Abstract
Purpose:
To determine the prevalence and morphological features of reticular pseudodrusen (RPD) and their association with participant demographics and AMD status in the Carotenoids in Age-Related Eye Disease Study 2 (CAREDS2) sample, an ancillary study of the Women’s Health Initiative Observational Study.
Design
Cross-sectional, multicenter, natural history study.
Participants
946 eyes from 473 female postmenopausal participants aged 69 to 101 years old
Methods
Multimodal imaging including spectral domain optical coherence tomography (SD OCT) and infrared reflectance (IR) were used to identify RPD characteristics, such as location (within or outside the 6 mm diameter circle centered at the macula), presence of peripapillary RPD, pattern of RPD, and RPD area. AMD features from SD OCT, IR, and color photographs were also assessed and AMD severity was categorized.
Main Outcome Measures
RPD prevalence using SD OCT and IR imaging, and AMD status.
Results
RPD were present in 130 eyes (14% of eyes, 16% of participants), with increasing prevalence with age; 7% in < 78 years, 14% in 78-83 years and 30% in > 83 years. Using clinical classification of AMD with color photography, RPD were seen in 2.4% of eyes with no AMD/aging changes, 11.5% in early AMD, 25.1% in intermediate AMD and 51.1% in late AMD. Mean RPD area was 17.4 (14.7) mm2. Ribbon morphology (53%) was more common than dot morphology RPD (36%). RPD were mostly located both within and outside the 6 mm circle with primarily superior retinal distribution. RPD were visualized with corresponding color fundus photography in only 38 eyes (4% of total eyes). Participants with and without RPD had a visual acuity ± standard error of 77.9 (1.4) and 81.3 (0.4) letters, respectively (P = 0.02).
Conclusion
The prevalence of RPD in CAREDS2 increased with age and was associated with AMD severity. RPD was detected in eyes without other features of AMD and could represent an earlier disease state. Multimodal imaging with SD OCT and IR has significantly greater sensitivity for visualizing RPD than color fundus photography.
Keywords: Age-related macular degeneration, Reticular pseudodrusen, Optical Coherence Tomography, Carotenoids in Age-Related Eye Disease Study 2
Precís
Reticular pseudodrusen were assessed with multimodal imaging on SD OCT, infrared reflectance, and color fundus photography in older women of CAREDS2. There were associations between RPD, advancing age, and AMD severity.
Age-related macular degeneration (AMD) is the leading cause of vision loss in developed nations, and the prevalence of AMD is expected to increase with the rise of aging populations.1,2 The first clinical manifestations of AMD are drusen, which are extracellular deposits underlying the retinal pigment epithelium – basal laminar layer and the inner collagenous layer of Bruch’s membrane.3 In contrast, reticular pseudodrusen (RPD) refer to reflective material accumulating above the retinal pigment epithelium in the subretinal space, hence the more accurate nomenclature “subretinal drusenoid deposits (SDD)” .4-6 RPD have been identified as an independent risk factor in the progression of AMD, particularly in the development of late-stage dry AMD, geographic atrophy.7-9
RPD can be identified on several imaging modalities, such as color fundus photography, fundus autofluorescence, near infrared reflectance (IR), and optical coherence tomography (OCT) (Figure 1). However, multimodal imaging with IR and OCT has become the preferred method for detecting RPD due to its high sensitivity (84.6 – 100%) and specificity (95.5 – 100%).10,11 With greater accuracy detecting RPD, we can more precisely determine the prevalence of RPD and its relationship with other ocular and systemic diseases.
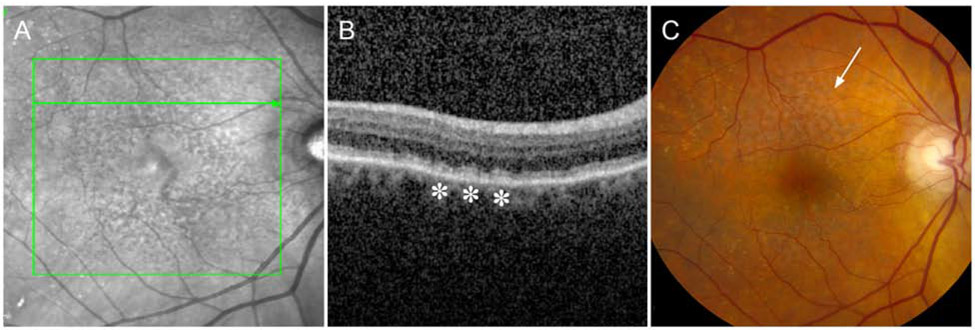
Figure 1.
RPD visualized with different imaging modalities. A, RPD are detected as hyporeflective dots and/or confluent ribbons on infrared imaging. B, RPD appear as hyperreflective deposits between the ellipsoid zone and RPE-BrM (asterisks). C, On color fundus photography RPD appear as an ill-defined network of white-to-yellow broad interlacing ribbons (arrow).
In this study we aim to determine the prevalence of RPD in the second Carotenoids in Age-Related Eye Disease Study (CAREDS2) sample, an ancillary study to the Women’s Health Initiative, a study of post-menopausal women in the US, and assess associations with demographics, comorbidities, visual acuity, and AMD status. The secondary aims include describing the characteristics of RPD including location, morphology, and area of RPD in the CAREDS2 participants.
