Abstract
Background
In ulcerative colitis (UC) patients who have achieved mucosal healing, active microscopic colonic mucosal inflammation is commonly observed. We aimed to assess the association between histological activity and disease relapse in endoscopically quiescent UC.
Methods
Ulcerative colitis patients with endoscopically quiescent disease and ≥12 months of follow-up were included. Biopsies were reviewed for the presence of basal plasmacytosis (BPC) and active histological inflammation, defined as a Geboes score (GS) ≥3.2. Primary outcome measures were disease relapse at 18 months and time to first relapse after index colonoscopy.
Results
Seventy-six UC patients (51% male; mean age, 38.6 years; median follow-up [range], 75.2 [2–118] months) were included. Sixty-two percent had an endoscopic Mayo score of 0 at index colonoscopy. Basal plasmacytosis was present in 46% and active histological inflammation in 30% of subjects. Presence of BPC was associated with a significantly shorter time to disease relapse (P = 0.01). Active histological inflammation was significantly associated with clinical relapse at 18 months (P = 0.0005) and shorter time to clinical relapse (P = 0.0006). Multivariate analysis demonstrated active histological inflammation to be independently associated with clinical relapse at 18 months and time to clinical relapse.
Conclusions
In endoscopically quiescent UC, active histological inflammation and the presence of BPC are adjunctive histological markers associated with increased likelihood of disease relapse. Although prospective studies are required, the presence of these histological markers should be a factor considered when making therapeutic decisions in UC.
Keywords: ulcerative colitis, clinical relapse, histology, basal plasmacytosis, Geboes score
In ulcerative colitis (UC) patients achieving mucosal healing, histological activity is commonly observed. We demonstrate an association between histological activity and clinical relapse in endoscopically quiescent disease. Histological activity provides valuable clinical information in patients with endoscopically quiescent UC.
BACKGROUND
Mucosal healing (MH) is considered an important end point in the treatment of inflammatory bowel disease (IBD). Mucosal healing is generally defined using the Mayo endoscopic subscore, which grades degree of mucosal inflammation based on an ascending 4-point scale (0–3).1 Mucosal healing has been defined as a Mayo endoscopic subscore of 0 to 1. Clinical trials of therapies in patients with ulcerative colitis (UC) now routinely include endoscopically assessed MH as an end point, and expert consensus recommends MH as an important treatment target in clinical practice.2 Mucosal healing in UC is associated with improved clinical outcomes, including a reduced risk for hospitalization and need for colectomy.3
Despite improved therapeutic options for UC and a focus on achieving objective treatment targets such as MH, a significant proportion of patients with UC relapse. Although the achievement of MH is associated with a reduced risk of disease relapse, patients without significant macroscopic mucosal inflammation are known to also subsequently experience disease exacerbations.3 In patients who have achieved MH, active microscopic colonic mucosal inflammation is commonly observed. A meta-analysis demonstrated the persistence of microscopic inflammation in 16%–100% of individuals with endoscopically quiescent disease, suggesting that endoscopic assessment of the mucosa alone may not fully characterize inflammatory burden in UC.4 Although histological activity in endoscopically quiescent disease is common, data remain sparse on the clinical significance of microscopic inflammation in the setting of endoscopically quiescent disease. Furthermore, the predictive role of histological activity on clinical outcomes in UC remains incompletely assessed.
We aimed to determine the association between histological markers and disease relapse in UC patients with endoscopically quiescent disease. We also aimed to assess the association between histological markers and other important clinical outcomes in UC, including corticosteroid and biological exposure, hospitalization, and colectomy.
METHODS
Study Design and Definitions
A retrospective cohort study was conducted. Patients with UC attending Mount Sinai Hospital, Toronto, Canada (MSH), between 2000 and 2014 were identified from an institutional database. Only individuals over the age of 18 with a confirmed diagnosis of UC based on accepted endoscopic, radiologic, and histologic criteria were considered eligible.5 Patients were included in the study if they were in clinical remission for at least 1 month, had endoscopically quiescent disease on their index study colonoscopy, and had a follow-up period of at least 12 months after this colonoscopy. The index study colonoscopy was defined as the first colonoscopy performed after UC diagnosis or the first colonoscopy performed at MSH after transfer of patient care to MSH from another institution. Endoscopically quiescent disease was defined as a UC endoscopic Mayo subscore of 0 or 1. Exclusion criteria were a diagnosis of IBD type unclassified, previous surgical resection, incomplete baseline colonoscopy, and prolonged disease remission >10 years.6 The local ethics committee of the hospital approved the study.
