ABSTRACT
Impairment of intestinal barrier function is linked to certain pathologies and to aging, and can be a cause of bacterial infections, systemic and hepatic inflammation, food allergies, and autoimmune disorders. The formation and maintenance of intestinal tight junctions is supported by glucagon-like peptide-2 (GLP-2), which via insulin-like growth factor I activity boosts phosphoinositide 3-kinase/Akt/mammalian target of rapamycin complex 1 (PI3K/Akt/mTORC1) signaling in enterocytes. 5′-AMP-activated protein kinase (AMPK) activity as well as estrogen receptor-β (ERβ) activity are also protective in this regard. Conversely, activation of mitogen-activated protein kinases (MAPKs) and cellular Src (c-Src) under inflammatory conditions can induce dissociation of tight junctions. Hence, nutraceuticals that promote GLP-2 secretion from L cells—effective pre/probiotics, glycine, and glutamine—as well as diets rich in soluble fiber or resistant starch, can support intestinal barrier function. AMPK activators—notably berberine and the butyric acid produced by health-promoting microflora—are also beneficial in this regard, as are soy isoflavones, which function as selective agonists for ERβ. The adverse impact of MAPK and c-Src overactivation on the intestinal barrier can be combatted with various antioxidant measures, including phycocyanobilin, phase 2–inducer nutraceuticals, and N-acetylcysteine. These considerations suggest that rationally designed functional foods or complex supplementation programs could have clinical potential for supporting and restoring healthful intestinal barrier function.
Keywords: nutraceuticals, intestinal permeability, tight junction, intestinal barrier, glucagon-like peptide 2, AMPK, phycocyanobilin, N-acetylcysteine, berberine, butyric acid
Intestinal barrier dysfunctions exist in multiple pathologies and during aging. Rationally designed functional foods or complex supplementation programs could have clinical potential for supporting and restoring healthful intestinal barrier functions.
Introduction
The enterocyte tight junction
The intestinal epithelial monolayer represents the body's largest interface with the external environment. It serves dual opposing functions. It selectively absorbs needed nutrients while preventing absorption of detrimental luminal components, including antigenic peptides, proinflammatory factors, oxidants, toxins, bacteria, yeasts, parasites, microbial components or their secreted mobilome, and various allergens and carcinogens (1). Adjacent intestinal enterocytes form tight junctions that are an integral part of the physical intestinal barrier, regulating the paracellular traffic. The tight junctions represent evolutionarily well-conserved sealing complexes between adjacent enterocytes. Also called occluding junctions or zonulae occludentes, they are composed of a branching network of sealing strands, acting independently from each other. Each one of them is composed of a row of transmembrane proteins embedded in both adjacent enterocytes’ plasma membranes, with extracellular extensions joining one another. There are ≥40 different proteins in the tight junction complex, each containing both cytoplasmic domains and transmembranous elongations. The 3 major ones are occludin, claudins, and junction adhesion molecule proteins. The parallel strands are attached to zona occludens-1 (ZO-1), located in the enterocyte's cytoplasm, which anchors the strands to the actin component of the cytoskeleton. Thus, tight junctions are fully integrated with the cytoskeletons of adjacent enterocytic cells.
Not surprisingly, the Hippocratic quote “all disease begins in the gut” is proving to be true. Two millennia later, it appears that dysfunction of the tight junctions is associated with numerous pathological conditions. Gastrointestinal infections, allergic, autoimmune, cancerous, and metabolic diseases have been linked to increased intestinal permeability (1–5). Even the elderly's senescent gut is leaky (6). The term “leaky gut” refers to the failure of tight junctions to execute their multiple homeostatic functions. Loss or impairment of intestinal barrier function owing to a failure to form or maintain tight junctions can lead to infections, an increase in systemic and hepatic inflammation reflecting LPS absorption, and induction of food allergies or autoimmune disorders. Hence, it is pertinent to examine what safe nutraceutical measures might be useful for maintaining an effective intestinal barrier.
Regulation of intestinal tight junctions
Formation of tight junctions requires synthesis and translocation to the enterocyte apical membrane of a range of proteins, including claudins, occludin, junction adhesion molecules, and ZO-1, which integrate to form junctional complexes that, as noted, form tight links to neighboring enterocytes and are linked via actin to the cell's actomyosin cytoskeleton. Signaling mechanisms that can promote tight junction formation and maintenance have been characterized, though the precise details of this induction require much further clarification. Specifically, as discussed below, the phosphoinositide 3-kinase/Akt/mammalian target of rapamycin complex 1 (PI3K/Akt/mTORC1) pathway, 5′-AMP-activated protein kinase (AMPK), and estrogen receptor-β (ERβ) function to promote tight junction formation. In contrast, inflammatory circumstances that activate the mitogen-activated protein kinases (MAPKs)—c-Jun N-terminal kinase (JNK), p38 MAP kinase (p38), and extracellular signal-related kinases 1 and 2 (ERK1/2)—and cellular Src (c-Src) tend to promote the disaggregation of tight junctions. Hence, nutraceuticals that promote the activity of PI3K, AMPK, or ERβ might be expected to aid intestinal barrier function whereas, when enterocyte MAPKs are activated in pathological conditions, nutraceuticals that inhibit MAPK activation might likewise have a favorable impact in this regard.
Trophic Impact of Glucagon-Like-Peptide 2 on Enterocytes is Mediated by Insulin-Like Growth Factor I and PI3K/Akt/mTORC1 Signaling
A key mediator of protective PI3K/Akt/mTORC1 activation in enterocytes is the hormone glucagon-like-peptide 2 (GLP-2), produced in response to various signals by special neuroendocrine L cells in the intestinal mucosa (7–9). GLP-2 does not act directly on enterocytes, but rather acts on intestinal subepithelial fibroblasts, which respond by secreting insulin-like growth factor I (IGF-I) (10). The latter acts on enterocytes to stimulate the PI3K/Akt/mTORC1 pathway, thereby promoting enterocyte proliferation, inhibiting enterocyte apoptosis, and supporting the formation and maintenance of tight junctions (7, 11–14). GLP-2 fails to exert these effects on enterocytes that lack IGF-I receptors, so IGF-I is an essential mediator of the trophic impact of GLP-2 on intestinal epithelium (12). The PI3K/Akt/mTORC1signaling pathway triggered by IGF-I activity on enterocytes can induce expression at the mRNA and protein level of a range of proteins required for tight junction formation, including occludin, claudins, and ZO-1 (15–20). Inhibitors of any of these 3 kinases block IGF-I–mediated induction of these proteins.
Pre/Probiotics, Glycine, and Glutamine Can Promote GLP-2 Secretion
Agents that stimulate L-cell secretion of GLP-2 appear to be identical to those that stimulate secretion of the better-studied GLP-1, because these 2 hormones are stored in the same secretory granules (21). The best-known stimulants of L-cell secretion are SCFAs, primarily butyrate and propionate, produced by healthful intestinal flora from inefficiently absorbed carbohydrate or soluble fiber that reaches the proximal intestine or colon. These SCFAs activate a Gq-coupled receptor expressed by L cells, free fatty acid receptor 2 (FFAR2), to provoke a release of endoplasmic reticulum calcium to the cytoplasm, increasing cytoplasmic free calcium; this in turn induces secretory granules to merge with plasma membranes, provoking release of GLP-1, -2, and other hormones that promote satiety, slow gastric emptying, and exert protective trophic effects on pancreatic β cells (22, 23). Probiotics are nutraceutical bacterial cultures capable of promoting a healthful intestinal microflora proficient at generating SCFAs or lactic acid; the latter is used by some bacteria as substrate for production of SCFAs (24–28). Prebiotics are poorly digested carbohydrates, such as inulin, that can reach the proximal intestine or colon, where they can serve as substrate for SCFA or lactic acid generation. Diets rich in soluble fiber or resistant starch can function as prebiotics.