Methods
Study Population
The study population has previously been described in detail.12 Briefly, 2,004 of 3,143 participants of the Women’s Health Initiative Observational Study (WHI-OS) from three WHI sites (Madison, WI; Iowa City, Iowa; Portland, Oregon), with intakes of lutein plus zeaxanthin that were above the 78th or below the 28th percentiles, were enrolled in baseline CAREDS between 2001 and 2004. Of the 895 surviving women who were not lost to follow-up and consented to contact, 685 women were enrolled in CAREDS2 2016-2019.12 The participants were aged 69 to 101 years (mean age ± standard deviation: 81 ± 5.7 years) and 98% were Caucasian (Mares et al. 2020, manuscript in preparation). The CAREDS study sample was broadly representative of the demographics of the larger WHI population and reported identical rates of AMD diagnoses from their doctors (5%) at WHI baseline (1994-1998). Relative to similarly aged women in the US population participating in the Third National Health and Examination Survey in 1988-1994, CAREDS participants were more likely to have some college education (79% in CAREDS vs. 29% in Americans over 50 years of age), and family incomes > $50,000 (37% vs. 19%), and be married (70%. vs 61%).12 The analysis dataset for the present report includes data from 473 of the 487 participants who participated in-person and provided gradable retinal images. This study was conducted under the Declaration of Helsinki and approved by institutional review boards at all participating clinics. Written informed consent was obtained from all study participants.
Covariates
Diabetes, hypertension, and cardiovascular disease (including heart disease, cerebrovascular disease, and peripheral vascular disease) diagnoses were acquired from information collected at WHI baseline, CAREDS baseline, WHI extension I, and/or WHI extension II. Study visits and questionnaires provided updated physical measurements (blood pressure, body mass index, waist circumference), as previously described.13,14 A measure of average annual ocular visible light exposure was determined from a sunlight exposure questionnaires in CAREDS1 and CAREDS2. This queried outdoor activities during routine and vacation periods, living location and use of protective gear (hats, sunglasses).15 Serum markers were previously analyzed in fasting blood samples collected at WHI baseline. Serum 25-hydroxyvitamin D and high sensitivity C-reactive protein RP were determined by radioimmunoassay, serum lutein plus zeaxanthin determined by high performance liquid chromatography, and serum total triglycerides and cholesterol were measured by an automated chemistry analyzer based on the cholesterol oxidase method, as previously described.13,16
Collection and Evaluation of Images
In person study visits were conducted at the University of Iowa, Department of Ophthalmology, Oregon Health Science University, Casey Eye Institute, and University of Wisconsin, Department of Ophthalmology and Visual Sciences. Study visits included assessment of best-corrected visual acuity (BCVA), in each eye, by certified examiners, using the standardized Early Treatment Diabetic Retinopathy Study (ETDRS) protocol modified for the Age-Related Eye Disease Study (AREDS) trials.17 Stereoscopic 30° fundus digital images, centered on the macula, and SD OCT and IR images, were also obtained by certified photographers using the CAREDS2 reading center (Fundus Photograph Reading Center, University of Wisconsin – Madison) approved protocols.12 Participants underwent SD OCT imaging in both eyes using a 20° x 20° high speed volume protocol with 97 B-scans, using the Heidelberg Spectralis (Heidelberg Engineering Inc, Heidelberg, Germany). The ART was set to a minimum of 9 and a maximum of 15 with a signal threshold Q score of > 20 for each individual scan during acquisition.
RPD were graded as definite if visualized on both SD OCT and IR imaging. On SD OCT, RPD were identified as hyperreflective material located between the ellipsoid zone (EZ) and the retinal pigment epithelial (RPE) layer (Figure 1).18,19 The appearance of RPD on OCT could belong to any of the four patterns as described by Zweifel et al., diffuse deposition, mounds altering the EZ contour, conical appearance breaking through the EZ, and fading within the inner retinal layers.18,20 All images were assessed by two independent graders (S.C., K.L., and C.H.) for the presence of RPD; grading discrepancies were adjudicated by a senior grader (J.P.).RPD were graded as definite if visualized on both SD OCT and IR imaging. On SD OCT, RPD were identified as hyperreflective material located between the ellipsoid zone (EZ) and the retinal pigment epithelial (RPE) layer (Figure 1).18,19
RPD on IR images presented with two distinct patterns: dot pattern RPD appear as clusters of discrete hyporeflective dots with occasional targetoid appearance, and ribbon pattern RPD appear as a network of confluent hyporeflective cords (Figure 2).5,11,21 A minimum area of ½ disc area (~5 lesions) was required for designating the presence of RPD.8 RPD location was identified as within, outside, or within and outside the Early Treatment in Diabetic Retinopathy Study (ETDRS) grid 6 mm outer circle. Distribution of RPD was categorized based on predominant quadrant of the ETDRS grid involved as superior, nasal, inferior, and temporal. If more than 50% of two or more quadrants contained RPD, the distribution was considered global. Peripapillary RPD was documented if RPD was visible outside the grid, superior or inferior to the optic nerve. Since this region was not included in the OCT scan, presence of peripapillary RPD was based upon infrared images only.
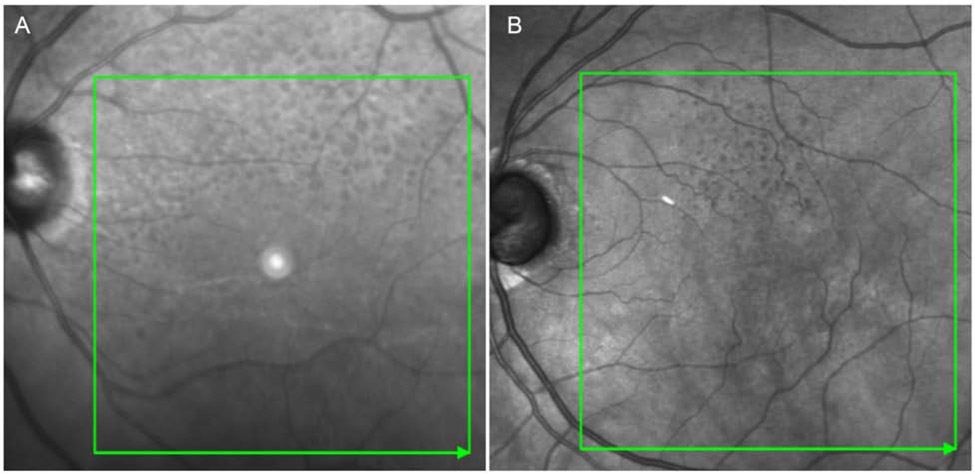
Figure 2.