Study Population
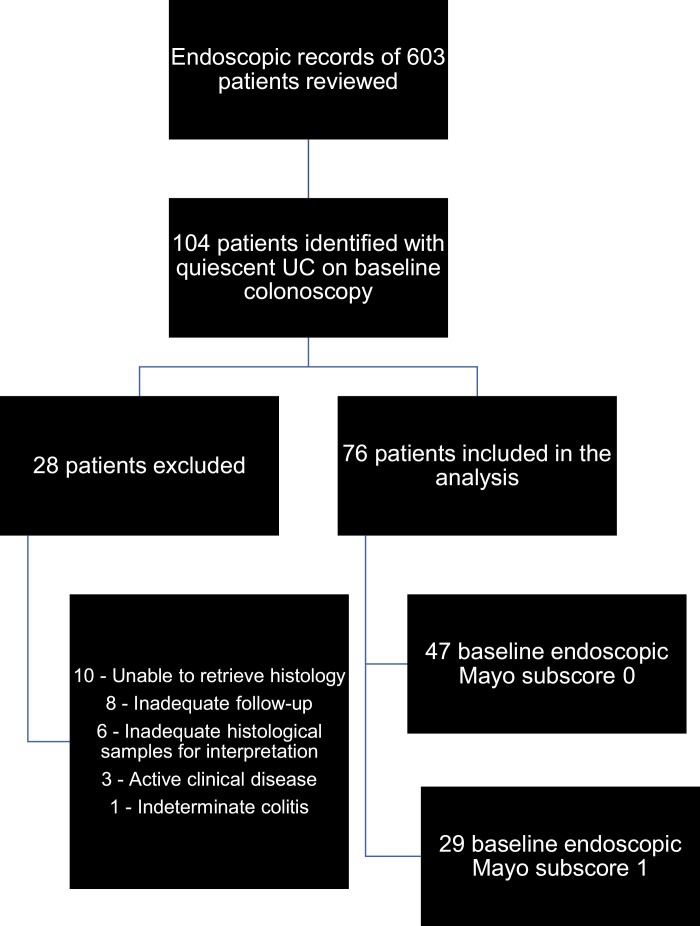
The endoscopic records of 603 patients with UC were reviewed for study eligibility. One hundred four patients were identified with endoscopically quiescent disease on their index study colonoscopy. Of these 104 patients, 28 were excluded, as original histological specimens could not be retrieved (n = 10), there was inadequate follow-up (n = 8), there were inadequate histological specimens for interpretation (n = 6), there was clinically active disease (n = 3), or they were classified on review as IBD type unclassified (n = 1). After, exclusions a final study cohort of 76 patients was available for analysis (Fig. 1).
FIGURE 1.
Flow diagram of final study population (n = 76).
Clinical, Laboratory, and Endoscopic Parameters Assessed
All demographic, clinical, and laboratory variables were extracted from a combination of electronic and paper medical records. Demographic and clinical variables collected included age, sex, age at UC diagnosis, months in clinical remission, smoking status, history of appendectomy, disease extent at baseline, and number and location of colonic biopsies taken at index study colonoscopy. Medical therapy at the time of index study colonoscopy was determined. Laboratory variables at the time of index study colonoscopy were collected, including hemoglobin, white blood cell count, platelet count, and C-reactive protein (CRP). Index study colonoscopy reports were reviewed, and endoscopic activity was assessed using the endoscopic Mayo subscore.1 An endoscopic Mayo subscore of 0 to 3 was assigned (0: normal; 1: erythema, decreased vascular pattern, mild friability; 2: marked erythema, absent vascular pattern, friability, erosion; 3: spontaneous bleeding, ulcerations). If the endoscopic Mayo subscore was not explicitly stated or apparent on review of the report, then a second reviewer independently assessed the report. If a consensus was not reached, then a third reviewer was used to arrive at a consensus score.