The amino acids glutamine and glycine also can function as GLP-2 secretagogues. Glycine activates a chloride channel in L cells that is strychnine-inhibitable (29). Although this receptor has a hyperpolarizing impact in many tissues, L cells concentrate chloride against a gradient; hence, glycine channel activation in L cells cause chloride to stream out of these cells, causing a depolarization that induces calcium uptake through voltage-sensitive calcium channels. This calcium influx likewise induces secretory granules to fuse with the plasma membrane, resulting in secretion of GLP-1 and -2. Because the EC50 for activation of glycine receptors is similar to plasma glycine concentrations, it follows that a moderate elevation of plasma glycine achieved through glycine supplementation could be expected to boost GLP-2 secretion and thereby promote effective intestinal barrier function (30, 31). Surprisingly, however, glycine's potential to promote intestinal health appears so far to have received little if any study. When improvement of intestinal barrier function is desired to counter systemic or hepatic inflammation, it is pertinent to note that supplemental glycine can act via its receptor to exert anti-inflammatory effects on a range of cell types, and that it also serves as a substrate for synthesis of the protective cellular antioxidant glutathione (31, 32).
In contrast, glutamine, which is a key substrate for enterocyte energy metabolism in addition to acting as a secretagogue for GLP-2, is well known to aid intestinal health (33, 34). Indeed, it is commonly employed as an adjuvant to cancer chemotherapy or radiotherapy to minimize their toxic impact on the intestinal tract (35). The mechanism whereby glutamine evokes secretory granule release in L cells involves both an influx of calcium and a boost in cAMP concentrations (35, 36). Uptake of glutamine by L cells is required for this response, which is not mediated by Gq and is still inadequately characterized.
Berberine and SCFA-Induced AMPK Activity Promotes Tight Junction Formation
The favorable impact of AMPK activation on intestinal barrier function is well established, and is mediated at least in part by increased expression of caudal type homeobox 2 (Cdx2), a master transcription factor driving differentiation of intestinal enterocytes (37–40). This increased expression reflects increased transcription of the CDX2 gene, though how AMPK promotes this is still unclear. An additional effect of AMPK favorable to tight junction maintenance is its ability to confer an inhibitory phosphorylation (Ser815) on myosin light chain kinase (MLCK) (41). This prevents the latter from phosphorylating myosin light chain 2 (MLC-2). Tight junctions are linked to actomyosin rings that form part of the cellular cytoskeleton; activating phosphorylation of MLC-2 causes a contraction of these rings, which can cause dissociation of tight junctions (42). Hence, AMPK activity helps to maintain cellular actomyosin in a relatively relaxed condition, favorable to the maintenance of tight junctions. Modulation of the contractile state of actomyosin rings is a key mechanism whereby various measures promote the maintenance or the dissociation of tight junctions (42).
The diabetic drug metformin is believed to exert its favorable impact on glycemic control via activation of AMPK (43–45). The nutraceutical berberine, a compound found in various Chinese medicinal herbs, can likewise activate AMPK and is widely employed for diabetes management in China (46–48). Not surprisingly, both metformin and berberine are reported to have favorable effects on the intestinal barrier and tight junction maintenance (49–54).
The SCFA butyrate likewise can activate AMPK in enterocytes (55). This effect is mediated by store-operated calcium entry (how butyrate provokes this remains unclear); this increase in cytosolic calcium activates calmodulin-activated kinase kinase β, which then confers an activating phosphorylation on AMPK (55). Hence, pre/probiotics and fiber-rich diets can also help to maintain the intestinal barrier via butyrate-mediated activation of AMPK in enterocytes.
Soy Isoflavones Aid Intestinal Barrier Function Via ERβ
Colonic epithelium expresses the β but not the α isoform of ER. This is suspected to mediate the favorable impact of postmenopausal hormone replacement on colorectal cancer risk (56, 57). ERβ activity also promotes an effective intestinal barrier and aids tight junction formation (58). Intestinal ERβ expression tends to be decreased in patients with inflammatory bowel disease, and in rodent models of this disorder. Exposure of enterocyte cell cultures to estrogen or to specific agonists for ERβ has been reported to boost epithelial barrier function in vitro—an effect that is blocked by coincubation with an estrogen receptor antagonist (59–61). Conversely, ovariectomy adversely affects intestinal barrier function (62). How ERβ achieves this protective effect remains unclear, but increased expression of occludin at the mRNA and protein level plays a part in this effect (59, 61).
The favorable impact of ERβ activity on intestinal permeability is not solely of interest to women, insofar as soy isoflavones, in free unconjugated plasma concentrations achievable with feasible intakes of dietary soy products or soy isoflavone supplements, can stimulate ERβ activity while minimally impacting ERα (63). Genistein acts directly as an ERβ agonist, whereas daidzein can be converted by the gut bacteria of some individuals to S-equol, a compound that likewise acts as a selective ERβ agonist at physiological concentrations (64–66). Consistent with these findings, soy isoflavone administration has been found to promote a healthy intestinal barrier in rodent studies (67, 68).
Antioxidants Can Aid Tight Junction Maintenance by Downregulating MAPK Activity
In many cell culture or rodent models of intestinal barrier breakdown, activation of MAPKs—JNK2, p38, and/or ERK1/2—has been shown to play a mediating role; the tyrosine kinase c-Src also disrupts tight junctions (69–79). p38 and ERK1/2 do so, in part, by increasing expression of MLCK; p38 accomplishes this by conferring an activating phosphorylation on the transcription factor activating transcription factor 2 (ATF2), whereas ERK1/2 does so by phosphorylating and activating the transcription factor ETS-like 1 (Elk-1) (80, 81). In addition, these kinases suppress the activity of myosin light chain phosphatase, further upregulating activating phosphorylation of MLC-2 (82).
The impact of JNK2 could largely reflect its ability to enable autoactivation of c-Src by conferring a threonine phosphorylation on it (69). c-Src activity is capable of breaking down tight junctions through tyrosine phosphorylation of occludin and ZO-1; these phosphorylations prevent the association of occludin and ZO-1, such that tight junctions cannot form or be maintained (83).
Oxidant stress impedes tight junction formation, and this could in large part reflect upregulated activity of MAPKs and c-Src (84–89). Hydrogen peroxide reversibly inhibits dual-specificity phosphatases that deactivate the MAPKs (90–93). It can also promote activation of JNK and p38 MAPKs by oxidizing thioredoxin and thereby dis-inhibiting the kinase apoptosis signal-regulating kinase 1 (ASK1), which functions as a MAPKKK upstream from both JNK and p38 (94–97). Hydrogen peroxide can also assist the formation of signaling complexes upstream from JNK/p38 activation in certain proinflammatory signaling pathways (98, 99). And hydrogen peroxide also promotes c-Src activation, in part via JNK2 activation as described above (100–103). Hence, nutraceuticals that can suppress oxidant production, promote catabolism of hydrogen peroxide, or reverse the sulfhydryl-oxidizing effects of hydrogen peroxide on signaling proteins, have potential for supporting intestinal barrier function in the context of inflammation.