RPD morphology visualized on IR. A, Predominantly ribbon RPD with a superior distribution and peripapillary involvement. B, Dot morphology with a superior distribution.
RPD area was evaluated on IR imaging with planimetry. In regions with both RPD and drusen, RPD were included in the area measurement only if they were the predominant feature in the area. Although the planimetry drawing was performed on IR images, presence of RPD on SD OCT was colocalized for the corresponding areas. If RPD extended beyond the SD OCT scan field, the RPD must have a similar and congruent appearance within and outside the SD OCT scan field to be included in the RPD area measurement. In cases with multifocal lesions, the RPD area was calculated as a cumulative area.
AMD status was assessed on SD OCT images based on the presence of late AMD, drusen >125 μm in diameter, hyperreflective foci and atrophic changes. Incomplete RPE and outer retinal atrophy (iRORA) was identified based on the presence of signal hypertransmission, attenuation or disruption of the RPE, and overlying photoreceptor degeneration, as previously described by Guymer et al.22 Hyperreflective foci were identified on SD OCT as discrete intraretinal lesions above or adjacent to the RPE of equal or higher reflectivity than the RPE band causing an underlying shadow effect.23,24 AMD classification included intermediate (presence of large drusen with or without hyperreflective foci and iRORA) and late AMD (identified by features of neovascular AMD or geographic atrophy ) on SD OCT.
Evaluation of Color Fundus Photographs
Color fundus photographs were obtained by certified photographers per a standardized three-field stereoscopic imaging protocol developed at the CAREDS2 reading center. RPD were identified on color fundus photographs as white-to-yellow lesions forming an ill-defined network of broad interlacing ribbons, which was adapted from the Wisconsin Age-Related Maculopathy Grading System definition (Figure 1C).25 In addition, AMD severity was classified as no or normal aging change, early AMD, intermediate AMD and late AMD using a clinical classification commonly known as the Beckman scale.26
Statistical Analysis
Characteristics of the CAREDS2 participants were tabulated for participants with and without RPD on IR and SD OCT. The CAREDS2 population was divided based on age tertiles and RPD characteristics within each group was compared. AMD characteristics were analyzed in participants with and without RPD. A generalized estimating equation (GEE) was applied to enable the use of scans from both study eyes of each participant. Empirical standard error estimates were used for GEE parameter estimation considering within person correlation. A two-tailed P-value <0.05 was considered significant. Concordance between graders was assessed with inter-grader percentage agreement. All statistical analysis was performed using SAS 9.4 (SAS Institute Inc. NC, USA).
Results
RPD Prevalence
927 eyes from 466 female participants were evaluated with SD OCT, IR, and color fundus photographs. 19 eyes from 12 participants were excluded due to poor image quality or confounding pathology, including retinal vein occlusion, severe peripheral retinal dystrophy, and disciform scar. RPD were identified in 130 eyes (14% of eyes, 16% of participants) using multimodal SD OCT and IR imaging (Table 1). Among participants with RPD, 76% had bilateral involvement. RPD were visualized on color fundus photography in 38 eyes (4%) There was one eye with RPD visible on color fundus photography and RPD not identified on SD OCT and IR. The intergrader-agreement for the presence of RPD was 97.1% (kappa 0.90, 95% confidence interval 0.86, 0.94) on SD OCT and IR combination.
Table 1.
CAREDS2 demographic characteristics of participants with and without RPD. Standard error (SE).