Histological Assessments
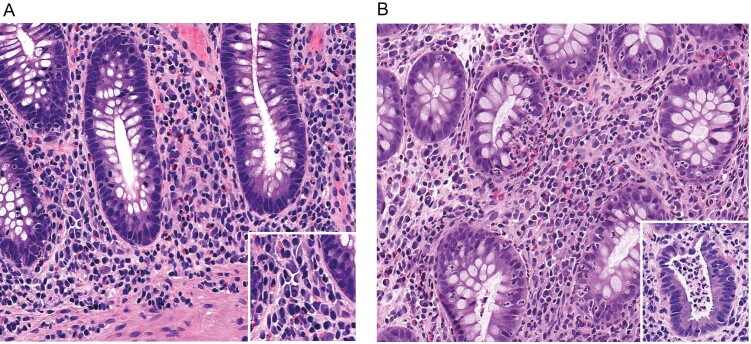
A gastrointestinal pathologist with expertise in IBD, blinded to clinical information, assessed colonic biopsy samples taken at the index study colonoscopy. Histological disease activity was characterised utilizing the Geboes score (GS), which has been described previously.7 All colonic biopsies were graded for inflammatory activity, and the biopsy with the highest degree of activity was used to calculate the GS. A number of previous reports have utilized a GS threshold of 3.1 to define active histological inflammation.8, 9 A GS of 3.1 represents active inflammation involving <5% of crypts, which may not be clinically significant. We hypothesized that a higher GS score threshold would have a better discriminatory value for clinical relapse and therefore defined active histological inflammation as a GS of ≥3.2. The presence of basal plasmacytosis (BPC) was also documented. Basal plasmacytosis was defined as dense infiltrate of plasma cells around the deep part of the lamina propria or at the base of the crypts, as described in a previous study (Fig. 2). A localized infiltrate was indicative of focal basal plasmacytosis, whereas a multifocal distribution represented a diffuse pattern. For the purposes of analyses, focal and diffuse basal plasmacytosis were grouped together.
FIGURE 2.
Representative images of basal plasmacytosis (A) and active histological inflammation (B). The inset in A is a higher-power image of plasma cells in the lamina propria adjacent to a crypt base. In B, the main image shows cryptitis (neutrophil infiltration of crypt epithelium), and the inset shows a crypt abscess.
Study End Points
The primary study end points were clinical relapse rate at 18 months and time to clinical relapse. Clinical relapse after index study colonoscopy was defined as the occurrence of UC-related symptoms with a partial Mayo score of ≥3; or where, in the opinion of the treating physician, a clinical relapse had occurred and a therapy escalation was required. Secondary end points included time to first corticosteroid or biological prescription, UC-related hospitalization, and colectomy.
Statistical Analysis
Baseline demographic and clinical data were presented as mean and standard deviation for continuous variables and frequencies and percentages for categorical variables. An a priori subgroup analysis was performed to assess differential rates of clinical relapse between subgroups, with endoscopic Mayo subscores of 0 and 1 on index study colonoscopy. The Kruskal-Wallis test was used to compare continuous variables between individuals by relapse status at 18 months. The Fisher exact test was used to compare frequency data. The log-rank test was used to compare individual variables and time to clinical relapse. Kaplan-Meier survival functions were also generated. The combined effects of individual predictors on relapse at 18 months and time to relapse were assessed with multivariate models. Logistic regression was used for the multivariate model with a binary outcome for relapse at 18 months and Cox regression with the Breslow method for ties when the outcome of interest was time to clinical relapse during follow-up. Due to the high correlation between BPC and active histological inflammation, the most significant of these variables in the univariate models was included in subsequent multivariate models. In addition, sex, age, and exposure to biologic therapy were also included in multivariate models. False discovery rate–corrected P values are reported, with a P value <0.05 considered significant.