The oxidant stress that can impair tight junction formation and maintenance in enterocytes often stems from NAD(P)H oxidase complexes (89, 104–106). The unconjugated bilirubin generated by heme oxygenase–mediated catabolism of heme functions as a direct inhibitor of such complexes (107–111). Unconjugated bilirubin has been found to improve intestinal barrier function in a rat model of ulcerative colitis, and to alleviate the loss of barrier functions associated with bile duct ligation (112, 113). The biliverdin metabolite phycocyanobilin (PCB), which within cells is reduced by biliverdin reductase activity to the bilirubin homolog phycocyanorubin, can mimic the inhibitory impact of bilirubin on NAD(P)H oxidase activity (114–116). PCB functions as a light-harvesting chromophore in cyanobacteria (such as the food and nutraceutical spirulina) and certain blue-green algae; its ability to inhibit NAD(P)H oxidase activity could rationalize many of the antioxidant/anti-inflammatory effects of spirulina ingestion (or of phycocyanin, the spirulina protein to which PCB is covalently bound) in rodent studies (114, 117–119). Hence, spirulina or PCB-enriched spirulina extracts could have clinical potential for supporting intestinal barrier function in certain inflammatory circumstances in which NAD(P)H oxidase is activated in enterocytes. Spirulina feeding has been reported to help preserve the intestinal barrier in rats fed a high-fat diet (120).
Clinically useful phase 2–inducing nutraceuticals, such as lipoic or ferulic acids, can promote induction of heme oxygenase-1, and hence inhibit NAD(P)H oxidase activity via intracellular bilirubin generation (121–125). Moreover, they can also induce peroxidases, which catabolize hydrogen peroxide, induce thioredoxin and thioredoxin reductase, which function to suppress ASK1 activation, and also help to reverse the hydrogen peroxide–induced oxidation of protein sulfhydryl groups via induction of γ-glutamylcysteine synthase, rate-limiting for glutathione synthesis (126–128). Concurrent supplementation with N-acetylcysteine (NAC) can aid the latter mechanism, because cysteine is a rate-limiting substrate for glutathione synthesis (129). Consistent with these considerations, lipoic and ferulic acids as well as NAC have shown favorable effects on intestinal barrier function in experimental studies (130–136). With respect to ferulic acid, it has a poorly understood anti-inflammatory effect that might enable it to suppress JNK/p38 MAPK activity in some circumstances (137–139). Hence, it is proposed that PCB (or spirulina), phase 2 inducers, and NAC can collaborate in suppressing the adverse impact of oxidant stress on tight junction formation and intestinal permeability.
A Nutraceutical Program for Promoting Intestinal Barrier Function
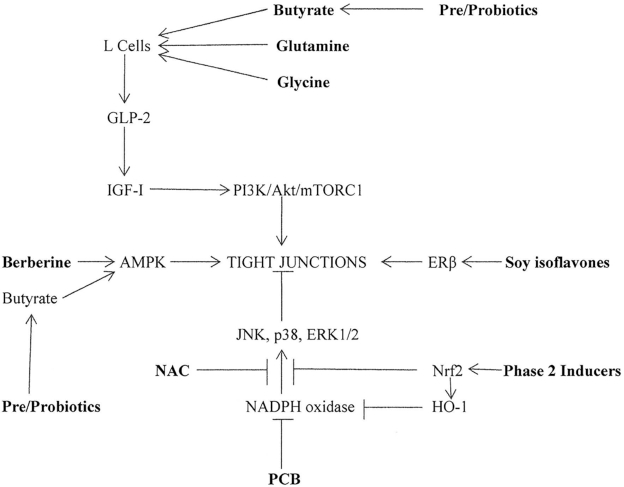
Figure 1 depicts suggested mechanisms whereby the nutraceuticals glutamine, glycine, berberine, soy isoflavones, PCB, phase 2 inducers (e.g., lipoic or ferulic acids), NAC, and pre- and probiotics could be expected to aid tight junction formation and maintenance in enterocytes, thereby supporting intestinal barrier function. Functional foods or complex supplementation programs incorporating several of these agents can be envisioned as aids to intestinal health.
FIGURE 1.
Nutraceutical strategies for promoting tight junction formation in enterocytes. Nutraceuticals are highlighted in bold. AMPK, 5′-AMP-activated protein kinase; ERK, extracellular signal-regulated kinase; ERβ, estrogen receptor-β; GLP-2, glucagon-like-peptide-2; HO-1, heme oxygenase-1; IGF-I, insulin-like growth factor I; JNK, c-Jun N-terminal kinase; NAC, N-acetylcysteine; Nrf2, nuclear factor erythroid 2-related factor 2; p38, p38 MAP kinase; PCB, phycocyanobilin; PI3/Akt/mTORC1, phosphoinositde 3-kinase/Akt/mammalian target of rapamycin complex 1.
Conclusions
A failure of intestinal barrier function reflecting inefficient formation or maintenance of the tight junctions linking enterocytes can lead to unregulated absorption of bacteria, yeast, parasites, bacterial toxins, and intact proteins or peptides derived from food or microbes. Such a failure can lead to infections and systemic and hepatic inflammation, and is suspected to trigger allergic and autoimmune disorders. Hence, promoting effective intestinal barrier function is an important goal for preventive medicine. An analysis of the enterocyte signaling mechanisms that either promote or oppose effective tight junction function—notably the PI3K/Akt/mTORC1 axis, AMPK, ERβ, the MAPKs, and c-Src—enables us to pinpoint certain nutraceutical and dietary measures that could be expected to aid intestinal barrier function. In particular, effective pre- and probiotics, the amino acids glycine, glutamine, and cysteine (provided as NAC), the herbal compound berberine, soy isoflavones, the spirulina antioxidant PCB, and phase 2–inducing nutrients or phytochemicals such as lipoic acid or ferulic acid, appear to have practical potential in this regard.
It should also be noted that those measures that protect the intestinal barrier by boosting L-cell secretion of GLP-2 could also be expected to boost production of GLP-1, and thereby aid effective β-cell function while acting in multiple other ways to promote leanness and metabolic, vascular, and neurological health (140–144).
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—MFM: screened the literature, and designed and wrote the manuscript; AL: edited, revised, and wrote the manuscript; and both authors: read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: MFM is coinventor and co-owner of a US patent on PhyCB oligopeptides derived from spirulina, as well as a European patent application on parenteral use of PhyCB in oxidative stress emergencies. AL reports no conflicts of interest.
Perspective articles allow authors to take a position on a topic of current major importance or controversy in the field of nutrition. As such, these articles could include statements based on author opinions or point of view. Opinions expressed in Perspective articles are those of the author and are not attributable to the funder(s) or the sponsor(s) or the publisher, Editor, or Editorial Board of Advances in Nutrition. Individuals with different positions on the topic of a Perspective are invited to submit their comments in the form of a Perspectives article or in a Letter to the Editor.
Abbreviations used: AMPK, 5′-AMP-activated protein kinase; ASK, apoptosis signal-regulating kinase; CDX, caudal type homeobox; c-Src, cellular Src; ERβ, estrogen receptor-β; ERK, extracellular signal-related kinase; GLP-2, glucagon-like-peptide-2; IGF-I, insulin-like growth factor I; JNK, c-Jun N-terminal kinase; MLC-2, myosin light chain 2; MLCK, myosin light chain kinase; NAC, N-acetylcysteine; p38, p38 MAP kinase; PCB, phycocyanobilin; PI3K/Akt/mTORC1, phosphoinositide 3-kinase/Akt/mammalian target of rapamyin complex 1; ZO-1, zona occludens-1.
Contributor Information
Mark F McCarty, Catalytic Longevity, San Diego, CA, USA.
Aaron Lerner, Chaim Sheba Medical Center, Zabludowicz Center for Autoimmune Diseases, Tel-Hashomer, Israel.
