ABSTRACT
Oral ketone supplements have gained popularity in recent years. There is biological rationale for a potential ergogenic effect of this type of supplement, as they might not only alter muscle fuel preference during exercise (and promote glycogen sparing, with potential benefits for endurance performance) but also favor cognition performance during exertion or muscle glycogen synthesis after exercise. However, as discussed in this Perspective, evidence to date does not support a benefit of acute ketone supplementation on sports performance, cognition, or muscle recovery [although further research with long-duration exercise (i.e., >60 min), is needed], and the evidence for chronic supplementation is sparse. In addition, acute intake of ketone supplements might be associated with gastrointestinal symptoms, and further research is warranted on the long-term safety of repeated use of ketone supplements. In summary, there is currently insufficient evidence to support the overall effectiveness of ketone supplements in sports.
Keywords: endurance, sports nutrition, ergogenic, supplementation, ketogenic
Introduction
Myofiber glycogen is the main energetic substrate for muscle work during intense endurance exercise (e.g., in the last mountain ascent of a Tour de France stage). As such, depletion of muscle glycogen stores accelerates fatigue onset during such strenuous tasks (1) and carbohydrate loading has been traditionally viewed as the best nutritional strategy before and during endurance exercise, in order to preserve muscle glycogen deposits and thus to prevent premature fatigue (2). However, in recent years, ketone supplementation has been proposed as an alternative (or complementary) strategy that might also boost endurance sports performance, yet through different metabolic pathways (3). The purported ergogenic effect of ketone supplementation is attracting scientific (1, 4, 5) as well as mass media attention, with these supplements allegedly used by numerous elite endurance athletes, notably professional cyclists.
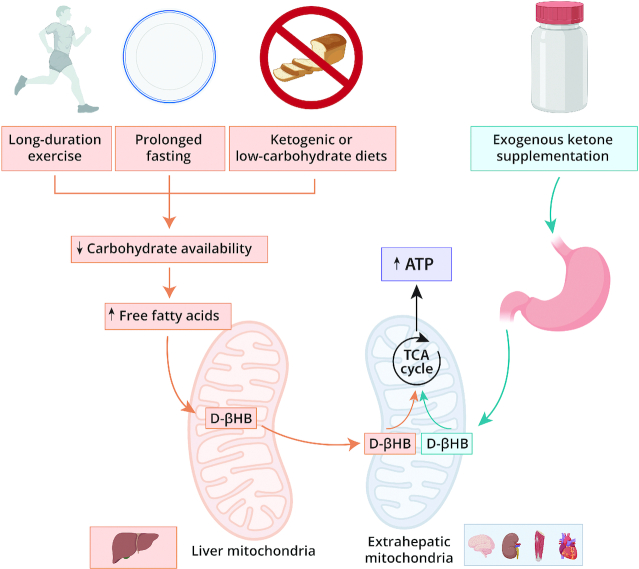
There is a biological rationale to support a potential ergogenic effect of ketone supplementation (Figure 1). Ketone bodies (acetoacetate, acetone), as well as β-hydroxybutyrate (βHB) synthesized from acetoacetate (all 3 herein considered as ketone bodies for the sake of simplicity), are produced from fatty acids by the liver mitochondria during periods of energy deficiency [prolonged fasting, ketogenic (low-carbohydrate) diets, long-duration exercise] to replace glucose as the primary energy substrate for extrahepatic tissues like the brain, heart, or skeletal muscle (1). Ketone bodies seem to inhibit glycolytic flux (6), and therefore oral acute ketone supplementation before or during exercise (which results in increased concentrations of ketone bodies in plasma) might represent an extra energy supply for muscle work, potentially sparing muscle glycogen stores (1, 4). In addition, ketone bodies would represent a more efficient energy substrate than glucose or fatty acids because the activation of the former into an oxidizable form (in a reaction catalyzed by succinyl-CoA:3-oxoacid CoA transferase) does not require ATP (7), thereby enabling generation of more muscle work for a given energy cost (5).
FIGURE 1.
Ketosis and ketones: effects on mitochondrial metabolism. D-βHB, D-β-hydroxybutyrate; TCA cycle, tricarboxylic acid cycle.
Despite the purported benefits of ketone body supplements (herein simply “ketone supplements”) on sports performance the actual evidence is controversial, and indeed, a recent systematic review concluded that the results provided across studies are mixed and equivocal (8). A recent meta-analysis by our research group concluded that acute (i.e., before or during an exercise bout) ketone supplementation seems to exert no consistent effects on exercise performance (9), although no information was provided regarding the effects on other important outcomes for sports such as cognitive performance or postexercise recovery. Moreover, the effects of chronic (i.e., repeated daily over a period of time) ketone supplementation were not assessed. Under this context, the present Perspective aims to summarize and discuss the evidence that is currently available on the physiological and exercise performance effects of both acute and chronic oral ketone supplementation on exercise performance in humans (as typically assessed in healthy, usually well-trained individuals) (Table 1). The potential effects on cognitive performance during or after fatiguing exercise and on postexercise muscle recovery are also discussed, as well as avenues of future research.
TABLE 1.
