Abstract
Background and Objective
Diabetic retinopathy, a microvascular complication of diabetes mellitus, is one of the most important causes of visual loss in developed countries. Our objective is to evaluate the efficacy of intensive versus conventional glycemic control of type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM) patients in terms of ophthalmologic outcome, pathogenesis of the early worsening of diabetic retinopathy, risk factors for early worsening and diabetic retinopathy progression.
Methods
A literature search on publications concerning glycaemic control in diabetic retinopathy and management of newly diagnosed diabetes mellitus by intensive versus conventional glycaemic control.
Results
A total of 22 articles were reviewed after curation by the authors for relevance. Nineteen articles are randomized control trial, 2 articles are observational studies and 1 is clinical trial. Fifteen articles investigated the glycaemic control in T1DM-related diabetic retinopathy and 8 on T2DM-related diabetic retinopathy. The level of glycemia (in terms of HbA1c level) is significantly related to the diabetic retinopathy progression in both T1DM and T2DM. Intensive glycemic control was found to reduce the development of severe diabetic retinopathy, including severe non-proliferative diabetic retinopathy, neovascularization, clinically significant macular edema and loss of vision. Early worsening of diabetic retinopathy commonly occurs during the first year of intensive treatment, especially those initially present with proliferative or severe non-proliferative retinopathy. However, most patients with early worsening can recover and their long-term ophthalmologic outcomes are better when compared to conventional glycemic control.
Conclusion
The current guideline on HbA1c level is considered sufficient for the minimization of diabetic retinopathy progression. More frequent monitoring for early worsening should be recommended for newly diagnosed diabetes cases already presenting with retinopathy.
Keywords: diabetic retinopathy, insulin dependent diabetes mellitus, non insulin dependent diabetes mellitus, intense glycemic control, conventional glycemic control
Introduction
Diabetic retinopathy is a microvascular complication of diabetes mellitus, that can result in significant visual loss.1 The prevalence of diabetes mellitus has been increasing worldwide, and diabetic retinopathy is one of the leading causes of visual loss in various countries.1,2 Microvascular complications of diabetes mellitus include diabetic neuropathy, diabetic retinopathy and diabetic nephropathy.3–5 Correlation between diabetic neuropathy and diabetic retinopathy is well known in patients with type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM).6,7 T1DM Patients with the development of diabetic nephropathy were found to have a higher risk of diabetic retinopathy progression and the severity of diabetic retinopathy was predictive of diabetic nephropathy.7–9 For T2DM patients, diabetic nephropathy was found to have a unidirectional correlation with diabetic retinopathy.6,7
Diabetic retinopathy is the most frequent cause of preventable blindness in adults.2 40% of patients with T1DMand 86% of patients with T2DM are estimated to develop diabetic retinopathy in the USA.2 The Wisconsin Epidemiologic Study of Diabetic Retinopathy has suggested that the prevalence of diabetic retinopathy in patients diagnosed diabetes mellitus at 30 years old or older ranged from 28.8% (diagnosed less than five years) to 77.8% (diagnosed for 15 years or above)10 Various studies have shown that the control of blood glucose level can prevent the development of diabetic retinopathy, suggesting that hyperglycaemia is a major risk factor for the development of diabetic retinopathy.1,11
The comparison between intensive and conventional glycemic control in the reduction of diabetes complications is always an important topic in the field of diabetes treatments. However, the definition of intensive and conventional glycemic control varies among different guidelines and clinical trials. Diabetes Control and Complications Trial (DCCT) defined intensive and conventional therapy according to the quantity of insulin injection given,12 whereas Veterans Affairs Diabetes Trial (VADT) focused on the dose of oral antihyperglycemics administered.13 The definition in Action to Control Cardiovascular Risk in Diabetes (ACCORD) was based on target HbA1c level, with target HbA1c <6% and 7.0–7.9% in intensively and conventionally treated groups, respectively.14 In general, intensive glycemic control refers to the use of insulin/oral antihyperglycemics to achieve a lower glycemic level (HbA1c <6.5% or <7%),15,16 whereas conventional glycemic control typically utilizes less diabetic medication with a less stringent requirement on target glycemic level. This review aims to summarize and compare the efficacy of intensive therapy versus conventional therapy in the management of T1DM and T2DM patients in terms of ophthalmologic outcome. This review also tries to understand more about several phenomena related to intensive glycemic control and diabetic retinopathy, including the pathogenesis of early rapid development of diabetic retinopathy, the metabolic memory of intensive glycaemic control, and the risk factors for diabetic retinopathy progression. By using the results of this review, we hope to provide some insights on the current treatment of newly diagnosed diabetes mellitus to optimize the ophthalmologic outcomes.
Methods
Eligibility Criteria
Clinical studies published between January 1 1975 and December 31 2019, in English language, comparing the retinopathy-related outcomes (including retinopathy incidence, progression, development of severe non-proliferative diabetic retinopathy, proliferative diabetic retinopathy, need for diabetic-related ocular surgery, or vision loss) between intensive and standard glycemic control were included. The study samples were general populations with insulin-dependent or non-insulin-dependent diabetes. Baseline glycaemic control and severity of retinopathy were stated.
Information Sources
The literature search was performed on PubMed by two of the authors (PYL and SCC) on 25 March 2020.
Search Strategy
Search terms used were “diabetic control”, “diabetes control”, “control of diabetes”, “glycaemic control”, “glycemic control” and “diabetic retinopathy”. The search was limited to human and English studies.
Study Selection
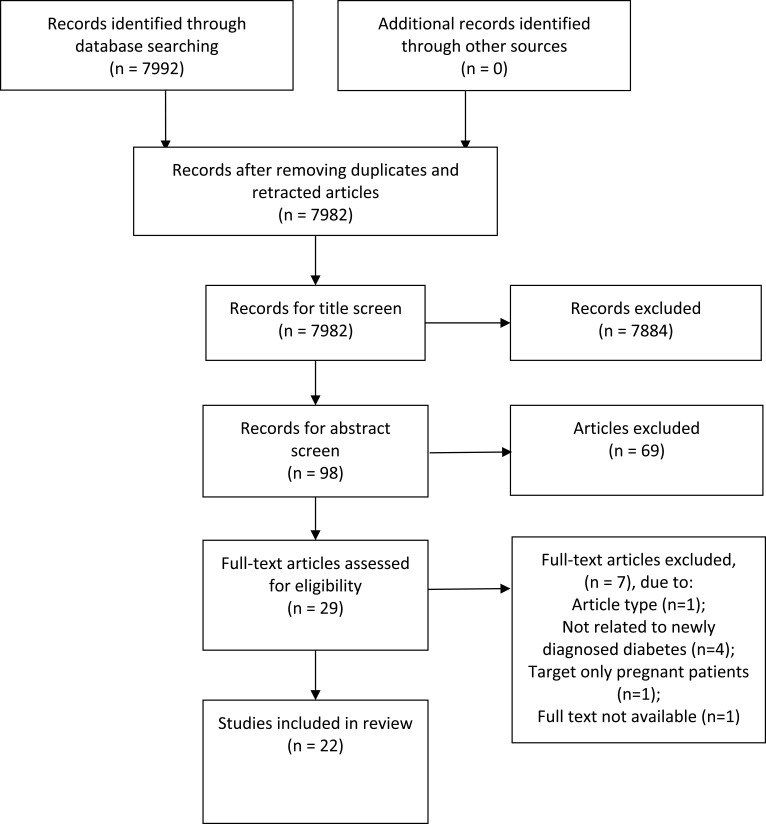
A total of 7982 articles were yielded with the aforementioned search strategy. Figure 1 describes the selection process for identified studies. Two authors (PYL and SCC) then selected the papers. Papers were screened for eligibility by title and, if necessary, by examining the abstract. Only randomized controlled trials, prospective studies, or observational studies were included. Case reports, expert opinions, or case series were all excluded. Full text of one of the included articles was not available for purchase.17 As important study data were missing, we decided to exclude this paper from our review.
Figure 1.