| Patient Characteristics | Eyes without RPD N (%) |
Eyes with RPD N (%) |
P Value* |
|---|---|---|---|
| All Participants | 797 (86%) | 130 (14%) | |
| Age (mean ± standard deviation) | 80.8±0.3 | 81.1±0.3 | 0.001 |
| Age Categories | <0.0001 | ||
| <78 | 309 (39%) | 20 (15%) | |
| 78 – 83 | 294 (37%) | 39 (30%) | |
| >83 | 194 (24%) | 71 (55%) | |
| Visual Acuity: Letters Read, mean (SE) | 81.3 (0.4) | 77.9 (1.4) | 0.02 |
| BMI category (%) | 0.44 | ||
| < 18.5 (underweight) | 15 (2%) | 1 (1%) | |
| 18.5 to < 25.0 (normal) | 299 (38%) | 71 (55%) | |
| 25.0 to < 30.0 (overweight) | 292 (37%) | 25 (19%) | |
| ≥ 30.0 (obese) | 181 (23%) | 33 (25%) | |
| Diabetes1, yes, % | 112 (14%) | 15 (12%) | 0.46 |
| Hypertension1, yes, % | 492 (62%) | 92 (71%) | 0.62 |
| Cardiovascular disease1, yes, % | 215 (27%) | 45 (35%) | 0.87 |
| Average annual ocular visible sunlight exposure (Maryland sun years): | |||
| Recent Pasta: | 0.006 | 0.006 | 0.42 |
| Distant Pastb: | 0.69 | 0.69 | 0.58 |
| Serum lutein/zeaxanthin, μmol/Lc | 0.35 | 0.35 | 0.59 |
| Serum 25 (OH) vitamin D, nmol/Lc | 60.5 | 60.5 | 0.10 |
| Hs C- Reactive Protein, mg/Lc | 4.4 | 4.4 | 0.99 |
| Serum triglycerides, mmol/Lc | 1.7 | 1.7 | 0.17 |
| Serum cholesterol, mmol/Lc | 5.7 | 5.7 | 0.25 |
Adjusted for age
Between CAREDS1 (2001-2004) and CAREDS2(2016-2019)
In the 20 years prior to CAREDS baseline
At WHI baseline – 1994-1998
Baseline demographics for the participants are presented in Table 1. The presence of RPD was significantly associated with participant age (P < 0.0001). When the sample was divided in tertiles, there were 329 participants with gradable images in < 78 year age group, 333 in the 78-83 years and 265 in the group > 83 years. The prevalence of RPD was 7% in participants less than 78 years old; 14% in ages 78-83; and 30% if greater than 83 years old (P < 0.0001) (Table 2). RPD was also significantly associated with lower visual acuity (P = 0.02). However, the difference was confounded by AMD status; when assessing without AMD (N = 675), the mean letters read and standard error of visual acuity for eyes with RPD (n = 49) and without RPD (n = 626) are 82.9 ± 1.0 and 82.2 ± 0.4, respectively (P = 0.53). There were also no significant differences between individuals with and without RPD for previously considered risk factors for AMD in this and other studies. (body mass index, history of diabetes mellitus, hypertension, cardiovascular disease. serum lipids, serum lutein and zeaxanthin, sunlight exposure)
Table 2.
Summary of RPD characteristics with patients categorized by age tertile. Early Treatment of Diabetic Retinopathy Study (EDTRS).
| RPD Characteristics | <78 Years Old, N (%) |
78 – 83 Years Old, N (%) |
>83 Years Old, N (%) |
Total N (%) |
|---|---|---|---|---|
| RPD Presence | ||||
| Number of eyes (%) | 20 (6%) | 39 (12%) | 71 (27%) | 130 (14%) |
| Number of participants (%) | 11 (7%) | 23 (14%) | 40 (30%) | 74 (16%) |
| Distribution per eye | ||||
| ETDRS Grid Distribution | ||||
| Within grid | 11 (55%) | 14 (37%) | 17 (26%) | 42 (34%) |
| Within and outside grid | 9 (45%) | 23 (60%) | 49 (74%) | 81 (65%) |
| Outside grid | 0 (0%) | 1 (3%) | 0 (0%) | 1 (1%) |
| Quadrant | ||||
| Superior | 11 (55%) | 13 (34%) | 17 (26%) | 41 (33%) |
| Temporal | 0 (0%) | 3 (8%) | 1 (2%) | 4 (3%) |
| Inferior | 1 (5%) | 1 (3%) | 0 (0%) | 2 (2%) |
| Nasal | 2 (10%) | 6 (16%) | 14 (21%) | 22 (18%) |
| Global | 6 (30%) | 15 (39%) | 34 (52%) | 55 (44%) |
| Peripapillary RPD | 2 (10%) | 18 (37%) | 37 (40%) | 57 (35%) |
| RPD Pattern | ||||
| Predominantly Dots | 5 (25%) | 14 (37%) | 26 (39%) | 45 (36%) |
| Predominantly Ribbon | 15 (75%) | 17 (45%) | 34 (52%) | 66 (53%) |
| Mixed (50:50) | 0 (0%) | 7 (18%) | 6 (9%) | 13 (10%) |
| RPD on Color Fundus Photography | 11 (3%) | 13 (4%) | 14 (5%) | 38 (4%) |
| RPD Mean Area (mm2) (mean ± standard deviation) | 13.0 ± 13.4 | 18.9 ± 16.3 | 17.8 ± 14.1 | 17.4 ± 14.7 |
RPD Characteristics
The RPD characteristics are summarized in Table 2. The majority of RPD were located both within and outside the ETDRS grid (65%). The remainder (34%) were eyes where RPD was limited to within the ETDRS grid. There was only one eye that had RPD limited to outside the ETDRS grid. However, distribution varied with each age tertile; 45% in the <78years, 60% in 73-83 years and 74% in >83 years group. The distribution of RPD involved two or more quadrants in 44% of eyes. The superior quadrant was most frequently involved at 33%. Ribbon RPD morphology (53%) was more common than dot morphology (36%). Ten percent of subjects had roughly equal amounts of dot and ribbon morphology. There was no age-related trend observed for the patterns. Thirty-five percent of eyes with RPD had peripapillary involvement and showed an increase with age. The mean area of RPD was 17.4 (SD, 14.7) mm2, and was not significantly associated with age (P = 0.10), or with the presence of late AMD vs. non late AMD (P=0.60).
Association with AMD
Intermediate and late AMD was identified in 240 (27%) of eyes using SD OCT. Of 121 eyes with RPD on SD OCT/IR, 74 (61%) eyes also had evidence of AMD using SD OCT (Table 3) compared to 21% in eyes without RPD. (P < 0.0001). RPD was also related to a two- to six- fold higher prevalence of specific coexisting AMD characteristics including late AMD (P < 0.0001), large drusen (P < 0.0001), hyperreflective foci (P <0.0001), and/or iRORA (P < 0.02). Thirty-nine percent of eyes with RPD had no AMD or early AMD on OCT.
Table 3.