Summary of the human studies assessing the effects of ketone supplements on exercise performance, cognition during or after exertion, or postexercise recovery
| Study (reference) | Participants | Acute/chronic1 | Supplementation | Control intervention | Exercise | Main results (ketones vs. control) |
|---|---|---|---|---|---|---|
| Cox et al. (3)2 | 8 endurance athletes | Acute | Ketone ester (573 mg/kg body weight) and dextrose before exercise, under fasting conditions | Taste- and color-matched CHO drink | 1-h steady-state workload at 75% of Wmax followed by a 30-min TT | ↑ Performance;↑ plasma D-βHB,↓ FFA, glucose, and lactate during exercise |
| Dearlove et al. (10) | 12 trained participants | Acute | Ketone ester [330 mg/kg body weight of (R)-3-hydroxybutyrate (R)-1,3-butanediol] before exercise, under fasting conditions | Noncaloric drink (bitter-flavored water) | Incremental cycle test to exhaustion | ↔ Performance;↑ plasma D-βHB after exercise;↑ workload at OBLA;↓ plasma glucose and lactate during exercise |
| Evans et al. (21) | 19 trained cyclists | Acute | Ketone salts (380 mg/kg body weight) 60 and 15 min before exercise, under fasting conditions | Water | Incremental cycle exercise up to 80% of VO2peak | ↑ Plasma D-βHB;↓ glucose before and during exercise;no changes in indirect markers of performance |
| Evans and Egan (11) | 11 male team sport athletes | Acute | Ketone ester [750 mg/kg body weight of R-βHB (R)1,3-butanediol] and CHO before exercise, under fasting conditions | Taste-matched CHO solution | Loughborough intermittent shuttle test [5 × 15 min of intermittent activity followed by shuttle run to exhaustion (lasting ∼4 min)] | ↔ Performance;↑ plasma D-βHB;↓ plasma glucose and lactate during exercise;↑ cognition |
| Evans et al. (12) | 8 endurance-trained runners | Acute | Ketone ester (573 mg/kg body weight) and CHO before and during exercise, under fed conditions | Taste-matched CHO solution | 1 h of submaximal exercise [∼65% of VO2max) followed by a 10-km running TT (∼40 min)] | ↔ Performance;↑ plasma D-βHB during exercise;↔ cognition |
| Faull et al. (13) | 12 trained participants | Acute | Ketone ester [330 mg/kg body weight of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate] before exercise, under fasting conditions | Noncaloric drink (bitter-flavored water) | Incremental cycle test to exhaustion | ↔ Performance;↑ plasma D-βHB before exercise |
| Holdsworth et al. (22) | 12 well-trained male athletes | Acute | Ketone ester [0.573 mL/kg body weight of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate] under fasting conditions after a glycogen-depleting exercise protocol | Noncaloric drink with sweeteners and citrus flavoring | Intermittent exercise alternating 2-min intervals at 90% and 50% PPO; workload was decreased progressively by 10% PPO as fatigue appeared, until exhaustion (60% PPO) | ↑ Plasma D-βHB after exercise;↑ glucose uptake and endogenous insulin concentration;↑ muscle glycogen content |
| James and Kjerulf Greer (23) | 10 non–aerobically trained participants | Acute | 11,700 mg of ketone salts taken together with decarbonated, noncaffeinated diet cola before exercise after a fast ≥4 h | Decarbonated, noncaffeinated diet cola | Two 6-min stages of the Bruce protocol | ↔ Performance;↑ plasma D-βHB concentration before and after exercise |
| Leckey et al. (19) | 11 male internationally competitive cyclists | Acute | Ketone ester (250 mg/kg body weight of 1,3-butanediol acetoacetate diester) 30 min and immediately before exercise, under fed conditions | Viscosity- and color-matched drink with bitter flavor | 31-km TT on a cycling ergometer (∼50-min duration) | ↓ Performance;↓ plasma FFAs, glucose and lactate;↑ plasma acetoacetate and D-βHB during exercise |
| O'Malley et al. (20) | 10 healthy, recreationally active males | Acute | Ketone salts (300 mg/kg body weight) with lemon juice and stevia 30 min before exercise, under fasting conditions | Lemon juice and stevia | Steady-state exercise at 30%, 60%, and 90% of VT followed by a 150-kJ cyclingTT (∼11-min duration) | ↓ Performance;↑ D-βHB and total fat oxidation during exercise;↓ plasma glucose and total CHO oxidation during exercise |
| Poffé et al. (24) | 18 physically active males | Chronic | Ketone ester [25 g of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate] after each exercise session and 30 min presleep during an intensive 3-wk training program, provided along with a 500-mL high-dose protein-carbohydrate drink following exercise | Isocaloric bitter drink containing16.4 g pure medium-chain triglycerides, provided along with a 500-mL high-dose protein-CHO drink following exercise | 3 wk of exercise training that aimed at inducing functional overreaching (2 sessions/d, 6 d/wk) | ↑ Plasma D-βHB 30 min postexercise↑ training workload in week 3;↑ TT performance;↔ body composition;↓ markers of overreaching (e.g., plasma GDF15 concentrations, urinary catecholamine excretion, and heart rate decrease) |
| Poffé et al. (14) | 12 highly trained male cyclists | Acute | Ketone ester [65 g of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate] provided in 3 boluses ingested 60 and 20 min before exercise, and at minute 30 during the exercise protocol; supplement was provided under fed conditions and subjects ingested CHO before and during exercise (60 g/h) | Viscosity- and taste-matched supplement (with less caloric content than the ketone supplement); subjects ingested CHO before and during exercise | Simulated cycling race consisting of 3 h of submaximal intermittent cycling followed by a 15-min TT and an all-out sprint | ↔ Performance;↑ plasma and urine D-βHB;↓ plasma glucose following ketone ingestion;↓ FFAs during exercise;↔ plasma lactate;↓ blood pH and bicarbonate concentration during submaximal exercise;↔ muscle glycogen and intramyocellular triglyceride content and breakdown |
| Poffé et al. (25) | 9 well-trained male cyclists | Acute | Ketone ester [65 g of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate] provided in 3 boluses | Viscosity- and taste-matched supplement; subjects ingested CHO before and during exercise (60 g/h) | Simulated cycling race consisting of 3 h of submaximal intermittent cycling followed by a 15-min | ↑ TT performance (only when provided with bicarbonate);↔ sprint performance;↑ plasma and urine D-βHB; |
| ingested 60 and 20 min before exercise, and at minute 30 during the exercise protocol; supplement was provided under fed conditions and subjects ingested CHO before and during exercise (60 g/h); the ketone ester supplement was provided with and without bicarbonate (300 mg/kg) | TT and an all-out sprint | ↔ blood glucose and lactate;↓ blood pH and bicarbonate concentration, but prevented with bicarbonate | ||||