PRISMA chart, curation process for studies identified. Notes: PRISMA figure adapted from Liberati A, Altman D, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of clinical epidemiology. 2009;62(10). Creative Commons.52
Data Analysis
The following information was extracted from the selected articles independently by the two authors: year of publication, study type, sample size, interventions used, study arms, study duration, ocular efficacy outcomes, overall findings, risks of bias and participant characteristics (diabetes type, baseline retinopathy status).
Results
A total of 22 articles were reviewed after manual curation, with 19 randomised controlled trials (RCTs),12,13,18–34 2 observational studies35,36 and 1 clinical trial37 (Table 1). There were 15 articles which investigated the glycaemic control in T1DM-related diabetic retinopathy,12,18–23,25,26,28–33 and 8 on T2DM-related diabetic retinopathy.1,13,24,33–37
Table 1.
Summary of Articles Review on the Effect of Intensive Glycemic Control on Diabetic Retinopathy
| Studies | Age of Participants | Study Type | Participants | Diabetes Type | Baseline Retinopathy Status | Interventions | Study Arms | Duration | Ocular Efficacy Outcome | Significant Findings | Limitations |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Diabetic retinopathy after two years of intensified insulin treatment. Follow-up of the Kroc Collaborative Study. The Kroc Collaborative Study Group (1988, North America and England)18 | N/A | Multicenter RCT | 68 patients | IDDM | Mild to moderate | Receiving either intensified diabetic control with continuous subcutaneous insulin infusion (CSII) or unchanged conventional insulin treatment (CIT) | i. CSII ii. CIT | 2 years | Mean retinopathy level (MRL) and the score keyed to the worse eye (Worse Eye Score) based on ETDRS protocol | With tightened control of diabetes in patients with mild to moderate nonproliferative retinopathy, no initiation of vasproliferative deterioration in retinopathy was found. | Small sample size |
| Early Worsening of Diabetic Retinopathy in the Diabetes Control and Complications Trial (1998)21 | 13 to 39 years old | Multicenter RCT | 1441 patients | IDDM | 726 (no), 715 (mild to moderate) | Receiving either intensified diabetic control (at least 3 injections per day) or conventional insulin treatment (1 or 2 injections per day) | i. intensive insulin injection ii: conventional insulin injection | 3–9 years follow-up | Retinopathy grading based on ETDRS protocol | Intensive insulin treatment provides patient with long term benefits and outweight the risks of worsening in early phrase. | N/A |
| Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: Diabetes Control and Complications Trial. Diabetes Control and Complications Trial Research Group (1994)26 | 13 to 39 years old | Multicenter RCT | 195 patients | IDDM | 125 (no), 70 (mild) | Receiving either intensified diabetic control (at least 3 injections per day) or conventional insulin treatment (1 or 2 injections per day) | i. intensive insulin injection ii: conventional insulin injection | 4–9 years follow-up | Retinopathy grading based on ETDRS protocol | The progression of diabetic retinopathy in adolescent patients became slower with intensive therapy | N/A |
| Progression of retinopathy with intensive versus conventional treatment in the Diabetes Control and Complications Trial. Diabetes Control and Complications Trial Research Group (1995)20 | 13 to 39 years old | Multicenter RCT | 1441 patients | IDDM | 726 (no), 715 (mild to moderate) | Receiving either intensified diabetic control (at least 3 injections per day) or conventional insulin treatment (1 or 2 injections per day) | i. intensive insulin injection ii: conventional insulin injection | 3–9 years follow-up | Retinopathy grading based on ETDRS protocol | After receiving 3 years of Intensive insulin therapy, a reduction in the need of laser treatment and prevention of blindness is found in patients with mild to moderate diabetic retinopathy | N/A |
| The effect of intensive diabetes treatment on the progression of diabetic retinopathy in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial (1995)19 | 13 to 39 years old | Multicenter RCT | 1441 patients | IDDM | 726 (no), 715 (mild to moderate) | Receiving either intensified diabetic control (at least 3 injections per day) or conventional insulin treatment (1 or 2 injections per day) | i. intensive insulin injection ii: conventional insulin injection | 3–9 years follow-up | Retinopathy grading based on ETDRS protocol | Intensive therapy is found to be able to decrease the progression of retinopathy after 8.5 years in diabetic patients with or without diabetic retinopathy. | N/A |
| Aiello (2015)22 | 13 to 39 years old | Multicenter RCT | 1441 patients | IDDM | 726 (no), 715 (mild to moderate) | Receiving either intensified diabetic control (at least 3 injections per day) or conventional insulin treatment (1 or 2 injections per day) | i. intensive insulin injection ii: conventional insulin injection | Follow up of patients from 1983–1989 until 2015 | Self report of ocular surgical procedures annually | A reduction in the risk of ocular surgery was reported in type 1 diabetes patients who received intensive therapy. | Ocular operations based on patients’ self report only |
| Azad (2014)13 | 60 ± 9 years old | Multicenter RCT | 858 patients | NIDDM | 263 (no), 595 (yes but not specified) | Receiving either intensive or standard glycemic control | i. intensive insulin/drug treatment ii: standard insulin/drug treatment | 5 years | Retinopathy grading based on ETDRS protocol | Intensive insulin therapy was found to be related with lowering of diabetic retinopathy in young patients but increasing risk in older patients. | Participants are mostly men with advanced NIDDM, no investigator blinding |
| Brinchmann-Hansen (1992)23 | Male: 26 (18–36) years old Female: 13 (6–23) years old |
RCT | 45 patients | IDDM | Mean count of microaneurysms and hemorrhages=17 | Receiving insulin pumps (continuous subcutaneous insulin infusion), multiple injections (four to six times daily), and conventional insulin treatment (twice daily) | i. insulin pumps; ii. multiple injections; iii. conventional insulin treatment | 7 years | Retinopathy score based on the number of microaneurysms and haemorrhages | Non proliferative retinopathy is beneficial by lowering glycated haemoglobin in long term. | Small sample size |
| Chew (2010)24 | 26 (18–36) years old | Multicenter RCT | 2856 patients | NIDDM | N/A | Receiving intensive glycemic/lipid/BP control or standard therapy | i. intensive glycemic control (target HbA1c <6.0%) ii.standard therapy (target HbA1c7.0 to 7.9%); i. intensive lipid control by fibrate and statin ii.: standard lipid control by statin only; i. intensive BP control (SBP<150mmHg) ii. standard BP control (SBP< 180mmHg) | 4 years | Retinopathy grading based on ETDRS protocol, development of proliferative diabetic retinopathy necessitating photocoagulation therapy or vitrectomy. | The rate of progression of diabetic retinopathy can be reduced by the use of intensive glycaemic control and intensive treatment of dyslipidemia | Retinopathy data collected only every 2 year |
| Crofford (1995)25 | 13 to 39 years old | Multicenter RCT | 1441 patients | IDDM | 726 (no), 715 (mild to moderate) | Receiving either intensified diabetic control (at least 3 injections per day) or conventional insulin treatment (1 or 2 injections per day) | i. intensive insulin injection ii: conventional insulin injection | 3–9 years follow-up | Retinopathy grading based on ETDRS protocol | Intensive treatment is probably recommended to most patients with IDDM, though the implementation is difficult. | N/A |
| Davis (1998)33 | 18–69 years old | Multicenter RCT | 3680 patients | Both IDDM and NIDDM | From mild NPDR to moderate PDR | Receiving either aspirin or placebo/early photocoagulation on one eye and deferral of photocoagulation unless high-risk PDR on the other eye | i. aspirin ii. placebo; i. early photocoagulation ii. photocoagulation unless high risk PDR | 5 years | Development of high-risk PDR and of severe visual loss or vitrectomy (SVLV) | Better glycemic control can reduce retinopathy progression in both diabetes types, in all age groups and in all retinopathy stages including the severe nonproliferative and early proliferative stages | N/A |
