient trajectories among persons hospitalized for COVID-19. Ann Intern Med 2021; 174:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of Covid-19 in New York City. New Engl J Med 2020; 382:2372–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hendren NS, de Lemos JA, Ayers C, et al. Association of body mass index and age with morbidity and mortality in patients hospitalized with COVID-19. Circulation 2021; 143:135–44. [DOI] [PubMed] [Google Scholar]
- 26. Garibaldi BT, Wang K, Robinson ML, et al. Comparison of time to clinical improvement with vs without remdesivir treatment in hospitalized patients with COVID-19. JAMA Netw Open 2021; 4:e213071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Charles BA, Doumatey A, Huang H, et al. The roles of IL-6, IL-10, and IL-1RA in obesity and insulin resistance in African-Americans. J Clin Endocrinol Metab 2011; 96:E2018–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schmidt FM, Weschenfelder J, Sander C, et al. Inflammatory cytokines in general and central obesity and modulating effects of physical activity. PLoS One 2015; 10:e0121971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Juge-Aubry CE, Somm E, Giusti V, et al. Adipose tissue is a major source of interleukin-1 receptor antagonist: upregulation in obesity and inflammation. Diabetes 2003; 52:1104–10. [DOI] [PubMed] [Google Scholar]
- 30. World Health Organization. R&D Blueprint: novel coronavirus: COVID-19 therapeutic trial synopsis. 2020.
- 31. Cox AL, Netski DM, Mosbruger T, et al. Prospective evaluation of community-acquired acute-phase hepatitis C virus infection. Clin Infect Dis 2005; 40:951–8. [DOI] [PubMed] [Google Scholar]
- 32. Dugas AF, Hsieh YH, LoVecchio F, et al. ; Emergency Department National Influenza Network Investigators. Derivation and validation of a clinical decision guideline for influenza testing in 4 US emergency departments. Clin Infect Dis 2020; 70:49–58. [DOI] [PubMed] [Google Scholar]
- 33. R: The R Project for Statistical Computing. Available at: https://www.r-project.org/. Accessed 4 January 2021.
- 34. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Stat Soc Series B (Methodological) 1995; 57:289–300. [Google Scholar]
- 35. Kuhn M, Wing J, Weston S, et al. caret: classification and regression training. 2020. Available at: https://CRAN.R-project.org/package=caret. Accessed 4 January 2021.
- 36. Liaw A, Wiener M. C lassification and regression by randomForest. R News 2002; 2:5. [Google Scholar]
- 37. Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. mediation: R Package for causal mediation analysis. Journal of Statistical Software 2014; 59:1–38.26917999 [Google Scholar]
- 38. Chattergoon MA, Levine JS, Latanich R, Osburn WO, Thomas DL, Cox AL. High plasma interleukin-18 levels mark the acute phase of hepatitis C virus infection. J Infect Dis 2011; 204:1730–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Blanco-Melo D, Nilsson-Payant BE, Liu WC, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 2020; 181:1036–1045.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Broggi A, Ghosh S, Sposito B, et al. Type III interferons disrupt the lung epithelial barrier upon viral recognition. Science 2020; 369:706–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol 2014; 5: 491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schulert GS, Grom AA. Pathogenesis of macrophage activation syndrome and potential for cytokine-directed therapies. Annu Rev Med 2015; 66:145–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Evavold CL, Kagan JC. Inflammasomes: threat-assessment organelles of the innate immune system. Immunity 2019; 51:609–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mantovani A, Dinarello CA, Molgora M, Garlanda C. Interleukin-1 and related cytokines in the regulation of inflammation and immunity. Immunity 2019; 50:778–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kuriakose T, Kanneganti TD. Regulation and functions of NLRP3 inflammasome during influenza virus infection. Mol Immunol 2017; 86:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chattergoon MA, Latanich R, Quinn J, et al. HIV and HCV activate the inflammasome in monocytes and macrophages via endosomal Toll-like receptors without induction of type 1 interferon. PLoS Pathog 2014; 10:e1004082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Galani IE, Rovina N, Lampropoulou V, et al. Untuned antiviral immunity in COVID-19 revealed by temporal type I/III interferon patterns and flu comparison. Nat Immunol 2021; 22:32–40. [DOI] [PubMed] [Google Scholar]
- 48. Lu L, Zhang H, Dauphars DJ, He Y-W. A potential role of interleukin-10 in COVID-19 pathogenesis. Trends Immunol 2021; 42:3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Laing AG, Lorenc A, Del Molino Del Barrio I, et al. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat Med 2020; 26:1623–35. [DOI] [PubMed] [Google Scholar]