| Rodger et al. (15) | 12 highly trained male cyclists | Acute | Ketone salts (11,700 mg) with sugar-free lemonade 20 min before and during exercise, after a fast ≥2.5 h | Taste-matched placebo diluted in sugar-free lemonade | Submaximal cycling (80% VT2) for 90 min followed by a 4-min TT | ↔ Performance;↑ plasma D-βHB and RER during exercise |
| Scott et al. (16) | 11 male runners | Acute | Ketone ester (500 mg/kg body weight of 1,3-butanediol) and CHO, under fasting conditions75% was taken before submaximal exercise, and 25% before the TT | Caloric-matched CHO drink | 60 min of submaximal running (75% VO2max) followed by a 5-km running TT (∼21-min duration) | ↔ Performance;↑ plasma D-βHB;↓ lactate during exercise |
| Shaw et al. (17) | 9 trained male cyclists | Acute | Ketone ester (350 mg/kg body weight of 1,3-butanediol) with an orange-flavored drink 30 min before exercise and again 60 min during submaximal exercise, after a fast ≥4 h | Orange-flavored drink | 85 min of submaximal exercise (85% of VT2) followed by a 7-kJ/kg TT (∼29 min) | ↔ Performance;↑ plasma D-βHB after exercise |
| Vandoorne et al. (26) | 8 healthy trained males | Acute | Ketone ester [0.5 g/kg body weight of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate] immediately after a glycogen-depleting exercise protocol and 0.25 g/kg/h thereafter for 5 h; the supplement was provided in the fed state and along with a CHO/protein recovery drink | Isocaloric supplement of long-chain triglycerides that was similar in taste and appearance, provided along with the same recovery drink as in the experimental condition | Unilateral knee extensions at the highest power output possible for 5 min, 9 series of 30 extensions at 30%1RM, and 5 series of 6 extensions at 70%1RM | ↑ Plasma D-βHB;↓ plasma glucose;↔ plasma insulin;↑ mTORC1 activation (higher phosphorylation status ofS6K1 and 4EBP1);↓ AMPK activation;↔ glycogen resynthesis |
| Waldman et al. (18) | 15 healthy, recreationally active males | Acute | Ketone salts (11,380 mg of D-ßHB) 30 min before exercise, under fed conditions | Caloric-matched drink with similar taste and smell | Four 15-s cycling sprints | ↔ Performance;↑ plasma D-βHB after exercise;↔ cognition |
Note: for simplicity purposes, only the main results related to performance and to significant changes in physiological changes are shown. AMPK, AMP-activated protein kinase; CHO, carbohydrate; D-βHB, D-β-hydroxybutyrate; FFA, free fatty acid; GDF15, growth differentiation factor 15; mTOR1, mammalian target or rapamycin; OBLA, onset of blood lactate accumulation; PPO, peak power output; RER, respiratory exchange ratio; RPE, rating of perceived exertion; S6K1 (ribosomal protein S6 kinase β1, also known as p70S6 kinase); TT, time trial; VO2max, maximal oxygen uptake; VO2peak, peak oxygen uptake; VT, ventilatory threshold; VT2, second ventilatory threshold; Wmax, maximal power output; 1RM, 1 repetition maximum; 4EBP1, eukaryotic translation initiation factor 4E (eIF4E)-binding protein 1; ↑, increase; ↔, no change; ↓, decrease.
“acute” refers to supplementation before or during an acute exercise bout only, whereas “chronic” refers to repeated supplementation over days.
The study by Cox et al. included several experiments, but only the one reporting performance measures is shown in the table.
Current Status of Knowledge
Sports performance
A seminal study by Cox et al. (3) reported an improvement in simulated endurance bicycling performance with acute administration of ketone esters along with carbohydrates (dextrose) in trained athletes, as reflected by a slight (∼2%), albeit significant, increase in the total distance covered during a fixed time (i.e., 30 min) on a cycloergometer compared with the administration of carbohydrates alone. These results must be contextualized in the sports competition setting, with the percentage difference in total performance time between the 1st and the 10th rider in the general classification of the Tour de France usually <1%. The study by Cox et al. indeed contributed to the growing popularity of ketone supplements. However, subsequent studies have failed to replicate performance benefits with acute ketone supplementation (10, 11–13, 14, 15–17, 18) and some authors have in fact reported decrements in performance (19, 20). In addition, a recent meta-analysis by our group—including 13 studies in total—found that acute ketone supplementation has no effect on exercise performance (Hedges’ g = −0.05; 95% CI: −0.30, 0.20; P = 0.682) (9).
Numerous factors could potentially influence the effects of ketone supplements on performance—whether these are positive, neutral, or negative—notably the type and dose of the supplement ingested. However, studies using both ketone esters and salts at different dosages (e.g., 330 vs. 750 mg/kg of body weight) have failed to demonstrate beneficial effects on performance. Moreover, some studies have reported no benefits on sports performance despite acute supplementation resulting in similar [∼3 mmol/L; range: 2.6 to 5.2 mmol/L (14)] if not higher [3.7 mmol/L (10)] plasma ketone concentration than that observed by Cox et al. (∼2–3.5 mmol/L) (3).
The effects of acute ketone supplements might also vary depending on the test used for assessing sports performance. In this regard, different endurance exercise modalities have been studied, notably cycloergometer-simulated time trials (lasting 4–50 min) with performance assessed as maximal distance covered in a given time (3) or total time (or average power output) to complete a fixed distance (12, 19, 20, 15–17), incremental cycloergometer (10) or shuttle run tests to exhaustion (11), as well as protocols with a predominant anaerobic component, such as 15-m running sprints (11) or the Wingate anaerobic test (18). Yet, the Cox et al. study was the only one to report performance benefits. The studies that applied the more “ecologically valid” time trials found no beneficial effects after acute oral intake of ketone supplements, such as in the study by Leckey et al. (19), who found a detrimental performance effect (−2%) after acute ingestion of ketone esters in professional cyclists during a 31-km time trial that simulated a road-cycling world championship course. Moreover, we have found no meta-analytical evidence for an effect of acute ketone supplementation on time trial performance (9).