| Emanuele (1996)34 | 40–69 years old | Multicenter RCT | 153 patients | NIDDM | 38% had no retinopathy, 5 of them had PDR | Receiving either intensive diabetic control (target HbA1c 4.0–6.1%) or standard insulin treatment (1injection per day) | i. 4-step intensive glycemic control ii: standard insulin injection | 2 years | Retinopathy grading based on ETDRS protocol | Intensive control did not cause transient worsening of retinopathy, though no improvement was seen in one-year time | Only men included, short follow-up time |
| Henricsson (1999)35 | Progression of retinopathy ≤ 2 level: 61.4 ±7.6 years old Progression of retinopathy ≥ 3 level: 64 ± 6.3 years old |
Prospective observational study | 45 patients | NIDDM | 21 (no), 17 (mild NPDR), 5 (moderate NPDR) | Receiving insulin therapy | i. diabetic retinopathy progression <2 levels after intervention ii. diabetic retinopathy progression > 3 years after intervention | 24 months | Change of retinopathy level, change in HbA1c, hemostatic variables and IGF-1 | In type 2 diabetes, the improvement in glycosylated hemoglobin is associated with the reduced retinopathy progression in 2-year time | Small sample size |
| Kayashima (1995)37 | Insulin withdrawal cases without shift to oral hypoglycemic agents: 55.3± 11.9 insulin withdrawal cases with shift to oral hypoglycemic agents: 57.0 ± 7.6 | Prospective study | 171 patients | NIDDM | N/A | Receiving obseration period for 2–5 days, insulin therapy with strict diet therapy. Insulin therapy was discontinued in patients with excellent level of glycemic control for more than 7 days. Patients with blood glucose concentrion more than 180 mg/dL within 7 days after after withdrawal of insulin therapy was given oral hypoglycaemic agents. | i. Insulin withdrawal cases without shift to oral hypoglycemix agents ii. insulin withdrawal cases with shift to oral hypoglycaemic agent iii. cases continuing with insulin | 1 year | Glycaemic control in terms of HbA1c, FBG, PPG, MDBG | Insulin therapy is advised to commence at the early stage in NIDDM patients without retinopathy. | N/A |
| Kilpatrick (2012)29 | 13 to 39 years old | Multicenter RCT | 1441 patients | IDDM | 726 (no), 715 (mild to moderate) | Receiving either intensive or conventional blood glucose management | i. intensive glycaemic control ii. conventional glycaemic control | 3–9 years follow-up | Retinopathy grading based on ETDRS protocol | The frequency of exposure to severe hypoglycaemia was not predictive of the risk of developing retinopathy at any given HbA1c | N/A |
| Lachin (2000)30 | 13 to 39 years old | Multicenter RCT | 1441 patients | IDDM | 726 (no), 715 (mild to moderate) | Receiving either intensified diabetic control (at least 3 injections per day) or conventional insulin treatment (1 or 2 injections per day) | i. intensive insulin injection ii: conventional insulin injection | 4 years | Retinopathy grading based on ETDRS protocol assessed by fundus photography, number of patients received panretinal scatter photocoagulation therapy during DCCT | In type 1 diabetes, intensive therapy resulted in reduced risk of progressive retinopathy despite increased incidence of hyperglycemia. | N/A |
| Molyneaux (1998)36 | 57.5 (50.0–64.6) years old | Observational study | 963 patients | NIDDM | Without any retinopathy | Receiving diet modification, oral agent and insulin treatment | i. type 2 diabetic patients ii. type 1 diabetic patients in DCCT | 7 years | Retinal findings of direct fundoscopy | In type 2 diabetes, the development of retinopathy is associated with the magnitude of hypoglycaemia although the association is weaker than that of type 1 diabetes | N/A |
| Nathan (1993)31 | 13 to 39 years old | Multicenter RCT | 1441 patients | IDDM | 726 (no), 715 (mild to moderate) | Receiving either intensified diabetic control (at least 3 injections per day) or conventional insulin treatment (1 or 2 injections per day) | i. intensive insulin injection ii: conventional insulin injection | 3–9 years follow-up | Retinopathy grading by seven field stereoscopic fundus photographs | Intensive therapy was useful in delaying the onset and slowing the progression of retinopathy | N/A |
| Nathan (2014)12 | 13 to 39 years old | Multicenter RCT | 1441 patients | IDDM | 726 (no), 715 (mild to moderate) | Receiving either intensified diabetic control (at least 3 injections per day) or conventional insulin treatment (1 or 2 injections per day) | i. intensive insulin injection ii: conventional insulin injection | DCCT (1982–1993); EDIC (1994–2012) | Retinopathy grading based on ETDRS protocol | Intensive glycemia control can reduce the long-term complications of diabetes. | N/A |
| Pettitt (2005)1 | Intervention group: 55.0±11.6 Control group: 55.5 ±12.9 | Multicenter RCT | 200 patients | NIDDM | 110 (no), 76 (mild), 14 (severe) | Receiving either intensive diabetes management in addition to standard care or standard care | i. intensive management ii. standard treatment | 2 years | Effects and progression of diabetic retinopathy by retinal photographs | Starting intensive treatment before clinically significant retinopathy develops can reduce the risk of retinopathy progression in type 2 diabetes. | N/A |
| Service (2001)32 | 13 to 39 years old | Multicenter RCT | 565 patients | IDDM | 268 (no), 265 (mild NPDR), 32 (moderate NPDR) | Receiving wither intensive therapy or conventional therapy | i. intensive therapy ii. conventional therapy | 4 years | Retinopathy grading based on ETDRS protocol in patients with seven point capillary glucose data | The major risk for progression of retinopathy is conveyed by updated mean blood glucose especially above 8.3 mmol/L | N/A |
| White (2008)28 | 13 to 39 years old | Multicenter RCT | 1441 patients | IDDM | 726 (no), 715 (mild to moderate) | Receiving either intensified diabetic control (at least 3 injections per day) or conventional insulin treatment (1 or 2 injections per day) | i. intensive insulin injection ii: conventional insulin injection | 10 years | Retinopathy status and progression assessed by 7 field sterofundus photography | The benefits of intensive therapy on retinopathy progression continues for at least 10 years | N/A |
Abbreviations: RCT, randomized controlled trials; IDDM, insulin-dependent diabetes; CSII, continuous subcutaneous insulin infusion; CIT, conventional insulin treatment; ETDRS, Early Treatment Diabetic Retinopathy; NIDDM, non-insulin-dependent diabetes; NPDR, non proliferative diabetic retinopathy; PDR, proliferative diabetic retinopathy; DCCT, Diabetes Control and Complications Trial.
Nine studies investigated the ophthalmologic efficacy in T1DM patients receiving either intensive treatment or conventional treatment,12,18–21,26,28,30,31 3 studies investigated on T2DM patients receiving either intensive treatment or conventional treatment.1,24,34 One study investigated the risk factors for early worsening of diabetic retinopathy21 and 8 studies investigated the risk factors for diabetic retinopathy progression.1,13,19,22,23,28,32,33
Ophthalmologic Outcomes in Intensive Treatment versus Conventional Treatment of Type 1 Diabetes Mellitus
A total of 9 studies investigated the efficacy in managing T1DM patients with intensive treatment or conventional treatment in terms of ophthalmologic outcomes.12,18–21,26,28,30,31
Progression of Diabetic Retinopathy in T1DM Patients with or without Baseline Retinopathy Receiving Intensive Treatment or Convention Treatment
Nine studies have demonstrated ophthalmological outcome of intensive treatment versus conventional treatment in insulin-dependent diabetes mellitus.12,18–21,26,28,30,31 All studies suggested that intensive therapy has a beneficial effect in long-term management of diabetic retinopathy when compared with conventional therapy. Early worsening of diabetic retinopathy has been demonstrated in T1DM patients receiving intensive therapy; however, the short-term adverse effect is outweighed by the long-term benefits.
Five studies demonstrated the results based on the Diabetes Control and Complications Trial (DCCT study), with a total of 1441 participants.19–21,26,31 T1DM Patients were assigned to receive either conventional therapy or intensive therapy. Intensive treatment group received insulin injection 3 times a day while conventional treatment group consisted of one or two daily injections. The primary prevention cohort studied patients without baseline retinopathy and a duration of diabetes of 1 to 5 years. The secondary prevention cohort studied patients with baseline of mild to moderate non-proliferative diabetic retinopathy and a duration of 1 to 15 years diabetes mellitus. The long-term effect of intensive therapy was shown to be more effective than conventional therapy in both prevention cohort.