References
- 1. Assimakopoulos SF, Triantos C, Maroulis I, Gogos C. The role of the gut barrier function in health and disease. Gastroenterol Res. 2018;11:261–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lerner A, Matthias T. Changes in intestinal tight junction permeability associated with industrial food additives explain the rising incidence of autoimmune disease. Autoimmun Rev. 2015;14:479–89. [DOI] [PubMed] [Google Scholar]
- 3. Lerner A, Matthias T. GUT—the Trojan horse in remote organs' autoimmunity. J Clin Cell Immunol. 2016;7:401. [Google Scholar]
- 4. Lerner A, Matthias T. Extraintestinal manifestations of CD: common pathways in the gut-remote organs' axes. Int J Celiac Dis. 2017;5:24–7. [Google Scholar]
- 5. Lerner A, Neidhofer S, Matthias T. The gut microbiome feelings of the brain: a perspective for non-microbiologists. Microorganisms. 2017;5:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Soenen S, Rayner CK, Jones KL, Horowitz M. The ageing gastrointestinal tract. Curr Opin Clin Nutr Metab Care. 2016;19:12–8. [DOI] [PubMed] [Google Scholar]
- 7. Brubaker PL. Glucagon-like peptide-2 and the regulation of intestinal growth and function. Compr Physiol. 2018;8:1185–210. [DOI] [PubMed] [Google Scholar]
- 8. Drucker DJ, Yusta B. Physiology and pharmacology of the enteroendocrine hormone glucagon-like peptide-2. Annu Rev Physiol. 2014;76:561–83. [DOI] [PubMed] [Google Scholar]
- 9. Ren W, Wu J, Li L, Lu Y, Shao Y, Qi Y, Xu B, He Y, Hu Y. Glucagon-like peptide-2 improves intestinal mucosal barrier function in aged rats. J Nutr Health Aging. 2018;22:731–8. [DOI] [PubMed] [Google Scholar]
- 10. Leen JL, Izzo A, Upadhyay C, Rowland KJ, Dubé PE, Gu S, Heximer SP, Rhodes CJ, Storm DR, Lund PKet al. . Mechanism of action of glucagon-like peptide-2 to increase IGF-I mRNA in intestinal subepithelial fibroblasts. Endocrinology. 2011;152:436–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dong CX, Zhao W, Solomon C, Rowland KJ, Ackerley C, Robine S, Holzenberger M, Gonska T, Brubaker PL. The intestinal epithelial insulin-like growth factor-1 receptor links glucagon-like peptide-2 action to gut barrier function. Endocrinology. 2014;155:370–9. [DOI] [PubMed] [Google Scholar]
- 12. Markovic MA, Srikrishnaraj A, Tsang D, Brubaker PL. Requirement for the intestinal epithelial insulin-like growth factor-1 receptor in the intestinal responses to glucagon-like peptide-2 and dietary fat. FASEB J. 2020;34:6628–40. [DOI] [PubMed] [Google Scholar]
- 13. Zhao TY, Su LP, Ma CY, Zhai X-H, Duan Z-J, Zhu Y, Zhao G, Li C-Y, Wang L-X, Yang D. IGF-1 decreases portal vein endotoxin via regulating intestinal tight junctions and plays a role in attenuating portal hypertension of cirrhotic rats. BMC Gastroenterol. 2015;15:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lorenzo-Zúñiga V, Rodríguez-Ortigosa CM, Bartolí R, Martínez-Chantar ML, Martínez-Peralta L, Pardo A, Ojanguren I, Quiroga J, Planas R, Prieto J. Insulin-like growth factor I improves intestinal barrier function in cirrhotic rats. Gut. 2006;55:1306–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang CY, Hsiao JK, Lu YZ, Lee TC, Yu LC. Anti-apoptotic PI3K/Akt signaling by sodium/glucose transporter 1 reduces epithelial barrier damage and bacterial translocation in intestinal ischemia. Lab Invest. 2011;91:294–309. [DOI] [PubMed] [Google Scholar]
- 16. Shao Y, Wolf PG, Guo S, Guo Y, Gaskins HR, Zhang B. Zinc enhances intestinal epithelial barrier function through the PI3K/AKT/mTOR signaling pathway in Caco-2 cells. J Nutr Biochem. 2017;43:18–26. [DOI] [PubMed] [Google Scholar]
- 17. Yan H, Ajuwon KM. Butyrate modifies intestinal barrier function in IPEC-J2 cells through a selective upregulation of tight junction proteins and activation of the Akt signaling pathway. PLoS One. 2017;12:e0179586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shiou SR, Yu Y, Chen S, Ciancio MJ, Petrof EO, Sun J, Claud EC. Erythropoietin protects intestinal epithelial barrier function and lowers the incidence of experimental neonatal necrotizing enterocolitis. J Biol Chem. 2011;286:12123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yu C, Jia G, Deng Q, Zhao H, Chen X, Liu G, Wang K. The effects of glucagon-like peptide-2 on the tight junction and barrier function in IPEC-J2 cells through phosphatidylinositol 3-kinase-protein kinase B-mammalian target of rapamycin signaling pathway. Asian Australas J Anim Sci. 2015;29:731–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang H, Ji Y, Wu G, Sun K, Sun Y, Li W, Wang B, He B, Zhang Q, Dai Zet al. . l-Tryptophan activates mammalian target of rapamycin and enhances expression of tight junction proteins in intestinal porcine epithelial cells. J Nutr. 2015;145:1156–62. [DOI] [PubMed] [Google Scholar]
- 21. Nishimura K, Hiramatsu K, Monir MM, Takemoto C, Watanabe T. Ultrastructural study on colocalization of glucagon-like peptide (GLP)-1 with GLP-2 in chicken intestinal L-cells. J Vet Med Sci. 2013;75:1335–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61:364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yadav H, Lee JH, Lloyd J, Walter P, Rane SG. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J Biol Chem. 2013;288:25088–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. De Prater V, Hamer HM, Windey K, Verbeke K. The impact of pre- and/or probiotics on human colonic metabolism: does it affect human health?. Mol Nutr Food Res. 2011;55:46–57. [DOI] [PubMed] [Google Scholar]
- 25. Bird AR, Conlon MA, Christophersen CT, Topping DL. Resistant starch, large bowel fermentation and a broader perspective of prebiotics and probiotics. Beneficial Microbes. 2010;1:423–31. [DOI] [PubMed] [Google Scholar]
- 26. Rios-Covian D, Ruas-Madiedo P, Margolles A, Gueimonde M, de Los Reyes-Gavilán CG, Salazar N. Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol. 2016;7:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Akram W, Garud N, Joshi R. Role of inulin as prebiotics on inflammatory bowel disease. Drug Discov Ther. 2019;13:1–8. [DOI] [PubMed] [Google Scholar]
- 28. Green M, Arora K, Prakash S. Microbial medicine: prebiotic and probiotic functional foods to target obesity and metabolic syndrome. Int J Mol Sci. 2020;21:2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gameiro A, Reimann F, Habib AM, O'Malley D, Williams L, Simpson AK, Gribble FM. The neurotransmitters glycine and GABA stimulate glucagon-like peptide-1 release from the GLUTag cell line. J Physiol. 2005;569(3):761–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lynch JW. Molecular structure and function of the glycine receptor chloride channel. Physiol Rev. 2004;84:1051–95. [DOI] [PubMed] [Google Scholar]
- 31. McCarty MF, O'Keefe JH, DiNicolantonio JJ. Dietary glycine is rate-limiting for glutathione synthesis and may have broad potential for health protection. Ochsner J. 2018;18:81–7. [PMC free article] [PubMed] [Google Scholar]