It must be noted, however, that most studies have assessed performance using tests with a duration <60 min. In this effect, although the glucose-sparing effect of ketone supplementation could be potentially beneficial for performance, ketones could also impair carbohydrate metabolism and consequently reduce performance during intense endurance exercise, which relies mostly on muscle glycolytic flux for rapid provision of energy. Indeed, muscle glycogen–derived ATP plays a key role in cellular functions whose failure might accelerate onset of fatigue, notably calcium handling by the sarcoplasmic reticulum (27). In fact, other strategies aiming at reducing reliance on muscle glycogen during exercise (e.g., low-carbohydrate, high-fat diets) have failed to provide clear benefits on competitive performance, and might reduce the capacity to sustain high-intensity exercise (28). Burke et al. (29) reported that a ketogenic diet increased muscle fat oxidation rate in elite race walkers but, on the other hand, impaired exercise economy and performance at race intensities. These results have been replicated in a different cohort of elite race walkers, with ketogenic diet impairing endurance performance in a 10,000-m test even if coupled with carbohydrate loading during the days before testing (30). McArdle's disease (glycogenosis type V) can provide mechanistic insight into the importance of muscle glycogen for intense endurance exercise performance. This condition is a myopathy caused by an inherited deficiency of the skeletal‐muscle isoform of glycogen phosphorylase, “myophosphorylase.” Because this enzyme catalyzes the breakdown of glycogen into glucose 1‐phosphate in muscle fibers, patients are unable to obtain energy from their muscle glycogen stores (31) and show very poor aerobic capacity (32) and extremely low muscle efficiency during endurance exercise (33).
In turn, it has been reported that muscle fat oxidation rate is higher in “keto-adapted” athletes than in those who consume a high-carbohydrate diet (34), with this adaptation being potentially beneficial for moderate-intensity exercise performance (e.g., ultra-endurance exercise). Indeed, greater fat oxidation rates during a simulated 100-km cycloergometer time trial as well as a higher power output during a subsequent critical power test were reported in endurance athletes who followed a 12-wk ketogenic diet compared with their peers who followed a high-carbohydrate diet (35). A positive association was recently found between the increase in plasma ketone concentrations during a multistage ultramarathon (240 km) and average running speed in the race (36), suggesting that acute ketone supplementation might be beneficial during long-duration low/moderate-intensity endurance exercise. Indeed, in the latter, reliance on muscle glycogenolysis for energy provision is expected to be lower than in shorter, more intense events. In this regard, a recent study in highly trained cyclists by Poffé et al. (14) assessed the effects of acute ketone ester supplementation on performance during both a 15-min time trial and a maximal sprint following 3 h of intermittent cycling, which aimed to replicate actual cycling racing. The authors found no benefits on performance indicators (14). Further research is needed to determine if acute ketone supplementation might exert some benefits on long-duration endurance exercise performance.
It must be noted that some authors supplied less carbohydrate along with the ketone supplement than in the control (non-ketone) supplement in order to match the energy intake of both acute interventions (60 vs. 110 g of carbohydrate for the ketone ester and control supplement, respectively) (16). It can be thus hypothesized that the lower availability of carbohydrates can potentially impair high-intensity performance. However, in the abovementioned study by Scott et al. (16), which did not find significant benefits with acute ketone supplementation, all participants ingested a relatively high carbohydrate intake (≥60 g/h) together with the ketone supplement, which would meet current recommendations for carbohydrate intake for an effort of such duration (<1 h) (37). Moreover, other studies that supplied the same amount of carbohydrates in both the ketone and control intervention also failed to report an ergogenic effect of acute ketone supplementation (11, 12, 19, 14).
It has also been hypothesized that acid-base disturbances after supplementation with ketone esters (as reflected by reductions in blood pH and bicarbonate concentration) might overshadow the ergogenic potential of exogenous ketosis (14). In this regard, a recent study reported that the ingestion of oral sodium bicarbonate along with a ketone ester supplement prevented the drop in blood pH, resulting in an improved time trial performance (by ∼5%) compared with the ingestion of placebo, bicarbonate, or ketone ester alone, respectively (25).
Metabolic effects
Controversy exists as to whether oral ketone supplements can produce a meaningful metabolic effect during exertion. The study by Cox et al. reported that, along with increased plasma concentrations of D-β-hydroxybutyrate (D-βHB; ∼2–3.5 mmol/L) and the abovementioned small but significant performance improvement (∼2%), ketone ester supplementation induced a shift in metabolic fuel preference during exercise, with reduced muscle carbohydrate oxidation and subsequent preservation of muscle glycogen stores as compared with the ingestion of a carbohydrate drink (3). It can, however, be discussed that when participants ingested the ketone ester supplement they consumed less carbohydrate. This, together with the fact that exercise was performed in the fasted state, could have likely contributed to a lower utilization of carbohydrates (2). Mixed evidence also exists for the effects of ketone supplements on plasma concentrations of ketone bodies, with mean plasma D-βHB concentrations reported to range between ∼0.3 (21) and 0.7 (23) mmol/L after ketone salt ingestion and between ∼0.3 (19) and 3.5 (3) mmol/L for ketone esters. Whether the fact that circulating ketone bodies do not consistently show a high increase after supplementation reflects that they are utilized by gut cells and/or rapidly oxidized by other tissues (e.g., brain) remains to be determined. In addition, the reported effects of ketone supplements on blood lactate concentration during exercise are not consistent, with some authors finding lower concentrations (3, 10, 11, 19, 16)—which would reflect a slower rate of muscle glycogen metabolism—but others finding no effects (21, 12, 20, 15, 17, 18). In the same line, some studies (3, 10, 11, 21, 19, 20, 23,) have found lower plasma glucose concentrations with ketone supplementation, but others (12, 15, 17, 18) have reported no differences compared with a control intervention. In addition, 1 study found a lower respiratory exchange ratio (RER) at light exercise intensities (thereby reflecting a lower rate of carbohydrate oxidation compared with fat oxidation) after acute supplementation with ketone salts, but not at high intensities (20), and others have reported no significant effects (3, 12, 16, 17) or even higher RER values (i.e., higher carbohydrate oxidation rate) after acute ketone supplementation (21, 23, 15). A recent study by Poffé et al. (14) found no differences between acute ketone ester supplementation and a placebo drink on muscle glycogen breakdown during 3 h of intermittent exercise or during a subsequent 15-min time trial, which suggests that this strategy had no glycogen-sparing effects.