DM patients receiving intensive therapy tend to have an early worsening of retinopathy. Two studies showed that in general, there were early worsening of retinopathy in the intensive therapy patients at the 6 and 12 month visits.19,21 In the primary prevention cohort of the DCCT study, intensive therapy showed a slightly higher mean of progression steps at one-year time (0.32 progression steps), when compared to that of the conventional therapy (0.24 progression steps). After one year, they started to have a beneficial effect.21 The risk of progression by 3 or more steps with regard to Early Treatment Diabetic Retinopathy Study (ETDRS) retinopathy severity scale was found to be five times lower in the intensive treatment group, compared with the conventional therapy group after 3.5 years.21 Another study performed by the DCCT research group showed a similar trend in adolescent subjects with type one diabetes mellitus.26
In the primary prevention cohort, the cumulative incidence of retinopathy was similar in two groups until 3 years.31 The estimated 5-year cumulative incidence rate in patients receiving intensive therapy was 50% less than those receiving conventional therapy.31 The estimated 8.5 year cumulative incidence rate of progression of patients receiving conventional therapy was much higher than patients receiving intensive treatment (54.1% vs 11.5%).19 The 9 year cumulative incidence rate was found to be 13% in the intensive treatment group and 55% in the conventional treatment group.20 In the secondary prevention cohort, patients receiving intensive therapy showed a higher incidence of progression during the first year.31 After 3 years, the cumulative incidence rate in intensive therapy group became lower than the conventional therapy group.31 The estimated 8.5 cumulative incidence rate of progression showed a similar result as the primary prevention cohort, conventional treatment group had a higher rate than intensive treatment group. (49.2% vs 17.1%)19 The 9 year cumulative incidence rate was found to be 56% in intensive treatment patient and 78% in those treated conventionally.20
White and Lachin demonstrated results based on the Epidemiology of Diabetes Interventions and Complications (EDIC) study, which is an observational study, recruited from 97% of patients who completed the DCCT study.28,30 By comparing the EDIC baseline with EDIC year 4, patients receiving intensive therapy had 71% odds reduction of prevalence of retinopathy when compared with conventional therapy (6.6% vs 21.8%, respectively). The odds reduction comparing the two groups lowers to 50% after ten years (24.2% vs 40.8%, respectively).28 The cumulative incidence rate of progression in the intensive therapy was 70% lower than the conventional therapy group at the EDIC year 4 (P<0.001).12
The study by Kroc Collaborative study group demonstrated deterioration of mean retinopathy level in T1DM patients, baseline mild to moderate diabetic retinopathy, receiving intensified diabetic control in the first 8 months, when compared with conventional injection treatment.18 The trend reversed after 8 months. Patients with intensified diabetic control had a significantly better mean retinopathy level when compared with the conventional group.
Development of Severe Non-Proliferative Diabetic Retinopathy in T1DM Patients with Intensive Therapy or Conventional Therapy
In general, T1DM patients treated with intensive therapy had a lower incidence rate of developing severe non-proliferative diabetic retinopathy, when compared with patients treated with conventional therapy.
Three studies investigated about the development of severe non-proliferative diabetic retinopathy based on the results of DCCT and EDIC clinical trial. Two studies presented data based on the DCCT.19,20 In the secondary intervention cohort, which included patients with mild or moderate diabetic retinopathy, it showed that the 9 year cumulative incidence rate of severe non-proliferative diabetic retinopathy (NPDR) was significantly lower with 9.2% in the intensive therapy group and 26% in the conventional treatment (P<0.001), an average risk reduction of 47%.19,20 For patients in the primary intervention cohort, the development of severe NPDR was too infrequent to compare. Only four cases in the conventional group and two cases in the intensive therapy group developed NPDR.19
In the EDIC trial, after 4 years, the prevalence of severe NPDR in patients receiving intensive therapy or conventional therapy was found to be 4.6% and 17.4%, respectively (P<0.001).28 The adjusted odds reduction (intensive therapy compared with conventional therapy) was 68%. After 10 years, the prevalence of severe NPDR was 9.1% in intensive therapy and 23% in conventional therapy (P<0.001). The adjusted off reduction was 58%.
Development of Neovascularization in T1DM Patients with Intensive Therapy or Conventional Therapy
IDDM patients treated with intensive therapy generally have a lower incidence rate of neovascularization in the retina when compared with those treated with conventional therapy. In the DCCT trial, in the primary prevention cohort, the nine-year incidence rate of developing neovascularization is significantly lower in patients treated with intensive therapy, when compared with patients receiving conventional treatment. (24% vs 8%) (P<0.02).19,20 The risk reduction was 48% in patients receiving intensive therapy.20 The number of patients developed neovascularization was too infrequent in the primary cohort for comparison between intensive therapy and conventional therapy group.19
The prevalence of proliferative diabetic retinopathy in the EDIC trial was found to be significantly lower in T1DM patients receiving intensive therapy (P,0.001) after 4 years of the trial, when compared with patients receiving conventional therapy (4.3% vs 15.7%).28 The adjusted odds reduction was 65%. After 10 years, the prevalence was 8.9% in the intensive therapy group and 24.7% in the conventional therapy group, with 58% reduction.28
Development of Clinically Significant Macular Edema in T1DM Patients with Intensive Therapy or Conventional Therapy
Four studies demonstrated the development of clinically significant macular edema (CSME) in T1DM patients treated with either intensive therapy or conventional therapy. The EDIC study suggested that the prevalence of CSME is significantly lower in the intensive treatment group, when compared with the conventional treatment group after 4 and 10 years of the trial.
Two studies based on the DCCT trial19,20 showed that the incidence rate curves in the two groups (intensive treatment group and conventional treatment group) increased rapidly within the first 5 years, reaching 12% in primary prevention cohort. After 5 years, the rate in the intensive treatment group decreased while the conventional treatment remained the same. However, no significant decrease in the 9 year cumulative incidence rate was found between the two groups (P=0.215).19,20 CSME is too infrequent for the comparison between two groups.
In the EDIC study, the prevalence of CSME was significantly lower in intensive therapy group, when compared with conventional therapy group at 4 years and 10 years after the start of the trial.28,30 The prevalence rate was 3.8% in patients who received intensive therapy and 13.3% in patients who received conventional therapy after 4 years. After 10 years, the prevalence was 9% in the intensive therapy group and 19% in the conventional therapy group. The adjusted off reduction was 38% (P<0.009).30
Requirement of Diabetic Related Ocular Surgery in T1DM Patients with Intensive Therapy or Conventional Therapy
Panretinal photocoagulation is a type of laser treatment, recommended for patients with high risk proliferative diabetic retinopathy.20 Study by Davis performed a life table analysis in T1DM patients, with 9 years of follow up, showing that about 7.9% subjects with intensive treatment and 30% subjects with conventional treatment would require at least one episode of laser treatment.20 The risk reduction in patients with intensive therapy was found to be 59%.
Lachin showed that after the first 4 years of the EDIC study, only 1% of patients with intensive therapy group require laser therapy, which is much lower than the 6% patients with conventional therapy require laser therapy.30 The adjusted odds reduction was 77%, comparing intensive therapy group with conventional therapy group.30 Another study, based on the DCCT/EDIC trial, showed that 8.9% of patients with intensive therapy and 13.4% of patients with conventional therapy performed diabetic related ocular operations (cataract extraction, vitrectomy, retinal detachment surgery), after a follow up of 23 years. A significantly lower rate was found in patients who received intensive therapy, when compared with conventional therapy (P=0.001).22
These findings show that patients with intensive therapy tend to require less diabetic-related ocular surgery, when compared with conventional therapy group.