RESULTS
Baseline Characteristics
The baseline characteristics of the cohort are described in Table 1. Study participant median (range) age was 38.0 (18–65) years, 51% were male, and the median duration of remission before study entry was 24.8 (2–118) months. Nine percent, 61%, and 32% of subjects had proctitis, left-sided colitis, and extensive colitis, respectively. Sixty-two percent and 38% of subjects, respectively, had endoscopic Mayo scores of 0 and 1 at index study colonoscopy. The mean number of endoscopic biopsies taken at index study colonoscopy was 13.8. The location of the biopsies with the highest degree of inflammatory activity (used to generate GS) was rectal in 49% (36/74), sigmoid in 24% (18/74), left colonic in 15% (11/74), and proximal colonic in 12% (9/74) of subjects, respectively. Two included subjects did not have the colonic location of their biopsies documented. Medication use at the time of index colonoscopy is described in Table 1. At the time of index colonoscopy, 30% of patients had active histological inflammation, whereas BPC was present in 46% of patients.
TABLE 1.
Baseline Characteristics of Study Cohort (n = 76)
| Age, median (range), y | 38.5 (18–65) |
| Duration of remission, median (range), mo | 24.8 (2–118) |
| Sex (male), % | 51 |
| Disease extent, % | |
| Proctitis | 9 |
| Left-sided colitis | 61 |
| Extensive colitis | 32 |
| Oral 5-ASA, % | 67 |
| Topical 5-ASA, % | 20 |
| Immunomodulator, % | 28 |
| Biologics, % | 12 |
| Index colonoscopy endoscopic Mayo subscore 0, % | 62 |
| Index colonoscopy endoscopic Mayo subscore 1, % | 38 |
| Active histological inflammation, % | 30 |
| Basal plasmacytosis, % | 46 |
Association Between Clinical and Histological Variables and Clinical Relapse at 18 Months
Twenty-five patients (33%) experienced disease relapse by 18-month follow-up. A univariate analysis was performed to evaluate variables associated with clinical relapse at 18 months (Table 2). A shorter duration of remission before study entry (P = 0.03) and female sex (P = 0.03) were associated with a significantly higher rate of clinical relapse at 18 months. Neither colitis extent nor maintenance medication at the time of index study colonoscopy was found to be significantly associated with disease relapse at 18 months. There was no difference in clinical relapse rates at 18 months comparing individuals with an endoscopic Mayo subscore of 0 vs 1 on index study colonoscopy (P = 0.62). There was a nonsignificant trend toward an association between the presence of BPC and clinical relapse at 18 months, with BPC present in 64% of individuals with clinical relapse compared with 37% of individuals who did not relapse (P = 0.07). There was a significant association between active histological inflammation and clinical relapse at 18 months, with 60% compared with 16% of individuals with and without active histological inflammation, respectively, experiencing clinical relapse at 18 months (P = 0.0005) (Table 2). Multivariate analysis demonstrated active histological inflammation and female sex to be independently associated with clinical relapse at 18 months (odds ratio [OR], 8.29; 95% confidence interval [CI], 2.49–27.61; P = 0.001; and OR, 3.65; 95% CI, 1.11–11.91; P = 0.032, respectively) (Table 3).
TABLE 2.
Univariate Analysis of Variables Associated With Clinical Relapse at 18 Months
| No Relapse (n = 51) | Relapse (n = 25) | P | |
|---|---|---|---|
| Age, median, y | 39 | 33 | 0.38 |
| Duration of remission, median, mo | 13 | 8 | 0.03 |
| Male, % | 61 | 32 | 0.03 |
| Proctitis, % | 10 | 8 | 1.00 |
| Left-sided colitis, % | 59 | 60 | |
| Extensive colitis, % | 31 | 32 | |
| No medication, % | 10 | 12 | 1.00 |
| Oral 5-ASA, % | 61 | 60 | 1.00 |
| Topical 5-ASA, % | 16 | 28 | 0.23 |
| Immunomodulator, % | 31 | 20 | 0.42 |
| Biologic, % | 12 | 12 | 1.00 |
| Endoscopic Mayo subscore 0, % | 65 | 56 | 0.62 |
| Endoscopic Mayo subscore 1, % | 35 | 44 | |
| Basal plasmacytosis present, % | 37 | 64 | 0.07 |
| Active histological inflammation, % | 16 | 60 | 0.0005 |
TABLE 3.