Cognitive performance
Cognitive performance is of major relevance in some sports, particularly in those where the ability for fast decision making can influence performance (e.g., team, combat, or racquet sports). Glucose is the preferred energy source for the brain, but strenuous exercise can reduce bloodborne glucose, thereby contributing to the development of “central fatigue” (38). In situations of prolonged starvation, ketones can substitute glucose as the main energy source for the brain, thereby preventing cognitive impairment (39). Ketone bodies can cross the blood–brain barrier, stimulate acetylation of histones at the brain-derived neurotrophic factor (Bdnf) gene promoters, and induce the production of hippocampal BDNF, a neurotrophin that is crucial for brain plasticity and regulation of cognitive function (40–42). Under this context, although controversy exists on the actual association between acute BDNF increases and their immediate effects on cognitive performance (43, 44), it has been proposed that ketone supplements might reduce brain reliance on glucose, increase BDNF production, and thus improve cognitive performance (or attenuate cognitive impairment) during and after strenuous exercise.
Evidence on the effects of ketone supplements on cognitive function is, however, scarce and mixed. On one hand, preclinical data (not related to sports) show that nutritional ketosis (i.e., induced by a ketogenic diet) increases cognitive function in rats (45, 46), and indeed moderate nutritional ketosis has been recommended for people with cognitive impairment (e.g., Alzheimer disease) (47). Moreover, adoption of a ketogenic diet has been recently reported to increase brain ketone utilization and to stabilize brain networks in healthy younger adults, with these effects also corroborated after acute supplementation with an exogenous ketone ester (D-βHB) bolus (48). In the same line, some data suggest that ketone supplements might attenuate the transient impairment in cognitive performance that is frequently observed upon termination of strenuous exercise. For instance, Evans and Egan (11) reported that acute supplementation with a ketone ester reduced the number of incorrect responses during a cognitive test performed after an intermittent exercise protocol until exhaustion compared with a placebo supplement (0 vs. 1.8% of wrong responses, respectively). However, Evans et al. (12) also reported no benefits of acute ketone monoester supplementation on reaction time and a multitasking test assessed before and after a 10-km running trial, and Waldman et al. (18) observed no benefits of acute ketone salt supplementation on cognitive performance after high-intensity intermittent exercise. There is therefore insufficient evidence on the effectiveness of ketone supplements to improve cognitive performance in sports.
Recovery postexertion
Ketone supplementation has been proposed to expedite recovery after exercise, which is paramount in multistage events (e.g., Tour de France). Postexercise administration of ketones together with carbohydrates can increase the conversion rate of glucose to glycogen, thereby promoting muscle glycogen replenishment (1, 6). In addition, ketone bodies could potentially attenuate protein oxidation and thus favor muscle repair (1).
Incubation of mouse skeletal muscle with high doses of ketone bodies after 1 h of exercise activated and inhibited protein kinase B (Akt) and AMP-activated protein kinase (AMPK) pathways, respectively, thereby increasing glycogen repletion during the first 2 h following exercise (49). In humans, Holdsworth et al. (22) reported that acute supplementation with a ketone monoester drink along with a glucose clamp after a strenuous interval exercise session increased insulin concentrations, glucose uptake, and muscle glycogen synthesis in humans compared with the ingestion of a control drink and the same glucose clamp (i.e., under conditions of matched glucose availability). Other authors have reported no effects of acute oral ketone ester supplementation after strenuous exercise on muscle glycogen resynthesis in humans, although they found a higher and lower activation of anabolic (e.g., mammalian target of rapamycin complex-1) and catabolic (AMPK) pathways, respectively, in human muscle tissue along with increased levels of protein synthesis assessed in vitro in murine skeletal muscle myoblasts (26). A recent study found that, compared with no supplementation, chronic ketone ester supplementation (i.e., daily ingestion after exercise and before sleeping for 3 wk) prevented the development of overreaching during a strenuous endurance training program in physically active individuals—as reflected by lower nocturnal concentrations of plasma adrenaline and noradrenaline compared with the control group, as well as higher ability to reach high heart rate values during exercise (24). Moreover, supplementation with the ketone ester allowed for a higher training workload to be tolerated during the third week, tended to prevent the depletion of muscle glycogen (albeit statistical significance was not reached), and elicited greater improvements in time trial performance (24). However, some debate has been raised around these findings due to methodological and statistical concerns (no registration of the trial, potential selective reporting, high risk of type I error, or no information on individual responses) (50, 51). Evidence for the effectiveness of ketone supplements for the enhancement of postexercise recovery is, therefore, still scarce and inconclusive.
Safety
Some critics propose that the World Anti-Doping Agency (WADA) should prohibit ketone supplements, with 1 criterion for a substance to be banned by WADA being the possibility that it might represent an actual/potential health risk to the athlete. This question has not been answered in the case of ketone supplements. Different studies have shown a high incidence of gastrointestinal symptoms (e.g., nausea, abdominal cramps, diarrhea) with acute ketone supplementation (11, 12, 21, 19). Indeed, gut disturbances such as those reported by all participants in the study by Leckey et al. (19) (including one with prolonged vomiting and dizziness, and others with mild to severe symptoms such as nausea or reflux) have been proposed as a potential explanation for the absence of performance benefits with ketone supplements. Controversy exists, however, regarding the comparison of gastrointestinal symptoms during exercise with oral ketones versus carbohydrate drinks, and some studies have found minimal differences between both types of supplements (19–25, 16). Stubbs et al. (52) recently reported that, at rest, the former result in greater gastrointestinal symptoms, but this difference disappeared if the 2 types of supplements were consumed during exercise. The authors also reported that symptoms seemed to depend on the type and dose of ketone supplement. Indeed, salts would induce greater symptoms than esters (probably due to the coingestion with the former of the inorganic cations sodium and potassium, leading to a hyperosmolar gut lumen and a greater gut water retention) and symptoms seem to increase with higher doses. Further evidence is needed to confirm if acute ketone supplementation–associated gastrointestinal symptoms are comparable to those produced by carbohydrate drinks, as well as to determine if these symptoms might play a role in the lack of performance benefits, and if so, if they could be avoided by modulating the supplementation strategy—for instance, by reducing dosage or using a different type of ketone supplement.