Visual Loss in T1DM Patients with Intensive Therapy or Conventional Therapy
Three studies compared the visual acuity in patients with intensive therapy or conventional therapy.20,28,30 By combining DCCT and EDIC, patients with best corrected visual acuity (BCVA) worse than 0.2 were observed in intensive therapy group and conventional therapy group (20 vs 21).20,38 Fourteen patients with BCVA worse than 0.1 were found in the intensive therapy group and conventional therapy group.20 After 10 years of EDIC follow up, only 4 former intensive therapy patients had a BCVA worse than 0.1 in 1 eye, none was so affected in both eyes.28,39 Only 1 of these 4 patients lost vision owing to diabetic retinopathy. One former conventional therapy group patient had a visual acuity worse than 0.1 in 1 eye at EDIC year 10 owing to proliferative diabetic retinopathy (PDR). No significant difference is found in terms of the development of poor visual acuity when comparing the two treatment groups.
Ophthalmological Outcome in Intensive Treatment versus Conventional Treatment of Type 2 Diabetes Mellitus
A total of three studies investigated in the ophthalmologic outcome of the usage of intensive therapy or conventional therapy in the management of T2DM. Further studies are needed to investigate the efficacy of intensive therapy in T2DM patients.
Progression of Diabetic Retinopathy in T2DM Patients Receiving Intensive Treatment or Convention Treatment
Three studies investigated the progression of diabetic retinopathy in T2DM patients. Results varied in the limited number of studies. A long-term benefit of intensive therapy is demonstrated in the study by Action to Control Cardiovascular Risk in Diabetes (ACCORD) study group, showing a lower prevalence of diabetic retinopathy in T2DM patients treated with intensive therapy.24 However, the short-term effect of intensive therapy varied in the two studies performed by Pettitt and Emanuele.1,34
Study by Pettitt demonstrated that there was no significant difference in the progression of retinopathy between the intensive diabetes case management group and traditional conventional treatment group after two years of intervention in T2DM patients with or without baseline diabetic retinopathy.1 The odds ratio for progression was found to be −0.65 by comparing the two groups (P=0.226). However, by comparing patients without baseline retinopathy while receiving either intensive management or conventional treatment, the former showed a significantly lower chance of progression of diabetic retinopathy after 2 years (P=0.028).1 Emanuele defined progression of retinopathy as two steps or more deterioration in retinopathy status. When considering patients without baseline retinopathy after 12 months of follow up, 18.5% of patients treated with standard therapy and 26.9% of patients treated with intensive therapy had worsened retinopathy.34 After two months, 20% of patients with standard therapy and 29.6% of patients treated with intensive therapy had progression of retinopathy. No significant difference was found between the two treatment groups. In patients with baseline retinopathy, the prevalence was 20.5% and 35.1% in standard and intensive therapy group, respectively, after 12 months. After two years, the prevalence of progression of retinopathy was 40.5% vs 36.1%. No significant difference was found between the two groups.
For the long-term benefit, the ACCORD study showed that by comparing T2DM patients receiving either intensive or standard glycemia therapy, patients with intensive glycaemia therapy have a significantly lower prevalence of development of diabetic retinopathy when compared with standard glycemia therapy group after 4 years of follow up (7.3% vs 10.4%) (P=0.003).24
Visual Loss in T2DM Patients with Intensive Therapy or Conventional Therapy
For the visual loss, ACCORD study group demonstrated that after 4 years of follow-up in patients with T2DM receiving either intensive therapy or standard therapy, 23.8% of the intensive therapy group had moderate vision loss while 26.3% patients receiving standard glycemia therapy had moderate vision loss, approaching a significant difference (P=0.06).24
Emanuele demonstrated that 9% of patients in the standard group and 6.7% of patients in the intensive group developed unilateral or bilateral impairment after 2 years of follow up.34
Development of Clinically Significant Macular Edema in T2DM Patients with Intensive Therapy or Conventional Therapy
Limited study has investigated the development of CSME in T2DM patients. Emanuele reported that a non-significant trend towards the development of macular edema in patients receiving standard therapy. In patients receiving intensive therapy, a non-significant decreasing trend is observed.34
Pathophysiology of Several Phenomena Associated with Intensive Glycemic Control
Early worsening of retinopathy has long been a unique phenomenon associated with intensive glycemic control, yet its pathogenesis still remains debatable Jingi gave a preliminary suggestion to the possible mechanism of early worsening.40 A significant reduction in HbA1c level lowers the intravascular osmotic pressure. This leads to a flow of water into the intravascular compartment, with the retinal vessels being a susceptible region. A few other possible mechanisms of early worsening were mentioned in DCCT, including the reduction in nutrient substrate, increase in growth factors and weakened ability of the retinal circulation to autoregulate.21 Recent in vitro studies also shed light on the pathogenesis of early worsening. Bain S.C. summarized numerous hypotheses, including the synergistic action of insulin and vascular endothelial growth factor (VEGF) on retinal vessels, the blood‐retinal barrier breakdown theory and the VEGF upregulation theory.41 However, these hypotheses are all tentative and inconclusive.
As mentioned in the previous section, intensive glycemic control was associated with at least 5-fold risk reduction in the long term.19 It was postulated that this significant risk reduction was related to patients’ better recovery from retinopathy if they are under intensive glycemic control. For patients who experienced 3-step progression in DCCT, those in the intensive treatment group would have more frequent recovery when compared to the conventional treatment group. The importance of the recovery factor was highlighted in the subgroup with more advanced retinopathy (ETDRS level 43/43+). Judging from the cumulative incidence of 3-step retinopathy progression, this subgroup showed similar results regardless of which treatment arms they were in.20 However, when considering the final retinopathy status of this subgroup, the proportion of patients with no retinopathy worsening was significantly lower with intensive therapy than with conventional treatment. This showed that recovery is an important factor in determining the final outcome of patients. Within the advanced retinopathy subgroup, despite having similar incidence of retinopathy progression with both treatments, the group with intensive therapy was able to recover better, leading to a more favourable final retinopathy status.
Another interesting phenomenon was observed in the DCCT/EDIC study.12,28 After being allocated to one treatment group (either intensive or conventional glucose control) for an average of 6.5 years in DCCT, even though they were allowed to freely switch treatments afterwards in EDIC, prolonged beneficial effects were demonstrated in the intensively treated group with continuous reduction in 3-step progression. This phenomenon was known as metabolic memory and could last for 10 years after DCCT. Metabolic memory existed in all microvascular complications of diabetes including retinopathy. The slow accumulation and degradation of advanced glycation end products (AGEs) may explain the concept of metabolic memory. Intensively treated patients were found to have had lower AGEs concentrations in skin collagen than patients in the conventional therapy group. Moreover, the AGEs concentrations in skin collagen continued to correlate with the subsequent incidence of retinopathy progression during the first 10 years of EDIC. However, the waning of the metabolic memory effect appeared starting from the fourth year of EDIC, possibly due to the clearance of the long-lasting AGEs in the former conventional group and the accumulation of AGEs in the former intensive treatment group, after patients were allowed to switch treatments freely.
The lower AGEs concentration after intensive therapy was also associated with reduced need for cataract surgery as suggested by Aiello.22 Cataractous lenses had a generally higher level of AGEs than normal lenses. Thus, intensive glycemic control may delay the pathogenesis of cataract via reduction in AGEs production.
Even though intensive therapy is effective in slowing down retinopathy progression, ophthalmologists should keep in mind that the retinopathic process can only be stopped or reversed by intensive therapy only after a considerable delay. The retinopathic process appeared to extend through the first 3 years of follow-up for severe NPDR or PDR and the first 5 years for macular edema.19 Thus, only sustained intensive therapy can benefit patients, especially those with advanced retinopathy.
Risk Factors for Early Worsening
Although the early worsening of retinopathy can be largely mitigated by long-term intensive glycemic control, we would still like to explore the risk factors associated with early worsening. There have been beliefs that early worsening is caused by a sudden significant reduction in hyperglycemia. To be specific, the degree of HbA1c reduction can be assessed by either the magnitude or rapidity of reduction. DCCT suggested that the magnitude, but not the rapidity, of reduction to be associated with increased incidence of early worsening. DCCT compared the retinopathy status of patients who achieved all reduction of HbA1c in 3 months and those who achieved gradually within 6–9 months.21 The incidences of early worsening were similar in both groups. Thus, HbA1c reduction across a longer interval was not associated with lower risk of early worsening, when compared to a reduction across a shorter interval.