Multivariate Analysis of Variables Associated With Clinical Relapse at 18 Months
| Relapse at 18 mo | Relapse Over Length of Follow-up | |||
|---|---|---|---|---|
| OR (95% CI) | P | HR (95% CI) | P | |
| Active histological inflammation | 8.29 (2.49–27.61) | 0.001 | 2.8 (1.5–5.0) | 0.001 |
| Sex (female) | 3.65 (1.12–11.90) | 0.032 | 1.97 (1.12–3.48) | 0.019 |
| Duration of remission | 0.97 (0.95–1.00) | 0.070 | 0.98 (0.97–0.99) | 0.003 |
Association Between Clinical at Histological Variables and Time to Clinical Relapse
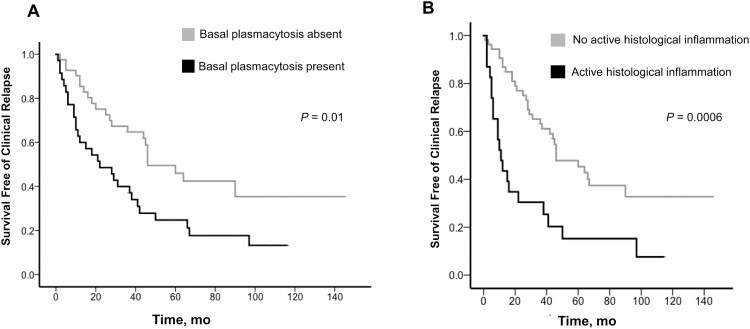
The average length of follow-up in the study cohort (range) was 75.2 (2–118) months. Over the length of follow-up, 67% of patients experienced disease relapse, at an average of 26.7 months from study entry. The presence of BPC was associated with a significantly shorter time to disease relapse (median time to relapse, 22.0 months; 95% CI, 5.7–38.2 months; vs median time to relapse, 46.0 months; 95% CI, 27.0–65.0 months; P = 0.01) (Fig. 3A). Active histological inflammation was associated with a shorter time to clinical relapse (median time to relapse, 11.0 months; 95% CI, 6.3–15.7 months; vs median time to relapse, 46.0 months; 95% CI, 26.1–65.9 months; P = 0.0006) (Fig. 3B). Multivariate analysis demonstrated active histological inflammation to be independently associated with time to clinical relapse (hazard ratio [HR], 2.8; 95% CI, 1.5–5.0; P = 0.001). Female sex (P = 0.019) and a shorter duration of remission before index colonoscopy (P = 0.003) were also independently associated with time to clinical relapse (Table 3).
FIGURE 3.
Association between histological markers and time to clinical relapse.
Association Between Histological Variables and Requirement for Corticosteroid Prescription, Biologic Therapy, Hospitalization, and Colectomy
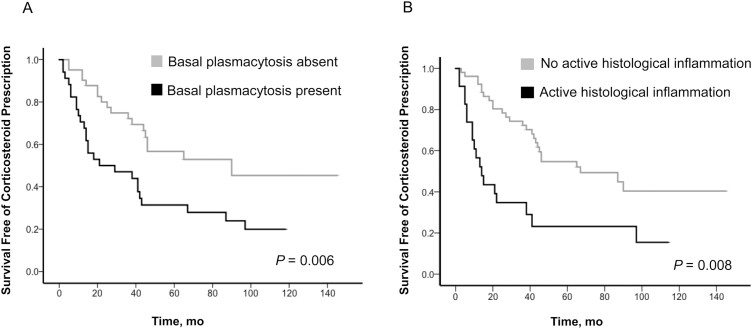
Over the length of follow-up after index study colonoscopy, 39 patients required corticosteroid prescription. Basal plasmacytosis on baseline histology was associated with a significantly shorter time to corticosteroid prescription (median time to corticosteroid prescription, 21.0 months; 95% CI, 0–53 months; vs median time to corticosteroid prescription, 90.0 months; 95% CI, not calculable for this variable; P = 0.006) (Fig. 4A). Active histological inflammation was significantly associated with a shorter time to corticosteroid prescription (median time to corticosteroid prescription, 14.0 months; 95% CI, 7.7–20.3 months; vs median time to corticosteroid prescription, 67.0 months; 95% CI, 24.9–109.1 months; P = 0.0008) (Fig. 4B). Seven patients required a biologic prescription (infliximab), 13 patients had a UC-related hospitalization, and 17 patients had a colectomy (12 for active inflammation, 3 for dysplasia, 2 for colorectal cancer). Neither the presence of BPC nor active histological inflammation was associated with requirement for a biologic prescription (P = 0.71), UC-related hospitalization (P = 0.85), or colectomy (P = 0.94) (Supplementary Table 1).