- 32. Zhong Z, Wheeler MD, Li X, Froh M, Schemmer P, Yin M, Bunzendaul H, Bradford B, Lemasters JJ. L-Glycine: a novel antiinflammatory, immunomodulatory, and cytoprotective agent. Curr Opin Clin Nutr Metab Care. 2003;6:229–40. [DOI] [PubMed] [Google Scholar]
- 33. Kim MH, Kim H. The roles of glutamine in the intestine and its implication in intestinal diseases. Int J Mol Sci. 2017;18:1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Anderson PM, Lalla RV. Glutamine for amelioration of radiation and chemotherapy associated mucositis during cancer therapy. Nutrients. 2020;12:1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tolhurst G, Zheng Y, Parker HE, Habib AM, Reimann F, Gribble FM. Glutamine triggers and potentiates glucagon-like peptide-1 secretion by raising cytosolic Ca2+ and cAMP. Endocrinology. 2011;152:405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nakamura T, Harada K, Kamiya T, Takizawa M, Küppers J, Nakajima K, Gütschow M, Kitaguchi T, Ohta K, Kato Tet al. . Glutamine-induced signaling pathways via amino acid receptors in enteroendocrine L cell lines. J Mol Endocrinol. 2020;64:133–43. [DOI] [PubMed] [Google Scholar]
- 37. Sun X, Yang Q, Rogers CJ, Du M, Zhu MJ. AMPK improves gut epithelial differentiation and barrier function via regulating Cdx2 expression. Cell Death Differ. 2017;24:819–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sun X, Du M, Navarre DA, Zhu MJ. Purple potato extract promotes intestinal epithelial differentiation and barrier function by activating AMP-activated protein kinase. Mol Nutr Food Res. [Internet]2018;62(4). doi:10.1002/mnfr.201700536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Suh E, Traber PG. An intestine-specific homeobox gene regulates proliferation and differentiation. Mol Cell Biol. 1996;16:619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Saad RS, Ghorab Z, Khalifa MA, Xu M. CDX2 as a marker for intestinal differentiation: its utility and limitations. World J Gastrointest Surg. 2011;3:159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Horman S, Morel N, Vertommen D, Hussain N, Neumann D, Beauloye C, El Najjar N, Forcet C, Viollet B, Walsh MPet al. . AMP-activated protein kinase phosphorylates and desensitizes smooth muscle myosin light chain kinase. J Biol Chem. 2008;283:18505–12. [DOI] [PubMed] [Google Scholar]
- 42. Jin Y, Blikslager AT. The regulation of intestinal mucosal barrier by myosin light chain kinase/rho kinases. Int J Mol Sci. 2020;21:3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii Net al. . Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Musi N, Hirshman MF, Nygren J, Svanfeldt M, Bavenholm P, Rooyackers O, Zhou G, Williamson JM, Ljunqvist O, Efendic Set al. . Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002;51:2074–81. [DOI] [PubMed] [Google Scholar]
- 45. Hawley SA, Gadalla AE, Olsen GS, Hardie DG. The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes. 2002;51:2420–5. [DOI] [PubMed] [Google Scholar]
- 46. Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, Ye J-M, Lee CH, Oh WK, Kim CTet al. . Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55:2256–64. [DOI] [PubMed] [Google Scholar]
- 47. Turner N, Li JY, Gosby A, To SWC, Cheng Z, Miyoshi H, Taketo MM, Cooney GJ, Kraegen EW, James DEet al. . Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: a mechanism for the action of berberine to activate AMP-activated protein kinase and improve insulin action. Diabetes. 2008;57:1414–8. [DOI] [PubMed] [Google Scholar]
- 48. Liang Y, Xu X, Yin M, Zhang Y, Huang L, Chen R, Ni J. Effects of berberine on blood glucose in patients with type 2 diabetes mellitus: a systematic literature review and a meta-analysis. Endocr J. 2019;66:51–63. [DOI] [PubMed] [Google Scholar]
- 49. Chen L, Wang J, You Q, He S, Meng Q, Gao J, Wu X, Shen Y, Sun Y, Wu Xet al. . Activating AMPK to restore tight junction assembly in intestinal epithelium and to attenuate experimental colitis by metformin. Front Pharmacol. 2018;9:761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. He Y, Yuan X, Zuo H, Li X, Sun Y, Feng A. Berberine induces ZIP14 expression and modulates zinc redistribution to protect intestinal mucosal barrier during polymicrobial sepsis. Life Sci. 2019;233:116697. [DOI] [PubMed] [Google Scholar]
- 51. Shan CY, Yang JH, Kong Y, Wang XY, Zheng MY, Xu YG, Wang Y, Ren HZ, Chang BC, Chen LM. Alteration of the intestinal barrier and GLP2 secretion in berberine-treated type 2 diabetic rats. J Endocrinol. 2013;218:255–62. [DOI] [PubMed] [Google Scholar]
- 52. Hou Q, Zhu S, Zhang C, Huang Y, Guo Y, Li P, Chen X, Wen Y, Han Q, Liu F. Berberine improves intestinal epithelial tight junctions by upregulating A20 expression in IBS-D mice. Biomed Pharmacother. 2019;118:109206. [DOI] [PubMed] [Google Scholar]
- 53. Cao M, Wang P, Sun C, He W, Wang F. Amelioration of IFN-γ and TNF-α -induced intestinal epithelial barrier dysfunction by berberine via suppression of MLCK-MLC phosphorylation signaling pathway. PLoS One. 2013;8:e61944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gu L, Li N, Gong J, Li Q, Zhu W, Li J. Berberine ameliorates intestinal epithelial tight-junction damage and down-regulates myosin light chain kinase pathways in a mouse model of endotoxinemia. J Infect Dis. 2011;203:1602–12. [DOI] [PubMed] [Google Scholar]
- 55. Miao W, Wu X, Wang K, Wang W, Wang Y, Li Z, Liu J, Li L, Peng L. Sodium butyrate promotes reassembly of tight junctions in caco-2 monolayers involving inhibition of MLCK/MLC2 pathway and phosphorylation of PKCβ2. Int J Mol Sci. 2016;17:1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Delellis HK, Duan L, Sullivan-Halley J, Ma H, Clarke CA, Neuhausen SL, Templeman C, Bernstein L. Menopausal hormone therapy use and risk of invasive colon cancer: the California Teachers Study. Am J Epidemiol. 2010;171:415–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Williams C, DiLeo A, Niv Y, Gustafsson JÅ. Estrogen receptor beta as target for colorectal cancer prevention. Cancer Lett. 2016;372:48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Langen ML, Hotte N, Dieleman LA, Albert E, Mulder C, Madsen KL. Estrogen receptor-β signaling modulates epithelial barrier function. Am J Physiol Gastrointest Liver Physiol. 2011;300:G621–6. [DOI] [PubMed] [Google Scholar]
- 59. Song CH, Kim N, Sohn SH, Lee SM, Nam RH, Na HY, Lee DH, Surh Y-J. Effects of 17β-estradiol on colonic permeability and inflammation in an azoxymethane/dextran sulfate sodium-induced colitis mouse model. Gut Liver. 2018;12:682–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tao Y, Yue M, Lv C, Yun X, Qiao S, Fang Y, Wei Z, Xia Y, Dai Y. Pharmacological activation of ERβ by arctigenin maintains the integrity of intestinal epithelial barrier in inflammatory bowel diseases. FASEB J. 2020;34:3069–90. [DOI] [PubMed] [Google Scholar]