Age-Related Macular Degeneration (AMD) characteristics in SD-OCT images, and color fundus photographs by the presence of reticular pseudodrusen in SD-OCT at CAREDS2 2016-2019 (n= 903 eyes graded with both imaging modalities).
| AMD Characteristics | Overall Eyes (n=903) |
Reticular Pseudodrusen Presence | ||
|---|---|---|---|---|
| Absent (%) (n=782 eyes) |
Present (%) (n = 121 eyes) |
P Value1 | ||
| AMD graded using OCT | ||||
| No AMD | 663 (73%) | 616 (79%) | 47 (39%) | <0.0001 |
| Any AMD | 240 (27%) | 166 (21%) | 74 (61%) | <0.0001 |
| Intermediate AMD | 211 (23%) | 152 (19%) | 59 (49%) | 0.0001 |
| Large drusen (>125 μm) | 203 (22%) | 145 (19%) | 58 (55%) | <0.0001 |
| Hyperreflective foci | 40 (4%) | 23 (3%) | 17 (16%) | <0.0001 |
| iRORA2 | 6 (1%) | 3 (0.4%) | 3 (3%) | 0.02 |
| Late AMD3 | 29 (3%) | 14 (2%) | 15 (12%) | 0.0001 |
| Ellipsoid Zone Changes | <0.0001 | |||
| Normal | 789 (90%) | 724 (94%) | 65 (61%) | |
| Absent | 11 (1%) | 7 (1%) | 4 (4%) | |
| Patchy | 75 (9%) | 38 (5%) | 37 (35%) | |
| AMD graded using color fundus photographs (Beckman scale) | ||||
| No or normal aging change | 373 (41%) | 364 (47%) | 9 (7%) | <0.0001 |
| Early AMD | 244 (27%) | 216 (28%) | 28 (23%) | |
| Intermediate AMD | 239 (26%) | 179 (23%) | 60 (50%) | |
| Late AMD3 | 47 (5%) | 23 (3%) | 24 (20%) | |
Adjusted for age
Late AMD refers to eyes with either geographic atrophy or neovascular AMD
Incomplete RPE and retinal atrophy (iRORA).
Table 3 compares RPD detection from SD OCT/IR with AMD features determined from color fundus photography, in the 903 eyes which were graded with both modalities. The current clinical classification for detecting early to late stages of AMD utilizes color fundus photographs. AMD was classified on color photography in 530 eyes (59%). Of 121 eyes which had RPD characteristics in SD- OCT/IR images, 93% had AMD defined by the Beckman Scale; early (23%), intermediate (50%) and late (20%) AMD. RPD prevalence increased with AMD severity on the Beckman scale. Eyes with early, intermediate, and late AMD, had RPD prevalence of 11%, 25% and 51% respectively. RPD were identified in about 2% of eyes with no signs of AMD or normal aging changes.
Discussion
This study provides insights into the prevalence and characteristics of RPD in the well-characterized aging female postmenopausal sample of CAREDS2. Amongst 466 female participants in the CAREDS2 population, 16% were found to have RPD using SD OCT and IR imaging. RPD prevalence increased with advancing age and AMD status.
AMD has been demonstrated to be strongly associated with RPD in clinic-based studies which often include a large proportion of the study sample with AMD.5,6,8 To date, there have been very few published epidemiologic studies assessing RPD with SD OCT and IR imaging, which have been shown to the most sensitive and specific imaging modalities for detecting RPD.10,11 The ALIENOR Study, a population-based study conducted in France with a mean age of 83.3 ± 3.8 years and 494 participants, described a similar RPD prevalence to that reported in the CAREDS2 sample (16%), at 13.4% overall and 15.6% amongst women.11 Consistent with the present study, the ALIENOR Study, also required RPD to be visible on 2 or more of several possible imaging modalities including SD OCT, IR, fundus autofluorescence, and color fundus photography.11 The Rotterdam study is another population based study where IR imaging was performed at a follow up visit in 2774 participants.27 Prevalence of RPD was 4.9% in this population aged 65 years and older. The mean age of the participants with RPD was 82.1 years with 71.5% female. The lower prevalence in the Rotterdam study could be due to lack of OCT imaging and lack of a standardized definitions in multimodal assessment when the study was conducted. The Rotterdam study did provide further insights in population prevalence of RPD.
Most previous epidemiological-based studies have used color photography for assessment of RPD. Using color fundus photography, only 4% of CAREDS2 participants had RPD. However, this is still significantly higher than 0.7% reported in the Beaver Dam Study, 1.95% in the Blue Mountain Eye Study, and 0.49% in the Melbourne Collaborative Cohort Study, which also used color fundus photographs.28-30 This difference could be attributed to the older female participants comprising the CAREDS2 sample; both age and female gender have been associated with higher rates of RPD.6,8,11,28-30 Also, the CAREDS2 study sample may be broadly representative of the demographics of the WHI population as a whole but may be comprised of women who are most interested in eye studies, because of their own eye health or family history of eye health.
The CAREDS2 sample demonstrated a strong association between RPD and advancing age, with more than three-fold increase in prevalence between participants younger than 78 years old and those aged more than 83. Previous reports have also shown a strong correlation between age and RPD.6,8,11,28-30 While women with RPD read an average of three more letters (Table 1), the 3.4 letter difference is not typically considered clinically significant, and the statistical significance did not persist in women across AMD severity tertiles. Systemic comorbidities, including obesity, diabetes mellitus, hypertension, and cardiovascular disease, which are often associated with AMD in epidemiological studies, were not found to be significantly associated with RPD. Measures of visible sunlight exposure, serum lutein and zeaxanthin, serum 25 (OH) vitamin D status, inflammation or lipids were also unrelated to RPD. Risk factors predictive of RPD, and which reflect the underlying pathogenesis require further investigation.