FIGURE 4.
Association between histological markers and time to corticosteroid prescription.
DISCUSSION
This report assessed the association between histological activity and clinical relapse in UC patients with clinically and endoscopically quiescent disease. We demonstrate, in concordance with previous studies, that a significant proportion of patients with endoscopically quiescent disease have active histological inflammation and evidence of basal plasmacytosis.4 We demonstrate the presence of BPC to be significantly associated with a shorter time to clinical relapse. We also show that active histological inflammation is significantly associated with clinical relapse at 18 months and a shorter time to clinical relapse. The findings from this study provide further evidence that, in endoscopically quiescent UC, histological markers provide valuable information that has clinical relevance and may guide therapeutic decision-making.
The histological findings in UC are characterized by a diffuse mucosal inflammatory infiltrate and evidence of previous crypt destruction, which may manifest with architectural distortion, crypt atrophy, and Paneth cell metaplasia. Inflammatory changes are generally continuous and maximal distally. Active disease manifests as the presence of cryptitis and crypt abscesses, which is reflected by neutrophils within the crypt epithelium and crypt lumen. Significant chronic inflammation in the lamina propria is often associated with the presence of BPC, which refers to the presence of a plasma cell infiltrate in the lower third of the lamina propria immediately above the muscularis mucosae and between crypt bases. Basal plasmacytosis may be observed in all forms of longstanding colitis and therefore has a high discriminating value for a diagnosis of IBD.4 Many indices to assess disease activity in UC have been described, though none are fully validated. The most widely used histological indices of disease activity in UC are the Riley Index and the GS. The Riley Index evaluates 6 features (acute inflammatory infiltrate, crypt abscesses, mucin depletion, epithelial integrity, chronic inflammatory infiltrate, and crypt architectural abnormalities).10 The Geboes Index includes 5 features (architectural change, lamina propria neutrophils and eosinophils, neutrophils in epithelium, crypt destruction, and erosion or ulceration).7 The GS is the best validated (interobserver variability κ, 0.59–0.70) and was therefore utilized in this study.7 A significant deficiency in current histological scoring systems is the lack of inclusion of BPC as a variable.
In patients with endoscopically quiescent UC, microscopic inflammation has been reported to persist in 16%–100% of patients. Persistent histological activity in the setting of MH is therefore common in clinical practice.4 The widely varying reported prevalence of persistent microscopic inflammation in UC patients with endoscopically quiescent disease relates to a number of factors, including the use of differing histological scoring systems in studies and the lack of a standardized definition for persistent microscopic inflammation. A number of studies have evaluated the association between histological disease activity and the outcome of endoscopically quiescent UC. Riley et al. demonstrated that that the presence of an acute inflammatory cell infiltrate in rectal biopsies was associated with increased risk of disease relapse.10 In a cohort of patients with clinically and endoscopically quiescent UC, Bitton et al. demonstrated that BPC, observed in rectal biopsies, was associated with a shorter time to disease relapse.11 Bessissow et al. evaluated the role of microscopic inflammatory activity in predicting disease relapse in UC patients with endoscopically inactive disease. The presence of BPC (P = 0.007) and a GS ≥3.1 (P = 0.007) were predictive of disease relapse.9 A report by Lobaton et al. evaluated asymptomatic UC patients in endoscopic remission (endoscopic Mayo score 0 or 1) undergoing surveillance colonoscopy and assessed the association between histological markers and disease outcome. Active histological inflammation (defined as a GS > 2.1 or >3.1) was demonstrated to be significantly associated with clinical relapse within 12 months. There was no association between BPC and clinical relapse demonstrated in this report.8 Ozaki et al. included patients with endoscopically quiescent UC (Endoscopic Mayo 0 or 1) in a study that evaluated the association between histological markers and disease relapse. Crypt architectural irregularities and mucin depletion were associated with time to disease relapse.12 Finally, a report by Calefat et al. examined patients with UC in clinical and endoscopic remission (endoscopic Mayo subscore ≤1) undergoing colonoscopy for dysplasia surveillance. Acute histological inflammatory activity, however (not BPC), was associated with clinical relapse within the first year of follow-up.13
In concordance with these previous reports, we demonstrated that a significant proportion of patients (33%) with endoscopically quiescent disease relapse over time. Supporting the previous observations of Bessisow et al., we demonstrated an association between the presence of BPC and a shorter time to clinical relapse. Active histological inflammation, defined as a GS ≥3.2, was even more strongly associated with disease relapse. Patients with active histological inflammation have significantly greater rates of clinical relapse at 18 months and a shorter time to clinical relapse. A novel aspect of this study was our assessment of the association between histological markers and other clinical markers of UC progression. The presence of BPC and active histological inflammation were associated with a shorter time to corticosteroid prescription in UC, but not with biologic prescription, UC-related hospitalization, or colectomy. Our study design provided for the use of the endoscopic biopsy with the highest degree of microscopic activity to generate the GS. In 88% of patients, the endoscopic biopsies with the highest degree of activity were located in the left colon. This finding suggests that a left-sided colonoscopy may be sufficient to assess for histological activity in the majority of patients with endoscopically quiescent disease.