One important caveat in the field is the lack of evidence on the long-term safety of ketone supplements. Physiological elevations in blood ketone concentrations have been potentially linked to a number of multisystemic benefits and, in fact, ketone supplementation has been proposed as a co-adjuvant treatment in several conditions including genetic myopathies or neurodegenerative disease (7). Nevertheless, above-normal concentrations such as those found in patients with uncontrolled diabetes, alcoholic ketoacidosis, or impairments in ketolysis pathways might be associated with higher oxidative stress and inflammation, thereby increasing the production of superoxide radicals and upregulating signaling pathways [e.g., mitogen-activated protein kinase (MAPK)/NF-κB] that induce the expression of adhesion molecules. This can, in turn, lead to the infiltration or transmigration of monocytes with subsequent tissue damage, particularly at the vascular and liver level (53). Moreover, the adaptation to ketosis induced by ketogenic diets is associated, particularly during the first week, with a variety of symptoms known together as “keto flu,” including headache, fatigue, nausea, dizziness, gastrointestinal discomfort, and decreased energy. Research should analyze whether repeated ketone supplement administration before exercise sessions could induce some of these symptoms (54).
Conclusions
There is a biological rationale to support a potential ergogenic effect of oral ketone supplementation, especially in its acute form. However, evidence to date shows no clear physiological or performance effects with acute supplementation, and the evidence for a potential benefit on cognition or postexercise recovery is scarce and mixed (Figure 2). Moreover, there is scarce evidence available on the effects of chronic supplementation, and more importantly, evidence is needed regarding the safety of the long-term use of ketone supplements. To date, there is not sufficient evidence to support the effectiveness of ketone supplementation in athletes.
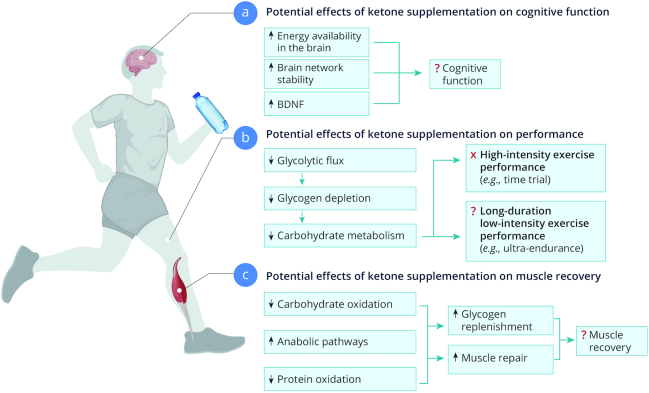
FIGURE 2.
Potential mechanisms by which acute ketone supplementation might influence cognitive function, sports performance, or muscle recovery, respectively. BDNF, brain-derived neurotrophic factor.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—PLV: conceived the original idea and drafted the initial version of the manuscript; and all authors: contributed to the final version of the manuscript and read and approved the final manuscript.
Notes
PLV is supported by a predoctoral contract granted by the University of Alcalá (FPI2016). AL is supported by grants from the Spanish Ministry of Economy and Competitiveness and Fondos Feder (PI18/00139).
Author disclosures: The authors report no conflicts of interest.
Perspective articles allow authors to take a position on a topic of current major importance or controversy in the field of nutrition. As such, these articles could include statements based on author opinions or point of view. Opinions expressed in Perspective articles are those of the author and are not attributable to the funder(s) or the sponsor(s) or the publisher, Editor, or Editorial Board of Advances in Nutrition. Individuals with different positions on the topic of a Perspective are invited to submit their comments in the form of a Perspectives article or in a Letter to the Editor.
Abbreviations used: AMPK, AMP-activated protein kinase; BDNF, brain-derived neurotrophic factor; D-βHB, D-β-hydroxybutyrate; RER, respiratory exchange ratio; WADA, World Anti-Doping Agency.
Contributor Information
Pedro L Valenzuela, Department of Systems Biology, University of Alcalá, Madrid, Spain.
Adrián Castillo-García, Fissac–Physiology, Health, and Physical Activity, Madrid, Spain.
Javier S Morales, Faculty of Sport Sciences, European University of Madrid, Madrid, Spain.
Alejandro Lucia, Faculty of Sport Sciences, European University of Madrid, Madrid, Spain.