- 61. Braniste V, Leveque M, Buisson-Brenac C, Bueno L, Fioramonti J, Houdeau E. Oestradiol decreases colonic permeability through oestrogen receptor beta-mediated up-regulation of occludin and junctional adhesion molecule-A in epithelial cells. J Physiol. 2009;587(13):3317–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Collins FL, Rios-Arce ND, Atkinson S, Bierhalter H, Schoenherr D, Bazil JN, McCabe LR, Narayanan Parameswaran N. Temporal and regional intestinal changes in permeability, tight junction, and cytokine gene expression following ovariectomy-induced estrogen deficiency. Physiol Rep. 2017;5:e13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. McCarty MF. Isoflavones made simple – genistein's agonist activity for the beta-type estrogen receptor mediates their health benefits. Med Hypotheses. 2006;66:1093–114. [DOI] [PubMed] [Google Scholar]
- 64. Nishimura Y, Mabuchi K, Takano A, Hara Y, Negishi H, Morimoto K, Ueno T, Uchiyama S, Takamata A. S-equol exerts estradiol-like anorectic action with minimal stimulation of estrogen receptor-α in ovariectomized rats. Front Endocrinol. 2017;8:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Muthyala RS, Ju YH, Sheng S, Williams LD, Doerge DR, Katzenellenbogen BS, Helferich WG, Katzenellenbogen JA. Equol, a natural estrogenic metabolite from soy isoflavones: convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg Med Chem. 2004;12:1559–67. [DOI] [PubMed] [Google Scholar]
- 66. Jackson RL, Greiwe JS, Schwen RJ. Emerging evidence of the health benefits of S-equol, an estrogen receptor β agonist. Nutr Rev. 2011;69:432–48. [DOI] [PubMed] [Google Scholar]
- 67. Al-Nakkash L, Kubinski A. Soy isoflavones and gastrointestinal health. Curr Nutr Rep. 2020;9:193–201. [DOI] [PubMed] [Google Scholar]
- 68. Wang B, Wu C. Dietary soy isoflavones alleviate dextran sulfate sodium-induced inflammation and oxidative stress in mice. Exp Ther Med. 2017;14:276–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Samak G, Chaudhry KK, Gangwar R, Narayanan D, Jaggar JH, Rao R. Calcium/Ask1/MKK7/JNK2/c-Src signalling cascade mediates disruption of intestinal epithelial tight junctions by dextran sulfate sodium. Biochem J. 2015;465:503–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Naydenov NG, Hopkins AM, Ivanov AI. c-Jun N-terminal kinase mediates disassembly of apical junctions in model intestinal epithelia. Cell Cycle. 2009;8:2110–21. [DOI] [PubMed] [Google Scholar]
- 71. Samak G, Suzuki T, Bhargava A, Rao RK. c-Jun NH2-terminal kinase-2 mediates osmotic stress-induced tight junction disruption in the intestinal epithelium. Am J Physiol Gastrointest Liver Physiol. 2010;299:G572–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Xiao YT, Yan WH, Cao Y, Yan JK, Cai W. P38 MAPK pharmacological inhibitor SB203580 alleviates total parenteral nutrition-induced loss of intestinal barrier function but promotes hepatocyte lipoapoptosis. Cell Physiol Biochem. 2017;41:623–34. [DOI] [PubMed] [Google Scholar]
- 73. Costantini TW, Peterson CY, Kroll L, Loomis WH, Eliceiri BP, Baird A, Bansal V, Coimbra R. Role of p38 MAPK in burn-induced intestinal barrier breakdown. J Surg Res. 2009;156:64–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wang Q, Guo XL, Wells-Byrum D, Noel G, Pritts TA, Ogle CK. Cytokine-induced epithelial permeability changes are regulated by the activation of the p38 mitogen-activated protein kinase pathway in cultured Caco-2 cells. Shock. 2008;29:531–7. [DOI] [PubMed] [Google Scholar]
- 75. Zhang Y, Wu S, Ma J, Xia Y, Ai X, Sun J. Bacterial protein AvrA stabilizes intestinal epithelial tight junctions via blockage of the C-Jun N-terminal kinase pathway. Tissue Barriers. 2015;3:e972849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Samak G, Narayanan D, Jaggar JH, Rao R. CaV1.3 channels and intracellular calcium mediate osmotic stress-induced N-terminal c-Jun kinase activation and disruption of tight junctions in Caco-2 cell monolayers. J Biol Chem. 2011;286:30232–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Carrozzino F, Pugnale P, Féraille E, Montesano R. Inhibition of basal p38 or JNK activity enhances epithelial barrier function through differential modulation of claudin expression. Am J Physiol Cell Physiol. 2009;297:C775–87. [DOI] [PubMed] [Google Scholar]
- 78. Lemieux E, Boucher MJ, Mongrain S, Boudreau F, Asselin C, Rivard N. Constitutive activation of the MEK/ERK pathway inhibits intestinal epithelial cell differentiation. Am J Physiol Gastrointest Liver Physiol. 2011;301:G719–30. [DOI] [PubMed] [Google Scholar]
- 79. Zhai Z, Ni X, Jin C, Ren W, Li J, Deng J, Deng B, Yin Y. Cecropin A modulates tight junction-related protein expression and enhances the barrier function of porcine intestinal epithelial cells by suppressing the MEK/ERK pathway. Int J Mol Sci. 2018;19:1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Al-Sadi R, Guo S, Ye D, Dokladny K, Alhmoud T, Ereifej L, Said HM, Ma TY. Mechanism of IL-1 β modulation of intestinal epithelial barrier involves p38 kinase and activating transcription factor-2 activation. J Immunol. 2013;190:6596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Al-Sadi R, Guo S, Ye D, Ma TY. TNF-α modulation of intestinal epithelial tight junction barrier is regulated by ERK1/2 activation of Elk-1. Am J Pathol. 2013;183:1871–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ihara E, Yu Q, Chappellaz M, MacDonald JA. ERK and p38MAPK pathways regulate myosin light chain phosphatase and contribute to Ca2+ sensitization of intestinal smooth muscle contraction. Neurogastroenterol Motil. 2015;27:135–46. [DOI] [PubMed] [Google Scholar]
- 83. Elias BC, Suzuki T, Seth A, Giorgianni F, Kale G, Shen L, Turner JR, Naren A, Desiderio DM, Rao R. Phosphorylation of Tyr-398 and Tyr-402 in occludin prevents its interaction with ZO-1 and destabilizes its assembly at the tight junctions. J Biol Chem. 2009;284:1559–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Maeda T, Miyazono Y, Ito K, Hamada K, Sekine S, Horie T. Oxidative stress and enhanced paracellular permeability in the small intestine of methotrexate-treated rats. Cancer Chemother Pharmacol. 2010;65:1117–23. [DOI] [PubMed] [Google Scholar]
- 85. Li H, Wu Q, Xu L, Li X, Duan J, Zhan J, Feng J, Sun X, Chen H. Increased oxidative stress and disrupted small intestinal tight junctions in cigarette smoke-exposed rats. Mol Med Rep. 2015;11:4639–44. [DOI] [PubMed] [Google Scholar]
- 86. Wang N, Han Q, Wang G, Ma W-P, Wang J, Wu W-X, Guo Y, Liu L, Jiang X-Y, Xie X-Let al. . Resveratrol protects oxidative stress-induced intestinal epithelial barrier dysfunction by upregulating heme oxygenase-1 expression. Dig Dis Sci. 2016;61:2522–34. [DOI] [PubMed] [Google Scholar]
- 87. Perez S, Talens-Visconti R, Rius-Perez S, Finamor I, Sastre J. Redox signaling in the gastrointestinal tract. Free Radic Biol Med. 2017;104:75–103. [DOI] [PubMed] [Google Scholar]