We observed female sex and shorter duration of clinical remission before index colonoscopy to be associated with likelihood of clinical relapse. The association between female sex and risk of UC disease relapse has previously been reported.14 Although the interaction between female sex and disease activity is not well understood, estrogens may play a role, as they are known to have both suppressive and stimulatory effects on autoimmune diseases.15 Shorter duration of disease remission has also previously been demonstrated to be associated with risk of future disease relapse.11
This study has a number of strengths. The report is based on a cohort of well-characterized UC patients, and follow-up was available for a considerable period of time (mean follow-up, 75.2 months). The biopsies in the study were reviewed in a systematic fashion by an experienced gastrointestinal pathologist to characterize histological findings. The limitations of the study are inherent to its retrospective design. The study cohort was derived from a tertiary referral center specializing in IBD care, and therefore may not be entirely representative of the general UC population with endoscopically quiescent disease. Patients were receiving heterogenous medical therapeutic regimens; however, this reflects real-world clinical practice. We defined endoscopically quiescent disease as an endoscopic Mayo score of 0 or 1. It could be argued that patients with an endoscopic Mayo score of 1 do not have truly endoscopically quiescent disease. The definition we used, however, has been accepted as a definition for endoscopically quiescent disease in expert consensus reviews and previous UC randomized controlled trials.16, 17 In addition, in a sensitivity analysis, we found no difference in rates of clinical relapse comparing individuals with an endoscopic Mayo score of 0 and 1. Finally, our study design provided for the use of the endoscopic biopsy with the highest degree of microscopic activity to generate the GS for a given study subject. It may be that a more global assessment of histological activity, with assessment of GS from serial endoscopic biopsies and the generation a mean histology score for the entire colon, is a superior approach. This limitation of our study should be considered in future study designs evaluating this question.
It is well established that achieving mucosal healing in UC is associated with improved long-term outcomes.3 The concept of histological healing as a therapeutic target in UC is based on the hypothesis that persistent histological inflammation in UC is associated with earlier relapse and adverse natural history of the disease, with increased risk of hospitalization, colectomy, and colorectal cancer.4 This study, along with previous reports, provides support for this concept, demonstrating that histological activity is associated with increased risk of disease relapse. Further study is required to determine whether titration of therapy to achieve histological healing will prolong remission and prevent disease-related complications.18 Another area where histology markers may be a valuable tool for clinicians in clinical practice is the identification of patients with clinically and endoscopically quiescent disease, in whom therapy de-escalation could be considered.
In conclusion, this report demonstrates that patients with endoscopically quiescent disease frequently relapse, highlighting the importance of additional markers of disease activity in this subgroup of UC patients. In concordance with previous reports, we demonstrated an association between histological activity and disease relapse in patients who have achieved mucosal healing. These findings highlight that histological markers have utility in the assessment of UC disease activity and can contribute to clinical decision-making, particularly in patients with endoscopically quiescent disease. Although further evaluation in prospective studies is required, histological activity is a marker that provides additional valuable information when making therapeutic decisions in patients with endoscopically quiescent UC.