References
- 1. Evans M, Cogan KE, Egan B. Metabolism of ketone bodies during exercise and training: physiological basis for exogenous supplementation. J Physiol. 2017;595:2857–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mata F, Valenzuela PL, Gimenez J, Tur C, Ferreria D, Domínguez R, Sanchez-Oliver AJ, Martínez Sanz JM. Carbohydrate availability and physical performance: physiological overview and practical recommendations. Nutrients. 2019;11:1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cox PJ, Kirk T, Ashmore T, Willerton K, Evans R, Smith A, Murray AJ, Stubbs B, West J, McLure SWet al. . Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Cell Metab. 2016;24:256–68. [DOI] [PubMed] [Google Scholar]
- 4. Pinckaers PJM, Churchward-Venne TA, Bailey D, van Loon LJC. Ketone bodies and exercise performance: the next magic bullet or merely hype?. Sport Med. 2017;47:383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Egan B, D'Agostino DP. Fueling performance: ketones enter the mix. Cell Metab. 2016;24:373–5. [DOI] [PubMed] [Google Scholar]
- 6. Maizels EZ, Ruderman NB, Goodman MN, Lau D. Effect of acetoacetate on glucose metabolism in the soleus and extensor digitorum longus muscles of the rat. Biochem J. 1977;162:557–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Puchalska P, Crawford PA.. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. 2017;25:262–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Margolis LM, O'Fallon KS.. Utility of ketone supplementation to enhance physical performance: a systematic review. Adv Nutr. 2019;11:412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Valenzuela PL, Morales JS, Castillo-García A, Lucia A. Acute ketone supplementation and exercise performance: a systematic review and meta-analysis of randomized controlled trials. Int J Sport Physiol Perform. In press. [DOI] [PubMed] [Google Scholar]
- 10. Dearlove DJ, Faull OK, Rolls E, Clarke K, Cox PJ. Nutritional ketoacidosis during incremental exercise in healthy athletes. Front Physiol. 2019;10:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Evans M, Egan B. Intermittent running and cognitive performance after ketone ester ingestion. Med Sci Sports Exerc. 2018;50:2330–8. [DOI] [PubMed] [Google Scholar]
- 12. Evans M, McSwiney FT, Brady AJ, Egan B. No benefit of ingestion of a ketone monoester supplement on 10-km running performance. Med Sci Sport Exerc. 2019;51:2506–15. [DOI] [PubMed] [Google Scholar]
- 13. Faull OK, Dearlove DJ, Clarke K, Cox PJ. Beyond RPE: the perception of exercise under normal and ketotic conditions. Front Physiol. 2019;10:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Poffé C, Ramaekers M, Bogaerts S, Hespel P. Exogenous ketosis impacts neither performance nor muscle glycogen breakdown in prolonged endurance exercise. J Appl Physiol. 2020;128:1643–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rodger S, Plews D, Laursen P, Driller M. Oral β-hydroxybutyrate salt fails to improve 4-minute cycling performance following submaximal exercise. J Sci Cycl. 2017;6:26–31. [Google Scholar]
- 16. Scott BE, Laursen PB, James LJ, Boxer B, Chandler Z, Lam E, Gascoyne T, Messenger J, Mears SA. The effect of 1,3-butanediol and carbohydrate supplementation on running performance. J Sci Med Sport. 2019;22:702–6. [DOI] [PubMed] [Google Scholar]
- 17. Shaw D, Merien F, Braakhuis A, Plews D, Laursen P, Dulson D. The effect of 1,3-butanediol on cycling time-trial performance. Int J Sport Nutr Exerc Metab. 2019;29:466–73. [DOI] [PubMed] [Google Scholar]
- 18. Waldman H, Basham S, Price F, Smith J, Chander H, Knight A, Krings B, McAllister M. Exogenous ketone salts do not improve cognitive responses after a high-intensity exercise protocol in healthy college-aged males. Appl Physiol Nutr Metab. 2018;43:711–7. [DOI] [PubMed] [Google Scholar]
- 19. Leckey JJ, Ross ML, Quod M, Hawley JA, Burke LM. Ketone diester ingestion impairs time-trial performance in professional cyclists. Front Physiol. 2017;8:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. O'Malley T, Myette-Cote E, Durrer C, Little JP. Nutritional ketone salts increase fat oxidation but impair high-intensity exercise performance in healthy adult males. Appl Physiol Nutr Metab. 2017;42:1031–5. [DOI] [PubMed] [Google Scholar]
- 21. Evans M, Patchett E, Nally R, Kearns R, Larney M, Egan B. Effect of acute ingestion of β-hydroxybutyrate salts on the response to graded exercise in trained cyclists. Eur J Sport Sci. 2018;18:376–86. [DOI] [PubMed] [Google Scholar]
- 22. Holdsworth DA, Cox PJ, Kirk T, Stradling H, Impey SG, Clarke K. A ketone ester drink increases postexercise muscle glycogen synthesis in humans. Med Sci Sports Exerc. 2017;49:1789–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. James S, Kjerulf Greer B. Influence of exogenous β-hydroxybutyrate on walking economy and rating of perceived exertion. J Diet Suppl. 2019;16:463–9. [DOI] [PubMed] [Google Scholar]
- 24. Poffé C, Ramaekers M, Van Thienen R, Hespel P. Ketone ester supplementation blunts overreaching symptoms during endurance training overload. J Physiol. 2019;597:3009–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Poffé C, Ramaekers M, Bogaerts S, Hespel P. Bicarbonate unlocks the ergogenic action of ketone monoester intake in endurance exercise. Med Sci Sports Exerc. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vandoorne T, De Smet S, Ramaekers M, Van Thienen R, De Bock K, Clarke K, Hespel P. Intake of a ketone ester drink during recovery from exercise promotes mTORC1 signaling but not glycogen resynthesis in human muscle. Front Physiol. 2017;8:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ørtenblad N, Westerblad H, Nielsen J. Muscle glycogen stores and fatigue. J Physiol. 2013;591:4405–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Burke LM. Re-examining high-fat diets for sports performance: did we call the “nail in the coffin” too soon?. Sport Med. 2015;45:S33–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Burke LM, Ross ML, Garvican-Lewis LA, Welvaert M, Heikura IA, Forbes SG, Mirtschin JG, Cato LE, Strobel N, Sharma APet al. . Low carbohydrate, high fat diet impairs exercise economy and negates the performance benefit from intensified training in elite race walkers. J Physiol. 2017;595:2785–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Burke LM, Sharma AP, Heikura IA, Forbes SF, Holloway M, McKay AKA, Bone J, Leckey JJ, Welvaert M, Ross MLR. Crisis of confidence averted: impairment of exercise economy and performance in elite race walkers by ketogenic low carbohydrate, high fat (LCHF) diet is reproducible. PLoS One. 2020;15:e0234027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lucia A, Nogales-Gadea G, Pérez M, Martín MA, Andreu AL, Arenas J. McArdle disease: what do neurologists need to know?. Nat Rev Neurol. 2008;4:568–77. [DOI] [PubMed] [Google Scholar]