On the contrary, the screening level of HbA1c and the magnitude of reduction in the first six monthly HbA1c levels were among the most important predictors of early worsening.21 The magnitude of reduction at month 4–5 demonstrated the most significant effect on risk of early worsening. For every unit reduction in mean HbA1c percentage at month 4–5, there is a 1.6-fold increase in the risk of any form of early worsening.21 The risk also increased with a longer duration of diabetes in both treatment arms and decrease in women in the conventional therapy group. However, there is currently no specific guideline on the reasonable magnitude of reduction to avoid early worsening.
Risk Factors for Diabetic Retinopathy Progression
The level of glycemia is a well-recognized risk factor for diabetic retinopathy progression. Aiello, Pettitt, Brinchmann-Hansen O, Molyneaux, Service and Brein, White, ETDRS and Veterans Affairs Diabetes Trial (VADT) all attributed the reduction in HbA1c level as the single most important factor contributing to the decreased risk of retinopathy.1,13,22,23,28,32,33,36 In DCCT, the risk reduction (in terms of the reduced need for ocular surgery) after intensive treatment was completely eliminated after adjustment for the HbA1c level, showing that a better glycemic control can largely explain the benefit of intensive therapy. In ETDRS, the beneficial effect of better glycemic control was found to extend across all ages, both diabetes types, and all retinopathy stages including the severe non-proliferative and early proliferative stages.33 Service and Brien showed significant associations of all the seven measured glycemic parameters with sustained 3-step retinopathy progression (p<0.01).32 In VADT, after adjustment for all covariates, risk of progression of DR increased by 30% for each unit increase in baseline HbA1c (p = 0.0004).33 In the study by Molyneaux, for every 10% decrease in HbA1c, the relative risk of retinopathy development diminished by 24%, about 2/3 of that reported for T1DM patients in DCCT.36 White even indicated a 1.9 time risk increase for every 10% increase in HbA1c.28 Furthermore, the study by Brinchmann-Hansen O. provided some hints on the target HbA1c to minimize retinopathy progression.23 HbA1c of over 10% was associated with increased risk of progression to NPSDR or PDR (p=0.0014). In contrast, a mean value <8–7% was associated with a diminished risk.
Those studies also tried to assess the relation of other factors with retinopathy progression. ETDRS pointed out that the duration of diabetes appeared to be a less significant determinant for retinopathy progression, especially when retinopathy was present initially.33 ETDRS also mentioned specific risk factors for the development of high-risk PDR, including baseline retinopathy, low visual acuity, higher HbA1c level, history of diabetic neuropathy, elevated triglycerides, low serum albumin and lower hematocrit.33 Henricsson pointed out that retinopathy progression was not associated with insulin growth factor-1 (IGF-1) level, but instead associated with hematological parameters, such as a higher prothrombin fragment 1+2 levels and factor VIII activity.35 Kilpatrick indicated that the occurrence and frequency of severe hypoglycemia was unrelated to the development of retinopathy.29 VADT found that pancreatic reserve capacity was inversely related to the incidence of retinopathy. Incidence reduced by 67.2% with each 1 pmol/mL increment in baseline C-peptide (p=0.0037).13 Age is also another risk factor pointed out by the same study, with the incidence of retinopathy greatest in those ≥70 years old (p=0.0043). On the contrary, the study by Brinchmann-Hansen O. claimed that age, urinary albumin excretion and blood pressure were not related to the incidence or progression of retinopathy.33 However, it is important to note that the study by Brinchmann-Hansen O. only had a sample size of 45 patients and may not be representative.
The ACCORD Eye Study is a recent multicenter RCT on whether intensive glycemic/lipid/blood pressure control can reduce retinopathy progression. At 4 years, the rates of 3-step progression of diabetic retinopathy were 7.3% with intensive glycemic control, versus 10.4% with standard therapy (odds ratio: 0.67; p=0.003); 6.5% with fenofibrate and simvastatin for intensive lipid control, versus 10.2% with simvastatin only (odds ratio: 0.60; p=0.006); and 10.4% with intensive blood pressure control, versus 8.8% with standard therapy (odds ratio: 1.23; p=0.29).24 In other words, intensive glycemic and lipid control, but not intensive blood pressure control, would result in better ocular outcomes for diabetic patients. The efficacy of fenofibrate in slowing retinopathy progression is also supplemented by Keech, which showed fenofibrate monotherapy significantly reduced the need for laser therapy for either macular edema or proliferative retinopathy (p<0.001).42 The mechanism of action of fenofibrate in controlling retinopathy is mainly via the reduction of high triglyceride level, which is a risk factor for high-risk PDR as previously pointed out by ETDRS.33
Discussion
According to the latest Consensus Statement by the American Association of Clinical Endocrinologists and American College of Endocrinology in 2020 and the American Diabetes Association guidelines in 2020, the optimal diabetes control target would be an HbA1c level of ≤6.5%, given if it can be achieved in a safe and affordable manner.43 It was believed that HbA1c level of ≤6.5% can reduce the lifetime risk of micro- and macrovascular complications in adults with recent T2DM onset and no clinically significant atherosclerotic cardiovascular disease (ASCVD).44,45 If a HbA1c target of ≤6.5% cannot be achieved, a level of 7% would be considered appropriate for most nonpregnant adults. The HbA1c target should be individualized and be less stringent according to numerous factors, such as advanced age, limited life expectancy, comorbidities (especially ASCVD), longer duration of diabetes and higher risk of hypoglycemia.
A stepwise approach should be implemented based on the level of hyperglycemia according to the current guideline. For patients with newly diagnosed T2DM or mild hyperglycemia (HbA1c <7.5%), lifestyle modification and antihyperglycemic monotherapy (preferably with metformin) are recommended. Patients with an HbA1c >7.5% should be started initially on metformin plus another antihyperglycemic agent apart from lifestyle modification. Symptomatic patients with A1C >9.0% would likely require insulin therapy for maximized relief and control.
The studies included in our review are consistent with the current guidelines on diabetes management that intensive therapy should be implemented to achieve an optimal HbA1c of 6.5%. The findings in DCCT strongly support the implementation of early intensive therapy for an extended period.30,31 It was expected that a prolonged period of nearly normoglycemia can minimize the risk of complications in patients with T1DM. Risk reduction in the development of severe diabetic retinopathy, including severe non-proliferative diabetic retinopathy, neovascularization, clinically significant macular edema and loss of vision, was observed in the intensively treated group. DCCT also advised ophthalmologists to closely monitor intensively treated patients’ retinopathy status for early worsening during the first year of treatment, especially those initially present with proliferative or severe non-proliferative retinopathy.19,21 Despite having an increased risk of early worsening with intensive therapy, many of these patients can recover from it. It is also important to note that apart from insulin used in DCCT, some other antihyperglycemic agents, such as semaglutide (GLP-1 receptor agonist), may cause early worsening of diabetic retinopathy if used to reduce HbA1c intensively.46
Although DCCT only studied T1DM, the general principles of glycemic control can be applied to T2DM as both types of diabetes share a common pathogenic mechanism of hyperglycemia. Later studies on T2DM, such as VADT, ACCORD and Molyneaux, also showed that the level of glycemia significantly affect retinopathy progression,13,24,36 and intensive therapy can reduce retinopathy progression in T2DM. In ACCORD Eye Study, keeping patients with a HbA1c <6.0% significantly reduced retinopathy progression, when compared to HbA1c of 7.0–7.9% in the standard treatment group. Hence, to optimize the ocular benefits, clinicians should adhere to the optimal target of 6.5%. Using a slightly higher target of 7% (the appropriate level as mentioned by American Diabetes Association 2020) may slightly worsen the retinopathic prognosis. However, in view of increased hypoglycemic episodes associated with intensive glycemic control,47 the target of HbA1c<7% may be acceptable if the diabetic retinopathy progression is well controlled and the glycemic control is associated with fewer adverse effects. Regarding whether intensive therapy leads to early worsening in T2DM, two studies demonstrated contrasting results. Further study is needed to conclude on the short-term efficacy of intensive therapy and conventional therapy in T2DM patients. Moreover, only a limited number of studies have investigated the development of severe outcomes such as neovascularization, CSME, and visual loss. Further study is needed to evaluate the likelihood of development of severe retinal outcome in the usage of intensive therapy versus conventional therapy in T2DM patients.