The CAREDS2 sample corroborated the strong association between RPD and AMD, which has been demonstrated in several studies using different imaging modalities. Interestingly, 39% of participants (47/121) with RPD had no evidence of AMD in either eye using SD OCT and 7% (9/121) using color photography. Reported prevalence of RPD without AMD ranges between 2.9 – 28.2% in other studies.11,18,31,32 Two main hypotheses exist on the solitary presence of RPD; one suggesting that this is a preclinical appearance of AMD, before drusen evolve and a second considering non AMD diseases.33 It is also possible that all drusen may not be detected with the current imaging technologies. Another explanation is that different criteria for AMD were used among studies. Assessment of AMD on SD OCT was limited to intermediate and late AMD in our study, whereas the color photography categorized AMD as early, intermediate, and late. Classification of AMD is important in interpreting reports as most clinical studies deal with intermediate and late AMD only. Another explanation is that older studies are limited by the lower RPD detection rates of different imaging methods. With AMD assessed using color photographs, the prevalence of RPD was 7% in the CAREDS2 sample, which is congruent with other studies. Further longitudinal studies are needed to improve our understanding of the temporal relationship between RPD and AMD development. Another interesting finding is the doubling in prevalence of RPD with AMD severity from 11% to 25% and 52% in early, intermediate, and late AMD, respectively. The association with AMD severity has also been shown in other studies.8,34
RPD in the CAREDS2 population were most frequently seen occupying two or more quadrants, or with a superior distribution. Other studies have shown that RPD location most commonly involves the superior arcade.6,28,32 When assessing the RPD location by age tertile, there was also a trend associating older age and global RPD, which was defined as RPD occupying two or more quadrants. However, RPD area was not significantly associated with greater age tertile (P = 0.10). Studies by Suzuki et al. and Elfandi et al. reported that RPD dot morphology was seen in 96.1 – 100% of eyes with coexisting ribbon morphology in 38% and 53% of eyes, respectively.21,35 Our study, which assessed the predominant RPD morphology, found ribbon RPD to be more common (53%). It is unclear whether the dot and ribbon morphologies represent different types or progressive stages of RPD presentation. Longitudinal studies will help determine the evolution of pattern in eyes with RPD.
There are limitations to this study. Although CAREDS is a prospective cohort study, the RPD analysis was conducted as a cross-sectional study with the 15-year follow-up visit imaging due to unavailability of SD OCT/ IR imaging at the baseline CAREDS visits (2001 – 2004). As such, we are unable to assess temporal relationships between RPD and AMD, and how RPD changes over time. Also, the sample, while generally similar to the larger WHI cohort overall, and having the same prevalence of physician diagnoses of AMD, is comprised of women who have higher incomes and education than the general US population, limiting generalizability to men and samples with lower income and education.12 The strength of this study is in its carefully characterized study sample and the standardized methods of RPD using the IR and OCT which are the current approved imaging methods for detecting RPD. In addition, AMD assessment has been performed at the reading center, with availability of data from the WHI to assess relationships of RPD with comorbid conditions.
In summary, our results provide data on the prevalence of RPD in the aging female population of CAREDS2 and their association with AMD, visual acuity, and systemic comorbidities, which have often been associated with AMD risk. The lack of relationships to comorbidities, and strong relationships with late AMD, suggests that RPD are an independent risk factor for the progression of AMD, and are particularly predictive of the later stages of AMD.7-9 Thus, RPD can be an important biomarker for clinicians and future epidemiologic investigations, which are now possible with the enhanced technology to detect RPD using SD-OCT and IR imaging.
Acknowledgements
The authors are grateful for the time and energy that the CAREDS2 participants devoted to collecting the data which informed this work.
D) Financial Support: This work was supported by National Eye Institute grants EY013018, EY016886 and EY025292, and a supplement ( EY025292-01S1) from the Office of Dietary Supplements. This work was also supported in part by an unrestricted grant from Research to Prevent Blindness, Inc. to the UW Madison Department of Ophthalmology and Visual Sciences, and in part by a National Eye Institute Vision Research Core grant (P30 EY016665) to the UW Madison Department of Ophthalmology and Visual Sciences. It is an ancillary study to WHI and the WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 2107-26, 42129-32, and 44221. The project described was supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR002373. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Appendix
*. The Second Carotenoids in Age-Related Eye Disease Study Research Group:
CAREDS2 Investigators:University of Wisconsin – Madison: Julie Mares PhD, Barbara Blodi MD, Yao Liu MD, Amitha Domalpally MD,PhD, Corinne Engelman PhD, Ronald Gangnon PhD, Gloria Sarto MD PhD; Oregon Health Sciences University: Steven Bailey MD, Erin LeBlanc (Kaiser-Permanente); University of Iowa: Karen Gehrs MD, Robert Wallace MD, Jennifer Robinson MD; Women’s Health Initiative: Lesley Tinker PhD, RD; University of Texas: D. Max Snodderly PhD; University of Georgia: Randy Hammond PhD; University at Buffalo: Amy Millen PhD; Tufts University: Elizabeth Johnson PhD; University of Wisconsin; Brown University: Bill Wooten, PhD
CAREDS 2 Examiners and Clinical Coordinators: Portland, OR: Jennifer Maykoski, BS; Ann Lundquist, BS; Madison, WI: Chris Smith, BS; Kim Wood, BS; Jennie Perry-Raymond, BS; Iowa City, IA: Heather Stockman, BS; Jean Walshire, BS; Christine Sinkey, BSN
CAREDS2 Coordinating Center Staff at theUniversity of Wisconsin – Madison. Thomas Lawler, MS; Courtney Blomme, MS. Kim Wood, BS.; Kristen Hall, BS, Diane Pauk, BS. Sherri Alexander, BS; Esther Mezhibovsky, MS; Scientists: Krista Christensen, PhD, Marine Nalbandyan, MD, PhD
The authors also thank Kristine Lang and Cynthia Hurtenbach for their assistance with image grading.