- 88. Gangwar R, Meena AS, Shukla PK, Nagaraja AS, Dorniak PL, Pallikuth S, Waters CM, Sood A, Rao R. Calcium-mediated oxidative stress: a common mechanism in tight junction disruption by different types of cellular stress. Biochem J. 2017;474:731–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wu S, Pan L, Liao H, Yao W, Shen N, Chen C, Liu D, Ge M. High-fat diet increased NADPH-oxidase-related oxidative stress and aggravated LPS-induced intestine injury. Life Sci. 2020;253:117539. [DOI] [PubMed] [Google Scholar]
- 90. Stone JR. An assessment of proposed mechanisms for sensing hydrogen peroxide in mammalian systems. Arch Biochem Biophys. 2004;422:119–24. [DOI] [PubMed] [Google Scholar]
- 91. Foley TD, Armstrong JJ, Kupchak BR. Identification and H2O2 sensitivity of the major constitutive MAPK phosphatase from rat brain. Biochem Biophys Res Commun. 2004;315:568–74. [DOI] [PubMed] [Google Scholar]
- 92. Seth D, Rudolph J. Redox regulation of MAP kinase phosphatase 3. Biochemistry. 2006;45:8476–87. [DOI] [PubMed] [Google Scholar]
- 93. Hou N, Torii S, Saito N, Hosaka M, Takeuchi T. Reactive oxygen species-mediated pancreatic beta-cell death is regulated by interactions between stress-activated protein kinases, p38 and c-Jun N-terminal kinase, and mitogen-activated protein kinase phosphatases. Endocrinology. 2008;149:1654–65. [DOI] [PubMed] [Google Scholar]
- 94. Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Nadeau PJ, Charette SJ, Toledano MB, Landry J. Disulfide bond-mediated multimerization of Ask1 and its reduction by thioredoxin-1 regulate H2O2-induced c-Jun NH2-terminal kinase activation and apoptosis. Mol Biol Cell. 2007;18:3903–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Soga M, Matsuzawa A, Ichijo H. Oxidative stress-induced diseases via the ASK1 signaling pathway. Int J Cell Biol. 2012;2012:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ogier JM, Nayagam BA, Lockhart PJ. ASK1 inhibition: a therapeutic strategy with multi-system benefits. J Mol Med. 2020;98:335–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Li Q, Harraz MM, Zhou W, Zhang LN, Ding W, Zhang Y, Eggleston T, Yeaman C, Banfi B, Engelhardt JF. Nox2 and Rac1 regulate H2O2-dependent recruitment of TRAF6 to endosomal interleukin-1 receptor complexes. Mol Cell Biol. 2006;26:140–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Li Q, Spencer NY, Oakley FD, Buettner GR, Engelhardt JF. Endosomal Nox2 facilitates redox-dependent induction of NF-kappaB by TNF-alpha. Antioxid Redox Signal. 2009;11:1249–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Suzaki Y, Yoshizumi M, Kagami S, Koyama AH, Taketani Y, Houchi H, Tsuchiya K, Takeda E, Tamaki T. Hydrogen peroxide stimulates c-Src-mediated big mitogen-activated protein kinase 1 (BMK1) and the MEF2C signaling pathway in PC12 cells: potential role in cell survival following oxidative insults. J Biol Chem. 2002;277:9614–21. [DOI] [PubMed] [Google Scholar]
- 101. Catarzi S, Biagioni C, Giannoni E, Favilli F, Marcucci T, Iantomasi T, Vincenzini MT. Redox regulation of platelet-derived-growth-factor-receptor: role of NADPH-oxidase and c-Src tyrosine kinase. Biochim Biophys Acta Mol Cell Res. 2005;1745:166–75. [DOI] [PubMed] [Google Scholar]
- 102. Basuroy S, Dunagan M, Sheth P, Seth A, Rao RK. Hydrogen peroxide activates focal adhesion kinase and c-Src by a phosphatidylinositol 3 kinase-dependent mechanism and promotes cell migration in Caco-2 cell monolayers. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2010;299:G186–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Garcia-Redondo AB, Briones AM, Martinez-Revelles S, Palao T, Vila L, Alonso MJ, Salaices M. c-Src, ERK1/2 and Rho kinase mediate hydrogen peroxide-induced vascular contraction in hypertension: role of TXA2, NAD(P)H oxidase and mitochondria. J Hypertens. 2015;33:77–87. [DOI] [PubMed] [Google Scholar]
- 104. Lindquist RL, Bayat-Sarmadi J, Leben R, Niesner R, Hauser AE. NAD(P)H oxidase activity in the small intestine is predominantly found in enterocytes, not professional phagocytes. Int J Mol Sci. 2018;19:1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Tesoriere L, Attanzio A, Allegra M, Gentile C, Livrea MA. Indicaxanthin inhibits NADPH oxidase (NOX)-1 activation and NF-kB-dependent release of inflammatory mediators and prevents the increase of epithelial permeability in IL-1β-exposed Caco-2 cells. Br J Nutr. 2014;111:415–23. [DOI] [PubMed] [Google Scholar]
- 106. Wang Z, Litterio MC, Müller M, Vauzour D, Oteiza PI. (-)-Epicatechin and NADPH oxidase inhibitors prevent bile acid-induced Caco-2 monolayer permeabilization through ERK1/2 modulation. Redox Biol. 2020;28:101360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Lanone S, Bloc S, Foresti R, Almolki A, Taillé C, Callebert J, Conti M, Goven D, Aubier M, Dureuil Bet al. . Bilirubin decreases NOS2 expression via inhibition of NAD(P)H oxidase: implications for protection against endotoxic shock in rats. FASEB J. 2005;19:1890–2. [DOI] [PubMed] [Google Scholar]
- 108. Matsumoto H, Ishikawa K, Itabe H, Maruyama Y. Carbon monoxide and bilirubin from heme oxygenase-1 suppresses reactive oxygen species generation and plasminogen activator inhibitor-1 induction. Mol Cell Biochem. 2006;291:21–8. [DOI] [PubMed] [Google Scholar]
- 109. Jiang F, Roberts SJ, Datla S, Dusting GJ. NO modulates NADPH oxidase function via heme oxygenase-1 in human endothelial cells. Hypertension. 2006;48:950–7. [DOI] [PubMed] [Google Scholar]
- 110. Datla SR, Dusting GJ, Mori TA, Taylor CJ, Croft KD, Jiang F. Induction of heme oxygenase-1 in vivo suppresses NADPH oxidase derived oxidative stress. Hypertension. 2007;50:636–42. [DOI] [PubMed] [Google Scholar]
- 111. Luo M, Tian R, Lu N. Nitric oxide protected against NADPH oxidase-derived superoxide generation in vascular endothelium: critical role for heme oxygenase-1. Int J Biol Macromol. 2019;126:549–54. [DOI] [PubMed] [Google Scholar]
- 112. Zheng JD, He Y, Yu HY, Liu Y-L, Ge Y-X, Li X-T, Li X, Wang Y, Guo M-R, Qu Y-Let al. . Unconjugated bilirubin alleviates experimental ulcerative colitis by regulating intestinal barrier function and immune inflammation. World J Gastroenterol. 2019;25:1865–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zhou K, Jiang M, Liu Y, Qu Y, Shi G, Yang X, Qin X, Wang X. Effect of bile pigments on the compromised gut barrier function in a rat model of bile duct ligation. PLoS One. 2014;9:e98905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. McCarty MF. Clinical potential of Spirulina as a source of phycocyanobilin. J Med Food. 2007;10:566–70. [DOI] [PubMed] [Google Scholar]
- 115. Terry MJ, Maines MD, Lagarias JC. Inactivation of phytochrome- and phycobiliprotein-chromophore precursors by rat liver biliverdin reductase. J Biol Chem. 1993;268:26099–106. [PubMed] [Google Scholar]
- 116. Zheng J, Inoguchi T, Sasaki S, Maeda Y, McCarty MF, Fujii M, Ikeda N, Kobayashi K, Sonoda N, Takayanagi R. Phycocyanin and phycocyanobilin from Spirulina platensis protect against diabetic nephropathy by inhibiting oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2013;304:R110–20. [DOI] [PubMed] [Google Scholar]