The United Kingdom Prospective Diabetes Study (UKPDS) has compared the outcome of T2DM patients who received intensive therapy (sulfonylurea or insulin or metformin) and patients who received conventional therapy.48 The conventional therapy is defined to be dietary restriction, which is different from our study’s definition. The study showed that intensive treatment was associated with a reduction of microvascular complications. Study by Holman investigated the 10 years post UKPDS trial follow up and showed a continued reduction in microvascular risk in patients receiving intensive therapy and the benefits of intensive therapy sustained for up to 10 years after the cessation of the treatment in the randomized trial.48 Extended effects of improved glycemic control are found in patients treated with intensive therapy. Similar results are also demonstrated by DCCT and EDIC, showing that intensive therapy received by T1DM patients had less microvascular complications development in long-term follow up. The concept of metabolic memory can explain the phenomenon. Recent studies have shown that metabolic memory may be related to oxidative stress, glycation of mitochondrial proteins, epigenetic changes and non-enzymatic glycation of proteins.49
The current management algorithm also advocated a multifaceted approach for T2DM control, including obesity care, blood pressure and lipid control.43 Regarding lipid control, a subgroup of participants in the ACCORD Eye study investigated the progression of diabetic retinopathy at 4 years, showing that fenofibrate plus simvastatin can reduce the progression in patients with mild non proliferative diabetic retinopathy, but have no effect in patients without diabetic retinopathy, moderate or severe non proliferative disease.50,51 For the FIELD study, fenofibrate was found to be able to lower the incidence of laser therapy for diabetic macular edema,42 however, this is not supported by ACCORD Eye study.24 The current AACE/ACE consensus does not recommend the routine combined use of fibrate and statin. The beneficial effects of the use of fibrate in diabetic retinopathy need to be further studied to make the conclusion.14,43
Conclusion
This review analysed 22 articles concerning the ophthalmologic outcomes of intensive versus standard glycemic control for newly diagnosed diabetes mellitus, with 19 of them being RCTs. The current guideline is considered adequate for the minimization of diabetic retinopathy progression. More frequent monitoring for early worsening should be required for newly diagnosed diabetes cases already presenting with retinopathy, with clear evidence showing association between early worsening and intensive therapy in T1DM.
Abbreviations
T1DM, type 1 diabetes mellitus; T2DM, type 2 dependent diabetes mellitus; RCT, randomized controlled trial; DCCT, diabetes control and complications trial; ETDRS, early treatment diabetic retinopathy study; EDIC, epidemiology of diabetes interventions and complications study; NPDR, non-proliferative diabetic retinopathy; CSME, clinically significant macular edema; BCVA, best corrected visual acuity; PDR, proliferative diabetic retinopathy; ACCORD, Action to Control Cardiovascular Risk in Diabetes; VEGF, vascular endothelial growth factor; AGE, advanced glycation end products; VADT, Veterans Affairs Diabetes Trial; ASCVD, atherosclerotic cardiovascular disease.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Pettitt DJ, Okada Wollitzer A, Jovanovic L, et al. Decreasing the risk of diabetic retinopathy in a study of case management: the California medi-cal type 2 diabetes study. Diabetes Care. 2005;28:2819–2822. doi: 10.2337/diacare.28.12.2819 [DOI] [PubMed] [Google Scholar]
- 2.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet (London, England). 2010;376:124–136. doi: 10.1016/s0140-6736(09)62124-3 [DOI] [PubMed] [Google Scholar]
- 3.Papatheodorou K, Papanas N, Banach M, et al. Complications of diabetes 2016. J Diabetes Res. 2016;2016:6989453. doi: 10.1155/2016/6989453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlienger JL. [Type 2 diabetes complications]. Presse medicale (Paris, France: 1983). 2013;42:839–848. French. doi: 10.1016/j.lpm.2013.02.313 [DOI] [PubMed] [Google Scholar]
- 5.Melendez-Ramirez LY, Richards RJ, Cefalu WT. Complications of type 1 diabetes. Endocrinol Metab Clin North Am. 2010;39:625–640. doi: 10.1016/j.ecl.2010.05.009 [DOI] [PubMed] [Google Scholar]
- 6.Pearce I, Simó R, Lövestam-Adrian M, et al. Association between diabetic eye disease and other complications of diabetes: implications for care. A systematic review. Diabetes Obes Metab. 2019;21:467–478. doi: 10.1111/dom.13550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotlarsky P, Bolotin A, Dorfman K, et al. Link between retinopathy and nephropathy caused by complications of diabetes mellitus type 2. Int Ophthalmol. 2015;35:59–66. doi: 10.1007/s10792-014-0018-6 [DOI] [PubMed] [Google Scholar]
- 8.El-Asrar AM, Al-Rubeaan KA, Al-Amro SA, et al. Retinopathy as a predictor of other diabetic complications. Int Ophthalmol. 2001;24:1–11. doi: 10.1023/a:1014409829614 [DOI] [PubMed] [Google Scholar]
- 9.Kramer CK, Retnakaran R. Concordance of retinopathy and nephropathy over time in Type 1 diabetes: an analysis of data from the Diabetes Control and Complications Trial. Diabetic Med. 2013;30:1333–1341. doi: 10.1111/dme.12296 [DOI] [PubMed] [Google Scholar]
- 10.Klein R, Knudtson MD, Lee KE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XXII the twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology. 2008;115:1859–1868. doi: 10.1016/j.ophtha.2008.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stratton IM, Kohner EM, Aldington SJ, et al. UKPDS 50: risk factors for incidence and progression of retinopathy in Type II diabetes over 6 years from diagnosis. Diabetologia. 2001;44:156–163. doi: 10.1007/s001250051594 [DOI] [PubMed] [Google Scholar]
- 12.Nathan DM. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37:9–16. doi: 10.2337/dc13-2112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azad N, Agrawal L, Emanuele NV, et al. Association of blood glucose control and pancreatic reserve with diabetic retinopathy in the Veterans Affairs Diabetes Trial (VADT). Diabetologia. 2014;57:1124–1131. doi: 10.1007/s00125-014-3199-7 [DOI] [PubMed] [Google Scholar]
- 14.Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Diabetes Association. 6. Glycemic targets: standards of medical care in diabetes-2020. Diabetes Care. 2020;43:S66–S76. doi: 10.2337/dc20-S006 [DOI] [PubMed] [Google Scholar]
- 16.Ceriello A, Colagiuri S. International Diabetes Federation guideline for management of postmeal glucose: a review of recommendations. Diabetic Med. 2008;25:1151–1156. doi: 10.1111/j.1464-5491.2008.02565.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Testa MA, Puklin JE, Sherwin RS, et al. Clinical predictors of retinopathy and its progression in patients with type I diabetes during CSII or conventional insulin treatment. Diabetes. 1985;34(Suppl 3):61–68. doi: 10.2337/diab.34.3.s61 [DOI] [PubMed] [Google Scholar]
- 18.The Kroc Collaborative Study Group. Diabetic retinopathy after two years of intensified insulin treatment. Follow-up of the Kroc Collaborative Study. JAMA. 1988;260:37–41. doi: 10.1001/jama.1988.03410010045032 [DOI] [PubMed] [Google Scholar]
- 19.Diabetes Control and Complications Trial Research Group. The effect of intensive diabetes treatment on the progression of diabetic retinopathy in insulin-dependent diabetes mellitus. Arch Ophthalmol. 1995;113:36–51. doi: 10.1001/archopht.1995.01100010038019 [DOI] [PubMed] [Google Scholar]
- 20.Diabetes Control and Complications Trial Research Group. Progression of retinopathy with intensive versus conventional treatment in the Diabetes Control and Complications Trial. Ophthalmology. 1995;102:647–661. doi: 10.1016/s0161-6420(95)30973-6 [DOI] [PubMed] [Google Scholar]