Short List of WHI Investigators: Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller
Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg
Investigators and Academic Centers: (Brigham and Women's Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker
Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker
Long List of WHI Investigators:
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
C) Meeting Presentation: This work was accepted as an abstract by the Association for Research in Vision and Ophthalmology (ARVO) Conference, 2020.
E) Conflict of Interest: The authors have no conflicts of interest to report.
References
- 1.Wong WL, Su X, Li X, et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob Heal. 2014;2(2):e106–e116. doi: 10.1016/S2214-109X(13)70145-1 [DOI] [PubMed] [Google Scholar]
- 2.Congdon N, O’Colmain B, Klaver C, et al. Causes and Prevalence of Visual Impairment Among Adults in the United States. Arch Ophthalmol. 2004;122(4):477–485. doi: 10.1001/archopht.122.4.477 [DOI] [PubMed] [Google Scholar]
- 3.Spaide RF, Curcio CA. Drusen characterization with multimodal imaging. Retina. 2010;30(9): 1441–1454. doi: 10.1097/IAE.0b013e3181ee5ce8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sadda SR, Guymer R, Holz FG, et al. Consensus Definition for Atrophy Associated with Age-Related Macular Degeneration on OCT. Classification of Atrophy Report 3. Ophthalmology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spaide RF, Ooto S, Curcio CA. Subretinal drusenoid deposits AKA pseudodrusen. Surv Ophthalmol. 2018;63(6):782–815. doi: 10.1016/j.survophthal.2018.05.005 [DOI] [PubMed] [Google Scholar]
- 6.Sivaprasad S, Bird A, Nitiahpapand R, Nicholson L, Hykin P, Chatziralli I. Perspectives on reticular pseudodrusen in age-related macular degeneration. Surv Ophthalmol. 2016;61(5):521–537. doi: 10.1016/j.survophthal.2016.02.005 [DOI] [PubMed] [Google Scholar]
- 7.Schmitz-Valckenberg S, Alten F, Steinberg JS, et al. Reticular drusen associated with geographic atrophy in age-related macular degeneration. Investig Ophthalmol Vis Sci. 2011;52(9):5009–5015. doi: 10.1167/iovs.11-7235 [DOI] [PubMed] [Google Scholar]
- 8.Domalpally A, Agron E, Pak JW, et al. Prevalence, Risk and Genetic Association of Reticular Pseudodrusen in Age-related Macular Degeneration. AREDS2 Report 21. Ophthalmology. 2019:1–8. doi: 10.1016/j.ophtha.2019.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gil JQ, Marques JP, Hogg R, et al. Clinical features and long-term progression of reticular pseudodrusen in agerelated macular degeneration: Findings from a multicenter cohort. Eye. 2017;31(3):364–371. doi: 10.1038/eye.2016.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Bats F, Mathis T, MAUGET-FAÿSSE M, Joubert F, Denis P, Kodjikian L. Prevalence of reticular pseudodrusen in age-related macular degeneration using multimodal imaging. Retina. 2016;36(1):46–52. doi: 10.1097/IAE.0000000000000648 [DOI] [PubMed] [Google Scholar]
- 11.Chan H, Cougnard-Grégoire A, Delyfer MN, et al. Multimodal imaging of reticular pseudodrusen in a population-based setting: The Alienor study. Investig Ophthalmol Vis Sci. 2016;57(7):3058–3065. doi: 10.1167/iovs.16-19487 [DOI] [PubMed] [Google Scholar]
- 12.Mares JA, Al E. The Second Carotenoids in Age-Related Eye Disease Study (CAREDS2) Rationale, Design and Methods. Prep. 2020. [Google Scholar]
- 13.Mares JA, LaRowe TL, Snodderly DM, et al. Predictors of optical density of lutein and zeaxanthin in retinas of older women in the Carotenoids in Age-Related Eye Disease Study, an ancillary study of the Women’s Health Initiative. Am J Clin Nutr. 2006;84(5): 1107–1122. doi: 10.1093/ajcn/84.5.1107 [DOI] [PubMed] [Google Scholar]
- 14.Parekh N, Voland RP, Moeller SM, et al. Association between dietary fat intake and age-related macular degeneration in the Carotenoids in Age-Related Eye Disease Study (CAREDS): An ancillary study of the women’s health initiative. Arch Ophthalmol. 2009; 127(11): 1483–1493. doi: 10.1001/archophthalmol.2009.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moeller SM, Parekh N, Tinker L, et al. Associations Between Intermediate Age-Related Macular Degeneration and Lutein and Zeaxanthin in the Carotenoids in Age-Related Eye Disease Study (CAREDS): Ancillary Study of the Women’s Health Initiative. Arch Ophthalmol. 2006;124(8): 1151–1162. doi: 10.1001/archopht.124.8.1151 [DOI] [PubMed] [Google Scholar]
- 16.Millen AE, Voland R, Sondel SA, et al. Vitamin D status and early age-related macular degeneration in postmenopausal women. Arch Ophthalmol (Chicago, Ill 1960). 2011; 129(4):481–489. doi: 10.1001/archophthalmol.2011.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Eye Institute (NEI) Age-Related Eye Disease Study (AREDS). Chapter 7: Examination Procedures. https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/document.cgi?study_id=phs000001.v3.p1&phd=7#Sec7.2.3.