- 117. Romay C, Gonzalez R, Ledon N, Remirez D, Rimbau V. C-phycocyanin: a biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Curr Protein Pept Sci. 2003;4:207–16. [DOI] [PubMed] [Google Scholar]
- 118. Liu Q, Huang Y, Zhang R, Cai T, Cai Y. Medical application of Spirulina platensis derived C-phycocyanin. Evid Based Complement Alternat Med. 2016;2016:7803846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Wu Q, Liu L, Miron A, Klímová B, Wan D, Kuča K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: an overview. Arch Toxicol. 2016;90:1817–40. [DOI] [PubMed] [Google Scholar]
- 120. Yu T, Wang Y, Chen X, Xiong W, Tang Y, Lin L. Spirulina platensis alleviates chronic inflammation with modulation of gut microbiota and intestinal permeability in rats fed a high-fat diet. J Cell Mol Med. 2020;24:8603–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Ogborne RM, Rushworth SA, O'Connell MA. Alpha-lipoic acid-induced heme oxygenase-1 expression is mediated by nuclear factor erythroid 2-related factor 2 and p38 mitogen-activated protein kinase in human monocytic cells. Arterioscler Thromb Vasc Biol. 2005;25:2100–5. [DOI] [PubMed] [Google Scholar]
- 122. Koriyama Y, Nakayama Y, Matsugo S, Kato S. Protective effect of lipoic acid against oxidative stress is mediated by Keap1/Nrf2-dependent heme oxygenase-1 induction in the RGC-5 cell line. Brain Res. 2013;1499:145–57. [DOI] [PubMed] [Google Scholar]
- 123. Lin YC, Lai YS, Chou TC. The protective effect of alpha-lipoic acid in lipopolysaccharide-induced acute lung injury is mediated by heme oxygenase-1. Evid Based Complement Alternat Med. 2013;2013:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Ma ZC, Hong Q, Wang YG, Liang QD, Tan HL, Xiao CR, Tang XL, Shao S, Zhou SS, Gao Y. Ferulic acid induces heme oxygenase-1 via activation of ERK and Nrf2. Drug Discov Ther. 2011;5:299–305. [DOI] [PubMed] [Google Scholar]
- 125. Yu CL, Zhao XM, Niu YC. Ferulic acid protects against lead acetate-induced inhibition of neurite outgrowth by upregulating HO-1 in PC12 cells: involvement of ERK1/2-Nrf2 pathway. Mol Neurobiol. 2016;53:6489–500. [DOI] [PubMed] [Google Scholar]
- 126. Brigelius-Flohe R, Muller M, Lippmann D, Kipp AP. The yin and yang of nrf2-regulated selenoproteins in carcinogenesis. Int J Cell Biol. 2012;2012:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Wild AC, Moinova HR, Mulcahy RT. Regulation of gamma-glutamylcysteine synthetase subunit gene expression by the transcription factor Nrf2. J Biol Chem. 1999;274:33627–36. [DOI] [PubMed] [Google Scholar]
- 128. Sakurai A, Nishimoto M, Himeno S, Imura N, Tsujimoto M, Kunimoto M, Hara S. Transcriptional regulation of thioredoxin reductase 1 expression by cadmium in vascular endothelial cells: role of NF-E2-related factor-2. J Cell Physiol. 2005;203:529–37. [DOI] [PubMed] [Google Scholar]
- 129. McCarty MF, DiNicolantonio JJ. An increased need for dietary cysteine in support of glutathione synthesis may underlie the increased risk for mortality associated with low protein intake in the elderly. Age (Dordr.). 2015;37:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Trivedi PP, Jena GB. Role of α-lipoic acid in dextran sulfate sodium-induced ulcerative colitis in mice: studies on inflammation, oxidative stress, DNA damage and fibrosis. Food Chem Toxicol. 2013;59:339–55. [DOI] [PubMed] [Google Scholar]
- 131. He S, Guo Y, Zhao J, Xu X, Song J, Wang N, Liu Q. Ferulic acid protects against heat stress-induced intestinal epithelial barrier dysfunction in IEC-6 cells via the PI3K/Akt-mediated Nrf2/HO-1 signaling pathway. Int J Hyperthermia. 2018;35:112–21. [DOI] [PubMed] [Google Scholar]
- 132. He S, Guo Y, Zhao J, Xu X, Wang N, Liu Q. Ferulic acid ameliorates lipopolysaccharide-induced barrier dysfunction via microRNA-200c-3p-mediated activation of PI3K/AKT pathway in Caco-2 cells. Front Pharmacol. 2020;11:376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Shukla PK, Gangwar R, Manda B, Meena AS, Yadav N, Szabo E, Balogh A, Lee SC, Tigyi G, Rao R. Rapid disruption of intestinal epithelial tight junction and barrier dysfunction by ionizing radiation in mouse colon in vivo: protection by N-acetyl-l-cysteine. Am J Physiol Gastrointest Liver Physiol. 2016;310:G705–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Lee SI, Kang KS. N-acetylcysteine modulates lipopolysaccharide-induced intestinal dysfunction. Sci Rep. 2019;9:1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Oliver SR, Phillips NA, Novosad VL, Bakos MP, Talbert EE, Clanton TL. Hyperthermia induces injury to the intestinal mucosa in the mouse: evidence for an oxidative stress mechanism. Am J Physiol Regul Integr Comp Physiol. 2012;302:R845–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Oz S, Okay E, Karadenizli A, Cekmen MB, Ozdogan HK. N-acetylcysteine improves intestinal barrier in partially hepatectomized rats. ANZ J Surg. 2007;77:173–6. [DOI] [PubMed] [Google Scholar]
- 137. Ren Z, Zhang R, Li Y, Li Y, Yang Z, Yang H. Ferulic acid exerts neuroprotective effects against cerebral ischemia/reperfusion-induced injury via antioxidant and anti-apoptotic mechanisms in vitro and in vivo. Int J Mol Med. 2017;40:1444–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. McCarty MF, Assanga SBI. Ferulic acid may target MyD88-mediated pro-inflammatory signaling – implications for the health protection afforded by whole grains, anthocyanins, and coffee. Med Hypotheses. 2018;118:114–20. [DOI] [PubMed] [Google Scholar]
- 139. Ma X, Guo Z, Zhang Z, Li X, Liu Y, Zhao L, Wang X. Ferulic acid protects against porcine parvovirus infection-induced apoptosis by suppressing the nuclear factor-kB inflammasome axis and Toll-like receptor 4 via nonstructural protein 1. Evid Based Complement Altern Med. 2020;2020:39436721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Chatterjee S, Ghosal S, Chatterjee S. Glucagon-like peptide-1 receptor agonists favorably address all components of metabolic syndrome. World J Diabetes. 2016;7:441–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Honigberg MC, Chang LS, McGuire DK, Plutzky J, Aroda VR, Vaduganathan M. Use of glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes and cardiovascular disease: a review. JAMA Cardiol. [Internet]2020. doi:10.1001/jamacardio.2020.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Grill HJ. A role for GLP-1 in treating hyperphagia and obesity. Endocrinology. 2020; Aug 1;161(8):bqaa093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Dong D, Xie J, Wang J. Neuroprotective effects of brain-gut peptides: a potential therapy for Parkinson's disease. Neurosci Bull. 2019;35:1085–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Grieco M, Giorgi A, Gentile MC, d'Erme M, Morano S, Maras B, Filardi T. Glucagon-like peptide-1: a focus on neurodegenerative diseases. Front Neurosci. 2019;13:1112. [DOI] [PMC free article] [PubMed] [Google Scholar]