- 21.Diabetes Control and Complications Trial Research Group. Early worsening of diabetic retinopathy in the Diabetes Control and Complications Trial. Arch Ophthalmol. 1998;116:874–886. doi: 10.1001/archopht.116.7.874 [DOI] [PubMed] [Google Scholar]
- 22.Aiello LP, Ayala AR, Antoszyk AN, et al. Assessing the effect of personalized diabetes risk assessments during ophthalmologic visits on glycemic control: a randomized clinical trial. JAMA Ophthalmol. 2015;133:888–896. doi: 10.1001/jamaophthalmol.2015.1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brinchmann-Hansen O, Dahl-Jørgensen K, Sandvik L, et al. Blood glucose concentrations and progression of diabetic retinopathy: the seven year results of the Oslo study. BMJ (Clinical Research Ed). 1992;304:19–22. doi: 10.1136/bmj.304.6818.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chew EY, Ambrosius WT, Davis MD, et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363:233–244. doi: 10.1056/NEJMoa1001288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crofford OB. Diabetes control and complications. Annu Rev Med. 1995;46:267–279. doi: 10.1146/annurev.med.46.1.267 [DOI] [PubMed] [Google Scholar]
- 26.Diabetes Control and Complications Trial Research Group. Effect of intensive diabetes treatment on the development and progression of long-term complications in adolescents with insulin-dependent diabetes mellitus: diabetes control and complications trial. J Pediatr. 1994;125:177–188. doi: 10.1016/s0022-3476(94)70190-3 [DOI] [PubMed] [Google Scholar]
- 27.Lachin JM, Orchard TJ, Nathan DM. Update on cardiovascular outcomes at 30 years of the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care. 2014;37:39–43. doi: 10.2337/dc13-2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White NH, Sun W, Cleary PA, et al. Prolonged effect of intensive therapy on the risk of retinopathy complications in patients with type 1 diabetes mellitus: 10 years after the diabetes control and complications trial. Arch Ophthalmol. 2008;126:1707–1715. doi: 10.1001/archopht.126.12.1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kilpatrick ES, Rigby AS, Atkin SL, et al. Does severe hypoglycaemia influence microvascular complications in Type 1 diabetes? An analysis of the Diabetes Control and Complications Trial database. Diabetic Med. 2012;29:1195–1198. doi: 10.1111/j.1464-5491.2012.03612.x [DOI] [PubMed] [Google Scholar]
- 30.Lachin JM, Genuth S, Cleary P, et al. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. N Engl J Med. 2000;342:381–389. doi: 10.1056/nejm200002103420603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nathan DM, Genuth S, Lachin J, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/nejm199309303291401 [DOI] [PubMed] [Google Scholar]
- 32.Service FJ, O’Brien PC. The relation of glycaemia to the risk of development and progression of retinopathy in the Diabetic Control and Complications Trial. Diabetologia. 2001;44:1215–1220. doi: 10.1007/s001250100635 [DOI] [PubMed] [Google Scholar]
- 33.Davis MD, Fisher MR, Gangnon RE, et al. Risk factors for high-risk proliferative diabetic retinopathy and severe visual loss: early Treatment Diabetic Retinopathy Study Report #18. Invest Ophthalmol Vis Sci. 1998;39:233–252. [PubMed] [Google Scholar]
- 34.Emanuele N, Klein R, Abraira C, et al. Evaluations of retinopathy in the VA Cooperative Study on Glycemic Control and Complications in Type II Diabetes (VA CSDM). A feasibility study. Diabetes Care. 1996;19:1375–1381. doi: 10.2337/diacare.19.12.1375 [DOI] [PubMed] [Google Scholar]
- 35.Henricsson M, Berntorp K, Berntorp E, et al. Progression of retinopathy after improved metabolic control in type 2 diabetic patients. Relation to IGF-1 and hemostatic variables. Diabetes Care. 1999;22:1944–1949. doi: 10.2337/diacare.22.12.1944 [DOI] [PubMed] [Google Scholar]
- 36.Molyneaux LM, Constantino MI, McGill M, et al. Better glycaemic control and risk reduction of diabetic complications in Type 2 diabetes: comparison with the DCCT. Diabetes Res Clin Pract. 1998;42:77–83. doi: 10.1016/s0168-8227(98)00095-3 [DOI] [PubMed] [Google Scholar]
- 37.Kayashima T, Yamaguchi K, Konno Y, et al. Effects of early introduction of intensive insulin therapy on the clinical course in non-obese NIDDM patients. Diabetes Res Clin Pract. 1995;28:119–125. doi: 10.1016/0168-8227(95)01066-m [DOI] [PubMed] [Google Scholar]
- 38.Lachin JM, White NH, Hainsworth DP, et al. Effect of intensive diabetes therapy on the progression of diabetic retinopathy in patients with type 1 diabetes: 18 years of follow-up in the DCCT/EDIC. Diabetes. 2015;64:631–642. doi: 10.2337/db14-0930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hollander P, Cooper J, Bregnhøj J, et al. A 52-week, multinational, open-label, parallel-group, noninferiority, treat-to-target trial comparing insulin detemir with insulin glargine in a basal-bolus regimen with mealtime insulin aspart in patients with type 2 diabetes. Clin Ther. 2008;30:1976–1987. doi: 10.1016/j.clinthera.2008.11.001 [DOI] [PubMed] [Google Scholar]
- 40.Jingi AM, Tankeu AT, Ateba NA, et al. Mechanism of worsening diabetic retinopathy with rapid lowering of blood glucose: the synergistic hypothesis. BMC Endocr Disord. 2017;17:63. doi: 10.1186/s12902-017-0213-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bain SC, Klufas MA, Ho A, et al. Worsening of diabetic retinopathy with rapid improvement in systemic glucose control: a review. Diabetes Obes Metab. 2019;21:454–466. doi: 10.1111/dom.13538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Keech AC, Mitchell P, Summanen PA, et al. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet (London, England). 2007;370:1687–1697. doi: 10.1016/s0140-6736(07)61607-9 [DOI] [PubMed] [Google Scholar]
- 43.Garber AJ, Handelsman Y, Grunberger G, et al. Consensus statement by the American Association of Clinical Endocrinologists and American College Of Endocrinology on the comprehensive type 2 diabetes management algorithm - 2020 executive summary. Endocr Pract. 2020;26:107–139. doi: 10.4158/cs-2019-0472 [DOI] [PubMed] [Google Scholar]
- 44.Anderson RT, Narayan KM, Feeney P, et al. Effect of intensive glycemic lowering on health-related quality of life in type 2 diabetes: ACCORD trial. Diabetes Care. 2011;34:807–812. doi: 10.2337/dc10-1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431 [DOI] [PubMed] [Google Scholar]
- 46.Vilsbøll T, Bain SC, Leiter LA, et al. Semaglutide, reduction in glycated haemoglobin and the risk of diabetic retinopathy. Diabetes Obes Metab. 2018;20:889–897. doi: 10.1111/dom.13172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Skyler JS, Bergenstal R, Bonow RO, et al. Intensive glycemic control and the prevention of cardiovascular events: implications of the ACCORD, ADVANCE, and VA diabetes trials: a position statement of the American Diabetes Association and a scientific statement of the American College of Cardiology Foundation and the American Heart Association. Circulation. 2009;119:351–357. doi: 10.1161/circulationaha.108.191305 [DOI] [PubMed] [Google Scholar]
- 48.Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470 [DOI] [PubMed] [Google Scholar]
- 49.Testa R, Bonfigli AR, Prattichizzo F, et al. The “metabolic memory” theory and the early treatment of hyperglycemia in prevention of diabetic complications. Nutrients. 2017;9:437. doi: 10.3390/nu9050437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Knickelbein JE, Abbott AB, Chew EY. Fenofibrate and diabetic retinopathy. Curr Diab Rep. 2016;16:90. doi: 10.1007/s11892-016-0786-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chew EY, Davis MD, Danis RP, et al. The effects of medical management on the progression of diabetic retinopathy in persons with type 2 diabetes: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) eye study. Ophthalmology. 2014;121:2443–2451. doi: 10.1016/j.ophtha.2014.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liberati A, Altman D, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Journal of clinical epidemiology.. 2009;62(10) [DOI] [PubMed] [Google Scholar]