- 18.Zweifel SA, Spaide RF, Curcio CA, Malek G, Imamura Y. Reticular Pseudodrusen Are Subretinal Drusenoid Deposits. Ophthalmology. 2010;117(2):303–312.e1. doi: 10.1016/j.ophtha.2009.07.014 [DOI] [PubMed] [Google Scholar]
- 19.Curcio CA, Messinger JD, Sloan KR, McGwin G, Medeiros NE, Spaide RF. Subretinal drusenoid deposits in non-neovascular age-related macular degeneration: morphology, prevalence, topography, and biogenesis model. Retina. 2013;33(2):1–24. doi: 10.1097/IAE.0b013e31827e25e0.Subretinal [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spaide RF. Outer retinal atrophy after regression of subretinal drusenoid deposits as a newly recognized form of late age-related macular degeneration. Retina. 2013;33(9): 1800–1808. doi: 10.1097/IAE.0b013e31829c3765 [DOI] [PubMed] [Google Scholar]
- 21.Suzuki M, Sato T, Spaide RF. Pseudodrusen subtypes as delineated by multimodal imaging of the fundus. Am J Ophthalmol. 2014;157(5): 1005–1012. doi: 10.1016/j.ajo.2014.01.025 [DOI] [PubMed] [Google Scholar]
- 22.Guymer RH, Rosenfeld PJ, Curcio CA, et al. Incomplete Retinal Pigment Epithelial and Outer Retinal Atrophy (iRORA) in Age-related Macular Degeneration: CAM Report 4. Ophthalmology. 2019:1–16. doi: 10.1016/j.ophtha.2019.09.035 [DOI] [Google Scholar]
- 23.Folgar FA, Chow JH, Farsiu S, et al. Spatial correlation between hyperpigmentary changes on color fundus photography and hyperreflective foci on SDOCT in intermediate AMD. Invest Ophthalmol Vis Sci. 2012;53(8):4626–4633. doi: 10.1167/iovs.12-9813 [DOI] [PubMed] [Google Scholar]
- 24.Ho J, Witkin AJ, Liu J, et al. Documentation of intraretinal retinal pigment epithelium migration via high-speed ultrahigh-resolution optical coherence tomography. Ophthalmology. 2011;118(4):687–693. doi: 10.1016/j.ophtha.2010.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein R, Davis MD, Magli YL, Segal P, Klein BEK, Hubbard L. The Wisconsin Age-related Maculopathy Grading System. Ophthalmology. 1991;98(7): 1128–1134. doi: 10.1016/S0161-6420(91)32186-9 [DOI] [PubMed] [Google Scholar]
- 26.Ferris FL, Wilkinson CP, Bird A, et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120(4):844–851. doi: 10.1016/j.ophtha.2012.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buitendijk GHS, Hooghart AJ, Brussee C, et al. Epidemiology of Reticular Pseudodrusen in Age-Related Macular Degeneration: The Rotterdam Study. Invest Ophthalmol Vis Sci. 2016;57(13):5593–5601. doi: 10.1167/iovs.15-18816 [DOI] [PubMed] [Google Scholar]
- 28.Klein R, Meuer SM, Knudtson MD, Iyengar SK, Klein BEK. The Epidemiology of Retinal Reticular Drusen. Am J Ophthalmol. 2008;145(2). doi: 10.1016/j.ajo.2007.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joachim N, Mitchell P, Rochtchina E, Tan AG, Wang JJ. Incidence and progression of reticular drusen in age-related macular degeneration: Findings from an older Australian cohort. Ophthalmology. 2014. doi: 10.1016/j.ophtha.2013.10.043 [DOI] [PubMed] [Google Scholar]
- 30.Finger RP, Chong E, McGuinness MB, et al. Reticular pseudodrusen and their association with age-related macular degeneration the melbourne collaborative cohort study. Ophthalmology. 2016;123(3):599–608. doi: 10.1016/j.ophtha.2015.10.029 [DOI] [PubMed] [Google Scholar]
- 31.Sarks J, Arnold J, Van Ho I, Sarks S, Killingsworth M. Evolution of reticular pseudodrusen. Br J Ophthalmol. 2011;95(7):979–985. doi: 10.1136/bjo.2010.194977 [DOI] [PubMed] [Google Scholar]
- 32.Lee MY, Yoon J, Ham D Il. Clinical characteristics of reticular pseudodrusen in Korean patients. Am J Ophthalmol. 2012;153(3):530–535. doi: 10.1016/j.ajo.2011.08.012 [DOI] [PubMed] [Google Scholar]
- 33.Spaide RF. Improving the Age-Related Macular Degeneration Construct: A New Classification System. Retina. 2018;38(5):891–899. doi: 10.1097/IAE.0000000000001732 [DOI] [PubMed] [Google Scholar]
- 34.Kovach JL, Schwartz SG, Agarwal A, et al. The Relationship Between Reticular Pseudodrusen and Severity of AMD. Ophthalmology. 2016;123(4):921–923. doi: 10.1016/j.ophtha.2015.10.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elfandi S, Ooto S, Ueda-Arakawa N, et al. Clinical and Genetic Characteristics of Japanese Patients with Age-Related Macular Degeneration and Pseudodrusen. Ophthalmology. 2016;123(10):2205–2212. doi: 10.1016/j.ophtha.2016.06.052 [DOI] [PubMed] [Google Scholar]