- 32. Santalla A, Nogales-Gadea G, Encinar AB, Vieitez I, González-Quintana A, Serrano-Lorenzo P, Consuegra IG, Asensio S, Ballester-Lopez A, Pintos-Morell Get al. . Genotypic and phenotypic features of all Spanish patients with McArdle disease: a 2016 update. BMC Genomics. 2017;18:819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Maté-Muñoz JL, Moran M, Pérez M, Chamorro-Viña C, Gómez-Gallego F, Santiago C, Chicharro L, Foster C, Nogales-Gadea G, Rubio JCet al. . Favorable responses to acute and chronic exercise in McArdle patients. Clin J Sport Med. 2007;17:297–303. [DOI] [PubMed] [Google Scholar]
- 34. Volek JS, Freidenreich DJ, Saenz C, Kunces LJ, Creighton BC, Bartley JM, Davitt PM, Munoz CX, Anderson JM, Maresh CMet al. . Metabolic characteristics of keto-adapted ultra-endurance runners. Metabolism. 2016;65:100–10. [DOI] [PubMed] [Google Scholar]
- 35. McSwiney FT, Wardrop B, Hyde PN, Lafountain RA, Volek JS, Doyle L. Keto-adaptation enhances exercise performance and body composition responses to training in endurance athletes. Metabolism. 2018;81:25–34. [DOI] [PubMed] [Google Scholar]
- 36. Edwards KH, Elliott BT, Kitic CM. Carbohydrate intake and ketosis in self-sufficient multi-stage ultramarathon runners. J Sports Sci. 2020;38:366–74. [DOI] [PubMed] [Google Scholar]
- 37. Jeukendrup A. A step towards personalized sports nutrition: carbohydrate intake during exercise. Sports Med. 2014;44:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meeusen R, Roelands B. Fatigue: is it all neurochemistry? Eur J Sport Sci. 2018;18:37–46. [DOI] [PubMed] [Google Scholar]
- 39. Owen OE, Morgan AP, Kemp HG, Sullivan JM, Herrera MG, Cahill Jr GF. Brain metabolism during fasting. J Clin Invest. 1967;46:1589–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hu E, Du H, Zhu X, Wang L, Shang S, Wu X, Lu H, Lu X. Beta-hydroxybutyrate promotes the expression of BDNF in hippocampal neurons under adequate glucose supply. Neuroscience. 2018;386:315–25. [DOI] [PubMed] [Google Scholar]
- 41. Sleiman SF, Henry J, Al-Haddad R, El Hayek L, Haidar EA, Stringer T, Ulja D, Karuppagounder SS, Holson EB, Ratan RRet al. . Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β- hydroxybutyrate. Elife. 2016;5:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Marosi K, Kim SW, Moehl K, Scheibye-Knudsen M, Cheng A, Cutler R, Camandola S, Mattson MP. 3-Hydroxybutyrate regulates energy metabolism and induces BDNF expression in cerebral cortical neurons. J Neurochem. 2016;139:769–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Piepmeier AT, Etnier JL. Brain-derived neurotrophic factor (BDNF) as a potential mechanism of the effects of acute exercise on cognitive performance. J Sport Heal Sci. 2015;4:14–23. [Google Scholar]
- 44. Borror A. Brain-derived neurotrophic factor mediates cognitive improvements following acute exercise. Med Hypotheses. 2017;106:1–5. [DOI] [PubMed] [Google Scholar]
- 45. Murray AJ, Knight NS, Cole MA, Cochlin LE, Carter E, Tchabanenko K, Pichulik T, Gulston MK, Atherton HJ, Schroeder MAet al. . Novel ketone diet enhances physical and cognitive performance. FASEB J. 2016;30:4021–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hernandez AR, Hernandez CM, Campos K, Truckenbrod L, Federico Q, Moon B, McQuail JA, Maurer AP, Bizon JL, Burke SN. A ketogenic diet improves cognition and has biochemical effects in prefrontal cortex that are dissociable from hippocampus. Front Aging Neurosci. 2018;10:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cunnane SC, Courchesne-Loyer A, St-Pierre V, Vandenberghe C, Pierotti T, Fortier M, Croteau E, Castellano CA. Can ketones compensate for deteriorating brain glucose uptake during aging? Implications for the risk and treatment of Alzheimer's disease. Ann N Y Acad Sci. 2016;1367:12–20. [DOI] [PubMed] [Google Scholar]
- 48. Mujica-Parodi LR, Amgalan A, Sultan SF, Antal B, Sun X, Skiena S, Lithen A, Adra N, Ratai EM, Weistuch Cet al. . Diet modulates brain network stability, a biomarker for brain aging, in young adults. Proc Natl Acad Sci U S A. 2020;117:6170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Takahashi Y, Terada S, Banjo M, Seike K, Nakano S, Hatta H. Effects of β-hydroxybutyrate treatment on glycogen repletion and its related signaling cascades in epitrochlearis muscle during 120 min of post-exercise recovery. Appl Physiol Nutr Metab. 2019;44:1311–9. [DOI] [PubMed] [Google Scholar]
- 50. Korevaar DA, Cohen JF, McInnes MDF. Ketone ester supplementation in endurance athletes: a miracle drink or “spin”?. J Physiol. 2019;597:4407–8. [DOI] [PubMed] [Google Scholar]
- 51. Bellinger P. Does ketone ester supplementation really blunt overreaching symptoms during endurance training overload? J Physiol. 2019;597:5307–8. [DOI] [PubMed] [Google Scholar]
- 52. Stubbs B, Cox P, Kirk T, Evans R, Clarke K. Gastrointestinal effects of exogenous ketone drinks are infrequent, mild and vary according to ketone compound and dose. Int J Sport Nutr Exerc Metab. In press. [DOI] [PubMed] [Google Scholar]
- 53. Kanikarla-Marie P, Jain S.. Hyperketonemia and ketosis increase the risk of complications in type 1 diabetes. Free Radic Biol Med. 2016;95:268–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bostock ECS, Kirkby KC, Taylor BV, Hawrelak JA. Consumer reports of “keto flu” associated with the ketogenic diet. Front Nutr. 2020;7:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]