Supplementary Material
Supported by: D.K. is a recipient of a Canadian Institutes of Health Research (CIHR)/Canadian Association of Gastroenterology (CAG)/AbbVie IBD Fellowship. M.S.S. is supported by the Gale and Graham Wright Chair in Digestive Diseases at Mount Sinai Hospital and by grants from the Crohn’s and Colitis Canada (CCFC) and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; DK-062423).
Conflicts of interest: The authors have no conflicts to disclose.
Author contributions: D.K.: study concept and design, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis. R.K.: study concept and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, study supervision. C.D.: study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content. B.K.: analysis and interpretation of data, critical revision of the manuscript for important intellectual content. R.R.: study concept and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, study supervision. M.S.S.: study concept and design, acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, study supervision.
REFERENCES
- 1. Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625–1629. [DOI] [PubMed] [Google Scholar]
- 2. Annese V, Daperno M, Rutter MD, et al. ; European Crohn’s and Colitis Organisation . European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis. 2013;7:982–1018. [DOI] [PubMed] [Google Scholar]
- 3. Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110:1324–1338. [DOI] [PubMed] [Google Scholar]
- 4. Bryant RV, Winer S, Travis SP, et al. Systematic review: histological remission in inflammatory bowel disease. Is ‘complete’ remission the new treatment paradigm? An IOIBD initiative. J Crohns Colitis. 2014;8:1582–1597. [DOI] [PubMed] [Google Scholar]
- 5. Dignass A, Eliakim R, Magro F, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohns Colitis. 2012;6:965–990. [DOI] [PubMed] [Google Scholar]
- 6. Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005;19(Suppl A):5A–36A. [DOI] [PubMed] [Google Scholar]
- 7. Geboes K, Riddell R, Ost A, et al. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut. 2000;47:404–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lobatón T, Bessissow T, Ruiz-Cerulla A, et al. Prognostic value of histological activity in patients with ulcerative colitis in deep remission: a prospective multicenter study. United European Gastroenterol J. 2018;6:765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bessissow T, Lemmens B, Ferrante M, et al. Prognostic value of serologic and histologic markers on clinical relapse in ulcerative colitis patients with mucosal healing. Am J Gastroenterol. 2012;107:1684–1692. [DOI] [PubMed] [Google Scholar]
- 10. Riley SA, Mani V, Goodman MJ, et al. Microscopic activity in ulcerative colitis: what does it mean? Gut. 1991;32:174–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bitton A, Peppercorn MA, Antonioli DA, et al. Clinical, biological, and histologic parameters as predictors of relapse in ulcerative colitis. Gastroenterology. 2001;120:13–20. [DOI] [PubMed] [Google Scholar]
- 12. Ozaki R, Kobayashi T, Okabayashi S, et al. Histological risk factors to predict clinical relapse in ulcerative colitis with endoscopically normal mucosa. J Crohns Colitis. 2018;12:1288–1294. [DOI] [PubMed] [Google Scholar]
- 13. Calafat M, Lobatón T, Hernández-Gallego A, et al. Acute histological inflammatory activity is associated with clinical relapse in patients with ulcerative colitis in clinical and endoscopic remission. Dig Liver Dis. 2017;49:1327–1331. [DOI] [PubMed] [Google Scholar]
- 14. Höie O, Wolters F, Riis L, et al. ; European Collaborative Study Group of Inflammatory Bowel Disease (EC-IBD) . Ulcerative colitis: patient characteristics may predict 10-yr disease recurrence in a European-wide population-based cohort. Am J Gastroenterol. 2007;102:1692–1701. [DOI] [PubMed] [Google Scholar]
- 15. Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–574. [DOI] [PubMed] [Google Scholar]
- 16. Travis SP, Higgins PD, Orchard T, et al. Review article: defining remission in ulcerative colitis. Aliment Pharmacol Ther. 2011;34:113–124. [DOI] [PubMed] [Google Scholar]
- 17. Reinisch W, Sandborn WJ, Hommes DW, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut. 2011;60:780–787. [DOI] [PubMed] [Google Scholar]
- 18. Pai RK, Jairath V, Vande Casteele N, et al. The emerging role of histologic disease activity assessment in ulcerative colitis. Gastrointest Endosc. 2018;88:887–898. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.