- 50. McGonagle D, Sharif K, O’Regan A, Bridgewood C. The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev 2020; 19:102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Meier CA, Bobbioni E, Gabay C, Assimacopoulos-Jeannet F, Golay A, Dayer JM. IL-1 receptor antagonist serum levels are increased in human obesity: a possible link to the resistance to leptin? J Clin Endocrinol Metab 2002; 87: 1184–8. [DOI] [PubMed] [Google Scholar]
- 52. Stienstra R, Tack CJ, Kanneganti TD, Joosten LA, Netea MG. The inflammasome puts obesity in the danger zone. Cell Metab 2012; 15:10–8. [DOI] [PubMed] [Google Scholar]
- 53. Mudd PA, Crawford JC, Turner JS, et al. Distinct inflammatory profiles distinguish COVID-19 from influenza with limited contributions from cytokine storm. Sci Adv 2020; 6:eabe3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395:1033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Liao M, Liu Y, Yuan J, et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med 2020; 26:842–4. [DOI] [PubMed] [Google Scholar]
- 56. Yap JKY, Moriyama M, Iwasaki A. Inflammasomes and pyroptosis as therapeutic targets for COVID-19. J Immunol 2020; 205: 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lee JS, Park S, Jeong HW, et al. Immunophenotyping of COVID-19 and influenza highlights the role of type I interferons in development of severe COVID-19. Sci Immunol 2020; 5:eabd1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rodrigues TS, de Sá KSG, Ishimoto AY, et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J Exp Med 2021; 218. Available at: 10.1084/jem.20201707. Accessed 28 July 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. The RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021; 384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gupta S, Wang W, Hayek SS, et al. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med 2021; 181:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Salama C, Han J, Yau L, et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. N Engl J Med 2021; 384:20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ignatius EH, Wang K, Karaba A, et al. Tocilizumab for the treatment of COVID-19 among hospitalized patients: a matched retrospective cohort analysis. Open Forum Infectious Diseases 2021; 8. Available at: 10.1093/ofid/ofaa598. Accessed 28 July 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cauchois R, Koubi M, Delarbre D, et al. Early IL-1 receptor blockade in severe inflammatory respiratory failure complicating COVID-19. Proc Natl Acad Sci U S A 2020; 117:18951–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dimopoulos G, de Mast Q, Markou N, et al. Favorable anakinra responses in severe Covid-19 patients with secondary hemophagocytic lymphohistiocytosis. Cell Host & Microbe 2020; 28:117–123.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Huet T, Beaussier H, Voisin O, et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol 2020; 2:e393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Guaraldi G, Meschiari M, Cozzi-Lepri A, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. The Lancet Rheumatology 2020; 2:e474–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Morena V, Milazzo L, Oreni L, et al. Off-label use of tocilizumab for the treatment of SARS-CoV-2 pneumonia in Milan, Italy. Eur J Intern Med 2020; 76:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hossen MdS, Barek MA, Jahan N, Safiqul Islam M. A review on current repurposing drugs for the treatment of COVID-19: reality and challenges. SN Compr Clin Med 2020; 2:1777–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Salvarani C, Dolci G, Massari M, et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med 2021; 181:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hermine O, Mariette X, Tharaux P-L, et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med 2021; 181:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Stone JH, Frigault MJ, Serling-Boyd NJ, et al. ; BACC Bay Tocilizumab Trial Investigators. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med 2020; 383:2333–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Popkin BM, Du S, Green WD, et al. Individuals with obesity and COVID-19: a global perspective on the epidemiology and biological relationships. Obes Rev 2020; 21:e13128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Rehani K, Wang H, Garcia CA, Kinane DF, Martin M. Toll-like receptor-mediated production of IL-1Ra is negatively regulated by GSK3 via the MAPK ERK1/2. J Immunol 2009; 182:547–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gabay C, Smith MF, Eidlen D, Arend WP. Interleukin 1 receptor antagonist (IL-1Ra) is an acute-phase protein. J Clin Invest 1997; 99:2930–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nature Reviews Cardiology 2018; 15:505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.